Abstract
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease and can lead to hepatocellular carcinoma and end-stage liver disease. The current FDA-approved treatment for HCV (pegylated interferon-alpha (IFNα) with ribavirin) is effective only in about 50% of patients. Epidemiological evidence suggests that obesity, alcohol, smoking and environmental pollutants may contribute to resistance to IFNα therapy in HCV. Acrolein, a ubiquitous environmental pollutant and major component of cigarette smoke, is also generated endogenously by cellular metabolism and lipid peroxidation. This study examines the effects of acrolein on (i) IFNα-mediated signaling and antiviral gene expression in cultured and primary human hepatocytes, and (ii) HCV replication in an HCV-replicon system. Our data demonstrate that non-toxic concentrations of acrolein significantly inhibited IFNα-induced tyrosine phosphorylation of both cytoplasmic and nuclear STAT1 and STAT2, without altering the total levels. Also, acrolein down-regulated IFNα stimulated gene transcription, resulting in reduced expression of antiviral genes. Importantly, acrolein abolished the IFNα mediated downregulation of HCV viral expression in the HCV-replicon system. This study defines mechanisms involved in resistance to IFNα and identifies the pathogenic role of acrolein, and potentially other environmental pollutants, in suppressing IFNα antiviral activity, and establishes their adverse impact on HCV therapy.
Keywords: Environmental pollutant, acrolein, hepatitis C virus, interferon alpha, JAK-STAT signaling, lipid peroxidation products
INTRODUCTION
Hepatitis C virus (HCV) infection is a leading cause of chronic liver disease (1). Chronic HCV infection can lead to cirrhosis, liver cancer and end-stage liver disease, making HCV an enormous burden on the health care system in the US and worldwide (2–5). The current FDA approved standard of care for chronic HCV is pegylated IFNα combined with ribavirin; however, treatment is ineffective in ∼ 50% of patients (particularly with HCV genotype 1, prevalent in USA) (6–8).
IFNα is a ubiquitously expressed, immunomodulatory cytokine that has antiviral and anti-HCV activity (9, 10). IFNα action in cells is mediated by specific cell surface receptors IFNAR1 and IFNAR2, and involves activation of the JAK-STAT (receptor associated Janus family tyrosine kinases - signal transducer and activator of transcription family of proteins) pathway (11, 12). Upon stimulation by IFNα, upstream kinases Jak1 and Tyk2 are activated by phosphorylation; they, in turn, phosphorylate STAT1 and STAT2. Tyrosine phosphorylated STAT1 and STAT2 heterodimerize, translocate into the nucleus, and together with nuclear p48/IRF-9 proteins form the ISGF3 (interferon stimulated gene factor-3) transcription factor complex (9–12). ISGF-3 binds to the interferon-stimulated response element (ISRE) in the promoters of IFNα stimulated genes, and induces the expression of several antiviral proteins, such as dsRNA-activated PKR, oligoadenylate synthetases (OAS), Mx proteins, and numerous IFNα stimulated genes (ISGs) (11, 12).
IFNα mediated antiviral gene expression is subject to negative regulation by dephosphorylation via serine/tyrosine protein phosphatases (PP2A, SHP-1, SHP-2, PTP1B) and by association with inhibitory proteins such as SOCS (suppressor of cytokine signaling proteins) or PIAS (protein inhibitor of activated STAT) (reviewed in 9). Upon stimulation by IFNα, STATs are rapidly phosphorylated, enabling nuclear translocation and DNA binding; this is followed by dephosphorylation of STATs by protein phosphatases. Thus, protein phosphatases control STAT activity by maintaining STAT in a latent cytosolic unphosphorylated form and by terminating nuclear STAT activity by dephosphorylation.
The molecular mechanisms underlying resistance to IFNα therapy are not fully understood and depend upon both viral and host-specific factors including HCV genotype, viral load, age, and genetic background. Epidemiological data have identified potentially modifiable cofactors that may contribute to the failure of HCV therapy, including obesity, diabetes, and external factors such as alcohol, smoking and pollutants (10). A recent study suggests that cigarette smoking may adversely influence HCV treatment outcomes, since smokers suffering from chronic hepatitis C have a lower response rate to IFNα compared to non-smokers (13). Also, increasing evidence suggests that increased lipid peroxidation and oxidative stress may play a pathogenic role in chronic hepatitis C and may be an important factor influencing IFNα therapy outcomes (14–19). Indeed, recent data show that H2O2 can directly inhibit IFNα signaling in a cultured hepatocyte cell line (20).
Acrolein is the most reactive and toxic aldehyde produced endogenously as a byproduct of cellular lipid peroxidation (LPO); it is also formed by the metabolism of polyamines, hydroxyl-amino acids, and anti-cancer drugs such as cyclophosphamide (21). Acrolein is a pervasive environmental and industrial pollutant, and is identified by the U.S. EPA as one of the 188 most hazardous air pollutants (22). Humans are exposed to acrolein via automobile exhaust, wood, plastic and cigarette smoke, overheated cooking fats and drinking water (21, 23). Importantly, acrolein forms irreversible, covalent carbonyl-retaining Michael adducts with proteins, phospholipids and nucleic acids, with deleterious consequences to their function (24–28). Moreover, protein adducts of acrolein (particularly, acrolein-FDP-lysine) have been detected in several chronic diseases, including Alzheimer’s disease (29), atherosclerosis and renal failure (30), and Type II diabetes (31).
This study tests the hypothesis that acrolein, a ubiquitous environmental pollutant and endogenous lipid peroxidation byproduct, contributes to poor response and resistance to IFNα therapy in hepatocytes. Our data demonstrate that non-lethal concentrations of acrolein significantly inhibit IFNα mediated JAK/STAT signaling, leading to reduced expression of antiviral proteins in HepG2 cells, and enhanced HCV viral expression in the HCV replicon system. The study identifies the pathogenic role of LPO products and environmental pollutants (such as acrolein) in repressing IFNα antiviral activity, and establishes their adverse impact on anti-HCV therapy.
