Abstract
The current study seeks to compare the effects of prenatal methamphetamine exposure (PME) on infant and child physical growth between the United States (US) and New Zealand (NZ). This cross-national comparison provides a unique opportunity to examine the potential impact of services provided to drug using mothers on child health.
Methods
The longitudinal Infant Development, Environment and Lifestyle (IDEAL) study of PME from birth to 36 months was conducted in the US and NZ. The US cohort included 204 children with PME and 212 non-PME matched comparisons (NPME); the NZ cohort included 108 children with PME and 115 NPME matched comparisons. Latent growth curve models were used to examine effects of PME, country of origin, and the country × PME interaction on growth in length/height and weight.
Results
In regard to length/height, PME and country of origin were associated with initial length and growth over time. There was also a significant interaction effect, such that children with PME in the US were shorter at birth than children with PME in NZ after controlling for other prenatal exposures, infant set, socioeconomic status, and maternal height. In regard to weight, there was only an effect of country of origin.
Conclusions
Effects of PME on infant and child growth were shown to differ across countries, with exposed children in NZ faring better than exposed children in the US. Implications for prevention programs and public policy are discussed.
Keywords: prenatal methamphetamine exposure, length/height, weight, cross-national research
Estimates from the 2010 National Surveys on Drug Use and Health indicate 353,000 individuals in the United States (US) used methamphetamine in the past month (SAMHSA, 2011), making it a serious public health concern. A similar concern is observed in New Zealand (NZ) (Ministry of Health, 2007), where more than 17,500 adults ages 15 to 45 (∼1% of this population) have used methamphetamine in the past year and more than 155,000 (∼9%) have used amphetamines as some point in their life (Wilkins & Sweetsur, 2008). Although the US and NZ are both industrialized, English speaking countries with relatively similar governments (e.g., two predominant political parties, democratically elected representatives with term limits, independent judiciary), work and education opportunities, and lifestyle, they do not share the same philosophy around drug and alcohol use. NZ takes a harm reduction approach in contrast to a more punitive approach common in the US (Mathews & Kenny 2008)
Methamphetamine use has been associated with a host of negative consequences for users including damage to the dopaminergic and serotonergic regions of the brain (Berman et al., 2008; Chang, Alicata, Ernst, & Volkow, 2007), respiratory problems (Havel, 1997; Wijetunga, Seto, Lindsay, & Schatz, 2003), poorer cognitive functioning (Block, Erwin, & Ghoneim, 2002), and increased violence (Sommers, Baskin, & Baskin-Sommers, 2006). In addition to the risk to users, pregnant women represent a sub-population of particular importance due to emerging reports of effects of prenatal methamphetamine exposure (PME) on child development outcomes mainly from our Infant Development, Environment and Lifestyle Study (IDEAL) (e.g., Derauf et al., 2012; LaGasse et al., 2011, 2012; Lester & LaGasse, 2010), which is the only large longitudinal study of PME and child development taking place in the US and NZ.
Research in the U.S. indicated that roughly 19,000 pregnant women use methamphetamine annually (Colliver, Kroutil, Dai, & Gfroerer, 2006), and methamphetamine use accounts for 24% of pregnant woman admissions to federally funded substance abuse treatment centers in the U.S. (Terplan, Smith, Kozloski, & Pollack, 2009). Although national data regarding maternal methamphetamine use while pregnant is not available in NZ, similar problems have been observed in regional research (Wouldes, LaGasse, Sheridan, & Lester, 2004). Findings from National Women's Hospital indicate that more than half of referrals to the hospital's Alcohol Drug and Pregnancy Team were due to methamphetamine use.
Several imaging studies of mostly school age children reported the association of PME and abnormal brain morphology (Chang et al., 2004; Cloak et al., 2009; Sowell et al., 2010), altered brain metabolism (Chang et al., 2009; Smith et al., 2001), impaired child executive functioning (Chang et al., 2004; Lu et al., 2009). In IDEAL-US, PME has been related to childhood behavioral dysregulation (Abar et al., 2012; LaGasse et al., 2012). This finding was also found in a small study of amphetamine exposure in Sweden (Billing et al., 1994). Relevant to this paper, PME has been also associated with infant and child growth decrements (Smith et al., 2003; Zabaneh et al., 2012).
Smith and colleagues (2003) found that infants prenatally exposed to methamphetamine throughout each trimester of pregnancy were significantly smaller at birth than infants whose mothers stopped using methamphetamine before the third trimester. There was also a greater proportion of ‘small-for-gestational age’ infants in the methamphetamine-exposed group than in the non-exposed group. This finding was replicated in the US cohort of the IDEAL study (Nguyen et al., 2010). Further, PME was associated with decreased linear length/height trajectory from birth to 3 years relative to non-exposed children, with no differences in linear weight trajectories (Zabaneh 2012). Similarly, exposure to amphetamine has been associated with below average height and weight at birth, 4 years, and 8 years (Eriksson, Jonsson, Steneroth, & Zetterström, 1994).
These studies on human growth are supported by experimental literature linking PME and growth deficits in rat pups (e.g., Acuff-Smith, Schilling, Fisher, & Vorhees, 1996; Šlamberová, Pometlová, & Charousová, 2006; Williams, Moran, & Vorhees, 2004). The current study, capitalizing on both the NZ and US cohorts of IDEAL, seeks to compare the effects of PME on trajectories of child growth between countries.
The majority of the work on PME (and other prenatal exposure variables; e.g., Eiden, Veira, & Granger, 2009; Chaplin, Frieburger, Mayes, & Sinha, 2010) in humans relies on the use of a host of covariates to equate exposed and non-exposed conditions as closely as possible (Lester & LaGasse, 2010). However, it is sometimes the case that suitable matches for exposed cases or suitable controls for preexisting differences cannot be found within a data set (Miller & Chapman, 2001; Rosenbaum, 2010). In the prenatal drug exposure literature, which is mostly from the US, characteristics like lack of proper pre- and postnatal care, poverty, and out-of-home placement due to mandatory reporting (to legal authorities) of illicit drug use during pregnancy are often closely linked to PME. In NZ, however, the universal, national healthcare system provides for free pre- and postnatal care and free visits to physicians during childhood (LaGasse et al., 2011, Wu et al., 2012). The NZ government also provides financial support for all citizens in need including drug addiction problems and does not require mandatory reporting of prenatal substance use (Mathews & Kenny 2008), which leads to greater engagement in prenatal services by drug abusing mothers (Wu et al., 2012). The common cross-national concerns regarding methamphetamine use among pregnant women, coupled with the differences in service provision to drug using mothers, provide a unique opportunity for a natural experiment (Bornstein, 2010; Harkness, 1992) on the impact of PME on child development.
