Abstract
The extracellular matrix (ECM) is a complex organization of structural proteins found within tissues and organs. Heterogeneous tissues with spatially and temporally modulated properties play an important role in organism physiology. Here we present a benzophenone (BP) based direct, photolithographic approach to spatially pattern solution phase biomolecules within collagen-GAG (CG) scaffolds and demonstrate creation of a wide range of patterns composed of multiple biomolecular species in a manner independent from scaffold fabrication steps. We demonstrate the ability to immobilize biomolecules at surface densities of up to 1000 ligands per square micron on the scaffold strut surface and to depths limited by the penetration depth of the excitation source into the scaffold structure. Importantly, while BP photopatterning does further crosslink the CG scaffold, evidenced by increased mechanical properties and collagen crystallinity, it does not affect scaffold microstructural or compositional properties or negatively influence cell adhesion, viability, or proliferation. We show that covalently photoimmobilized fibronectin within a CG scaffold significantly increases the speed of MC3T3-E1 cell attachment relative to the bare CG scaffold or non-specifically adsorbed fibronectin, suggesting that this approach can be used to improve scaffold bioactivity. Our findings, on the whole, establish the use of direct, BP photolithography as a methodology for covalently incorporating activity-improving biochemical cues within 3D collagen biomaterial scaffolds with spatial control over biomolecular deposition.
Keywords: Collagen, Scaffold, Micropatterning, Photolithography, Cell adhesion, Surface modification
1. Introduction
The extracellular matrix (ECM) is a complex organization of structural proteins such as collagens and proteoglycans. A wide variety of tissue engineering scaffolds have been created in attempts to mimic features of the native ECM. Notably, microstructural features [1–3], mechanical properties [4–6], and inclusion of soluble or insoluble biomolecules [7,8] have all been shown to significantly influence cell behaviors such as adhesion, growth, and differentiation as well as to affect material bioactivity for in vivo applications. Heterogeneous tissues with spatially and temporally modulated properties and their biomaterial mimics play an important role in organism physiology and regenerative medicine [9,10]. An example of particular relevance is the graded interfacial region found between bone and tendon in the musculoskeletal system, which contains complex compositional, microstructural, and mechanical patterns; the gradient interface reduces formation of interfacial stress concentrations that can lead to interface failure while maintaining distinct tendon and osseous compartments [11,12].
With the understanding that the microstructure, mechanics, and composition of the ECM is dynamic and often spatially patterned or heterogeneous over the length scale of traditional biomaterials, there has recently been significant effort aimed at moving away from static, monolithic biomaterials toward instructive biomaterials that provide specialized cell behavioral cues in spatially and temporally defined manners [13,14]. These materials hypothetically recapitulate aspects of the dynamic and spatially heterogeneous constellation of cues presented by the ECM. While majority of this effort has been applied toward strategies to create discrete and gradient patterns on two-dimensional substrates [15–17], recently a number of approaches to generate and then temporally modify and/or remove microenvironmental patterns in 3D hydrogel systems have been described [18–20]. However similar methods for achieving biomolecular patterning have yet to be achieved for scaffold-based biomaterials. Scaffolds offer advantages in the form of independent modulation of scaffold microstructural, mechanical, and compositional properties as well as the potential to separate scaffold fabrication and cell integration steps [21]. Their open-cell nature can enable improved cell infiltration and metabolite diffusion compared to hydrogels. Collagen-glycosaminoglycan (CG) scaffolds have long been utilized as ECM analogs for regenerative medicine applications [2,8,21–23]. Recently methods to create CG scaffolds with a range of controllable pore microstructures and mechanical properties have been developed to study the effect of scaffold microenvironment on cell attachment, migration, and contraction [1,3,24,25]. Experimental characterization and theoretical modeling techniques have also been developed to describe the microstructural (pore size, specific surface area, permeability) and mechanical properties as well as cell behavior (attachment, viability, differentiation) within these variants [1,3,24–27]. Growth factors, plasmids, and genes have been immobilized to collagen scaffolds [7,8], but these methods do not offer the capability to spatially modulate biomolecule distribution within a single construct.
The development of molecularly general approaches to spatially control the presentation of multiple biomolecules within porous scaffolds is an important goal for creating advanced biomaterials. While soluble and insoluble presentation of biomolecular factors have both been studied in the context of biomaterials for tissue engineering applications, here we concentrate on methods to create spatial patterns of surface-immobilized biomolecules within a CG scaffold. Additionally, many adhesion ligands, growth factors, and other biomolecules are typically sequestered as opposed to freely soluble within the ECM [28]. Biomolecule immobilization has further shown benefits relative to bolus or even controlled delivery of soluble growth factors [7]; explanations include extended biomolecule half-life, elimination of diffusive dilution [7], and avoidance of cellular uptake that limits long term bioactivity. While a number of methods have been developed for creating spatial patterns of surface-immobilized biomolecules on 2D surfaces [16,29–31], many of these approaches are not amenable to patterning within porous scaffolds where conformal contact and/or confinement of fluid flow cannot be readily achieved.
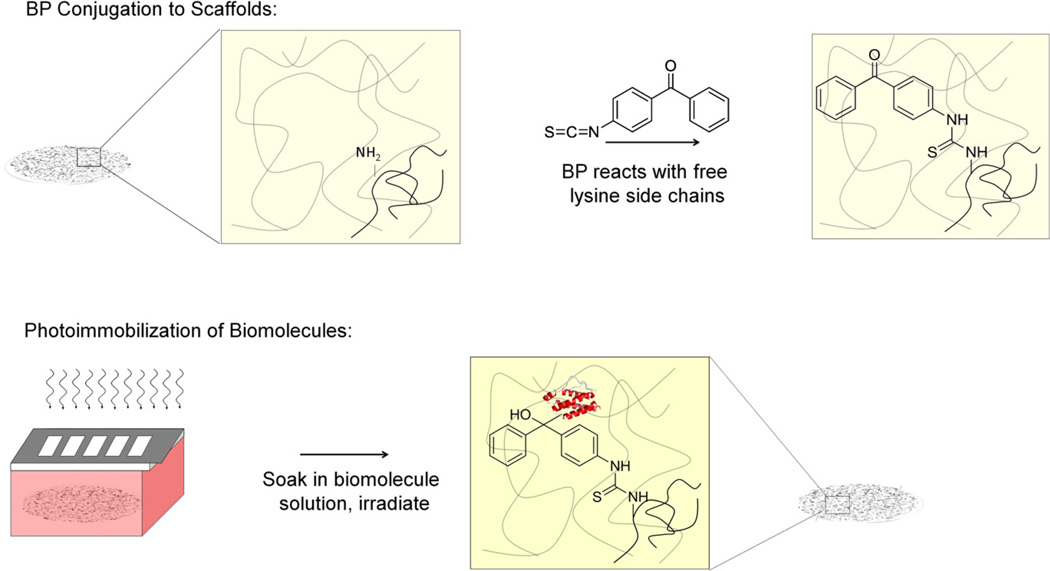
Recently, we reported a direct photolithographic method for covalently attaching biomolecules onto 2D surfaces; here substrates are uniformly immersed in the biomolecule of interest and immobilization is controlled solely by the presence (or absence) of incident light [32]. This method takes advantage of the photochemistry of surface-attached benzophenone (BP). Upon excitation with UV light, BP forms a transient diradical that can react with nearby C–H bonds from an adjacent biomolecule, forming a C–C covalent bond. Since the BP is surface-immobilized, the new C–C bond represents a covalent tether between the biomolecule and the substrate. Importantly, if BP does not react with a nearby molecule within the excited state lifetime it relaxes back to the ground state from which it can be re-excited with subsequent optical pumping. Since the attachment occurs only where light is incident, geometric patterns and gradients of biomolecules can be generated by controlling the spatial exposure of light across the substrate (Fig. 1). Since the spatial confinement of light is the only requirement for patterning, we reasoned that this technique could be extended to porous, 3D scaffolds.