MATERIALS AND METHODS
Reagents
Human interferon alpha (IFNα) was purchased from PBL Biomedical Laboratories (Piscataway, NJ). General chemicals, acrolein and (β-actin antibody were purchased from Sigma Aldrich (St.Louis, MO). Antibodies against phospho-STAT1 (Tyr701), STAT 1, phospho- STAT 2, and STAT 2 were purchased from Cell Signaling (Beverly, MA). Cell culture supplies were obtained from Invitrogen (Carlsbad, CA).
Cell culture
HepG2, a human hepatoma cell line obtained from American Type Culture Collection (Rockville, MD) was used as described previously (32). The HCV replicon (Clone B) was obtained from Apath LLC (St. Louis, MO) and used in accordance with company instructions. All treatments were performed on sub-confluent monolayers of cells. Primary human hepatocytes were obtained from ZenBio (Research Triangle Park, NC) and used in accordance with company instructions.
RNA isolation, RT- PCR and Real Time PCR analysis
Total RNA was isolated using TRIzol (Invitrogen, Carlsbad, CA) and subjected to real time PCR using SYBR green I dye reagents with an ABI prism 7500 sequence detection system (Applied Biosystems, Foster City, CA). The specific primers were obtained from Superarray (Frederick, MD). The relative gene expression was analyzed using 2−ΔΔCt method by normalizing with GAPDH gene expression in all the experiments.
Western Blot Analysis
Following treatment, cytosolic and nuclear protein extracts were analyzed by SDS-polyacrylamide gel electrophoresis and visualized using the enhanced chemiluminescence light (ECL) reagents (Amersham, Arlington Heights, IL).
Cell Viability-MTT assay
Cell survival/cell death was measured in cells treated for 24h by the MTT (3, (4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide) assay as described previously (32).
Luciferase Reporter Assays
HepG2 cells were transiently transfected for 24h with an ISRE cis-reporting system (Stratagene, La Jolla, CA) using the transfection reagent FugeneTM6 according to the manufacturer’s instructions (Roche, Indianapolis, IN). To control for transfection efficiency, cells were transfected in a single large batch and reseeded into 24-well plates for treatments. Total cell extracts were analyzed for luciferase activity using a commercial assay kit (Promega, Madison, WI). Results were normalized by protein concentration.
Protein phosphatase Assay
The Serine/Threonine or Tyrosine Phosphatase Assay System (Promega, Madison, Wisconsin) was used following the manufacturer’s instructions. In this assay, serine/threonine phosphatase activity was selectively measured using the serine phosphopeptide substrate, RRA(pT)VA peptide. Tyrosine phosphatases activity was measured using DADE(pY)LIPQQG peptide, which serves as substrate for most cellular tyrosine phosphatases.
Statistical Analysis
All data are expressed as mean ± SD. The method of analysis used was unpaired analysis of variance with the Student’s t-test, with data from three experiments. Differences were considered statistically significant for P < 0.05.
RESULTS
We studied the effects of acrolein, an LPO derivative and environmental pollutant, on IFNα signaling in human hepatoma cells (HepG2 cells), owing to the fact that they are a well-characterized, commonly used model system for human hepatocytes. However, we confirmed key findings in primary human hepatocytes. Additionally, we examined the effects of acrolein on HCV viral replication using an HCV replicon system.
Effect of acrolein on cell survival
Acrolein is known to be hepatotoxic and induces cell death in various cell types at high concentrations (22). To determine the appropriate non-lethal working concentrations of acrolein, we initially tested the cytotoxic effects of various concentrations of acrolein on HepG2, HCV replicon cells and primary human hepatocytes (Figure 1). We found that acrolein was relatively non-toxic at concentrations lower than 50µM (2.5µM - 50µM), and significant cell death (greater than 50%) was seen at doses higher than 75µM. As anticipated, primary hepatocytes were slightly more sensitive to acrolein compared to the established cell lines, and a statistically significant (greater than 40%) decrease in survival was seen at 50µM. Although we evaluated the effects of acrolein over a wide range of concentrations (both toxic and non-toxic), proteins and RNA were extracted only from the adherent (living) cells, and equal amounts of protein or RNA were used for further analysis.
Fig 1. Effect of Acrolein on Cell Survival.
HepG2, HCV replicon cells or primary human hepatocytes were untreated (UT) or exposed to acrolein (2.5µM - 100µM)for 30min in serum free media, after which serum was added back with further incubation for 24h. Cell viability was measured by MTT assay. Data are normalized to UT values and expressed as %UT. Data represent mean ± S.D. (n=3). * = p<0.05, compared with UT.
Effect of ACR on IFNα-induced activation of the STAT proteins in HepG2 cells
A key event in the antiviral signaling initiated by IFNα is activation by tyrosine phosphorylation of STAT1 and STAT2, followed by nuclear translocation and binding to the IFNα response elements on target genes. We evaluated the effect of acrolein on STAT1 and STAT2 tyrosine phosphorylation, which is an essential event and serves as an excellent marker for IFNα signaling. As shown in Fig. 2, IFNα stimulation resulted in tyrosine phosphorylation of both STAT1 and STAT2 in cytoplasmic extracts, while untreated HepG2 cells show no activation of STATs. A dose-dependent decrease in phosphorylated STAT1 (Fig. 2A) and STAT2 (Fig. 2B) was observed in cytoplasmic extracts of cells exposed to acrolein starting at a concentration of 10µM, without alteration of the total levels of STAT1 and STAT2. Additionally, nuclear localization of phosphorylated STAT1 and STAT2 was greatly reduced demonstrating that acrolein effectively suppressed IFNα signaling and decreased activity of STAT1 and STAT2. An important mechanism for down-regulation of the JAK/STAT pathway is the induction of newly synthesized inhibitory proteins such as SOCS and PIAS. This possibility was ruled out since inhibition by acrolein was rapid and effective within 30min, and pretreatment with the protein synthesis inhibitor cycloheximide did not abolish acrolein-mediated inhibition of STAT activation (data not shown). Additionally, protein levels of SOCS2 and PIAS1 were unchanged (data not shown).
