Abstract
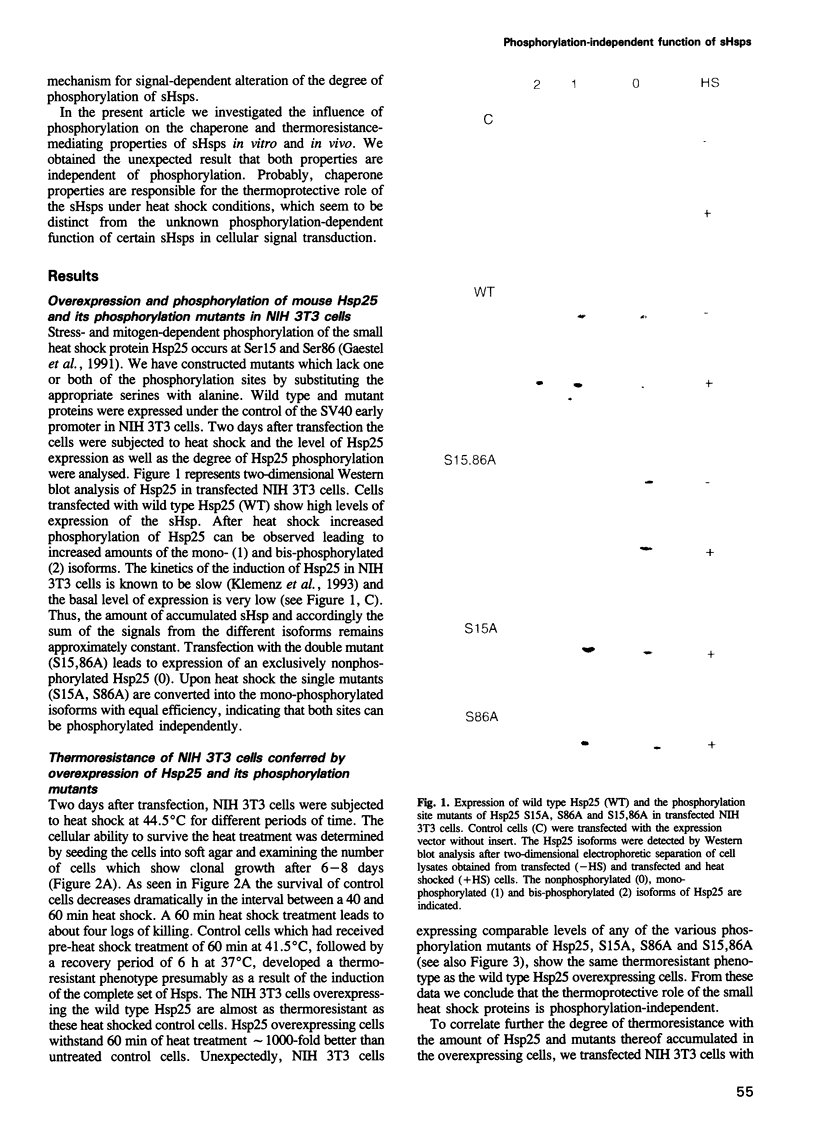
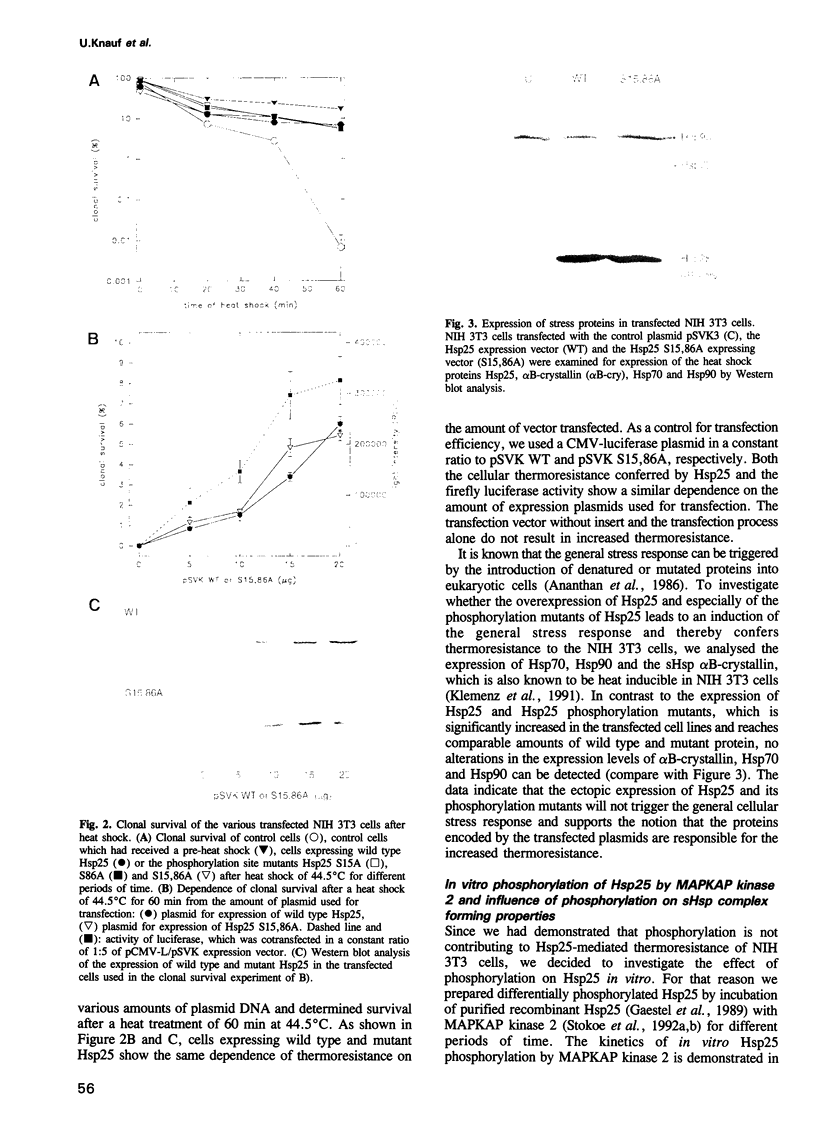
Small heat shock proteins (sHsps) show a very rapid stress- and mitogen-dependent phosphorylation by MAPKAP kinase 2. Based on this observation, phosphorylation of sHsps was thought to play a key role in mediating thermoresistance immediately after heat shock, before the increased synthesis of heat shock proteins becomes relevant. We have analysed the phosphorylation dependence of the chaperone and thermoresistance-mediating properties of the small heat shock protein Hsp25. Surprisingly, overexpression of Hsp25 mutants, which are not phosphorylated in the transfected cells, confers the same thermoresistant phenotype as overexpression of wild type Hsp25, which is either mono- or bis-phosphorylated at serine residues 15 and 86 within the cells. Furthermore, in vitro phosphorylated Hsp25 shows the same oligomerization properties and the same chaperone activity as the nonphosphorylated protein. No differences between phosphorylated and nonphosphorylated Hsp25 are detected in preventing thermal aggregation of unfolding proteins and assisting refolding of denatured proteins. The results suggest that chaperone properties of the small heat shock proteins contribute to the increased cellular thermoresistance in a phosphorylation-independent manner.