The current study examines differences in the impact of PME on growth from birth through three years of age in the US and NZ. Matched samples of PME children and children with no methamphetamine exposure (NPME) were recruited in the US and NZ, and these samples are modeled over time using latent growth curve analysis (Duncan, Duncan, & Strycker, 2006). We hypothesized that the growth trajectories of PME children in NZ would be more optimal than those of PME children in the US.
Method
Recruitment and Participants
Data come from the cross-cultural IDEAL Study of PME and child outcome in NZ and in the US. The IDEAL study was a prospective study of children with PME and matched comparisons, with participants recruited from 4 geographically representative sites known to have methamphetamine problems (Los Angeles, CA; Des Moines, IA; Tulsa, OK; Honolulu, HI; for more information on IDEAL, see Smith et al., 2007; 2008). The IDEAL cohort in NZ coms from a separate, related project on children with PME, with participants selected from the Auckland area of the north island due to its large urban population base (for more on the NZ IDEAL cohort, see LaGasse et al., 2011).
In the US, recruitment occurred postpartum, and the Institutional Review Boards at all participating sites approved the protocol and consent procedure. A National Institute on Drug Abuse Certificate of confidentiality was also obtained to allow participants to report on their drug use without fear of mandatory reporting of illegal substance use. However, mothers were informed that the certificate did not exclude reporting of evidence of child abuse or neglect. Mothers were contacted after giving birth, screened for eligibility, and, if interested, provided written consent.
In NZ, recruitment was performed during pregnancy, and approval for the study was granted by the Auckland District Health Board (DHB), Waitemata DHB, Northern Regional Ethics Committee (through the NZ Ministry of Health), and the Maori Ethics Committee at both the Auckland and Waitemata DHBs. There is very little child removal due to prenatal substance use in NZ, as there no statutes regarding mandatory reporting of prenatal substance-using mothers. In NZ, most pregnant mothers receive pre- and postnatal care through midwives subsidized through the country's universal health care plan. All participants in the NZ cohort were referred to study staff through independent or hospital employed midwives. Research staff met with mothers during the prenatal period to discuss the study and obtain written consent. When the child was born, staff returned, reviewed the study protocol with the mother, and performed the baseline interview. In both cohorts, meconium specimens were collected, and shipped to a central laboratory for analysis of drug metabolites using gas chromatography-mass spectrometry (U.S. Drug Testing Laboratory; Des Plains, IL; for more information, see LaGasse et al., 2011).
Mothers identified as a methamphetamine user by self-report and/or positive confirmation of amphetamines in meconium were assigned to PME group. Meconium samples were shipped within 2 days in the US but up to 6 months in NZ. Given the long delay for meconium results in NZ, methamphetamine use was mainly based on self-report. There were relatively few cases where meconium was positive for MA but prenatal use was denied; (this occurred in 8 participants in the US and 2 participants in NZ). To accommodate the unique drug use patterns of each country, inclusion to the PME group could include prenatal cocaine use in the US or prenatal opiate use in NZ. There were 17 mothers who used cocaine during pregnancy in the US and 12 mothers who used opiates during pregnancy in NZ.
Infants with no prenatal methamphetamine exposure (NPME) within each US site and NZ were matched to the exposed individuals based on race/ethnicity, infant birth weight (categorized as < 1500 g, 1500-2500 g, > 2500 g), and maternal educational level (in US, high school degree or greater vs. less than high school degree; in NZ, 5th form certificate achieved or not achieved, as this is the closest analog to high school in NZ). In the US, participants were also matched on private vs. public insurance status. Mothers in the US were provided with a $50 incentive for participation at each time point from birth, and mothers in NZ were paid an equivalent amount in NZ dollars. All data collection took place postpartum. Prenatal use of cocaine (US) or opiates (NZ) were excluded from the respective comparison groups.
Exclusion criteria included: non-English speaking (except Maori in NZ), maternal age < 18-years in US (age of consent in the US) and < 17.5-years in NZ (age of consent in NZ), multiple births, maternal cognitive or psychological impairment, overt psychotic behavior or documented history of psychosis, maternal use of LSD/hallucinogens/PCP, infant congenital anomalies/chromosomal abnormalities, infants unlikely to survive, overt TORCH infection, and infants with a sibling previously enrolled in the study. In the US, a total of 17,961 screened mother-infant dyads were eligible to participate, with 3,705 (21%) agreeing to participate; 204 infants were found to have PME and 208 NPME matched comparison participants were selected from the remaining potential participants. In NZ, data on total eligible participants and enrollment rates were not available given the recruitment procedure used, with midwives only referring expectant mothers who had expressed interest in participating. A total of 223 mother-infant dyads in NZ were enrolled, with 108 PME participants and 115 NPME comparisons. As such, the total sample size for the current study was 635 (PME n = 312; NPME n = 323). Table 1 presents the demographic characteristics of the sample by exposure status and overall.
Table 1. Demographic Characteristics by Exposure Status, Country, and Overall.
