Abstract
Background
The annual incidence of inflammatory breast cancer (IBC) in the United States reportedly increased during the last quarter of the twentieth century. We investigated whether that increase has continued into the twenty-first century.
Methods
We queried the Surveillance Epidemiology and End Results database for all cases of IBC in women age 20 and older between 1992 and 2009. Cases were breast tumors with at least one of the following codes: extent of disease size 998, extension 70, or ICD-3-O morphology 8530 or 8533. Age-adjusted incidence was also examined.
Results
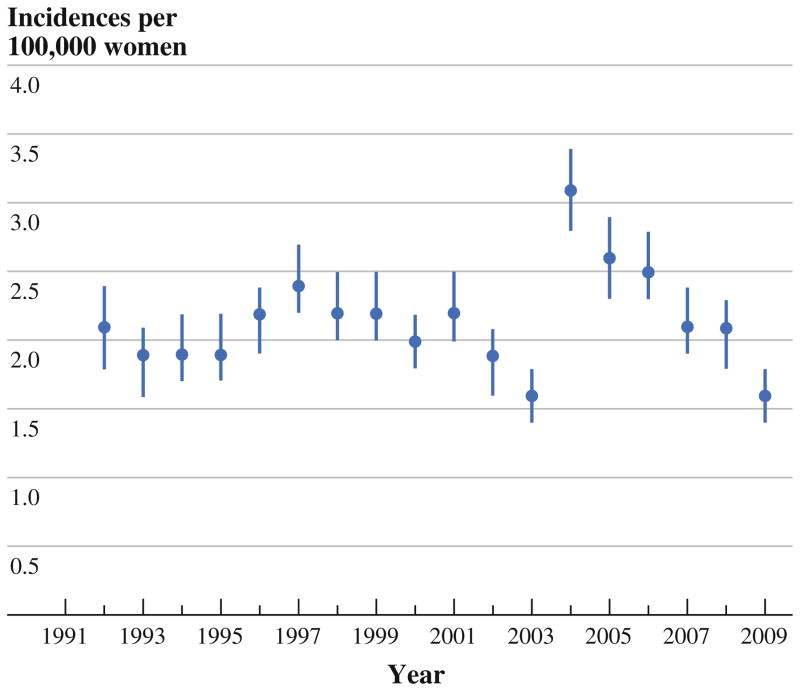
During 1992–2009, the annual incidence of IBC did not increase over time in any age group, nor did it vary significantly from year to year, except between 2003 and 2004, when there was a jump from 1.6 (95 % confidence interval 1.4–1.8) to 3.1 (2.8–3.4) cases per 100,000 women. Similar changes occurred in all age and racial groups before gradually returning to prejump levels. Overall, the incidence of IBC rose steeply with age until reaching a plateau at age 65. The incidence was greatest among black women (3.0; 2.8–3.2), intermediate among white women (2.1; 2.1–2.2), and lowest among Asian women (1.4; 1.3–1.6).
Conclusions
The incidence of IBC has remained essentially stable for nearly two decades. A transient jump in 2003–2004 occurred in all age and racial groups, suggesting adjustment to coding changes at that time. Often described as a disease of younger women, IBC in fact disproportionately affects older women. Racial/ethnic variation in the incidence of IBC suggests that dietary, lifestyle, or genetic factors contribute to its pathogenesis.
Inflammatory breast cancer (IBC) is a rare and aggressive disease within the spectrum of breast cancer. It is defined by the American Joint Committee on Cancer as a clinicopathologic entity characterized by diffuse erythema and edema (peau d’orange) of the breast, often without an underlying palpable mass.1 Other findings on clinical examination may include nodules, induration, increased breast size, or ulceration, but the key clinical finding is the erythema, which must affect greater than one-third of the area of the breast.2
Pathologically, IBC is characterized by dermal tumor emboli in the papillary and reticular dermis of the skin overlying the breast. The emboli are most commonly composed of ductal cells of high nuclear grade. It is important to note that the absence of these pathologic findings does not exclude the diagnosis of IBC.3
Because of its infrequent occurrence, as well as the presentation with erythema, edema, and induration, IBC is commonly mistaken for other processes. The differential diagnosis of IBC includes infectious causes (abscess or mastitis) and noninfectious causes (dermatitis, lymphomas, and, rarely, congestive heart failure). It is not uncommon for IBC to be misdiagnosed initially and treated as one of these other conditions.2 When the proper diagnosis is made, IBC is treated by a trimodal approach including chemotherapy, surgery, and radiation.4
IBC is currently perceived by many as a disease of younger women despite evidence to the contrary.2,5 The incidence of IBC in the United States has reportedly increased in recent years, during the period of 1975–1992 and also 1988–2000.5,6 The objective of our study was to investigate whether the incidence of IBC has continued to increase.