Fig. 1.
BP Conjugation and Biomolecular Conjugation. A) BP-isothiocyanate is conjugated through free amino-groups on the collagen-GAG scaffold. B) Scaffolds are immersed in a biomolecular solution of interest and exposed to UV light. Covalent immobilization of biomolecules is dependent on the incidence of light.
This manuscript describes a BP patterning approach to spatially modulate attachment of biomolecules within CG scaffolds. While CG scaffold microstructural, mechanical, and bulk compositional properties, as well as monolithic immobilization of growth factors, plasmid, and gene delivery vectors, have been shown to affect scaffold bioactivity, spatial patterning of key biomolecular regulators within the scaffold structure has not previously been achieved. Here we investigate the effects of BP photolithographic patterning on the microstructural and mechanical properties as well as the native bioactivity of a CG scaffold variant. We then probe the ability to create single and multi-component biomolecule patterns as well as the ability for BP photolithographic patterning to impart additional, specific bioactivity to the CG scaffold. Our findings, on the whole, establish the use of direct photolithography as a robust method for generating scaffolds with spatially patterned biomolecules.
2. Materials and methods
All reagents were purchased from Sigma–Aldrich (St. Louis, MO) unless otherwise noted.
2.1. CG scaffold fabrication
CG scaffolds were fabricated via freeze drying from a suspension of type I microfibrillar collagen from bovine dermis (Devro Inc., Columbia, SC) and chondroitin sulfate derived from shark in 0.05 m acetic acid [25]. This process has been previously optimized to produce a range of CG scaffold variants with a uniform pore microstructure with regular, polyhedral pores [1,25,26,33,34]. Briefly, a degassed CG suspension was added to an aluminum mold and placed in a freeze dryer (VirTis Genesis, Gardiner, NY) at room temperature. The suspension was cooled at a constant rate (1.0 °C/min) to −4 °C and held there for 2 h to allow the suspension temperature to equilibrate; the suspension was then further cooled to a final freezing temperature of −40 °C at 1.0 °C/min, resulting in a continuous, interpenetrating network of ice crystals surrounded by the CG co-precipitate. Ice crystals were removed via sublimation under vacuum (200 mTorr) to produce a highly porous CG scaffold structure defined by individual fibers of CG content, termed struts. Scaffolds were dehydrothermally crosslinked and sterilized at 105 °C for 24 h under vacuum (<25 torr) in a vacuum oven (Welch, Niles, IL) prior to use [35].
2.2. Chemical attachment of BP to CG scaffolds
A 20 mm solution of benzophenone-4-isothiocyanate containing 0.5 m N,N-diisopropylethylamine was prepared in dimethyl formamide (DMF) via an established method [36]. CG scaffolds were added to the solution and allowed to react at room temperature in the dark for 48 h. Scaffolds were thoroughly rinsed three times in DMF, 200 proof ethanol, and water (ELGA LabWater Reservoir, Veolia Water Systems, Buckinghamshire, UK) each for 1 h; BP-tethered CG scaffolds were then stored under water and in the dark.
2.3. Photoattachment of biomolecules onto BP-presenting CG scaffolds
Biotinylated concanavalin A (ConA-biotin) was purchased from Vector Laboratories (Burlingame, CA), fibronectin (FN) was purchased from Invitrogen (Carlsbad, CA) and N-Cadherin (NC) was purchased from R&D Systems (Minneapolis, MN). Stock solutions of biomolecules were prepared by resuspending the lyophilized proteins in the manufacturer’s recommended buffer solutions to a concentration of 1 mg/mL. For ConA-biotin and FN, the buffer was phosphate buffered saline, pH = 7.4 (PBS); PBS containing Ca2+ and Mg2+ was used for NC. The solutions were aliquotted and stored at −20 °C. Immediately prior to use, the stock solutions were diluted in the respective buffers to yield the empirically determined final solution concentrations of 5 µg/mL ConA-biotin or 100 µg/mL FN.
BP-modified scaffolds were placed onto a microscope slide and surrounded by a rubber o-ring. A 20 µL aliquot of protein solution was added to the top of the hydrated scaffolds and the chamber assembled by placing a glass coverslip on top. Scaffolds were soaked in protein solutions for 1 h at room temperature prior to photoimmobilization. Photoimmobilization was then performed with an argon ion laser (Coherent Innova 90-4, Laser Innovations, Santa Paula, CA) with UV optics providing illumination at 351.1–363.8 nm. The Gaussian beam profile was shaped and expanded to give a uniform illumination of the scaffold using a π-shaper (Molecular Technologies, Berlin, Germany) and beam expanding optics. The uniformity of illumination was ensured using beam profiler (Ophir-Spiricon, Logan, UT). The laser was adjusted to give a power of 14 mW/cm2 at the illumination scaffold surface. For biomolecular patterning, a chromium-coated quartz mask was placed metal side down onto the chamber housing the CG scaffold and the substrate irradiated for set times varying from 30 s to 5 min. Bulk exposure was achieved in the same way except without the photomask. Following irradiation, the scaffolds were immersed in a solution containing 0.2% pluronic F-127 in PBS for 1 h. For 2- component patterning, scaffolds were subsequently washed for 1 h in PBS and then incubated for 1 h in the appropriate secondary component protein solution prior to a second patterning step, performed as described above. After immobilization, scaffolds were either stored in PBS prior to compositional analysis, structural characterization, and cell experiments, or were incubated in 1% (w:v) bovine serum albumin in PBS prior to microscopic visualization of biomolecular patterns.
2.4. Fluorescent visualization of biomolecular patterns
Biomolecularly patterned scaffolds were removed from the storage buffer and placed in a solution containing a fluorescently-labeled binding partner for at least 1 h. Patterned ConA-biotin was visualized after incubation with a solution of 5 µm Qdot 525 conjugated streptavidin (Invitrogen, Carlsbad, CA) in 1% BSA-PBS. NC patterns were visualized with a pre-mixed cocktail of sheep anti-human NC (1 µg/mL, R&D Systems, Minneapolis, MN) and Alexa Fluor 647-conjugated donkey anti-sheep IgG (0.5 µg/mL, Invitrogen, Carlsbad, CA) in 1% BSA-PBS with Ca2+ and Mg2+. FN patterns were visualized with a pre-mixed cocktail of biotinylated rabbit anti-human FN (1 µg/mL, AbCam, Cambridge, MA) and Alexa Fluor 568-conjugated streptavidin (0.5 µg/ mL, Invitrogen, Carlsbad, CA) in 1% BSA-PBS with Ca2+ and Mg2+. Two-component patterns were simultaneously stained for both protein components. After staining, scaffolds were rinsed in PBS prior to imaging on a LSM 710 confocal microscope (Carl Zeiss Microimaging, GmbH, Germany). Image analysis was performed using Imaris 7.0 (Bitplane AG, Zurich, Switzerland) to render 3D fluorescent images.