| UnitedStates | New Zealand | Overall | ||||
|---|---|---|---|---|---|---|
| PME (n = 204) | NPME (n = 208) | PME (n = 108) | NPME (n = 115) | PME (n = 312) | NPME (n = 323) | |
| Child Sex | ||||||
| Boy | 110 (54%) | 110 (53%) | 60 (56%) | 60 (52%) | 170 (55%) | 170 (53%) |
| Girl | 94 (46%) | 98 (47%) | 48 (44%) | 55 (48%) | 142 (45%) | 153 (47%) |
| SES Category | ||||||
| 1 | 1 (1%) | 5 (2%) | 1 (1%) | 8 (7%) | 2 (1%) | 13 (4%) |
| 2 | 14 (7%) | 35 (17%) | 5 (5%) | 19 (17%) | 19 (6%) | 54 (17%) |
| 3 | 40 (20%) | 68 (33%) | 13 (12%) | 18 (16%) | 53 (17%) | 86 (27%) |
| 4 | 80 (39%) | 74 (36%) | 36 (34%) | 48 (42%) | 116 (37%) | 122 (38%) |
| 5 | 68 (34%) | 25 (12%) | 52 (49%) | 22 (19%) | 120 (39%) | 47 (15%) |
| Mean (Standard Deviation) | ||||||
| Prenatal Tobacco Exposure | 6.96 | 1.67 | 8.39 | 3.37 | 7.46 | 2.27 |
| (number of cigarettes/day) | (8.19) | (4.68) | (7.24) | (5.47) | (7.89) | (5.03) |
| Prenatal Alcohol Exposure | 0.13 | 0.00 | 0.31 | 0.12 | 0.19 | 0.04 |
| (ounces of absolute alcohol 1/day) | (0.49) | (0.02) | (0.80) | (0.28) | (0.62) | (0.17) |
| Prenatal Marijuana Exposure | 0.09 | 0.08 | 0.47 | 0.17 | 0.22 | 0.12 |
| (number of joints/day) | (0.25) | (1.04) | (0.99) | (0.64) | (0.64) | (0.92) |
| Maternal Height (feet) | 5.36 | 5.33 | 5.41 | 5.40 | 5.37 | 5.35 |
| (0.23) | (0.22) | (0.26) | (0.21) | (0.24) | (0.22) | |
| Maternal Weight (pounds) | 141.92 | 144.50 | 140.68 | 155.76 | 141.53 | 148.41 |
| (36.71) | (35.24) | (36.23) | (37.75) | (36.50) | (36.47) | |
Measures
Child Length/Height and Weight
Child height/length and weight were measured at birth, 12 months, 24 months, and 36 months. Height was measured in centimeters with children wearing socks and no shoes, and weight was measured in pounds with children wearing light clothing. Using normative values for boys and girls at each time point from the World Health Organization, heights and weights were transformed into z-scores. As such, a score of 0 represents the average height or weight of children at a given age across the world.
Covariates
Covariates were selected to account for factors that could affect growth in order to isolate the impact of PME. To account for potential effects of heredity, self-reported maternal height was included as a predictor of child length/height growth parameters, and maternal weight was included as a predictor of child weight growth parameters. Child sex was included to account for differences in growth trajectories for boys and girls, as was socioeconomic status (SES). Education and occupation information was collected to calculate the four-factor Hollingshead Index (Hollingshead, 1975; LaGasse et al., 1999). The continuous SES measure, index of social prestige, is categorized into 5 groups ranging from1 (high SES) to 5 (low SES). Prenatal exposure to tobacco (average cigarettes per day), alcohol (average ounces of absolute alcohol per day), and marijuana (average joints per day) were included as covariates on each growth parameter.
Plan of Analysis
All analyses were performed in Mplus 6.0 (Muthén & Muthén, 1998-2010) using a full information maximum likelihood estimator robust to non-normality to account for missing data over time. Unconditional latent growth curve (LGC) models were first performed in a structural equation modeling framework (Duncan, Duncan, & Strycker, 2006) to demonstrate the overall trajectories of infant and child growth in length/height and weight annually from birth through 36 months. Fixed chronometric factor loadings were used to represent the intercept (i.e., 1, 1, 1, and 1), linear slope (i.e., 0, 1, 2, and 3), and quadratic trend (i.e., 0, 1, 4, 9). Model fit was compared using χ², Comparative Fit Index (CFI; Bentler, 1990), and Root Mean Square Error of Approximation (RMSEA; Steiger & Lind, 1980).
We then performed conditional LGCs, including measures of PME and country of origin (0 = US; 1 = NZ) as predictors of growth parameters (i.e., intercept, linear slope, and quadratic trend). We also included the interaction between PME and country of origin, defined by the product of centered PME and centered country, as a predictor of growth. Prenatal exposure to other substances (tobacco, alcohol, and marijuana), child sex, and maternal height/weight were used as covariates. Associations between predictors/covariates and growth outcomes are presented using standardized betas (β).
Results
Preliminary Analysis
Table 1 presents sample demographic characteristics by country and exposure status. There was no difference across country and exposure status on child sex, χ² (1) = 0.01, p > 0.10; χ² (1) = 0.22, p > 0.05, respectively. Individuals with PME were more likely than those with NMPE to be in lower SES categories, χ² (4) = 64.54, p < 0.001. Individuals in NZ were also more likely to be in the lowest SES category than individuals in the US, χ² (4) = 20.45, p < 0.001. In regard to prenatal exposure to other substances, there was a consistent pattern of greater exposure in NZ than in the US, tobacco t (631) = 2.60, p < 0.01; alcohol t (632) = 3.91, p < 0.001; marijuana t (631) = 3.50, p < 0.01. Children with PME were also prenatally exposed to higher levels of tobacco and alcohol, tobacco t (631) = 9.89, p < 0.001; alcohol t (632) = 4.09, p < 0.001, but the difference in prenatal exposure to marijuana was not significant, t (631) = 1.70, p > 0.05.
In regard to maternal physical size, mothers in NZ were taller than mothers in the US, t (617) = 3.38, p < 0.01, but there was no country difference in maternal weight, t (608) = 1.77, p > 0.05. Mothers of children with PME tended to weigh less than mothers of children with NPME, t (608) = 2.33, p < 0.05, but there was no difference by exposure status in maternal height, t (617) = 1.08, p > 0.05.