METHODS
The study population was women aged 20 and older who were resident in the United States. The data source was the national sample (approximately 13.8 % of the United States) in the Surveillance Epidemiology and End Results (SEER) database (April 2012 release, 13 geographic regions, 1992–2009).7 Cases of IBC were defined as breast tumors with at least one of the following codes: extent of disease size 998, extension 70, or ICD-3-O morphology 8530 or 8533. The age-adjusted incidence of IBC (standardized against the year 2000 US population) was examined by calendar year, age, and race/ethnicity. Because our study used an existing publicly available database without protected health or identifying information, it was deemed exempt from review by our Center’s Institutional Review Board.
RESULTS
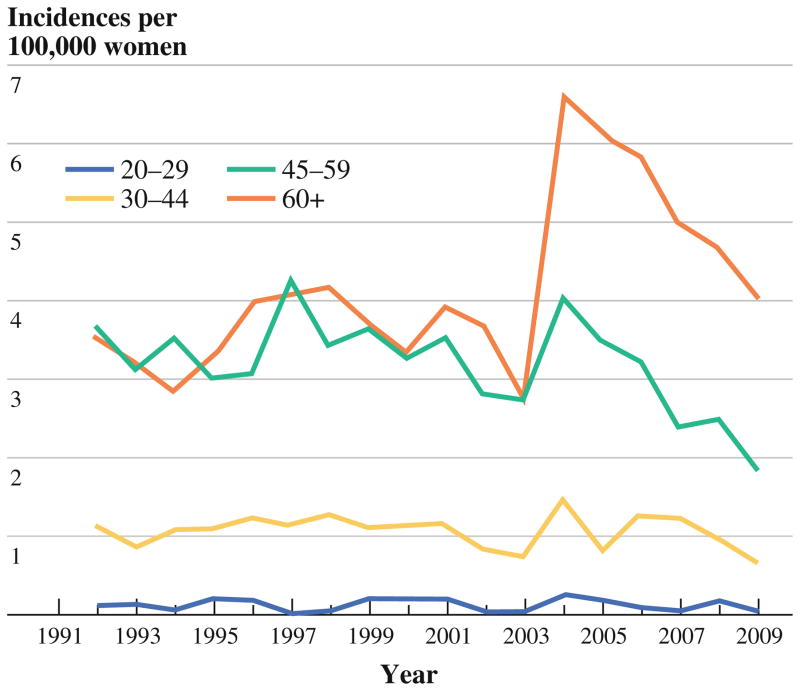
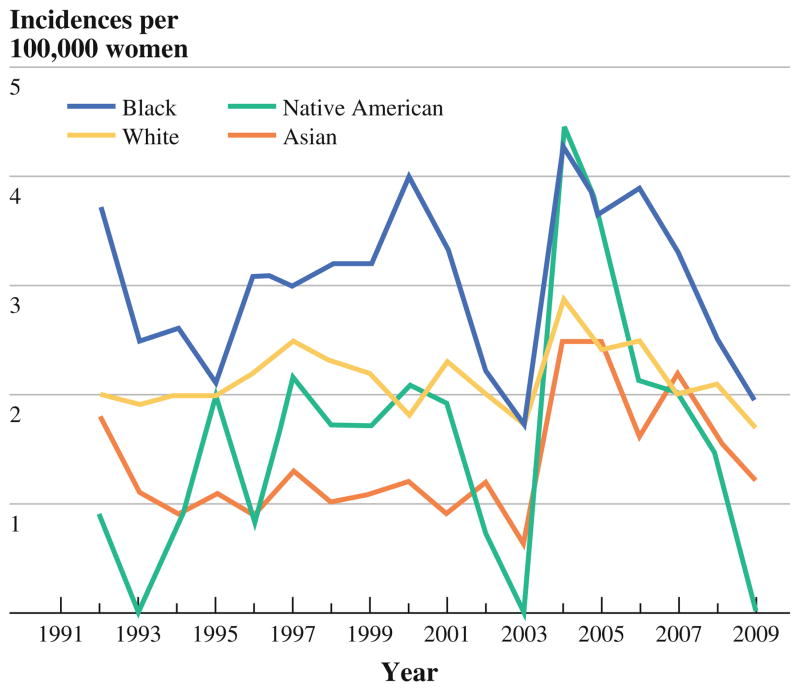
During the period of study, the annual incidence of IBC (Fig. 1) showed no clear trend, fluctuating modestly from year to year except between 2003 and 2004, when the overall annual incidence rose from 1.6 (95 % confidence interval, 1.4–1.8) to 3.1 (2.8–3.4) per 100,000 women. This transient spike in incidence occurred within all age and racial groups (Figs. 2 and 3) and gradually returned to previously seen levels.
FIG. 1.
Annual incidence of inflammatory breast cancer (IBC), United States, 1992–2009
FIG. 2.
Annual incidence of IBC, by age group