2.5. Determination of photoimmobilized ligand density
The amount of biomolecule photochemically attached to the scaffold was determined using a modified radioimmunoassay. Streptavidin was radiolabeled with [I125] using Pierce iodination tubes (Pierce, Rockford, IL), and purified using spin filter columns (Biorad, Hercules, CA). The percentage of free [I125] present in the sample was determined to be less than 3% and the concentration of protein in the sample was determined using a Bradford assay. Separately, a ConA-biotin-containing solution was introduced to the scaffolds before exposure (bulk, no patterning) for varying amounts of time. The modified scaffolds were then incubated with an excess of [I125]-streptavidin for 1 h before unattached streptavidin was removed by soaking in PBS for 1 h. The scaffold was then covered with scintillation fluid (ScintiSafe Econo 1, Fisher Scientific, Pittsburgh, PA) and the total radioactive counts per minute were measured from the scaffold using a Beckman LS 6500 liquid scintillation counter (Beckman Coulter, Brea, CA). This value was then converted to the number of streptavidin molecules on the scaffold via the specific activity and the amount of bound ConA-biotin determined by assuming a 1:1 binding relationship.
The number of immobilized proteins was converted to a ligand density by dividing the number of conjugated molecules by the scaffold surface area as determined by cellular solids modeling. Cellular solids modeling has been a useful tool for the microstructural characterization of low density, open-cell foams such as CG scaffolds [37]. The specific surface area (surface area/volume; SA/V) of the scaffold was calculated as a function of pore diameter (d) and the relative density (ratio of the scaffold density to the density of the solid material it is composed from: ρ*/ρs) [1]:
2.6. Microstructural, mechanical, and compositional analysis
2.6.1. Scaffold pore size and shape
Mean pore sizes and aspect ratios were calculated for CG and BP-tethered CG (CG-BP) scaffold variants to determine the effect of BP functionalization on CG scaffold microstructure. The size and shape of CG scaffold variants were determined via a previously described stereology approach [33]. Briefly, full thickness, 8 mm diameter samples were removed from the CG scaffolds with a biopsy punch (Miltex) and embedded in glycolmethacrylate. Longitudinal and transverse scaffold sections (5 µm thick) were cut on a microtome and stained with aniline blue to allow visualization of the CG strut network. Images were acquired from these specimens at 10× magnification on an optical microscope (Leica Microsystems, Germany) and analyzed using a linear intercept macro in Scion Image to determine both the mean pore size and the pore aspect ratio from a best fit ellipse representation of the average pore in each image.
2.6.2. Scaffold mechanical properties
Tensile tests were performed on hydrated CG and CG-BP scaffolds in order to determine the effect of BP functionalization on scaffold mechanical properties. Tests were performed on rectangular scaffold samples (8 mm × 25 mm × 3.5 mm thick); scaffolds were hydrated in PBS for 24 h and then tested in tension with an MTS Instron 2 (Eden Prairie, MN) using rubberized grips to prevent sample slip. Scaffolds were pulled to failure at a rate of 1 mm/min; scaffold tensile elastic modulus (Es, tension) was calculated from the slope of the stress-strain curve over a strain range of 5–10% [37].
2.6.3. Scaffold composition
X-ray diffraction (XRD) analyses were used to determine whether any significant changes in the crystallinity or chemical nature of the CG scaffold occurred with BP functionalization; XRD has previously been used to quantify CG scaffold composition following integration of variable levels of mineral content (0–80 wt.%) [22]. XRD analyses were performed using a Cu Kα radiation source with a Rigaku D-Max diffractometer. Diffraction patterns were acquired using a step size of 0.028° 2θ and a dwell time of 10 s.
2.7. Cell culture and scaffold seeding
MC3T3-E1 mouse clonal osteogenic cells were cultured in standard culture flasks in α-MEM supplemented with 10% fetal bovine serum, 1% l-glutamine, and 1% penicillin/streptomycin. Cells were fed every 3 days and cultured to confluence at 37 °C and 5% CO2. Prior to each experiment, full thickness (3.5 mm thick), 6 mm diameter CG scaffold samples were cut with a biopsy punch (Miltex) and then hydrated (>1 h) in culture media. MC3T3-E1 cells were then trypsinized and resuspended at a concentration of 3 × 105 cells per 20 µL media or 1 × PBS. 1.5 × 105 cells in 10 µL media were seeded onto each side of the hydrated scaffold disks in a previously described manner [3] to a final concentration of 3 × 105 cells per scaffold. Cell-seeded scaffolds were then incubated in ultra-low attachment 6-well plates (Corning Life Sciences, Lowell, MA) at 37 °C and 5% CO2 for the duration of all experiments. For longer (>30 min attachment) experiments, 4 mL of supplemented α-MEM was added to each well and changed every 3 days.
2.8. Quantifying cell attachment
A previously developed cell attachment assay was used to determine the total number of cells attached to the scaffold. At each time point, scaffolds were first washed in PBS to remove any unattached cells and then placed in a papain solution to digest the scaffold and lyse the cells to liberate their DNA. A Hoechst 33258 dye (Invitrogen, Carlsbad, CA) was used to fluorescently label double-stranded DNA [38] and fluorescence levels from each sample were read using a fluorescence spectrophotometer (Varian, Santa Clara, CA): 352 nm excitation, 461 nm emission. Experimental readings were then compared to a standard curve created by measuring the fluorescence levels for a range of known cell numbers to determine cell attachment at each time point as a percentage of the total number of seeded cells.
2.9. Characterizing cell bioactivity
A non-destructive alamarBlue approach was used to compare the metabolic activity of the cells in each cell-seeded scaffold over time. Healthy cells continuously reduce resazurin, the active ingredient in alamarBlue, to the highly fluorescent compound resorufin; consistent exposure times enable comparison of the gross metabolic activity in each cell-seeded construct. Cell-seeded scaffolds were incubated at 37 °C in alamarBlue (Invitrogen, Carlsbad, CA) solution with gentle shaking for 120 min [39]. Resorufin fluorescence was read at 570 nm excitation and 585 nm emission using a fluorescent spectrophotometer. A standard curve was created by measuring the metabolic activity of a range of known cell numbers. Scaffold fluorescence readings were interpolated on this curve to express results as a percentage of the total number of seeded cells.
2.10. Statistical analysis
One-way analysis of variance (ANOVA) was performed on cell bioactivity, cell number, and mechanical data sets respectively followed by Tukey-HSD post-hoc tests. Paired student t-tests were used to compare CG and CG-BP scaffold pore size and shape. Significance was set at p<0.05. At least n = 6 scaffolds were analyzed at each time point for cell bioactivity while n=6 scaffolds were digested and assayed at each time point for cell number. Each group for mechanical tests contained n = 6 scaffolds. Pore size and shape analysis was performed on transverse (n = 3) and longitudinal (n = 3) scaffold sections from each scaffold variant (36–54 discrete cross-sectional histology images). Error is reported as the standard error of the mean unless otherwise noted.
3. Results
Throughout, CG scaffold variants will be referred to as CG, CG-DMF, CG-BP, and CG-BP-X for unmodified CG scaffolds, CG scaffolds that have been exposed to the DMF solvent (but without BP conjugation), CG scaffolds with conjugated BP, and CG-BP scaffolds with photoimmobilized biomolecule X, respectively.