Unconditional LGC
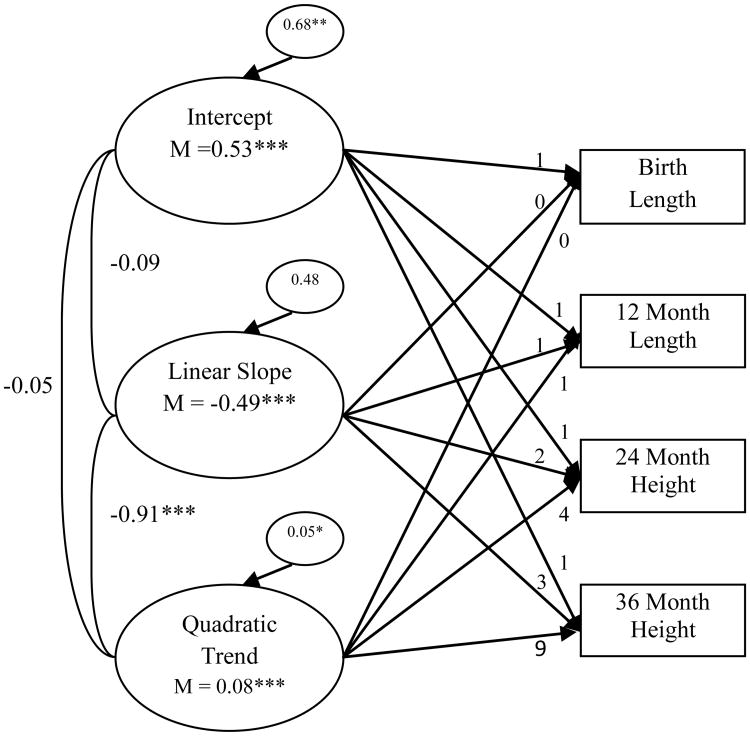
LGC models were then performed for child length/height and weight without the inclusion of PME and country factors or any covariates. In regard to child length/height, results indicated that a model with a random intercept, random linear slope, and random quadratic trend provided good fit to the data, χ² (1) = 4.75, p = 0.03, CFI = 0.99, RMSEA < 0.08 (see Figure 1). Relative to the WHO standards, individuals in the sample were significantly longer than average at birth and declined over time1. There was also a significantly positive quadratic trend representing an overall flattening in the growth curve from 12 months through 36 months.
Figure 1. Unconditional latent growth curve of child length/height.
Chronometric factor loadings from the intercept, linear slope, and quadratic trend were fixed. Correlations between factors are presented. Model fit the data well, χ² (1) = 4.75, p = 0.03; CFI = 0.99; RMSEA < 0.08. * p < 0.05, ** p < 0.01, ***p < 0.001
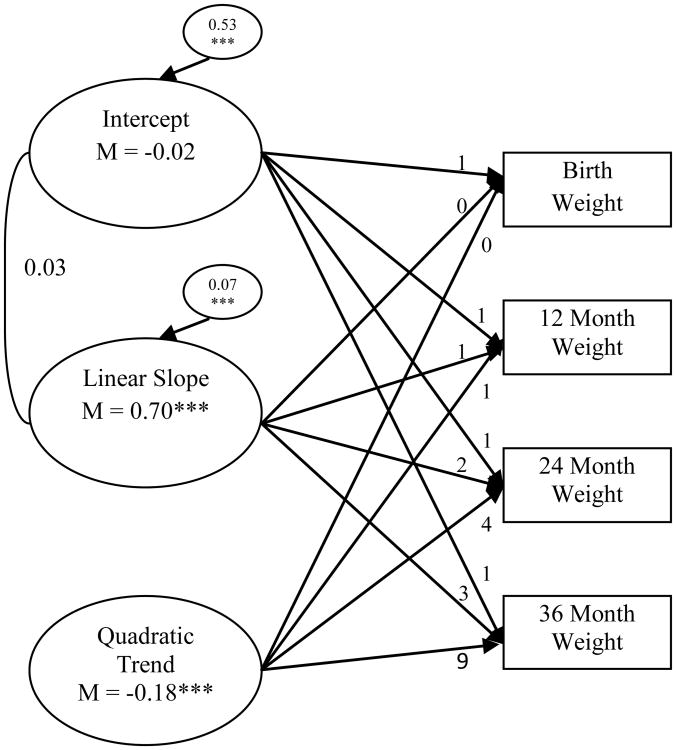
In regard to child weight, results indicated that a model with a random intercept, random linear slope, and constant quadratic trend also provided good fit to the data, χ² (4) = 13.70, p < 0.01, CFI = 0.96, RMSEA = 0.06 (see Figure 2). Modeling growth in weight with a random quadratic trend led to estimation problems (i.e., non-positive definite psi matrix), so the quadratic trend was held constant. Relative to the WHO standards, individuals in the sample were of average weight at birth and significantly increased over time. The quadratic trend was significantly negative, representing the overall increase until 24 months and decrease from 24 months to 36 months.
Figure 2. Unconditional latent growth curve of child weight.
Chronometric factor loadings from the intercept, linear slope, and quadratic trend were fixed. The correlation between intercept and linear slope is presented. The quadratic trend was held constant to facilitate model convergence with a positive definite psi matrix. Model fit the data well, χ2 (4) = 13.70, p < 0.01; CFI = 0.96; RMSEA = 0.06. * p < 0.05, ** p < 0.01, ***p < 0.001
Predicting LGC Parameters
Predictors of interest (exposure status, country of origin, exposure × country) and covariates (child sex, SES, other prenatal exposures, and maternal height/weight) were included predicting random growth parameters (intercept, linear slope, and quadratic trend for height/length; intercept and linear slope for weight).
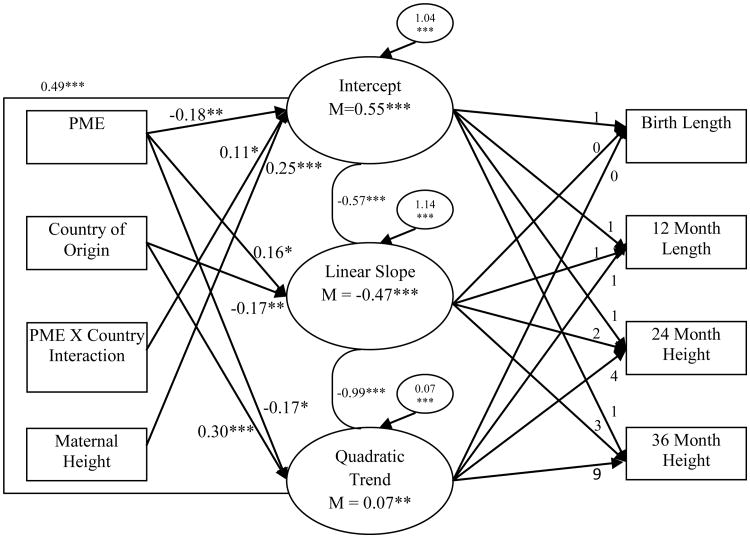
In regard to length/height, PME was significantly associated with each growth parameter (see Figure 3). Children with PME tended to be shorter at birth than children with NMPE and tended to decline, relative to WHO standards, at a slower rate (positive effect on linear slope) and continued to decline from 24 to 36 months (negative effect on quadratic trend; see Figure 4).2 Country of origin was associated with linear slope and quadratic trend, such that individuals in NZ declined at a faster rate (negative effect on linear slope) with a stronger increase from 12 to 36 months than individuals in the US (positive effect on quadratic trend). There was also an exposure × country effect on the intercept, such that children with PME in NZ were significantly longer at birth than children with PME in the US. Greater maternal height was also predictive of greater length at birth. There were no significant effects of child sex, SES, and prenatal exposure to tobacco, alcohol, and marijuana on height/length growth parameters.