Full text
PDF
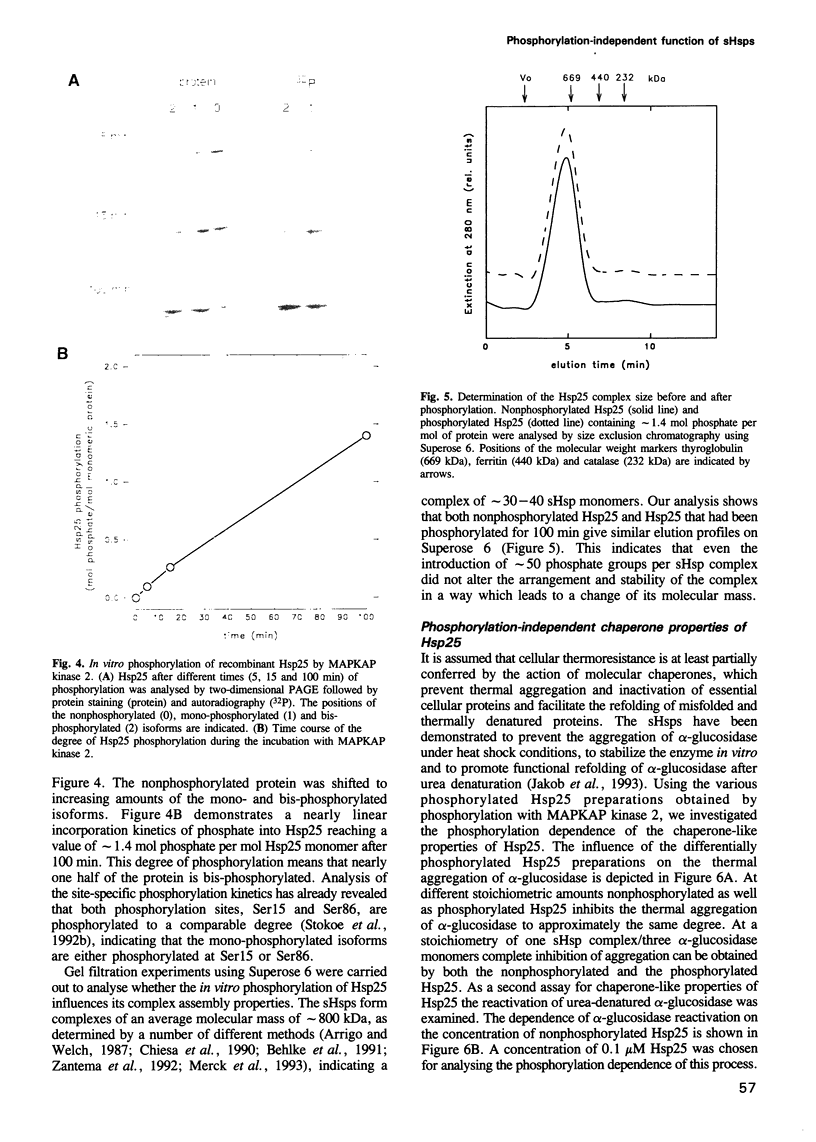
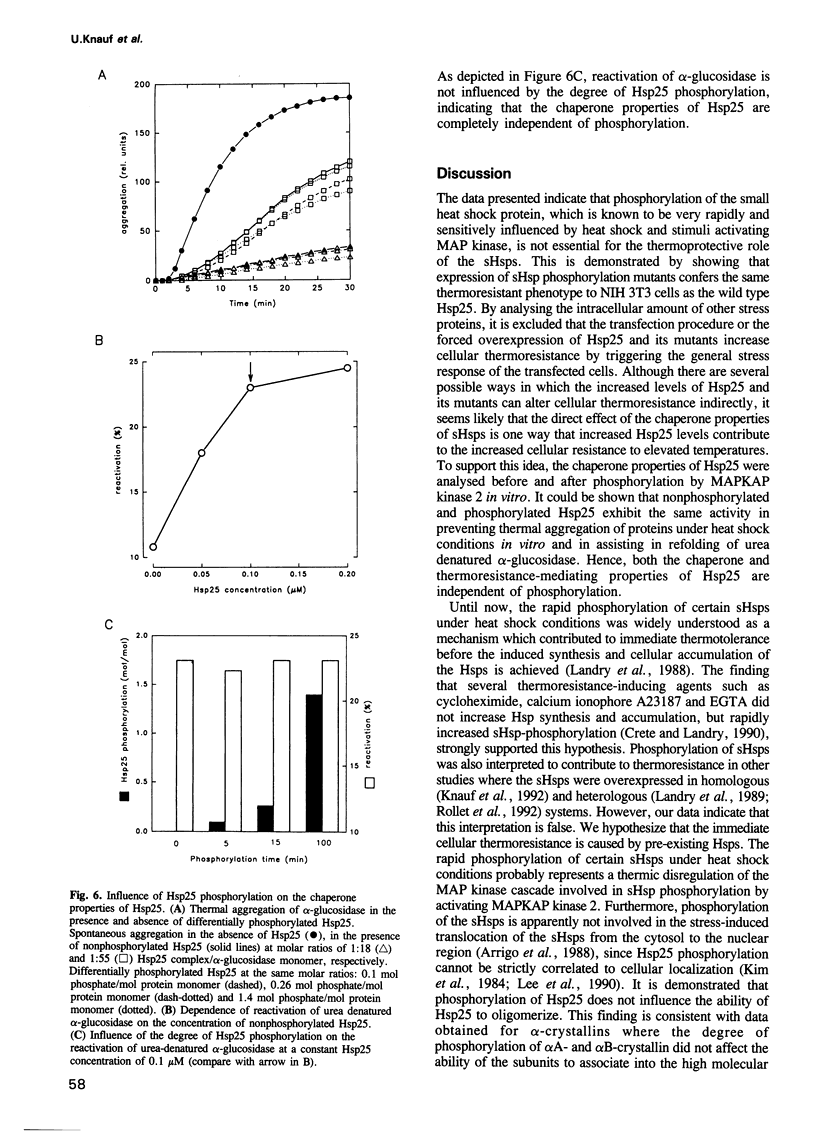


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ananthan J., Goldberg A. L., Voellmy R. Abnormal proteins serve as eukaryotic stress signals and trigger the activation of heat shock genes. Science. 1986 Apr 25;232(4749):522–524. doi: 10.1126/science.3083508. [DOI] [PubMed] [Google Scholar]
- Angelidis C. E., Lazaridis I., Pagoulatos G. N. Constitutive expression of heat-shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem. 1991 Jul 1;199(1):35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- Aoyama A., Fröhli E., Schäfer R., Klemenz R. Alpha B-crystallin expression in mouse NIH 3T3 fibroblasts: glucocorticoid responsiveness and involvement in thermal protection. Mol Cell Biol. 1993 Mar;13(3):1824–1835. doi: 10.1128/mcb.13.3.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Michel M. R. Decreased heat- and tumor necrosis factor-mediated hsp28 phosphorylation in thermotolerant HeLa cells. FEBS Lett. 1991 Apr 22;282(1):152–156. doi: 10.1016/0014-5793(91)80466-g. [DOI] [PubMed] [Google Scholar]
- Arrigo A. P., Suhan J. P., Welch W. J. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol Cell Biol. 1988 Dec;8(12):5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A. P., Welch W. J. Characterization and purification of the small 28,000-dalton mammalian heat shock protein. J Biol Chem. 1987 Nov 15;262(32):15359–15369. [PubMed] [Google Scholar]
- Augusteyn R. C., Koretz J. F., Schurtenberger P. The effect of phosphorylation on the structure of alpha-crystallin. Biochim Biophys Acta. 1989 Dec 21;999(3):293–299. doi: 10.1016/0167-4838(89)90012-5. [DOI] [PubMed] [Google Scholar]
- Behlke J., Lutsch G., Gaestel M., Bielka H. Supramolecular structure of the recombinant murine small heat shock protein hsp25. FEBS Lett. 1991 Aug 19;288(1-2):119–122. doi: 10.1016/0014-5793(91)81016-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chiesa R., McDermott M. J., Mann E., Spector A. The apparent molecular size of native alpha-crystallin B in non-lenticular tissues. FEBS Lett. 1990 Jul 30;268(1):222–226. doi: 10.1016/0014-5793(90)81013-e. [DOI] [PubMed] [Google Scholar]
- Chrétien P., Landry J. Enhanced constitutive expression of the 27-kDa heat shock proteins in heat-resistant variants from Chinese hamster cells. J Cell Physiol. 1988 Oct;137(1):157–166. doi: 10.1002/jcp.1041370119. [DOI] [PubMed] [Google Scholar]
- Crête P., Landry J. Induction of HSP27 phosphorylation and thermoresistance in Chinese hamster cells by arsenite, cycloheximide, A23187, and EGTA. Radiat Res. 1990 Mar;121(3):320–327. [PubMed] [Google Scholar]
- Gaestel M., Benndorf R., Hayess K., Priemer E., Engel K. Dephosphorylation of the small heat shock protein hsp25 by calcium/calmodulin-dependent (type 2B) protein phosphatase. J Biol Chem. 1992 Oct 25;267(30):21607–21611. [PubMed] [Google Scholar]