3.1. Visualization of biomolecularly patterned CG scaffolds
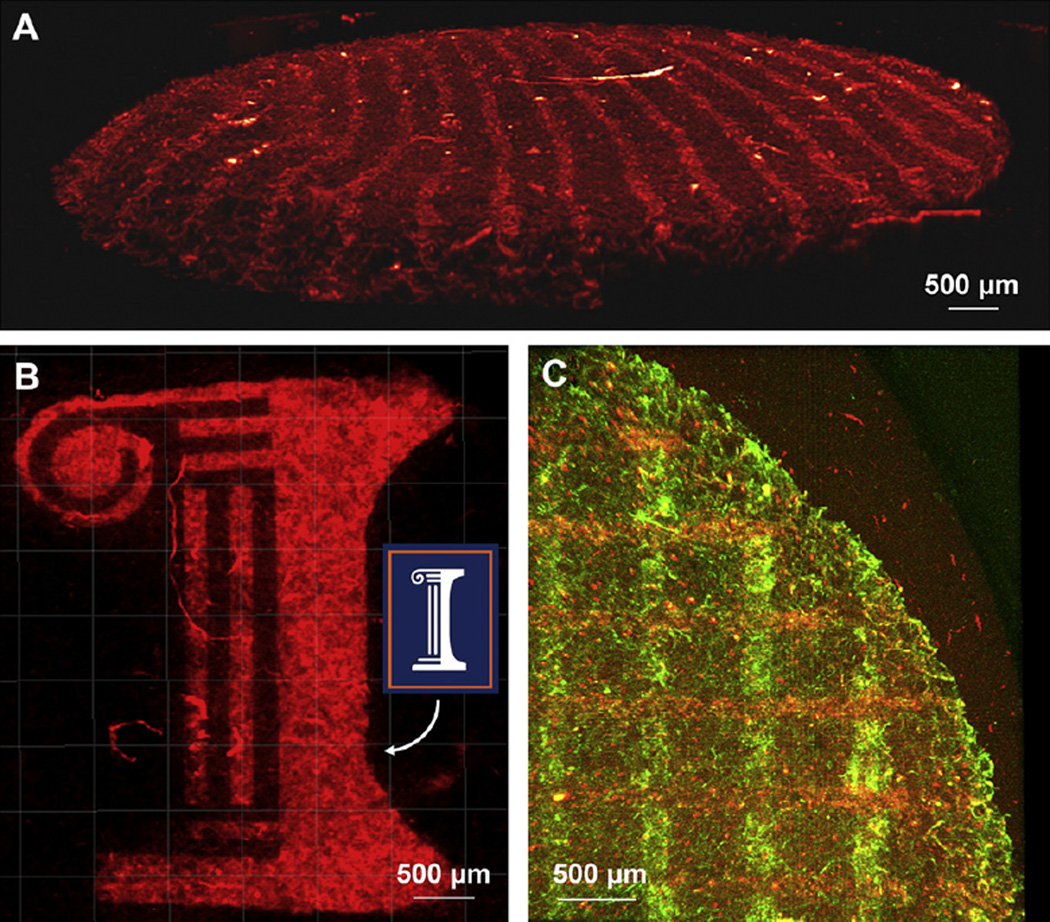
Single component ConA-biotin patterns of 100 µm stripes with 400 µmspacing, and the script “I” logo of the University of Illinois at Urbana-Champaign were visualized using confocal fluorescence microscopy (Fig. 2a,b). Patterns are clearly discernable from the background indicating successful photoimmobilization of ConA-biotin via the benzophenone groups presented on the CG scaffold. Multi-component patterns of FN and NC were photopatterned in two steps by sequential exposure of each biomolecule through the same photomask utilized for one component patterns (Fig. 2c). NC was patterned first (red, horizontal) followed by FN (green, vertical) with a 90° rotation of the photomask between exposures.
Fig. 2.
Photoimmobilization of Biomolecules. A) Photoimmobilized conA-biotin in 100 µm stripes with 400 µm spacing, visualized with Qdot 525-streptavidin. B) Photoimmobilized conA-biotin in the “Script I” logo of the University of Illinois at Urbana-Champaign to demonstrate patterns of different shapes. C) Overlapping patterns of 100 µm stripes with 400 µm spacing of N-cadherin (horizontal, red) and fibronectin (vertical, green). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.2. Determining the amount of biomolecular immobilization
Using cellular solids modeling tools to calculate scaffold specific surface area and a modified radioimmunoassay to determine the total amount of ConA-biotin immobilized to the scaffold surface, the surface density of this representative biomolecule bound to the CG scaffolds was determined to be approximately 1000 molecules/µm2 for maximum loading conditions.
3.3. Scaffold microstructural analysis
CG and CG-BP scaffolds were determined to have mean pore sizes of 86.6 ± 9.8 µm and 77.1 ± 9.7 µm (mean ± standard deviation) as well as mean pore aspect ratios (ratio of pore size for longitudinal vs. transverse sections taken from the scaffold) of 1.05 ± 0.03 and 1.06 ± 0.03, respectively. No significant difference was observed in scaffold mean pore size (p = 0.13) or aspect ratio (p = 0.32) for CG versus CG-BP scaffolds. No significant difference was observed in scaffold pore size in the longitudinal versus transverse planes for CG and CG-BP scaffolds (p = 0.14, 0.66 respectively). These pore diameters and longitudinal:transverse aspect ratios were also similar to previously characterized CG scaffolds fabricated via similar freezing conditions [1].
3.4. Mechanics of CG, CG-DMF, and CG-BP scaffolds
The DMF treatment stage of the BP functionalization process was observed to significantly increase scaffold tensile modulus (p = 0.0005, Fig. 3). The tensile modulus of CG scaffolds alone (54.7 ± 12.5 kPa; mean ± standard deviation) was significantly lower than that of the CG-DMF (87.6 ± 18.4 kPa) and CG-BP (102.8 ± 17.6 kPa) variants; no significant difference was observed between the tensile modulus of the CG-DMF and CG-BP scaffolds (p = 0.16).
Fig. 3.
Tensile Strength and Stress-Strain Curves. A) Mechanical testing of CG, CG-DMF, and CG-BP scaffolds shows that DMF treatment significantly increases tensile elastic modulus. B) Stress-strain curves for CG, CG-DMF, and CG-BP scaffolds.
3.5. Scaffold compositional analysis
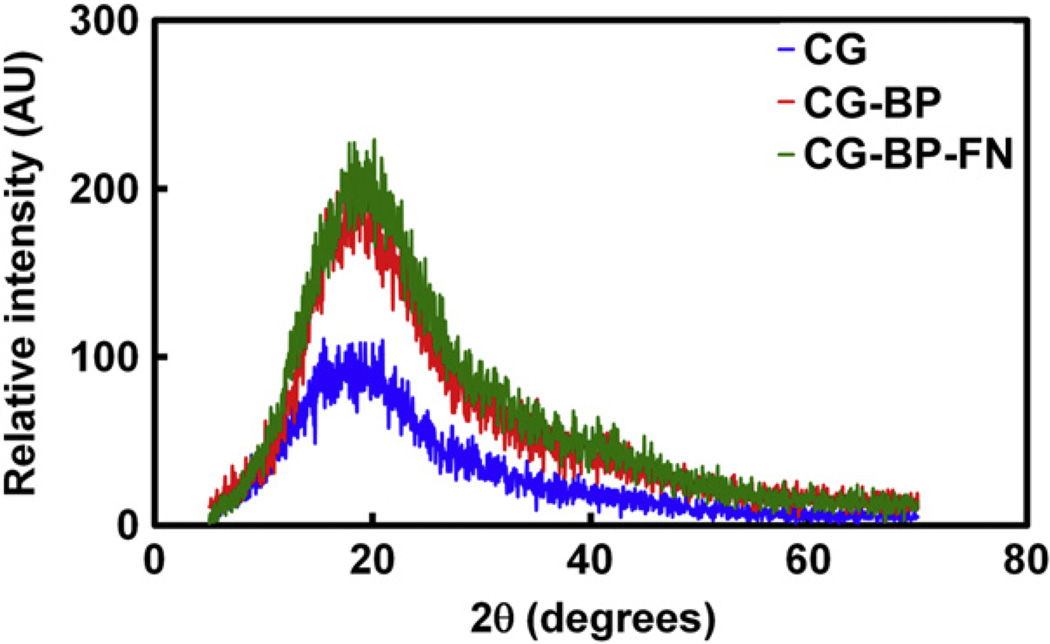
Fig. 4 shows characteristic XRD patterns for the CG, CG-BP, and CG-BP-FN scaffolds. All three variants display a broad peak at 2θ = 20° representing the characteristic interchain spacing of the collagen triple helix [40]. No sign of collagen denaturation (loss of the broad collagen peak) was observed for any of the samples; the increase in peak height for the CG-BP and CG-BP-FN variants is indicative of increased crystallinity of the samples relative to the original CG scaffold likely due to a crosslinking effect from DMF exposure, consistent with the observed increase in scaffold tensile modulus.