FIG. 3.
Annual incidence of IBC, by race/ethnicity
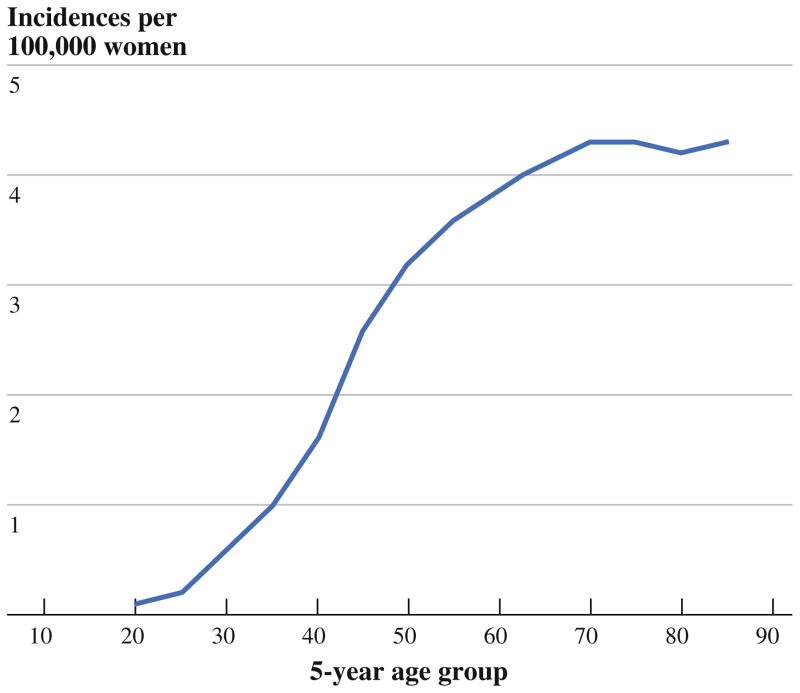
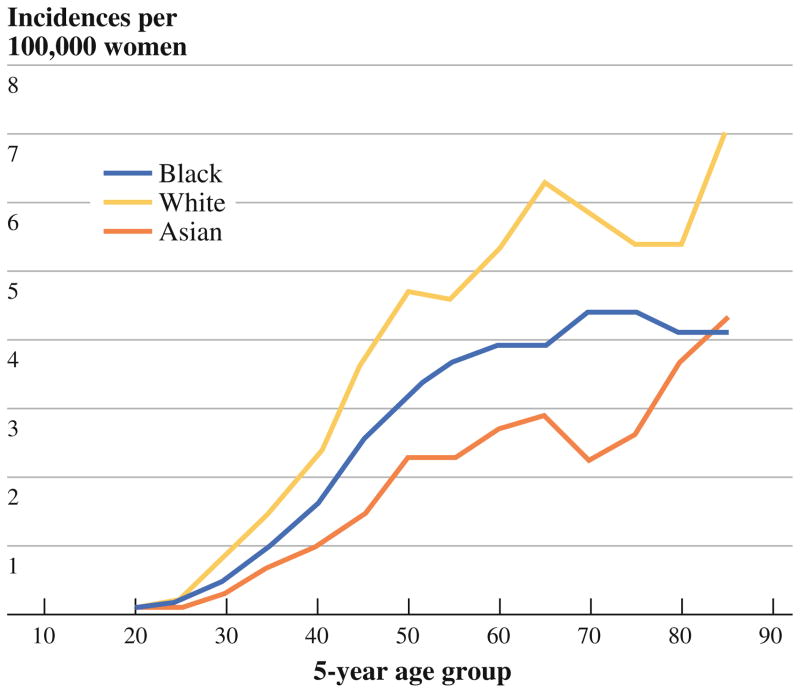
The incidence of IBC rose steeply with age until it plateaued after age 65 (Fig. 4). Incidence also varied by race/ethnicity (Fig. 5), being greatest among black women (3.0; 2.8–3.2), intermediate among white women (2.1; 2.1–2.2), and lowest among Asian women (1.4; 1.3–1.6). The elevated incidence of IBC in black women was apparent as early as age 30 (Fig. 5).
FIG. 4.
Age-specific incidence of IBC, United States, 1992–2009
FIG. 5.
Age-specific incidence of IBC, by race/ethnicity. Note: Data on IBC incidence among Native Americans were too sparse to permit their inclusion in this figure
DISCUSSION
According to the current analysis, widely held perceptions regarding the epidemiology of IBC require revision. One misperception is that the incidence of IBC is currently increasing. Chang and colleagues reported that the incidence of IBC doubled within the SEER database from 1975 to 1992, rising from 0.3 to 0.7 cases per 100,000 person-years for white women and from 0.6 to 1.1 cases per 100,000 person-years for black women during the study period.6 Likewise, Hance et al.5 reported a rise in IBC incidence between 1988–1990 and 1997–1999, from 2 to 2.5 cases per 100,000 person-years. In that study also, IBC incidence was higher in black women (3.1 cases per 100,000 person-years) than in white women (2.2 cases per 100,000 person-years). From those reports, one might expect IBC to be on the increase in the United States today.
According to the current study, however, the incidence of IBC is not increasing and has sustained only minor fluctuations over the past two decades. An apparent increase from 2003 to 2004 was a transient occurrence that involved all age and racial/ethnic groups, suggesting that it arose from changes in coding and classification rather than from an actual increase in cases. In fact, coincident with this transient spike in the incidence of IBC, there was a change in the SEER codes for IBC. Specific coding for IBC remained unchanged, but extension 50 was replaced by extensions 51 and 52. Extension 50 had specified “extensive skin involvement: skin edema, peau d’orange, ‘pigskin,’ en cuirasse, lenticular nodule(s), inflammation of skin, erythema, ulceration of skin of breast, satellite nod-ule(s) in skin of primary breast.” The new extension 51 is identical to the previous extension 50, but extension 52 adds for the first time the qualification “without a stated diagnosis of inflammatory carcinoma”.7 During the period when registrars would have adapted to the new coding system, there was a gradual return to the incidence recorded before 2004. With the addition of more recent data, the upward trend reported previously can now be recognized as random fluctuation within a stable range.