Fig. 2. Acrolein dose dependently downregulates tyrosine phosphorylation of STAT1 (A) and STAT2 (B) in HepG2 cells.
HepG2 cells were untreated (UT), pre-treated with 0, 5, 10, 25 or 50µM acrolein in serum-free media for 30 minutes and then stimulated with 1000 U/mL IFNα for 30 minutes. Equal amounts of cytoplasmic (CE) or nuclear lysates (NE) were analyzed by SDS-PAGE followed by immunobloting with specific antibodies against tyrosine-phosphorylated STAT1 (A) or STAT2 (B), with total STAT1 and STAT2 and β-actin as controls.
Effect of acrolein on IFNα-mediated ISRE transcriptional activation in HepG2 cells
To evaluate the effect of acrolein on IFNα dependent gene transcription, we analyzed IFNα mediated transcriptional activation of an ISRE-dependent luciferase reporter construct in transiently transfected HepG2 cells treated with increasing concentrations of acrolein followed by IFNα stimulation. The IFNα-responsive reporter yielded a significant, almost twenty-fold induction of luciferase activity upon IFNα treatment (Fig. 3), while no induction was observed with acrolein alone at 25µM or 50µM. Correspondent with the observed down regulation of STAT1 activation in acrolein treated cells, IFNα-dependent ISRE reporter activity was significantly and dose-dependently reduced by pretreatment with acrolein, starting from a concentration of 10µM (Fig. 3).
Fig. 3. Acrolein dose dependently inhibits IFNα inducible ISRE-luciferase activity in HepG2 cells.
HepG2 cells transiently transfected with an ISRE-luciferase reporter construct were untreated (UT), stimulated with 1000 U/mL IFNα for 6h (IFN), or pre-treated with 5, 10, 25, 50 µM acrolein in serum-free media for 30 minutes and then stimulated with 1000 U/mL IFNα for 6h (A-5+I thru A-50+I). Total cell extracts were analyzed for luciferase activity. Data are presented as mean ± SD, n=3. * = p<0.05, compared with IFN alone.
Effect of acrolein on mRNA levels of IFNα-inducible antiviral genes in HepG2 cells
We examined the effect of acrolein on IFNα-induced gene expression in HepG2 cells. The antiviral genes PKR, OAS, ISG15 and ISG54 were used as indicators of IFNα-induced gene expression and messenger RNA levels of PKR (A), OAS (B), ISG15 (C), and ISG54 (D) were measured in HepG2 cells. Treatment of HepG2 cells with IFNα caused a large increase in mRNA levels of all the antiviral genes; fold induction of the different genes varied from approximately 3-fold for PKR to 30-fold for ISG54. Pretreatment with acrolein (10µM - 100µM) dose-dependently reduced IFNα-mediated expression of all the genes, and statistically significant down regulation was observed at or above 25µM acrolein in each case (Fig. 4). In all experiments, mRNA levels of GAPDH were used for normalization; these levels were not affected by acrolein at any concentrations, indicating that the effect of acrolein was not caused by nonspecific toxicity (not shown).
Fig. 4. Acrolein dose-dependently inhibits IFNα inducible gene expression of antiviral genes in HepG2 cells.
Messenger RNA levels of PKR (A), OAS (B), ISG15 (C), and ISG54 (D) were determined by real time PCR using total RNA from HepG2 cells that were untreated (UT), stimulated with 1000 U/mL IFNα for 2h (IFN), or pre-treated with acrolein (5uM-100uM)for 30 minutes followed by 1000U/mL IFNα for 2h (A10+IFN – A100+IFN). Data are presented as mean ± SD, n=3. * = p<0.05, compared with IFN alone.
Effect of acrolein on protein levels of IFNα-inducible PKR and OAS in HepG2 cells
We examined the effect of acrolein on cellular levels of the antiviral proteins OAS and PKR. These proteins are induced by IFNα and are known to contribute to the antiviral effects of IFNα. Pretreatment with acrolein caused a significant down regulation of IFNα-mediated expression of both proteins (Fig 5A). Densitometric analysis revealed that IFNα induced a 1.3- and 1.2-fold upregulation in the protein levels of PKR and OAS, respectively, which was inhibited by acrolein (Fig 5B).
Fig 5. Effect of acrolein on IFNα -inducible antiviral proteins PKR and OAS in HepG2 cells.
HepG2 cells were untreated (UT), pre-treated with 25µM acrolein in serum-free media for 30 minutes and then stimulated with 1000 U/mL IFNα for 24h. Equal amounts of total cellular extracts were analyzed by SDS-PAGE followed by immunobloting with specific antibodies against OAS or PKR, and with β-actin as control.