Fig. 4.
Crystallinity of Modified Scaffolds. XRD of CG, CG-BP, and CG-BP-FN scaffold variants. All four scaffolds display a broad peak at 2θ = 20° representing the characteristic interchain spacing of the collagen triple helix.
3.6. MC3T3-E1 viability in CG-BP scaffolds
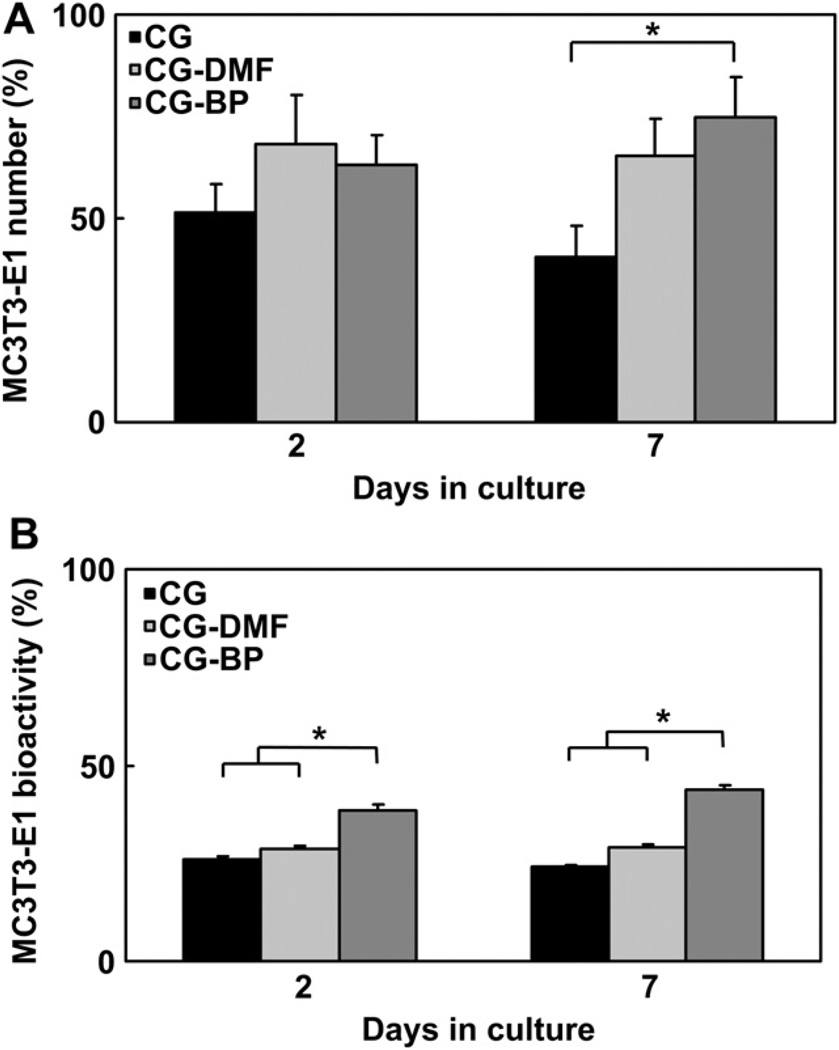
The long term viability of MC3T3-E1 cells on CG, CG-DMF, and CG-BP scaffolds was determined for up to 7 days of in vitro culture in complete α-MEM media in order to confirm that the BP immobilization process did not impart any inherent cytotoxicity. One-way ANOVA indicates significant differences in cell metabolic activity via alamarBlue assay at both day 2 and day 7 between the three groups (p < 0.0001) with the CG-BP group showing significantly higher bioactivity at both time points compared to the CG group (day 2: p = 0.0002, day 7: p < 0.0001). While there are no significant differences in total number of attached cells as determined via DNA assay at day 2 (p = 0.44), the CG-BP group showed significantly higher cell attachment at day 7 (p = 0.04) (Fig. 5). These data suggest that the BP functionalization process does not have a negative impact on the native bioactivity of CG scaffolds.
Fig. 5.
Metabolic Activity and Cell Attachment in Modified Scaffolds. A) MC3T3-E1 attachment in CG, CG-DMF, and CG-BP scaffolds after 2 and 7 days of in vitro culture. B) MC3T3-E1 bioactivity CG, CG-DMF, and CG-BP scaffolds after 2 and 7 days of in vitro culture. Conjugation of BP to the scaffolds or DMF exposure does not adversely affect cell activity or attachment.
3.7. MC3T3-E1 attachment and bioactivity in CG-BP-FN scaffolds
Previous work has demonstrated that fibronectin is the key adhesion ligand required to mediate early (<1 h) cell attachment to CG scaffolds [41]. Here, MC3T3-E1 cell attachment was assessed after 30 min in CG, CG-BP-FN, and CG-FN (unmodified CG scaffold soaked in the FN solution used to create CG-BP-FN scaffolds and then washed in PBS) scaffolds to assess the relative ability for BP functionalized CG scaffolds to alter the innate bioactivity of the CG scaffold. The MC3T3-E1 cells were washed and resuspended in PBS prior to being seeded into PBS hydrated scaffold variants, and the entire experiment was performed in PBS in order to prevent nonspecific adsorption of exogenous factors from the α-MEM media, thereby insuring that any change in cell attachment was due to the covalent immobilization of fibronectin rather than additional soluble proteins from the α-MEM media. Fibronectin treatment was observed to have a significant effect on early MC3T3-E1 attachment (p < 0.0001) (Fig. 6). Scaffolds with BP-immobilized fibronectin (CG-BP-FN) showed significantly higher levels of cell attachment after 30 min relative to CG scaffolds alone (CG, p < 0.0001) or CG scaffolds with passively adsorbed FN (CG-FN, p < 0.0001); no significant difference was observed in initial cell attachment for CG versus CG-FN groups (p = 0.12), suggesting non-specific FN adsorption to the CG scaffold does not mediate initial cell attachment.
Fig. 6.
Cell Attachment Assay. CG-BP-Fn scaffolds showed significantly higher MC3T3-E1 attachment compared to the control bare CG and CG-Fn scaffolds after 30 min.
4. Discussion
CG scaffolds have found useful application as ECM analogs for regeneration of a variety of tissue types [2,21,42] and as experimental substrates to investigate microenvironmental regulation of cell behavior [3,24]. This study describes the development and application of a direct, photolithographic method for covalently attaching multiple biomolecules onto CG scaffolds with spatial control over the immobilization. Here, we employed a benzophenone-based direct photolithographic method to spatially pattern biomolecules within defined CG scaffold microstructures, adding the capacity to provide spatially-tuned, instructive signals to cells within the scaffold microenvironment. Through a series of imaging and bioactivity assays we observed that BP photolithography methods can be applied to 3D biomaterials in order to spatially pattern immobilized biomolecules in the CG scaffold structure, and that these factors can induce a resultant change in cell bioactivity in response to factor presentation.