- Gaestel M., Gross B., Benndorf R., Strauss M., Schunk W. H., Kraft R., Otto A., Böhm H., Stahl J., Drabsch H. Molecular cloning, sequencing and expression in Escherichia coli of the 25-kDa growth-related protein of Ehrlich ascites tumor and its homology to mammalian stress proteins. Eur J Biochem. 1989 Jan 15;179(1):209–213. doi: 10.1111/j.1432-1033.1989.tb14542.x. [DOI] [PubMed] [Google Scholar]
- Gaestel M., Schröder W., Benndorf R., Lippmann C., Buchner K., Hucho F., Erdmann V. A., Bielka H. Identification of the phosphorylation sites of the murine small heat shock protein hsp25. J Biol Chem. 1991 Aug 5;266(22):14721–14724. [PubMed] [Google Scholar]
- Gernold M., Knauf U., Gaestel M., Stahl J., Kloetzel P. M. Development and tissue-specific distribution of mouse small heat shock protein hsp25. Dev Genet. 1993;14(2):103–111. doi: 10.1002/dvg.1020140204. [DOI] [PubMed] [Google Scholar]
- Heidecker G., Huleihel M., Cleveland J. L., Kolch W., Beck T. W., Lloyd P., Pawson T., Rapp U. R. Mutational activation of c-raf-1 and definition of the minimal transforming sequence. Mol Cell Biol. 1990 Jun;10(6):2503–2512. doi: 10.1128/mcb.10.6.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hepburn A., Demolle D., Boeynaems J. m., Fiers W., Dumont J. E. Rapid phosphorylation of a 27 kDa protein induced by tumor necrosis factor. FEBS Lett. 1988 Jan 25;227(2):175–178. doi: 10.1016/0014-5793(88)80892-5. [DOI] [PubMed] [Google Scholar]
- Higuchi R., Krummel B., Saiki R. K. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988 Aug 11;16(15):7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin can function as a molecular chaperone. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10449–10453. doi: 10.1073/pnas.89.21.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenicke R., Creighton T. E. Protein folding: junior chaperones. Curr Biol. 1993 Apr 1;3(4):234–235. doi: 10.1016/0960-9822(93)90342-l. [DOI] [PubMed] [Google Scholar]
- Jakob U., Gaestel M., Engel K., Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993 Jan 25;268(3):1517–1520. [PubMed] [Google Scholar]
- Kaur P., Welch W. J., Saklatvala J. Interleukin 1 and tumour necrosis factor increase phosphorylation of the small heat shock protein. Effects in fibroblasts, Hep G2 and U937 cells. FEBS Lett. 1989 Dec 4;258(2):269–273. doi: 10.1016/0014-5793(89)81671-0. [DOI] [PubMed] [Google Scholar]