Figure 3. Conditional latent growth curve of child length/height using PME, country of origin, PME × country interaction, and covariates.
Chronometric factor loadings from the intercept, linear slope, and quadratic trend were fixed. Correlations between factors are presented. All associations between predictors/covariates and growth factors were modeled, but only statistically significant paths are presented in the interest of clarity and parsimony. Prenatal exposure to tobacco, alcohol, and marijuana, child sex, and SES were included as covariates of growth parameters, but none of these effects were statistically significant. As such, these variables were not included in the figure. Model fit the data well, χ² (11) = 20.52, p = 0.04; CFI = 0.98; RMSEA < 0.05. * p < 0.05, ** p < 0.01, ***p < 0.001
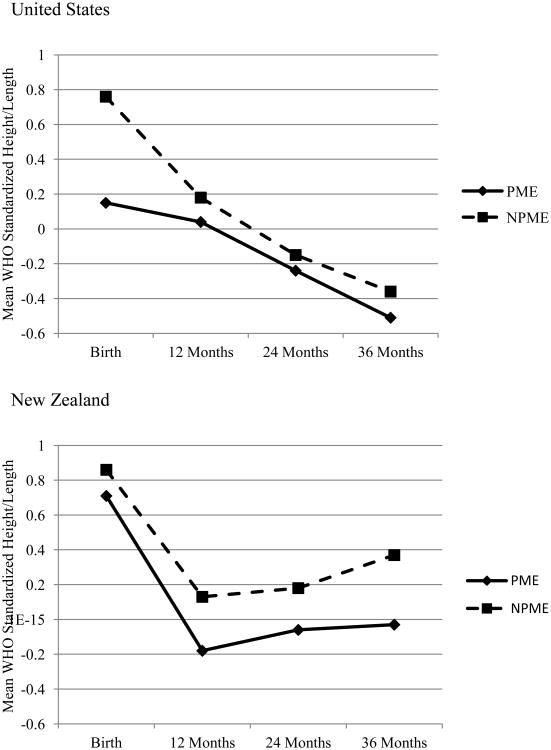
Figure 4. Child length/height by country and exposure status.
Values presented in the figure represent the unadjusted mean standardized height/length values using World Health Organization standards for birth through 36 months.
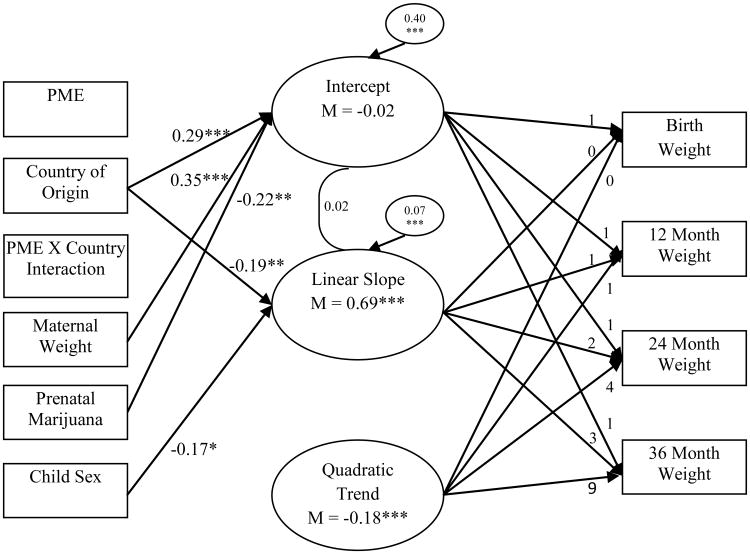
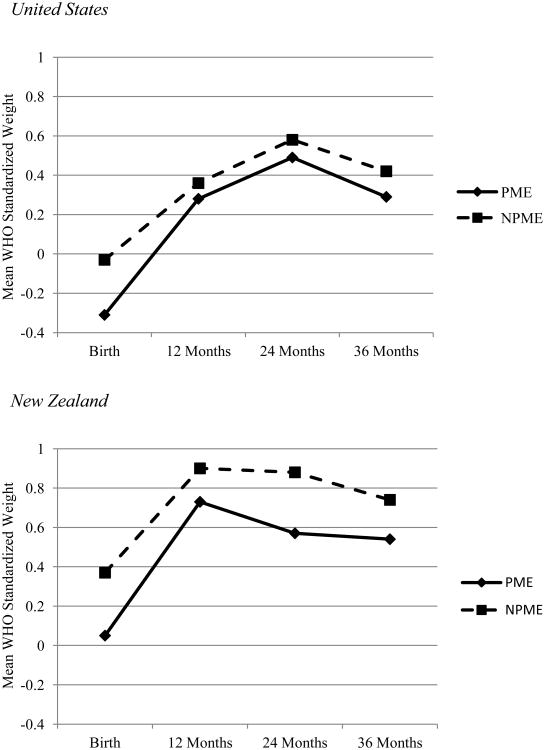
In regard to weight, country of origin was predictive of intercept and linear slope (see Figure 5). Individuals in NZ were heavier at birth with a slower linear rate of growth (negative effect on linear slope) than individuals in the US (see Figure 6). Greater maternal weight was predictive of greater weight at birth, while prenatal exposure to marijuana was predictive of lower weight at birth. Boys were also found to increase in weight slower than girls. There were no significant effects of PME, exposure × country interaction, SES, and exposure to tobacco and alcohol on weight growth parameters.