We used fluorescence microscopy approaches to validate the creation of a range of biomolecules patterns within CG scaffolds. We first created a single-phase, discrete geometric pattern in the form of repeating stripes (100 µm wide, 400 µm periodicity) of photoimmobilized ConA-biotin, visualized via Qdot 525 conjugated streptavidin (Red, Fig. 2a). Conversely, areas in between the stripes where UV light was blocked by the photomask are not fluorescent since the BP molecules were not excited and thus did not conjugate ConA-biotin. The technique was also successfully used to create a non-geometric pattern of ConA-biotin in the form of the University of Illinois script “I” logo (Fig. 2b). Again, a clear contrast was observed between areas exposed to light define the pattern while fluorescence is not observed from the areas blocked during illumination. Taking advantage of the depth profiling capability of confocal microscopy it was determined that currently BP photopatterns can be created to a depth of 300 µm into the scaffold (data not shown). Although the high porosity of the CG scaffold does allow for reasonable patterning depths, the scatter of photons at this wavelength currently limits the depth to which patterning can be achieved. Furthermore, this scatter also slightly diminishes the patterning resolution. We are currently working to better define the relationship between scaffold microstructure (pore size, relative density), UV intensity/exposure time, and resultant pattern depth and resolution and are exploring different UV exposure conditions as well as two photon patterning schemes which may improve our photopatterning capacity because the longer wavelengths are scattered to a lesser degree.
Sequential exposures of BP-presenting scaffolds in the presence of different biomolecules allow for the construction of multicomponent patterns. Fig. 2c shows an example of a CG scaffold patterned with vertical 100 µmstripes of FN and horizontal 100 µm stripes of NC. Notably, both FN and NC are co-immobilized at the intersections of the two stripe patterns, highlighting an interesting property of BP-photoattachment strategy as opposed to other schemes that rely upon deprotection or unmasking of reactive groups [43,44]. Because the diradical formed with UV light has a finite lifetime (80–100 µs) after which it relaxes back to the ground state [45], if an excited BP fails to react with a solution phase biomolecule it can be re-exposed in the presence of a second biomolecule, which allows for the creation of overlapping patterns. The relative yield of these photoreactions can be controlled by exposure intensity and time, as well as the solution phase biomolecule concentration, since the number of molecules in solution dictates the probability of interaction while the BP is in the excited state [32]. The conditions used for two-component patterning were controlled so that the first component exposure did not saturate the scaffold, facilitating the construction of overlapping patterns. Importantly, the ability to control the presented concentration of multiple biomolecules at a single spatial location will be of tremendous utility in downstream applications. The selection of FN and NC for two-component patterning was strategic in that both proteins are implicated in the proliferation and maintenance of the MC3T3 osteoblast-like cells used in this study [46,47]. While beyond the scope of this initial manuscript, multicomponent biomolecule patterns may prove particularly useful for engineering biomaterial mimics of tissue interfaces (e.g. osteochondral, ligament-bone, and tendon-bone) where the sub-millimeter gradients of chemical and mechanical properties found in vivo are difficult to recapitulate in synthetic model systems [48]. We are currently exploring immobilization of growth factor agonists of tenocyte and osteoblast bioactivity into tendinous and osseous compartments of a multiphase CG scaffold; we are also exploring BP photopatterning of the ECM proteins aggrecan and decorin at the interfacial zone of these same materials. Region-specific high concentration of native ECM proteins are difficult to fabricate via freeze drying approaches, but may have significant benefit for regenerative medicine applications.
Quantitative analysis of scaffold microstructural, mechanical, and chemical composition revealed that the processing steps required for BP conjugation to the CG scaffold induced some quantifiable changes in scaffold properties, but not in a manner that would suggest a negative biological response. By understanding the effects of DMF exposure and BP patterning on scaffold properties, we are able to assess the influence of photolithographically immobilized biomolecules within CG scaffolds variants on cell bioactivity. Scaffold microstructure (pore size, pore aspect ratio), a key parameter in determining overall scaffold bioactivity, was not influenced by BP conjugation. Exposure of the CG scaffold to the DMF buffer required to mediate BP conjugation likely increased the crosslinking density of the CG scaffold, as reflected by the increase in scaffold tensile modulus (Fig. 3) and material crystallinity (XRD, Fig. 4). However, XRD spectra did not exhibit a peak shift characteristic of collagen gelatinization (Fig. 4). These results suggest that the DMF buffer additionally crosslinks the CG scaffold, imparting improved mechanical properties, but does not fundamentally alter scaffold microstructural or compositional properties; in the future we can, adjust the crosslinking of CG scaffolds [25] to mimic the crosslinking effect of CG-BP scaffold variants in order to allow for additional comparative experiments. These results also suggest that photopatterning of biomolecules to the BP functionalized scaffold does not additionally alter scaffold properties; no change in scaffold modulus or material crystallinity was observed due to FN functionalization to the BP.
After demonstrating that CG scaffolds could be patterned using BP-based photolithography methods without significant deterioration of physical properties, we examined the effect of BP conjugation to CG scaffolds on overall material bioactivity. Even though extensive washing steps were utilized, we were particularly concerned with the cytotoxicity of DMF and BP chemistries, but found no decrease in MC3T3-E1 preosteoblast number or metabolic activity out to 7 days of culture in CG-DMF or CG-BP scaffolds relative to unmodified CG scaffolds (Fig. 5). These data indicate that BP conjugation and the organic solvents used in this process do not adversely influence cell attachment, bioactivity, and proliferative potential. The observed increase in MC3T3 number and bioactivity for CG-BP scaffolds relative to CG control is in fact likely due to differences in scaffold modulus. Though other groups have also used DMF-based solutions during fabrication of collagen-based biomaterials and demonstrated subsequent bioactivity after DMF treatment [49], ongoing work will include an alternative BP conjugation step that altogether avoids organic solvents.
We then examined the ability of BP photolithography to specifically alter a cellular response within the CG scaffold. Previous studies have shown that CG scaffold microstructure plays a significant role in influencing cell attachment [1,50] and that cell attachment to the scaffold is mediated by sequential utilization of vitronectin, fibronectin and collagen binding motifs [41]; however the fibronectin binding motif was specifically implicated in mediating the initial cell attachment events to the CG scaffold within the first 2 h after cell-seeding prior to formation of stable attachments directly to the collagen structure [41]. Here we examined the fraction of MC3T3-E1 preosteoblasts adhered after only 30 min to untreated CG scaffolds versus BP-conjugated CG scaffolds where we covalently immobilized fibronectin via photolithographic attachment to the entire scaffold (CG-BP-FN). As an additional control, we also tested CG scaffolds that were soaked in a fibronectin solution (CG-FN) and then washed in PBS, where passive FN physisorption was the only mechanism for surface patterning. The cell attachment study was performed in PBS rather than media to prevent non-specific adsorption of additional proteins from the media that could alter cell adhesion profiles. No significant difference in cell attachment was observed for the CG versus FN-physisorbed CG scaffold, while a greater than two-fold (p < 0.0001) increase in MC3T3-E1 cell attachment was observed for the CG-BP-FN scaffold versus the CG or CG-FN scaffolds (Fig. 6). These data demonstrate that BP photolithography can be used to immobilize biomolecules to CG scaffolds in order to elicit a specific biological response. These results also demonstrate the advantage of covalent immobilization (CG-BP-FN) rather than physical adsorption (CG-FN) of fibronectin to mediate initial cell attachment events within 3D biomaterials. Furthermore, preliminary results indicate that cells do preferentially adhere specifically to regions displaying adhesion ligands patterned within CG-BP scaffolds over areas that were not exposed to light during the immobilization process. An further advantage of our approach is that BP photopatterning at high surface densities allows us to sample a wide range potential biomolecule conformations. While not able to present a specific FN conformation, the C–H bond insertion mechanism that links the biomolecule (i.e. FN) to the scaffold via BP is not biased toward any particular C–H bonds and therefore it is almost certain that proteins are immobilized in random orientations across the surface. On a cellular length scale, which is significantly larger that the biomolecular length scale and the spacing between BP molecules on the scaffold surface, there statistically are likely many proteins that have a suitable configuration to engage cellular receptors [32].