- Kim Y. J., Shuman J., Sette M., Przybyla A. Nuclear localization and phosphorylation of three 25-kilodalton rat stress proteins. Mol Cell Biol. 1984 Mar;4(3):468–474. doi: 10.1128/mcb.4.3.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Andres A. C., Fröhli E., Schäfer R., Aoyama A. Expression of the murine small heat shock proteins hsp 25 and alpha B crystallin in the absence of stress. J Cell Biol. 1993 Feb;120(3):639–645. doi: 10.1083/jcb.120.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Fröhli E., Steiger R. H., Schäfer R., Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
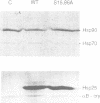
- Knauf U., Bielka H., Gaestel M. Over-expression of the small heat-shock protein, hsp25, inhibits growth of Ehrlich ascites tumor cells. FEBS Lett. 1992 Sep 14;309(3):297–302. doi: 10.1016/0014-5793(92)80793-g. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Lambert H., Hickey E., Weber L. A. Heat shock resistance conferred by expression of the human HSP27 gene in rodent cells. J Cell Biol. 1989 Jul;109(1):7–15. doi: 10.1083/jcb.109.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry J., Chrétien P., Laszlo A., Lambert H. Phosphorylation of HSP27 during development and decay of thermotolerance in Chinese hamster cells. J Cell Physiol. 1991 Apr;147(1):93–101. doi: 10.1002/jcp.1041470113. [DOI] [PubMed] [Google Scholar]
- Landry J., Crête P., Lamarche S., Chrétien P. Activation of Ca2+-dependent processes during heat shock: role in cell thermoresistance. Radiat Res. 1988 Mar;113(3):426–436. [PubMed] [Google Scholar]
- Lavoie J. N., Gingras-Breton G., Tanguay R. M., Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. HSP27 stabilization of the microfilament organization. J Biol Chem. 1993 Feb 15;268(5):3420–3429. [PubMed] [Google Scholar]
- Lee Y. J., Hou Z. Z., Curetty L., Borrelli M. J., Corry P. M. Correlation between redistribution of a 26 kDa protein and development of chronic thermotolerance in various mammalian cell lines. J Cell Physiol. 1990 Nov;145(2):324–332. doi: 10.1002/jcp.1041450218. [DOI] [PubMed] [Google Scholar]
- Li G. C., Li L. G., Liu Y. K., Mak J. Y., Chen L. L., Lee W. M. Thermal response of rat fibroblasts stably transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1681–1685. doi: 10.1073/pnas.88.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarty J. S., Walker G. C. DnaK as a thermometer: threonine-199 is site of autophosphorylation and is critical for ATPase activity. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9513–9517. doi: 10.1073/pnas.88.21.9513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merck K. B., Groenen P. J., Voorter C. E., de Haard-Hoekman W. A., Horwitz J., Bloemendal H., de Jong W. W. Structural and functional similarities of bovine alpha-crystallin and mouse small heat-shock protein. A family of chaperones. J Biol Chem. 1993 Jan 15;268(2):1046–1052. [PubMed] [Google Scholar]