Effect of acrolein on IFNα-inducible antiviral genes in primary human hepatocytes
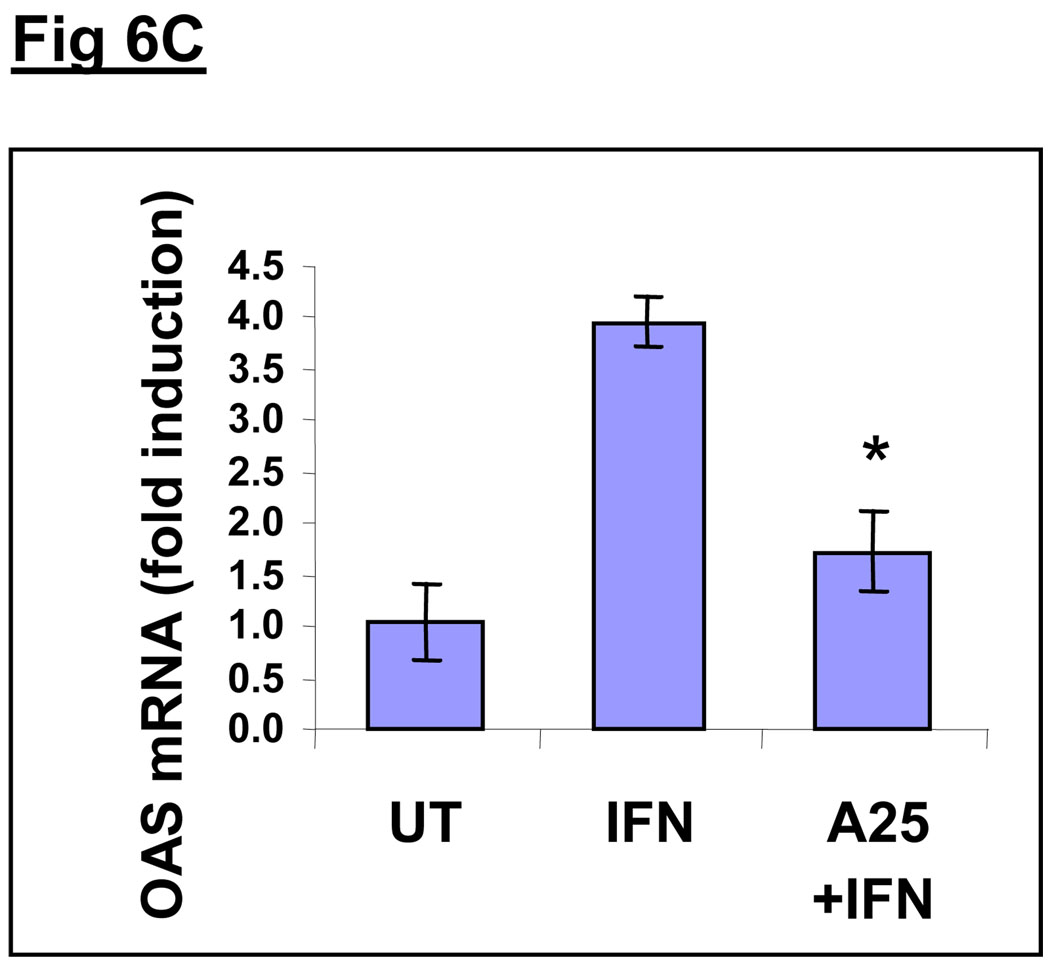
Although HepG2 cells are a well-established surrogate model system for human hepatocytes and have been extensively used for liver studies, they are immortalized, cultured hepatoma cells, which may not exhibit the same responses as primary hepatocytes. Hence, we used commercially available primary human hepatocytes to extend our HepG2 findings and validate the inhibitory effect of acrolein on IFNα-inducible antiviral genes (Fig. 6). We examined mRNA levels of the antiviral genes ISG15 (6A), ISG54 (6B) and OAS (6C) in primary human hepatocytes stimulated with IFNα without or with pretreatment with 25µM acrolein. Treatment of cells with IFNα led to a significant increase in mRNA levels of all antiviral genes. Although the magnitude of induction of each gene was different in the primary hepatocytes, the variability in magnitude was significantly less than that seen in HepG2 cells. There was an approximate 3–6 fold induction of all genes. Pretreatment with acrolein (25µM) significantly reduced IFNα-mediated expression of all antiviral genes. In all experiments, results were normalized with GAPDH mRNA levels, which were unaffected by acrolein.
Fig. 6. Acrolein dose-dependently inhibits IFNα inducible gene expression of antiviral genes in primary human hepatocytes.
Messenger RNA levels of ISG15 (A), ISG54 (B) and OAS (C) were determined by real time PCR using total RNA from primary human hepatocytes that were untreated (UT), stimulated with 1000 U/mL IFNα for 2h (IFN), or pre-treated with acrolein (25uM) for 30 minutes followed by 1000U/mL IFNα for 2h (A25+IFN). Data are presented as mean ± SD, n=3. * = p<0.05, compared with IFN.
Effect of acrolein on anti-HCV activity of IFNα in an HCV replicon system
Although the above experiments determined the effect of acrolein on hepatic anti-viral gene expression, they did not reveal any information about HCV viral replication. To determine the effect of acrolein on HCV viral replication and HCV-specific antiviral activity of IFNα, we used an HCV replicon expressing Huh7 cell line obtained from Apath LLC. This system has been well characterized and allows the direct assessment of effects on HCV viral replication. IFNα dose-dependently blocked HCV viral replication at concentrations that were much lower than those used in HepG2 cells and approximately 80% inhibition was seen at 100U/ml of IFNα (not shown). Based on this observation, we treated cells for 24h with 100U/ml IFNα, with a 30 min pretreatment with non-toxic concentrations of acrolein (5µM and 10µM), as determined by MTT-based cell survival data (Fig. 1). We observed that acrolein dose dependently reversed the antiviral effect of IFNα, allowing HCV replication to occur as efficiently as seen in UT (Fig. 7). In fact, 10µM acrolein pretreatment led to a small but reproducible increase in HCV RNA over the level seen in untreated cells. Thus, acrolein not only interfered with IFNα antiviral responses but slightly promoted HCV viral replication.
Fig. 7. Non-toxic cencentrations of acrolein dose-dependently reverse antiviral effects of IFNα on HCV Replicon.
Huh7 cells expressing the HCV replicon were untreated (UT), stimulated with100U/mL IFNα for 2h (IFN), or pre-treated with acrolein (5uM or 10uM) for 30 minutes followed by 100U/mL IFNα for 2h (A5+IFN and A10+IFN). Total cell RNA was isolated and subjected to real time PCR using HCV specific primers, with GAPDH for normalization. Data are presented as mean ± SD, n=3. * = p<0.05, compared to IFN.