BP-based, direct photolithography has previously been used to spatially pattern a range of biomolecules on 2D, planar surfaces. Here we show that this approach can be applied to the CG scaffold system to create a range of biomolecular patterns, that BP conjugation increased scaffold crosslinking but did not change scaffold microstructure or composition in a way that negatively affects cell viability, and that BP-based photolithography can be used to improve cell bioactivity by covalent immobilization of an adhesive factor. Ongoing work is using multiple (stripes and gradients) orthogonal patterns of fibronectin and N-cadherin in order to further probe the capacity for BP-driven biomolecular attachment to controllably regulate cellular activity. These proteins are differentially implicated in the proliferation and maintenance [46,47] of the MC3T3-E1 cells used in this study, and thus generating multiple orthogonal patterns of both proteins will provide a toolset to explore their separate and linked regulation of MC3T3 cell attachment and proliferation. We are also using BP-based photolithography to explore patterning multiple growth factors and ECM proteins for studies looking to create CG scaffolds to mimic complex orthopedic interfaces for regenerative medicine applications.
5. Conclusions
We have demonstrated the ability to conjugate benzophenone to CG scaffolds and photochemically generate patterns of biomolecules in a controlled and spatially well-defined manner. Since this immobilization approach only requires the presence of a C–H bond, it is biomolecularly general and has broad applicability to a range of classes of biomolecules. The method offers a direct, photolithographic approach to generate complex, multi-component patterns or gradients of biomolecules to more accurately mimic the heterogeneity of the native ECM using biologically mimetic collagen scaffolds. Importantly, this process allows separating scaffold fabrication, biomolecule patterning, and cell-seeding steps, thereby increasing the potential for independent factor modulation as well as reducing the potential for adverse (cytotoxic) effects on cell behavior due to fabrication and patterning. Future work will focus on generating multi-component patterns and gradients to investigate cell adhesion, migration, proliferation, differentiation, gene expression, and ECM biosynthesis in both single and multicompartment CG scaffolds.
Acknowledgments
The authors would like to acknowledge Dr. Mauro Sardela (Materials Research Laboratory, UIUC), Karen Doty (Veterinary Sciences, UIUC) for sectioning of GMA embedded samples, Dr. Hyun-Joon Kong (ChBE, UIUC) for the use of his mechanical tester, Dr. Charles Schroeder (ChBE, UIUC) for the use of his fluorescence spectrophotometer, Christine Herman (Chemistry, UIUC) for the synthesis of BP-NCS and assistance is laser beam homogenization, and Manuel Ramirez (BioE, UIUC) for assistance with mechanical tests. We are grateful for the funding for this study provided by the Chemical and Biomolecular Engineering Dept. (SRC, BAH), the Institute for Genomic Biology (SRC, BAH) at the University of Illinois at Urbana-Champaign, the Chemistry–Biology Interface Training Program NIH NIGMS T32GM070421 (SRC), the Roy J. Carver Charitable Trust and the Camille and Henry Dreyfus Foundation (TAM, RCB). This research was carried out in part in the Frederick Seitz Materials Research Laboratory Central Facilities, University of Illinois, which are partially supported by the U.S. Department of Energy under grants DE-FG02-07ER46453 and DE-FG02-07ER46471.
Contributor Information
Brendan A. Harley, Email: bharley@illinois.edu.
Ryan C. Bailey, Email: baileyrc@illinois.edu.
References
- 1.O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials. 2005;26:433–441. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 2.Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci U S A. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J. 2008;95:4013–4024. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tse JR, Engler AJ. Stiffness gradients mimicking in vivo tissue variation regulate mesenchymal stem cell fate. PLoS ONE. 2011;6:e15978. doi: 10.1371/journal.pone.0015978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 6.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, et al. Migration of tumor cells in 3D matrices is governed by matrix stiffness along with cell-matrix adhesion and proteolysis. Proc Natl Acad Sci U S A. 2006;103:10889–10894. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen YH, Shoichet MS, Radisic M. Vascular endothelial growth factor immobilized in collagen scaffold promotes penetration and proliferation of endothelial cells. Acta Biomater. 2008;4:477–489. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Capito RM, Spector M. Collagen scaffolds for nonviral IGF-1 gene delivery in articular cartilage tissue engineering. Gene Ther. 2007;14:721–732. doi: 10.1038/sj.gt.3302918. [DOI] [PubMed] [Google Scholar]
- 9.Mikos AG, Herring SW, Ochareon P, Elisseeff J, Lu HH, Kandel R, et al. Engineering complex tissues. Tissue Eng. 2006;12:3307–3339. doi: 10.1089/ten.2006.12.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Engelmayr GC, Jr, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Accordion-like honeycombs for tissue engineering of cardiac anisotropy. Nat Mater. 2008;7:1003–1010. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wopenka B, Kent A, Pasteris JD, Yoon Y, Thomopoulos S. The tendon-to-bone transition of the rotator cuff: a preliminary Raman spectroscopic study documenting the gradual mineralization across the insertion in rat tissue samples. Appl Spectrosc. 2008;62:1285–1294. doi: 10.1366/000370208786822179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moffat KL, Sun WH, Pena PE, Chahine NO, Doty SB, Ateshian GA, et al. Characterization of the structure-function relationship at the ligament-to-bone interface. Proc Natl Acad Sci U S A. 2008;105:7947–7952. doi: 10.1073/pnas.0712150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 14.Huebsch N, Mooney DJ. Inspiration and application in the evolution of biomaterials. Nature. 2009;462:426–432. doi: 10.1038/nature08601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang J, Peng R, Ding J. The regulation of stem cell differentiation by cell-cell contact on micropatterned material surfaces. Biomaterials. 2010;31:2470–2476. doi: 10.1016/j.biomaterials.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Ding J. Nanostructured interfaces with RGD arrays to control cell-matrix interaction. Soft Matter. 2010;6:3395–3401. [Google Scholar]
- 17.Gray DS, Liu WF, Shen CJ, Bhadriraju K, Nelson CM, Chen CS. Engineering amount of cell-cell contact demonstrates biphasic proliferative regulation through RhoA and the actin cytoskeleton. Exp Cell Res. 2008;314:2846–2854. doi: 10.1016/j.yexcr.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DeForest CA, Polizzotti BD, Anseth KS. Sequential click reactions for synthesizing and patterning three-dimensional cell microenvironments. Nat Mater. 2009;8:659–664. doi: 10.1038/nmat2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Luo Y, Shoichet MS. A photolabile hydrogel for guided three-dimensional cell growth and migration. Nat Mater. 2004;3:249–253. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 21.Harley BAC, Gibson LJ. In vivo and in vitro applications of collagen-GAG scaffolds. Chem Eng J. 2008;137:102–121. [Google Scholar]