- Miron T., Vancompernolle K., Vandekerckhove J., Wilchek M., Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991 Jul;114(2):255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parsell D. A., Taulien J., Lindquist S. The role of heat-shock proteins in thermotolerance. Philos Trans R Soc Lond B Biol Sci. 1993 Mar 29;339(1289):279–286. doi: 10.1098/rstb.1993.0026. [DOI] [PubMed] [Google Scholar]
- Petko L., Lindquist S. Hsp26 is not required for growth at high temperatures, nor for thermotolerance, spore development, or germination. Cell. 1986 Jun 20;45(6):885–894. doi: 10.1016/0092-8674(86)90563-5. [DOI] [PubMed] [Google Scholar]
- Plesofsky-Vig N., Vig J., Brambl R. Phylogeny of the alpha-crystallin-related heat-shock proteins. J Mol Evol. 1992 Dec;35(6):537–545. doi: 10.1007/BF00160214. [DOI] [PubMed] [Google Scholar]
- Robaye B., Hepburn A., Lecocq R., Fiers W., Boeynaems J. M., Dumont J. E. Tumor necrosis factor-alpha induces the phosphorylation of 28kDa stress proteins in endothelial cells: possible role in protection against cytotoxicity? Biochem Biophys Res Commun. 1989 Aug 30;163(1):301–308. doi: 10.1016/0006-291x(89)92135-9. [DOI] [PubMed] [Google Scholar]
- Rollet E., Lavoie J. N., Landry J., Tanguay R. M. Expression of Drosophila's 27 kDa heat shock protein into rodent cells confers thermal resistance. Biochem Biophys Res Commun. 1992 May 29;185(1):116–120. doi: 10.1016/s0006-291x(05)80963-5. [DOI] [PubMed] [Google Scholar]
- Saklatvala J., Kaur P., Guesdon F. Phosphorylation of the small heat-shock protein is regulated by interleukin 1, tumour necrosis factor, growth factors, bradykinin and ATP. Biochem J. 1991 Aug 1;277(Pt 3):635–642. doi: 10.1042/bj2770635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez Y., Lindquist S. L. HSP104 required for induced thermotolerance. Science. 1990 Jun 1;248(4959):1112–1115. doi: 10.1126/science.2188365. [DOI] [PubMed] [Google Scholar]
- Santell L., Bartfeld N. S., Levin E. G. Identification of a protein transiently phosphorylated by activators of endothelial cell function as the heat-shock protein HSP27. A possible role for protein kinase C. Biochem J. 1992 Jun 15;284(Pt 3):705–710. doi: 10.1042/bj2840705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakoori A. R., Oberdorf A. M., Owen T. A., Weber L. A., Hickey E., Stein J. L., Lian J. B., Stein G. S. Expression of heat shock genes during differentiation of mammalian osteoblasts and promyelocytic leukemia cells. J Cell Biochem. 1992 Mar;48(3):277–287. doi: 10.1002/jcb.240480308. [DOI] [PubMed] [Google Scholar]
- Sherman MYu, Goldberg A. L. Heat shock in Escherichia coli alters the protein-binding properties of the chaperonin groEL by inducing its phosphorylation. Nature. 1992 May 14;357(6374):167–169. doi: 10.1038/357167a0. [DOI] [PubMed] [Google Scholar]