Effect of acrolein on protein phosphatases in HepG2 cells
One of the ways that acrolein exposure may cause reduced IFNα signaling and gene expression is by up regulation of protein phosphatases leading to dephosphorylation of critical signaling components, such as STATs. We investigated the effects of acrolein on the enzymatic activity of serine/threonine and tyrosine-specific protein phosphatases in HepG2 cells (Fig. 8A). Cells were treated with a non-toxic concentration of acrolein (25µM) for 30min or 1h. Phosphatase activity was measured using serine- or tyrosine-specific phosphopeptide substrates. A significant increase in both serine and tyrosine phosphatase activity was seen in cells treated with acrolein for 30min and 1h. At 30min, a 1.5-fold increase was seen in serine/threonine-phosphatase activity, with an even greater rise (∼2.5-fold) in tyrosine-phosphatase activity. Parallel phosphatase assays were conducted in the presence of inhibitors to evaluate specificity using sodium orthovanadate (Na3VO4), a tyrosine phosphatase inhibitor and sodium fluoride (NaF), a serine/threonine phosphatase inhibitor. Additionally, the inhibitors were used to pretreat HepG2 cells to examine whether they would reverse the acrolein-induced inhibition of IFNα antiviral gene expression, using IFNα mediated up regulation of OAS mRNA as an indicator. As seen in Fig. 8B, IFNα induced a 13-fold increase in OAS message, which was decreased to less than 4-fold with acrolein exposure. Pretreatment of cells with NaF partially restored OAS mRNA levels to approximately 7-fold, while vanadate pretreatment raised OAS mRNA to about 9-fold. Although neither inhibitor completely restored OAS expression, partial protection was observed, suggesting that phosphatase activity contributes to the inhibitory effects of acrolein on IFNα mediated antiviral signaling. Further studies are needed to determine the exact contribution of phosphatases in the effect of acrolein.
Figure 8A. Acrolein increases protein phosphatase activity in HepG2 cells.
HepG2 cells were incubated with 25µM acrolein for 0.5h or 1h. Serine/threonine- (white bars) and tyrosine- (black bars) phosphatase activity was measured from total cell extracts as described in Materials and Methods. Parallel assays were conducted in the presence of inhibitors 100µM sodium orthovanadate (Van), a tyrosine phosphatase inhibitor and 100µM sodium fluoride (NaF), a serine/threonine phosphatase inhibitor. Each column represents the mean ± S.D. of triplicate assays. (*= p<0.05 compared to No inhibitor-0h; # = p<0.05, compared to No inhibitor-30min; ** = p<0.05 compared to 1h–No inhibitor).
Protein phosphatase inhibitors partially reverse the effect of acrolein.
Messenger RNA level of OAS was determined by real time PCR using total RNA from HepG2 cells that were untreated (UT), stimulated with 1000 U/mL IFNα for 2h, without (IFN) or with pre-treated for 30 minutes with 25µM acrolein (A25+IFN). Phosphatase inhibitors sodium floride and sodium orthovanadate were added 30min prior to 25µM acrolein, followed by 1000 U/mL IFNα for 2h (NaF+A+I and Van+A+I, respectively). Data are presented as mean ± SD, n=3. * = p<0.05, compared to A25+IFN.
DISCUSSION
Despite substantial research, the molecular mechanisms underlying resistance to IFNα therapy are not fully understood. Clinical studies have identified several modifiable cofactors that may contribute to the failure of anti-HCV therapy, including obesity, diabetes, alcohol abuse, smoking and environmental pollutants (34). Our study demonstrates that acrolein, which is both an environmental pollutant and a product of endogenous LPO, inhibits IFNα antiviral activity at non-lethal concentrations, and may have a significant impact on response to HCV therapy. Because acrolein is a reactive byproduct of LPO and can itself induce lipid peroxidation/oxidative stress, it may play a dual role in HCV disease progression by mediating oxidative injury of hepatocytes, and by actively inhibiting IFNα antiviral function.
The predominant route of environmental exposure to acrolein is inhalation of smoke or automotive exhaust. However, since acrolein is also a constituent of several food substances and has been detected in drinking water, high acrolein exposure via ingestion is also possible. Individuals likely to receive the highest exposures include smokers and those inhaling second-hand smoke, persons in close proximity to sources of wood and plastic smoke, including those in the forest products and firefighting communities, and populations living or working in areas of dense automotive traffic (22). Although acrolein exposure is expected to primarily affect respiratory organs, in vivo hepatic effects have also been reported (22). Moreover, in vitro hepatotoxicity of acrolein has been clearly documented (21). Elevated acrolein levels can also occur endogenously in the liver due to the metabolism of (i) allyl alcohol, which is widely used in food flavoring and fire retardant industries and is a well-known hepatotoxicant; (ii) anti-cancer drugs such as cyclophosphamide; and (iii) carbohydrates, lipids, polyamines, and hydroxyl-amino acids (reviewed in 21). In this study, we find that non-cytotoxic concentrations of acrolein can dramatically inhibit IFNα antiviral activity. Hence, although endogenous hepatic acrolein may not reach high cytotoxic levels, the effects on certain signaling pathways may be profound, and may lead to major defects with important disease consequences.
Acrolein has been linked to various disease conditions that exhibit enhanced lipid peroxidation and oxidative stress, including renal failure (30), Alzheimer’s disease (29, 35), diabetes (31), atherosclerosis (30, 36, 37). Our study suggests that HCV is another disease wherein acrolein may play a critical role in determining disease progression and therapy outcomes. Recent studies correlate elevated oxidative stress and lipid peroxidation in the pathogenesis of HCV (18, 19, 20). A role for oxidative stress in HCV is also supported by the fact that antioxidant therapy improves liver injury in some HCV patients (38). Indeed, the reported correlation between elevated oxidative stress/lipid peroxidation and HCV pathogenesis may be attributable, at least in part, to increases in acrolein concentrations. Importantly, acrolein adducts (specifically, acrolein-FDP-lysine adducts) have been used as a disease correlate in Alzheimer’s disease (29, 35), and type 2 diabetes (31). We are currently evaluating whether or not elevated serum levels of acrolein adducts are prognostic for poor IFNα antiviral function; these acrolein-adduct levels may serve as a potential biomarker for non-response IFNα therapy in HCV.