Figure 5. Conditional latent growth curve of child weight using PME, country of origin, PME × country interaction, and covariates.
Chronometric factor loadings from the intercept, linear slope, and quadratic trend were fixed. The correlation between intercept and linear slope is presented. The quadratic trend was held constant to facilitate model convergence with a positive definite psi matrix. All associations between predictors/covariates and growth factors were modeled, but only statistically significant paths are presented in the interest of clarity and parsimony. Prenatal exposure to alcohol and tobacco and SES were included as covariates of growth parameters, but none of these effects were statistically significant. As such, these variables were not included in the figure. Model fit the data well, χ2 (22) = 58.34, p < 0.001; CFI = 0.94; RMSEA = 0.05. * p < 0.05, ** p < 0.01, ***p < 0.001
Figure 6. Child weight by country and exposure status.
Values presented in the figure represent the unadjusted mean standardized weight values using World Health Organization standards for birth through 36 months.
Discussion
The current study sought to examine differences in the effects of PME on infant and child physical growth across the US and NZ using a large, prospective design. Given the considerable differences in governmental and healthcare responses to maternal drug use across countries (Wu et al., 2012), this comparison was a unique and informative way to account for these traditional confounds (e.g., inadequate pre/postnatal care, poverty, out-of-home placement due to maternal drug use) in prenatal drug exposure research.
Results indicated there was a stronger negative effect of PME on infant and child length/height in the US than in NZ, which was supportive of our hypothesis that exposed children in NZ would fare better than exposed children in the US. There were no significant PME effects observed on infant and child weight, which was not supportive of our hypothesis but was in line with previous work in the US alone (Zabaneh et al., 2012). It is important to note that, PME individuals in each country were consistently shorter and lighter than their matched comparisons, although not always to a significant degree. Each of the analyses described was performed while adjusting for other prenatal exposures, child sex, SES, and heredity (height/weight of mothers).
The observed PME effects on infant length/height again highlight the relevance of the prenatal period for postnatal development. Observed PME effects may be the result of altered placental blood flow due to the vasoconstrictive properties of methamphetamine restricting nutrient delivery, as well its anorexic effects on mothers (Plessinger, 1998; Salisbury, Ponder, Padbury, & Lester, 2009). The difference between the effects of PME in the US and PME in NZ highlights the potential importance of providing adequate prenatal care to drug-using mothers. Wu and colleagues (2012) showed that methamphetamine-using mothers in the US had much higher rates of inadequate prenatal care than their NZ counterparts, likely due to the duel effect of free health care and a lack of fear regarding one's child being taken away due to mandatory reporting by doctors and hospital staff.
These findings call attention to enhanced pre-and postnatal service provision to drug using mothers in the US as a potential way to prevent the growth decrements on the developing fetus/child. Effective prevention programs like the Nurse-Family Partnership (NFP; e.g., Olds, 2006) have been providing these types of services to first-time mothers in communities across the US, reaching approximately 170,000 families since 1996 (Nurse-Family Partnership, 2012). The NFP seeks to create a link between the healthcare system (i.e., nurses) and mothers most at risk for inappropriate or insufficient prenatal care, and support is continued through the first two years of life. Programs like this can be adapted for use specifically with drug-using pregnant mothers, thereby creating conditions more similar to those observed in NZ for this at-risk population, with the goal of improving developmental outcomes among exposed infants/children in the US.
Limitations
There are several limitations to the current study. First, the nature of the question regarding cross-national comparisons of the effects of PME on development necessitates the use of an observational design, such that causal relations cannot be defined. Future studies employing similar designs might benefit from statistical methods like propensity score matching (e.g., Harder, Stuart, & Anthony, 2010; Rutter, 2007) or graphical modeling techniques (e.g., Greenland, Pearl, & Robins, 1999; Robins, 2001) to provide more causal estimates. Second, although the samples from each country represent the largest prospective data collections on PME available, we were only able to collect data from four areas in the US and the greater Auckland area of NZ. As such, generalizability may not extend to all regions and ethnicities. Third, the differences in recruitment procedures between the countries may have impacted findings, as drug-using mothers in NZ were approached for participation before birth and may have altered their behaviors due to this contact. Replication work would benefit from the use of more consistent recruitment across sites. Fourth, despite the certificate of confidentiality, it remains possible that mothers in the US sample underreported their drug use, particularly comparison individuals, over concerns regarding child removal. Although this potential was lessened by the use of meconium testing and the fact that significant differences remained between PME and “NPME” participants, future work on prenatal exposure should seek more definitive measures of maternal use (i.e., meconium, maternal hair, and urine testing). Fifth, the current study only follows growth trajectories from birth through three years, such that longer-term follow-ups are required for a more complete understanding of differences in the effects of PME on infant and child growth. Finally, the current study does not address proposed mechanisms for the described country × exposure status interaction (e.g., health care, fear of mandatory reporting) due to data limitations. Future research should seek to quantify access, both actual and perceived, to health care services and concerns regarding child removal and/or prosecution due to mandatory reporting of prenatal drug exposure to explicitly test these mechanisms.
Conclusions
The effects of PME were shown to differ across countries, with less decrements in birth length observed in NZ than in the US. With sufficient replication, findings like these have the potential to influence public policy regarding the treatment of drug-using mothers. By addressing deficits specific to this sub-population of women, investigators and policy makers may be able to prevent much of the harm from drug use during pregnancy that is transferred onto the child.
Footnotes
It is important to note that this decline does not represent declining height, but rather a decline in height relative to the population of children worldwide.
Models were also run with cocaine- and opiate-exposed infants removed, and predictor/covariate results were nearly identical. As such, the analyses with the complete sample were presented and interpreted.