- Shibanuma M., Kuroki T., Nose K. Cell-cycle dependent phosphorylation of HSP28 by TGF beta 1 and H2O2 in normal mouse osteoblastic cells (MC3T3-E1), but not in their ras-transformants. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1418–1425. doi: 10.1016/0006-291x(92)90460-3. [DOI] [PubMed] [Google Scholar]
- Solomon J. M., Rossi J. M., Golic K., McGarry T., Lindquist S. Changes in hsp70 alter thermotolerance and heat-shock regulation in Drosophila. New Biol. 1991 Nov;3(11):1106–1120. [PubMed] [Google Scholar]
- Spector N. L., Samson W., Ryan C., Gribben J., Urba W., Welch W. J., Nadler L. M. Growth arrest of human B lymphocytes is accompanied by induction of the low molecular weight mammalian heat shock protein (Hsp28). J Immunol. 1992 Mar 15;148(6):1668–1673. [PubMed] [Google Scholar]
- Stahl J., Wobus A. M., Ihrig S., Lutsch G., Bielka H. The small heat shock protein hsp25 is accumulated in P19 embryonal carcinoma cells and embryonic stem cells of line BLC6 during differentiation. Differentiation. 1992 Sep;51(1):33–37. doi: 10.1111/j.1432-0436.1992.tb00677.x. [DOI] [PubMed] [Google Scholar]
- Stokoe D., Campbell D. G., Nakielny S., Hidaka H., Leevers S. J., Marshall C., Cohen P. MAPKAP kinase-2; a novel protein kinase activated by mitogen-activated protein kinase. EMBO J. 1992 Nov;11(11):3985–3994. doi: 10.1002/j.1460-2075.1992.tb05492.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokoe D., Engel K., Campbell D. G., Cohen P., Gaestel M. Identification of MAPKAP kinase 2 as a major enzyme responsible for the phosphorylation of the small mammalian heat shock proteins. FEBS Lett. 1992 Nov 30;313(3):307–313. doi: 10.1016/0014-5793(92)81216-9. [DOI] [PubMed] [Google Scholar]
- Voorter C. E., Wintjes L., Bloemendal H., de Jong W. W. Relocalization of alpha B-crystallin by heat shock in ovarian carcinoma cells. FEBS Lett. 1992 Sep 7;309(2):111–114. doi: 10.1016/0014-5793(92)81075-w. [DOI] [PubMed] [Google Scholar]
- Welch W. J. Phorbol ester, calcium ionophore, or serum added to quiescent rat embryo fibroblast cells all result in the elevated phosphorylation of two 28,000-dalton mammalian stress proteins. J Biol Chem. 1985 Mar 10;260(5):3058–3062. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wistow G. Domain structure and evolution in alpha-crystallins and small heat-shock proteins. FEBS Lett. 1985 Feb 11;181(1):1–6. doi: 10.1016/0014-5793(85)81102-9. [DOI] [PubMed] [Google Scholar]
- Zantema A., Verlaan-De Vries M., Maasdam D., Bol S., van der Eb A. Heat shock protein 27 and alpha B-crystallin can form a complex, which dissociates by heat shock. J Biol Chem. 1992 Jun 25;267(18):12936–12941. [PubMed] [Google Scholar]
- de Jong W. W., Leunissen J. A., Voorter C. E. Evolution of the alpha-crystallin/small heat-shock protein family. Mol Biol Evol. 1993 Jan;10(1):103–126. doi: 10.1093/oxfordjournals.molbev.a039992. [DOI] [PubMed] [Google Scholar]