- 22.Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold II: fabrication of a mineralized collagen-GAG scaffold. J Biomed Mater Res A. 2010;92:1066–1077. doi: 10.1002/jbm.a.32361. [DOI] [PubMed] [Google Scholar]
- 23.Harley BA, Lynn AK, Wissner-Gross Z, Bonfield W, Yannas IV, Gibson LJ. Design of a multiphase osteochondral scaffold III: fabrication of layered scaffolds with continuous interfaces. J Biomed Mater Res A. 2010;92:1078–1093. doi: 10.1002/jbm.a.32387. [DOI] [PubMed] [Google Scholar]
- 24.Harley BA, Freyman TM, Wong MQ, Gibson LJ. A new technique for calculating individual dermal fibroblast contractile forces generated within collagen-GAG scaffolds. Biophys J. 2007;93:2911–2922. doi: 10.1529/biophysj.106.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harley BA, Leung JH, Silva ECCM, Gibson LJ. Mechanical characterization of collagen-glycosaminoglycan scaffolds. Acta Biomater. 2007;3:463–474. doi: 10.1016/j.actbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 26.O’Brien FJ, Harley BA, Waller MA, Yannas IV, Gibson LJ, Prendergast PJ. The effect of pore size on permeability and cell attachment in collagen scaffolds for tissue engineering. Technol Health Care. 2007;15:3–17. [PubMed] [Google Scholar]
- 27.Farrell E, O’Brien FJ, Doyle P, Fischer J, Yannas I, Harley BA, et al. A collagen-glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006;12:459–468. doi: 10.1089/ten.2006.12.459. [DOI] [PubMed] [Google Scholar]
- 28.Liu HW, Chen CH, Tsai CL, Hsiue GH. Targeted delivery system for juxtacrine signaling growth factor based on rhBMP-2-mediated carrier-protein conjugation. Bone. 2006;39:825–836. doi: 10.1016/j.bone.2006.04.027. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Ling XY, van Bennekom J, Duan X, Ludden MJW, Reinhoudt DN, et al. Microcontact printing of dendrimers, proteins, and nanoparticles by porous stamps. J Am Chem Soc. 2009;131:797–803. doi: 10.1021/ja807611n. [DOI] [PubMed] [Google Scholar]
- 30.Song Y-F, McMillan N, Long D-L, Kane S, Malm J, Riehle MO, et al. Micro-patterned surfaces with covalently grafted unsymmetrical polyoxometalate-hybrid clusters lead to selective cell adhesion. J Am Chem Soc. 2009;131:1340–1341. doi: 10.1021/ja807091v. [DOI] [PubMed] [Google Scholar]
- 31.Irimia D, Liu S-Y, Tharp WG, Samadani A, Toner M, Poznansky MC. Microfluidic system for measuring neutrophil migratory responses to fast switches of chemical gradients. Lab a Chip. 2006;6:191–198. doi: 10.1039/b511877h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toh CR, Fraterman TA, Walker DA, Bailey RC. Direct biophotolithographic method for generating substrates with multiple overlapping biomolecular patterns and gradients. Langmuir. 2009;25:8894–8898. doi: 10.1021/la9019537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen-GAG scaffolds. Biomaterials. 2004;25:1077–1086. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 34.Haugh MG, Murphy CM, O’Brien FJ. Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng Part C Methods. 2010;16:887–894. doi: 10.1089/ten.TEC.2009.0422. [DOI] [PubMed] [Google Scholar]
- 35.Harley BA, Spilker MH, Wu JW, Asano K, Hsu HP, Spector M, et al. Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps. Cells Tissues Organs. 2004;176:153–165. doi: 10.1159/000075035. [DOI] [PubMed] [Google Scholar]
- 36.Sinsheimer JE, Jagodic V, Polak LJ, Hong DD, Burckhalter JH. Polycyclic aromatic isothiocyanate compounds as fluorescent labeling reagents. J Pharm Sci. 1975;64:925–930. doi: 10.1002/jps.2600640605. [DOI] [PubMed] [Google Scholar]
- 37.Gibson LJ, Ashby MF, Harley BA. Cellular materials in nature and medicine. Cambridge, U.K: Cambridge University Press; 2010. [Google Scholar]
- 38.Kim YJ, Sah RL, Doong JY, Grodzinsky AJ. Fluorometric assay of DNA in cartilage explants using Hoechst 33258. Anal Biochem. 1988;174:168–176. doi: 10.1016/0003-2697(88)90532-5. [DOI] [PubMed] [Google Scholar]
- 39.Tierney CM, Jaasma MJ, O’Brien FJ. Osteoblast activity on collagen-GAG scaffolds is affected by collagen and GAG concentrations. J Biomed Mater Res A. 2009;91:92–101. doi: 10.1002/jbm.a.32207. [DOI] [PubMed] [Google Scholar]
- 40.Davidenko N, Campbell JJ, Thian ES, Watson CJ, Cameron RE. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010 doi: 10.1016/j.actbio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Sethi KK, Yannas IV, Mudera V, Eastwood M, McFarland C, Brown RA. Evidence for sequential utilization of fibronectin, vitronectin, and collagen during fibroblast-mediated collagen contraction. Wound Repair Regen. 2002;10:397–408. doi: 10.1046/j.1524-475x.2002.10609.x. [DOI] [PubMed] [Google Scholar]
- 42.Yannas IV, Tzeranis DS, Harley BA, So PTC. Biologically active collagen-based scaffolds: advances in processing and characterization. Philosophical Trans Royal Soc A Math Phys Eng Sci. 2010;368:2123–2139. doi: 10.1098/rsta.2010.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan EW, Yousaf MN. A photo-electroactive surface strategy for immobilizing ligands in patterns and gradients for studies of cell polarization. Mol Biosyst. 2008;4:746–753. doi: 10.1039/b801394b. [DOI] [PubMed] [Google Scholar]
- 44.Petty RT, Li HW, Maduram JH, Ismagilov R, Mrksich M. Attachment of cells to islands presenting gradients of adhesion ligands. J Am Chem Soc. 2007;129:8966–8967. doi: 10.1021/ja0735709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dorman G, Prestwich GD. Benzophenone photophores in biochemistry. Biochemistry. 1994;33:5661–5673. doi: 10.1021/bi00185a001. [DOI] [PubMed] [Google Scholar]
- 46.Lee MH, Adams CS, Boettiger D, Degrado WF, Shapiro IM, Composto RJ, et al. Adhesion of MC3T3-E1 cells to RGD peptides of different flanking residues: detachment strength and correlation with long-term cellular function. J Biomed Mater Res A. 2007;81:150–160. doi: 10.1002/jbm.a.31065. [DOI] [PubMed] [Google Scholar]
- 47.Hay E, Laplantine E, Geoffroy V, Frain M, Kohler T, Muller R, et al. N-cadherin interacts with axin and LRP5 to negatively regulate Wnt/beta-catenin signaling, osteoblast function, and bone formation. Mol Cell Biol. 2009;29:953–964. doi: 10.1128/MCB.00349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lu HH, Jiang J. Interface tissue engineering and the formulation of multiple-tissue systems. Adv Biochem Eng Biotechnol. 2006;102:91–111. [PubMed] [Google Scholar]
- 49.Kim TG, Chung HJ, Park TG. Macroporous and nanofibrous hyaluronic acid/collagen hybrid scaffold fabricated by concurrent electrospinning and deposition/leaching of salt particles. Acta Biomater. 2008;4:1611–1619. doi: 10.1016/j.actbio.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 50.Murphy CM, Haugh MG, O’Brien FJ. The effect of mean pore size on cell attachment, proliferation and migration in collagen-glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials. 2010;31:461–466. doi: 10.1016/j.biomaterials.2009.09.063. [DOI] [PubMed] [Google Scholar]