References
- Abar B, LaGasse LL, Derauf C, Newman E, Shah R, Smith LM, et al. Lester BM. Examining the relationships between prenatal methamphetamine exposure, early adversity, and child neurobehavioral disinhibition. Psychology of Addictive Behaviors. 2012 doi: 10.1037/a0030157. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acuff-Smith KD, Schilling MA, Fisher JE, Vorhees CV. Stage-specific effects of prenatal d-methamphetamine exposure on behavioral and eye development in rats. Neurotoxicology & Teratology. 1996;18:199–215. doi: 10.1016/0892-0362(95)02015-2. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indices in structural models. Psychological Bulletin. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Berman S, O'Neill, Fears S, Bartzokis G, Londen ED. Abuse of amphetamines and structural abnormalities in the brain. Annals of the New York Academy of Sciences. 2008;1141:195–220. doi: 10.1196/annals.1441.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billing L, Eriksson M, Jonsson B, Steneroth G, Zetterström R. The influence of environmental factors on behavioural problems in 8-year-old children exposed to amphetamine during fetal life. Child Abuse & Neglect. 1994;18:3–9. doi: 10.1016/0145-2134(94)90091-4. [DOI] [PubMed] [Google Scholar]
- Block RI, Erwin WJ, Ghoneim MM. Chronic drug use and cognitive impairment. Pharmacological Biochemistry & Behavior Journal. 2002;73:491–504. doi: 10.1016/s0091-3057(02)00816-x. [DOI] [PubMed] [Google Scholar]
- Bornstein MH. Handbook of cross-cultural developmental science. New York, NY: Taylor & Francis; 2010. [Google Scholar]
- Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Supplement 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- Chang L, Cloak C, Jiang CS, Farnham S, Tokeshi B, Buchtal S, et al. Ernst T. Altered neurometabolites and motor integration in children exposed to methamphetamine in utero. Neuroimage. 2009;48:391–397. doi: 10.1016/j.neuroimage.2009.06.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L, Smith L, LoPresti C, Yonekura M, Kuo J, Walot I, et al. Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Research: Neuroimaging. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Chaplin TM, Freiburger MB, Mayes LC, Sinha R. Prenatal cocaine exposure, gender, and adolescent stress response: A prospective longitudinal study. Neurotoxicology and Teratology. 2010;32:595–604. doi: 10.1016/j.ntt.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloak CC, Ernst T, Fujii L, Hedemark B, Chang L. Lower diffusion in white matter of children with prenatal methamphetamine exposure. Neurology. 2009;72:2068–2075. doi: 10.1212/01.wnl.0000346516.49126.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colliver JD, Kroutil LA, Dai L, Gfroerer JC. Misuse of Prescription Drugs: Data from the 2002 2003 and 2004 Surveys on Drug Use and Health. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2006. [Google Scholar]
- Derauf C, LaGasse LL, Smith LM, Newman E, Shah R, Neal CR, et al. Lester BM. Prenatal methamphetamine exposure and inhibitory control among young school-age children. Journal of Pediatrics. 2012;161:452–459. doi: 10.1016/j.jpeds.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan TE, Duncan SC, Strycker LA. An introduction to latent variable growth curve modeling. 2nd. New York, NY: Lawrence Erlbaum Associates; 2006. [Google Scholar]
- Eiden RD, Veira Y, Granger DA. Prenatal cocaine exposure and infant cortisol reactivity. Child Development. 2009;80:528–543. doi: 10.1111/j.1467-8624.2009.01277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson M, Jonsson B, Steneroth G, Zetterström R. Cross-sectional growth of children whose mothers abused amphetamines during pregnancy. Acta Paediatrica. 1994;82:612–617. doi: 10.1111/j.1651-2227.1994.tb13091.x. [DOI] [PubMed] [Google Scholar]
- Greenland S, Pearl J, Robins JM. Confounding and collapsibility in causal inference. Statistical Science. 1999;14:29–46. [Google Scholar]
- Harder VS, Stuart EA, Anthony J. Propensity score techniques and the assessment of measured covariate balance to test causal associations in psychological research. Psychological Methods. 2010;15:234–249. doi: 10.1037/a0019623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness S. Human development in psychological anthropology. In: Schwartz T, White GM, Lutz CA, editors. New directions in psychological anthropology. New York, NY: Cambridge University Press; 1992. pp. 102–121. [Google Scholar]
- Havel M. Annual report of the centralized substance abuse assessment project. Report to the Governor's Alliance on Substance Abuse; Des Moines, IA: 1997. [Google Scholar]
- Hollingshead A. Four Factor Index of Social Status. New Haven, CT: Department of Sociology, Yale University; 1975. [Google Scholar]
- LaGasse LL, Derauf C, Smith LM, Newman E, Shah R, Neal C, et al. Lester BM. Prenatal methamphetamine exposure and childhood behavior problems at 3 and 5 years of age. Pediatrics. 2012;129:681–688. doi: 10.1542/peds.2011-2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaGasse L, Seifer R, Wright LL, Lester BM, Tronick EZ, Bauer CR, et al. Smeriglio VL. The Maternal Lifestyle (MLS): The caretaking environment of infants exposed to cocaine/opiates. Pediatric Research. 1999;45:247a. [Google Scholar]
- LaGasse L, Wouldes T, Newman E, Smith L, Shah R, Derauf C, et al. Lester B. Prenatal methamphetamine exposure and neonatal neurobehavioral outcome in the USA and New Zealand. Neurotoxicology & Teratology. 2011;33:166–175. doi: 10.1016/j.ntt.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester BM, LaGasse LL. Children of addicted women. Journal of Addictive Diseases. 2010;29:259–276. doi: 10.1080/10550881003684921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu LH, Johnson A, O'Hare ED, Bookheimer SY, Smith LM, O'Connor MJ, et al. Sowell ER. Effects of prenatal methamphetamine exposure on verbal member revealed with fMRI. Journal of Developmental and Behavioral Pediatrics. 2009;30:185–192. doi: 10.1097/DBP.0b013e3181a7ee6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews B, Kenny MC. Mandatory reporting legislation in the United States, Canada, and Australia: A cross-jurisdictional review of key features, differences, and issues. Child Maltreatment. 2008;13:50–63. doi: 10.1177/1077559507310613. [DOI] [PubMed] [Google Scholar]
- Miller GA, Chapman JP. Misunderstanding analysis of covariance. Journal of Abnormal Psychology. 2001;110:40–48. doi: 10.1037//0021-843x.110.1.40. [DOI] [PubMed] [Google Scholar]
- Ministry of Health. Drug use in New Zealand: Analysis of the 2003 New Zealand Health Behaviors Survey—Drug use. Wellington, NZ: Ministry of Health; 2007. [Google Scholar]
- Muthén LK, Muthén BO. Mplus User's Guide. 6th. Los Angeles, CA: Muthén & Muthén; 1998-2010. [Google Scholar]
- Nguyen D, Smith LM, LaGasse LL, Derauf C, Grant P, Shah R, et al. Lester BM. Intrauterine growth of infants exposed to prenatal methamphetamine: Results from the Infant Development, Environment, and Lifestyle (IDEAL) Study. Journal of Pediatrics. 2010;157:337–339. doi: 10.1016/j.jpeds.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse-Family Partnership. Nurse-Family Partnership Snapshot. 2012 Retrieved September 18, 2012 from http://www.nursefamilypartnership.org/assets/PDF/Fact-sheets/NFP_Snapshot.