Another misperception addressed by our study is that IBC is, as described by some, a disease of younger women.5,6,8 On the contrary, we and others have found that IBC, like other forms of breast cancer, disproportionately affects older women2,5 The mean age at IBC diagnosis has been estimated at 55 years (per the M. D. Anderson Inflammatory Breast Cancer Registry) and at 58.7 years (per the SEER 13 Registry, 1992–2002).2,9 Our current analysis of SEER 1992–2009 indicates that the incidence of IBC rises sharply with age until it plateaus after age 65. Il’Yasova and colleagues drew a similar observation from a subset of the same data (SEER 2004–2007).10
Consistent with previous reports, we observed a heightened incidence of IBC among black women, who tend to be diagnosed with IBC at an earlier age than other women.5,6,10 A similar finding was reported by Yang et al.11 whose analysis of the Florida cancer registry observed that a greater proportion of black than white women was diagnosed with IBC before age 45 (28.5 vs. 18.3 %, respectively; p = 0.003). That the incidence of IBC varies by race/ethnicity suggests that the pathogenesis of IBC involves one or more factors that have previously been reported to vary by race, such as genetics, age of menarche, body mass index, diet, and other lifestyle factors.9,10
We conclude that, according to the most recent SEER data, IBC in the United States is not increasing in incidence and disproportionately affects older women and black women. Thus, IBC should remain in the differential diagnosis of all women, regardless of age or race, who present with signs and symptoms of inflammation in the breast, to avoid delay in recognizing those IBC cases that do occur. The racial/ethnic differences in incidence that do exist suggest that future epidemiologic research into IBC should investigate potential risk factors that vary between Asian and black women and that could affect levels of sex steroid hormones during the premenopausal years.
Acknowledgments
The authors have no financial interests to disclose.
References
- 1.Green FL, Page DL, Fleming ID, et al. Breast. In: Green FL, Page DL, Fleming ID, et al., editors. AJCC cancer staging manual. 6. New York: Springer-Verlag; 2002. pp. 225–81. [Google Scholar]
- 2.Robertson FM, Bondy M, Yang W, et al. Inflammatory breast cancer: the disease, the biology, the treatment. CA Cancer J Clin. 2010;60:351–75. doi: 10.3322/caac.20082. [DOI] [PubMed] [Google Scholar]
- 3.Bonnier P, Charpin C, Lejeune C, et al. Inflammatory carcinomas of the breast: a clinical, pathological, or a clinical and pathological definition? Int J Cancer. 1995;62:382–5. doi: 10.1002/ijc.2910620404. [DOI] [PubMed] [Google Scholar]
- 4.Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515–23. doi: 10.1093/annonc/mdq345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hance KW, Anderson WF, Devesa SS, Young HA, Levine PH. Trends in inflammatory breast carcinoma incidence and survival: the Surveillance, Epidemiology, and End Results program at the National Cancer Institute. J Natl Cancer Inst. 2005;97:966–75. doi: 10.1093/jnci/dji172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang S, Parker SL, Pham T, Buzdar AU, Hursting SD. Inflammatory breast carcinoma incidence and survival: the Surveillance, Epidemiology, and End Results program of the National Cancer Institute, 1975–1992. Cancer. 1998;82:2366–72. [PubMed] [Google Scholar]
- 7.Surveillance, Epidemiology, and End Results (SEER) Program. Research Data (1973–2009) National Cancer Institute, DCCPS Surveillance Research Program, Surveillance Systems Branch; ( www.seer.cancer.gov) released April 2012, based on the November 2011 submission. [Google Scholar]
- 8.Levine PH, Steinhorn SC, Gloeckler Ries L, Aron JL. Inflammatory breast cancer: the experience of the Surveillance, Epidemiology, and End Results (seer) Program. J Natl Cancer Inst. 1985;74:291–7. [PubMed] [Google Scholar]
- 9.Anderson WF, Schairer C, Chen BE, Hance KW, Levine PH. Epidemiology of inflammatory breast cancer (IBC) Breast Dis. 2005;22:9–23. doi: 10.3233/bd-2006-22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Il’yasova D, Siamakpour-Reihani S, Akushevich I, Akushevich L, Spector N, Schildkraut J. What can we learn from the age- and race/ethnicity-specific rates of inflammatory breast carcinoma? Breast Cancer Res Treat. 2011;130:691–7. doi: 10.1007/s10549-011-1719-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang R, Cheung MC, Hurley J, et al. A comprehensive evaluation of outcomes for inflammatory breast cancer. Breast Cancer Res Treat. 2009;117:631–41. doi: 10.1007/s10549-009-0312-6. [DOI] [PubMed] [Google Scholar]