Acrolein is a major component of cigarette smoke (25–140 µg/cigarette). The adverse effects of smoking on the liver have recently been reviewed by El-Zayadi, and these include inflammation, apoptosis, excess iron deposition and oxidative stress and increased LPO (39). There is a growing association between smoking and severity of HCV, and associated HCC (40–44). Moreover, El-Zayadi et al. recently reported that smokers suffering from HCV tend to have a lower response rate to IFNα therapy (13). The concentrations of the acrolein metabolite S-(3-hydroxypropyl)mercaturic acid (HPMA) in the urine of smokers is shown to be 4-fold higher than in nonsmokers, where it is about 422µg/L (45), corresponding to a concentration of approximately 7.5µM of acrolein. Also, endogenous generation of acrolein in humans due to LPO is ∼10-fold higher than that of HNE. Undoubtedly, localized tissue-specific concentrations of acrolein at sites of generation or exposure are likely to be higher, particularly in disease conditions. Hence, the concentrations of acrolein used in this study are within the range of human exposure and levels of acrolein that are associated with pathological states. Our data demonstrate the direct inhibitory effects of acrolein on IFNα antiviral signaling in hepatocytes. The effects were seen at low, non-toxic levels of acrolein, suggesting that they are independent of apoptosis and the hepatotoxicity associated with acrolein. Thus, in addition to significantly contributing to cigarette smoke-induced inflammation and hepatic injury, acrolein may directly lower response to IFNα therapy, thereby promoting HCV disease progression.
In our study, we observed that acrolein up regulated the catalytic activity of both tyrosine and, to a lesser degree, serine/threonine phosphatases, suggesting one possible mechanism of inhibition of IFNα signaling by acrolein. Interestingly, another LPO aldehyde, 4-hydroxynonenal (HNE) is known to activate the serine/threonine phosphatase PP2A, albeit in a different cell type (46). We saw a 1.5-fold increase of ser/thr phosphatase activity and an increase in tyr-phosphatase of almost 2.5-fold. Since both sodium orthovanadate and sodium floride only partially reversed the effects of acrolein in this study, the exact mechanism of acrolein inhibition of IFNα antiviral activity may be multifactorial. Interestingly, oxidative stress induced by hydrogen peroxide inhibits IFNα mediated JAK–STAT signaling without activation of phosphatases (14). In seeming contrast to our observations, a recent report has demonstrated that acrolein, in fact, inactivates a tyrosine phosphatase (47). The authors observed that in vitro exposure to acrolein of the purified catalytic subunit of human protein tyrosine phosphatase 1B resulted in its potent time-dependent inactivation. However, the concentration of acrolein required to achieve a half-maximal rate of inactivation, Ki, was determined to be 2.3 ± 0.6 × 10−4 M, which is almost 10-fold higher than the effective acrolein concentration used here (25µM); this may explain the divergence of the results. Further studies using specific siRNA inhibition will be needed to unequivocally establish the contribution of phosphatases in the inhibition of IFNα antiviral function by acrolein.
Overall, our findings suggest that inhibition of the IFNα mediated JAK/STAT pathway due to increased acrolein concentrations in the liver could be one of the mechanisms explaining the resistance to antiviral action of IFNα in HCV infected patients with complicating conditions associated with high level of oxidative stress and LPO in the liver, such as advanced age, alcohol, iron overload, steatosis, fibrosis, as well as exposure to environmental and other hepatotoxins. Further studies are necessary to elucidate the exact mechanisms involved in the inhibition of IFNα signaling by acrolein in hepatocytes, and to determine whether or not acrolein adducts can serve as a biomarker for poor response to HCV therapy.
Acknowledgements
This research was supported by NIH grants (McClain and S Barve), VA (McClain) and AASLD-Sheila Sherlock Award (Cave).
List of Abbreviations
- HCV
Hepatitis C virus
- IFNα
interferon-alpha
- JAK
Janus kinase
- STAT
signal transducer and activator of transcription
- ISGF
interferon-stimulated gene factor
- ISG
interferon-stimulated genes
- ISRE
interferon-α-stimulated response elements
- IRF
interferon regulatory factor
- OAS
2’5’-oligoadenylate synthetase 1
- PKR
dsRNA-dependent protein kinase
- GSH
glutathione
- ELISA
Enzyme linked immunosorbant assay
- Mrna
messenger ribonucleic acid
- RT-PCR
real time polymerase chain reaction
- GAPDH
glyceraldehyde-3-phosphate-dehydrogenase
- LPO
lipid peroxidation, IFNAR1, Interferon-alpha receptor 1 and IFNAR2, Interferon-alpha receptor 2
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- 1.World Health Organization Fact Sheet #164. 2000 [PubMed] [Google Scholar]
- 2.Chou R, Clark EC, Helfand M. Screening for hepatitis C virus infection: a review of the evidence for the U.S. Preventive Services Task Force. Ann Intern.Med. 2004;140(6):465–479. doi: 10.7326/0003-4819-140-6-200403160-00014. [DOI] [PubMed] [Google Scholar]
- 3.Davis GL, ’ Albright JE, Cook SF, Rosenberg DM. Projecting future complications of chronic hepatitis C in the United States. Liver Transplant. 2003;9:331–338. doi: 10.1053/jlts.2003.50073. [DOI] [PubMed] [Google Scholar]