- Olds DL. The nurse-family partnership: An evidence-based preventive intervention. Infant Mental Health Journal. 2006;27:5–25. doi: 10.1002/imhj.20077. [DOI] [PubMed] [Google Scholar]
- Plessinger MA. Prenatal exposure to amphetamines: Risks and adverse outcomes in pregnancy. Obstetrics and Gynecology Clinics. 1998;25:119–138. doi: 10.1016/s0889-8545(05)70361-2. [DOI] [PubMed] [Google Scholar]
- Robins J. Data, design, and background knowledge in etiologic inference. Epidemiology. 2001;12:313–320. doi: 10.1097/00001648-200105000-00011. [DOI] [PubMed] [Google Scholar]
- Rosenbaum PR. Design of Observational Studies. New York, NY: Springer; 2010. [Google Scholar]
- Rutter M. Proceeding from observed correlation to causal inference: The use of natural experiments. Perspectives on Psychological Science. 2007;2:377–395. doi: 10.1111/j.1745-6916.2007.00050.x. [DOI] [PubMed] [Google Scholar]
- Salisbury AL, Ponder KL, Padbury JF, Lester BM. Fetal effects of psychoactive drugs. Clinical Perinatology. 2009;36:595–619. doi: 10.1016/j.clp.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamberova R, Pometlova M, Charousova P. Postnatal development of rat pups is altered by prenatal methamphetamine exposure. Progress in Neuro-psychopharmacology & Biological Psychiatry. 2006;30:82–88. doi: 10.1016/j.pnpbp.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Smith LM, Chang L, Yonekura MD, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy in children exposed to methamphetamine in utero. Neurology. 2001;57:255–260. doi: 10.1212/wnl.57.2.255. [DOI] [PubMed] [Google Scholar]
- Smith DK, Johnson AB, Pears KC, Fisher PA, DeGarmo DS. Child maltreatment and foster care: Unpacking the effects of prenatal and postnatal parental substance use. Child Maltreatment. 2007;12:150–160. doi: 10.1177/1077559507300129. [DOI] [PubMed] [Google Scholar]
- Smith LM, LaGasse LL, Derouf C, Grant P, Shah R, Arria A, et al. Lester BM. Prenatal methamphetamine use and neonatal neurobehavioral outcome. Neurotoxicology and Teratology. 2008;30:20–28. doi: 10.1016/j.ntt.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L, Vonekura ML, Wallace TL, Berman N, Kuo J, Berkowitz C. Effects of prenatal methamphetamine exposure on fetal growth and drug withdrawal symptoms in infants born at term. Journal of Developmental & Behavioral Pediatrics. 2003;24:17–23. doi: 10.1097/00004703-200302000-00006. [DOI] [PubMed] [Google Scholar]
- Sommers I, Baskin A, Baskin-Sommers A. Methamphetamine use among young adults: Health and social consequences. Addictive Behaviors. 2006;31:1469–1476. doi: 10.1016/j.addbeh.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Sowell E, Leow A, Bookheimer S, Smith L, O'Connor M, Kan E, et al. Thompson P. Differentiating prenatal exposure to methamphetamine and alcohol versus alcohol and not methamphetamine using tensor-based brain morphometry and discriminant analysis. The Journal of Neuroscience. 2010;30:3876–3885. doi: 10.1523/JNEUROSCI.4967-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2010 National Survey on Drug Use and Health: Summary of National Findings. Rockville, MD: SAMHSA; 2011. pp. 11–4658. HHS Publication No. (SMA) [Google Scholar]
- Steiger JH, Lind J. Statistically-based tests for the number of common factors; Paper presented at the Annual Spring Meeting of the Psychometric Society; Iowa City, IO. 1980. [Google Scholar]
- Terplan M, Smith EJ, Kozloski MJ, Pollack HA. Methamphetamine use among pregnant women. Obstetrics & Gynecology. 2009;113:1285–1291. doi: 10.1097/AOG.0b013e3181a5ec6f. [DOI] [PubMed] [Google Scholar]
- Wijetunga M, Seto R, Lindsay J, Schatz I. Crystal methamphetamine- associated cardiomyopathy: Tip of the iceberg? Journal of Toxicology – Clinical Toxicology. 2003;41:981–986. doi: 10.1081/clt-120026521. [DOI] [PubMed] [Google Scholar]
- Wilkins C, Sweetsur P. Trends in population drug use in New Zealand: Findings form national household surveying of drug use in 1998, 2001, 2003, and 2006. New Zealand Medical Journal. 2008;121:61–71. [PubMed] [Google Scholar]
- Williams MT, Moran MS, Vorhees CV. Behavioral and growth effects induced by low dose methamphetamine administration during the neonatal period in rats. International Journal of Developmental Neuroscience. 2004;22:273–283. doi: 10.1016/j.ijdevneu.2004.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouldes T, LaGasse L, Sheridan J, Lester B. Maternal methamphetamine use during pregnancy and child outcome: What do we know? Journal of the New Zealand Medical Association. 2004;117:U1180. [PubMed] [Google Scholar]
- Wu M, LaGasse LL, Wouldes TA, Arria AM, Wilcox T, Derauf C, et al. Lester BM. Predictors of inadequate prenatal care in methamphetamine-using mothers in New Zealand and the United States. Maternal and Child Health Journal. 2012 doi: 10.1007/s10995-012-1033-8. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabaneh R, Smith L, LaGasse L, Derauf C, Newman E, Shah R, et al. Lester B. The effects of prenatal methamphetamine exposure on childhood growth patterns from birth to 3 years. American Journal of Perinatology. 2012;29:203–210. doi: 10.1055/s-0031-1285094. [DOI] [PMC free article] [PubMed] [Google Scholar]