- 4.Benvegnu L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53(5):744–749. doi: 10.1136/gut.2003.020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ikeda K, Saitoh S, Suzuki Y, et al. Disease progression and hepatocellular carcinogenesis in patients with chronic viral hepatitis: a prospective observation of 2215 patients. J. Hepato.l. 1998;28(6):930–938. doi: 10.1016/s0168-8278(98)80339-5. [DOI] [PubMed] [Google Scholar]
- 6.Ferenci P. Pegylated interferon plus ribavirin for chronic hepatitis C: the role of combination therapy today, tomorrow and in the future. Minerva Gastroenterol Dietol. 2006;52(2):157–174. [PubMed] [Google Scholar]
- 7.Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis natural history, treatment, and prevention of hepatitis. C. Ann. Intern. Med. 2000;132(4):296–305. doi: 10.7326/0003-4819-132-4-200002150-00008. [DOI] [PubMed] [Google Scholar]
- 8.Fried MW, Shiffman ML, Reddy R, Smith C, Marino G, Goncales F, et al. Peg-interferon alfa-2a plus ribavirin for chronic hepatitis C. N. Engl. J. Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 9.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines. J.Biol.Chem. 2007;282(28):20059–20063. doi: 10.1074/jbc.R700016200. [DOI] [PubMed] [Google Scholar]
- 10.Feld JJ, Hoofnagle JH. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature. 2005;436(7053):967–972. doi: 10.1038/nature04082. [DOI] [PubMed] [Google Scholar]
- 11.Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu. Rev. Biochem. 1998;67:227–264. doi: 10.1146/annurev.biochem.67.1.227. [DOI] [PubMed] [Google Scholar]
- 12.Takaoka A, Yanai H. Interferon signaling network in innate defence. Cell Microbiol. 2006;8(6):907–922. doi: 10.1111/j.1462-5822.2006.00716.x. [DOI] [PubMed] [Google Scholar]
- 13.El-Zayadi A, Selim O, Hamdy H, El-Tawil A, Hanaa M. Impact of cigarette smoking on response to interferon therapy in chronic hepatitis C Egyptian patients. World J. Gastroenterol. 2004;10(20):2963–2966. doi: 10.3748/wjg.v10.i20.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahmood S, Kawanaka M, Kamei A, Izumi A, Nakata K, Niiyama G, et al. Immunohistochemical evaluation of oxidative stress markers in chronic hepatitis C. Antioxid. Redox. Signal. 2004;6(1):19–24. doi: 10.1089/152308604771978318. [DOI] [PubMed] [Google Scholar]
- 15.Konishi M, Iwasa M, Araki J, Kobayashi Y, Katsuki A, Sumida Y, et al. Increased lipid peroxidation in patients with non-alcoholic fatty liver disease and chronic hepatitis C as measured by the plasma level of 8-J isoprostane. J.Gastroenterol.Hepatol. 2006;21(12):1821–1825. doi: 10.1111/j.1440-1746.2006.04420.x. [DOI] [PubMed] [Google Scholar]
- 16.Naoki F, Shinichiro H, Ryosuke S, Hideaki T, et al. Hepatic oxidative DNA damage correlates with iron overload in chronic hepatitis C patients. Free Radical Biology & Med. 2007;42:353–362. doi: 10.1016/j.freeradbiomed.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 17.Paradis V, Mathurin P, Kollinger M, Imbert-Bismut F, Charlotte F, Piton A, et al. In situ detection of lipid peroxidation of chronic hepatitis C: correlation with pathological features. J. Clin. Pathol. 1997;50:401–406. doi: 10.1136/jcp.50.5.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larrea E, Beloqui O, Munoz-Navas MA, Civeira MP, Prieto J. Superoxide dismutase in patients with chronic hepatitis C virus infection. FreeRadic. Biol. Med. 1998;24:1235–1241. doi: 10.1016/s0891-5849(97)00437-1. [DOI] [PubMed] [Google Scholar]
- 19.Barbaro G, Di Lorenzo G, Ribersani M, Soldini M, Giancaspro G, Bellomo G, et al. Serum and hepatic glutathione concentrations in chronic hepatitis C patients related to hepatitis C virus genotype. J. Hepatol. 1999;30:774–782. doi: 10.1016/s0168-8278(99)80128-7. [DOI] [PubMed] [Google Scholar]
- 20.Di Bona D, Cippitelli M, Fionda C, Camma C, Licata A, Santoni A, Craxi A. Oxidative stress inhibits IFN-alpha-induced antiviral gene expression by blocking the JAK-STAT pathway. J Hepatol. 2006;45(2):271–279. doi: 10.1016/j.jhep.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 21.Stevens JF, Maier CS. Acrolein: Sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52(1):7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.EPA. Toxicological profile for acrolein. U.S. Department of Health and Human Services - Agency for Toxic Substances and Disease Registry; 2007. [Google Scholar]
- 23.Lambert C, McCue J, Portas M, Ouyang Y, Li J, Rosano TG, et al. Acrolein in cigarette smoke inhibits T-cell responses. J Allergy Clin Immunol. 2005;116(4):916–922. doi: 10.1016/j.jaci.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 24.Kehrer JP, Biswal SS. The molecular effects of acrolein, Toxicol. Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- 25.Biswal S, Acquaah-Mensah G, Datta K, Wu X, Kehrer JP. Inhibition of cell proliferation and AP-1 activity by acrolein in human A549 lung adenocarcinoma cells due to thiol imbalance and covalent modifications. Chem. Res. Toxicol. 2002;15:180–186. doi: 10.1021/tx015552p. [DOI] [PubMed] [Google Scholar]
- 26.Ranganna K, Yousefipour Z, Nasif R, Yatsu FM, Milton SG, Hayes BE. Acrolein activates mitogen-activated protein kinase signal transduction pathways in rat vascular smooth muscle cells. Mo.l Cell Biochem. 2002;240:83–98. doi: 10.1023/a:1020659808981. [DOI] [PubMed] [Google Scholar]
- 27.Luo J, Shi R. Acrolein induces axolemmal disruption, oxidative stress and mitochondrial impairment in spinal cord tissue. Neurochem. Int. 2004;44:475–486. doi: 10.1016/j.neuint.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Wu X, Choi YE, Kern JC, Kehrer JP. Effect of acrolein and glutathione, depleting agents on thioredoxin. Toxicology. 2004;204:209–218. doi: 10.1016/j.tox.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 29.Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer’s disease. J. Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- 30.Mercado C, Jaimes EA. Cigarette smoking as a risk factor for atherosclerosis and renal disease: novel pathogenic insights. Curr. Hypertens. Rep. 2007;9(1):66–72. doi: 10.1007/s11906-007-0012-8. [DOI] [PubMed] [Google Scholar]
- 31.Daimon M, Sugiyama K, Kameda W, Saitoh T, Oizumi T, Hirata A, et al. Increased urinary levels of pentosidine, pyrraline and acrolein adduct in type 2 diabetes. Endocr. J. 2003;50:61–67. doi: 10.1507/endocrj.50.61. [DOI] [PubMed] [Google Scholar]
- 32.Joshi-Barve S, Barve SS, Butt W, Klein J, McClain T, Hirata CJ. Inhibition of proteasome function leads to NF-KB-independent IL-8 expression in human hepatocytes. Hepatology. 2003;38(5):1178–1187. doi: 10.1053/jhep.2003.50470. [DOI] [PubMed] [Google Scholar]
- 33.Richie JP, Jr, Lang CA. The determination of glutathione cyst(e)ine,and other thiols and disulfides in biological samples using high-performance liquid chromatography with dual electrochemical detection. Anal. Biochem. 1987;163:9–15. doi: 10.1016/0003-2697(87)90085-6. [DOI] [PubMed] [Google Scholar]
- 34.De Francesco R, Migliaccio G. Challenges and successes in developing new therapies for hepatitis C. Nature. 2005;436:953–960. doi: 10.1038/nature04080. [DOI] [PubMed] [Google Scholar]
- 35.Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer’s disease brain and is toxic to primary hippocampal cultures. Neurobiol. Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- 36.Park YS, Taniguchi N. Acrole in induces inflammatory response underlying endothelial dysfunction: a risk factor for atherosclerosis. Ann. N. Y. Acad. Sci. 2008;1126:185–189. doi: 10.1196/annals.1433.034. [DOI] [PubMed] [Google Scholar]
- 37.Park YS, Kim J, Misonou Y, Takamiya R, Takahashi M, Freeman MR, Taniguchi N. Acrolein induces cyclooxygenase-2, and prostaglandin production in human umbilical vein endothelial cells: roles of p38 MAP kinase. Arterioscler. Thromb. Vasc. Biol. 2007;27(6):1319–1325. doi: 10.1161/ATVBAHA.106.132837. [DOI] [PubMed] [Google Scholar]
- 38.Mahmood S, Yamada G, Niiyama G, Kawanaka M, Togawa K, Sho M, Ito T, Sasagawa T, Okita M, Nakamura H, Yodoi J. Effect of vitamin E on serum aminotransferase and thioredoxin levels in patients with viral hepatitis. C.Free Radic. Res. 2003;37:781–785. doi: 10.1080/1071576031000102141. [DOI] [PubMed] [Google Scholar]
- 39.El-Zayadi AR. Heavy smoking and liver. World J Gastroenterol. 2006;12(38):6098–6101. doi: 10.3748/wjg.v12.i38.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pessione, Ramond MJ, Njapoum C, Duchatelle V, Degott C, Erlinger S, et al. Cigarette smoking and hepatic lesions in patients with chronic hepatitis C. Hepatology. 2001;34:121–125. doi: 10.1053/jhep.2001.25385. [DOI] [PubMed] [Google Scholar]
- 41.Fujita Y, Shibata A, Ogimoto I, Kurozawa Y, Nose T, Yoshimura T, et al. The effect of interaction between hepatitis C virus and cigarette smoking on the risk of hepatocellular carcinoma. Br. J. Cancer. 2006;94:737–739. doi: 10.1038/sj.bjc.6602981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dev A, Patel K, Conrad A, Blatt LM, McHutchison JG. Relationship of smoking and fibrosis in patients with chronic hepatitis C. Clin. Gastroenterol. Hepatol. 2006;4:797–801. doi: 10.1016/j.cgh.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 43.Hezode C, Lonjon I, Roudot-Thoraval F, Mavier JP, Pawlotsky JM, Zafrani ES, et al. Impact of smoking on histological liver lesions in chronic hepatitis C. Gut. 2003;52:126–129. doi: 10.1136/gut.52.1.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang CS, Wang ST, Chang TT, Yao WJ, Chou P. Smoking and alanine aminotransferase levels in hepatitis C virus infection: implications for prevention of hepatitis C virus progression. Arch. Int. Med. 2002;162:811–815. doi: 10.1001/archinte.162.7.811. [DOI] [PubMed] [Google Scholar]
- 45.Caramella SG, Chen M, Zhang Y, Zhang S, Hatsukami DK, Hecht SS. Quantitation of acrolein-derived (3-hydroxypropyl)mercapturic acid in human urine by liquid chromatography-atmospheric pressure chemical ionization tandem mass spectrometry: effects of cigarette smoking. Chem. Res. Toxicol. 2007;20:986–990. doi: 10.1021/tx700075y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu W, Akhand AA, Takeda K, Kawamoto Y, Itoigawa M, et al. Protein phosphatase 2A–linked and -unlinked caspase-dependent pathways for downregulation of Akt kinase triggered by 4-hydroxynonenal. Cell Death and Differentiation. 2003;10:772–781. doi: 10.1038/sj.cdd.4401238. [DOI] [PubMed] [Google Scholar]
- 47.Seiner DR, Labutti JN, Gates KS. Kinetics and mechanism of protein tyrosine phosphatase 1B inactivation by acrolein. Chem. Res. Toxicol. 2007;20:1315–1320. doi: 10.1021/tx700213s. [DOI] [PMC free article] [PubMed] [Google Scholar]