Abstract
Pathogenesis related (PR) proteins are one of the major sources of plant derived allergens. These proteins are induced by the plants as a defense response system in stress conditions like microbial and insect infections, wounding, exposure to harsh chemicals, and atmospheric conditions. However, some plant tissues that are more exposed to environmental conditions like UV irradiation and insect or fungal attacks express these proteins constitutively. These proteins are mostly resistant to proteases and most of them show considerable stability at low pH. Many of these plant pathogenesis related proteins are found to act as food allergens, latex allergens, and pollen allergens. Proteins having similar amino acid sequences among the members of PR proteins may be responsible for cross-reactivity among allergens from diverse plants. This review analyzes the different pathogenesis related protein families that have been reported as allergens. Proteins of these families have been characterized in regard to their biological functions, amino acid sequence, and cross-reactivity. The three-dimensional structures of some of these allergens have also been evaluated to elucidate the antigenic determinants of these molecules and to explain the cross-reactivity among the various allergens.
1. Introduction
Plants are one of the major sources of allergens which elicit allergenic response by immunoglobulin E (IgE) mediated allergies [1, 2]. These allergens may diffuse into the body from the upper respiratory tract or enter the body through intake of vast range of plant food or may cause external skin irritations [3–5]. Allergens present primarily in pollens, spores, and other plant associated products are responsible for symptoms like rhinoconjunctivitis, asthma, edema, urticarial, and anaphylaxis [6–8]. Allergens ingested as food result in responses like pruritus and swelling of lips, tongue, and soft palate, often accompanied by mild laryngeal symptoms as a sensation of tightness, itching, cough, gastrointestinal symptoms, rhinitis, asthma, cutaneous reactions, and more severe systemic anaphylaxis [9–12]. Some plant derived allergens result in contact dermatitis mostly in skin like itchy fingers, skin irritations, and so forth [13, 14]. The most widespread groups of plant allergens that are reported belong to the seed storage proteins, structural proteins, and pathogenesis related (PR) proteins [15–17].
Allergens are assigned names based on their accepted taxonomic nomenclature with the first three letters designating their genus and followed by the first letter of the species and an Arabic number denoting the order of the identification. The allergenicity of the protein is mostly dependent on the structural motifs present in the allergens which act as the allergenic determinants or the epitopes that are responsible for binding to B and T cells. B cell epitopes are mostly discontinuous motifs forming conformational epitopes whereas T cell epitopes are linear and continuous [18, 19]. It has been found that allergens from diverse species possess similar structural motifs that can be identified by the antibodies resulting in IgE cross-reactivity.
2. Plant Pathogenesis Related Proteins
Plant pathogenesis related (PR) proteins are generally induced by various types of pathogens such as viruses, bacteria, and fungi [20–22]. Some of these proteins are also expressed in response to some chemicals that act in a similar way as pathogen infection [23, 24]. However, some of the PR proteins are constitutively expressed in some organs or during certain developmental stages [25, 26]. They are regarded as PR-like proteins because of their sequence homology. However, some PR-like proteins are found to be strongly induced by infections and hence they are also designated mostly as PR proteins [27].
PRs were first identified from tobacco leaves (Nicotiana tabacum) infected with tobacco mosaic virus and later have been detected in numerous plants of different species [28]. They exhibit distinct biochemical characteristics which are necessary when the plant is under pathogenic infections or any unwanted stress. They are generally low-molecular weight proteins in the range 6 to 43 kDa, stable at low pH (<3), and are protease resistant which helps them survive in the harsh conditions like the vacuolar compartment, cell wall, or intercellular spaces [20]. Depending on their isoelectric points, PR proteins are either acidic or basic and are also found to be either vacuolar or apoplastic [29]. The acidic forms of PR proteins are mostly secreted to the extracellular space and the basic forms are transported to the vacuole by a signal located at the C-terminus [30]. However, such localization cannot be generalized for all PR proteins except in the case of certain tobacco PR family. Currently, PRs are found to be localized in almost all plant organs including leaves, stems, roots, and flowers, though maximum abundance of these proteins is found in the leaves [29].
Usually, upregulation of gene expression during pathogen attack takes place by various signaling molecules like salicylic acid [23, 31, 32] and reactive oxygen species [33] which mediate the expression of acidic PR genes. Induction of basic PR genes is mediated by gaseous phytohormone ethylene and methyl jasmonate [24]. Apart from various environmental factors that trigger the synthesis of these PR proteins, their expressions are also dependent on certain internal developmental stimuli of the plant.
Originally 5 groups of PR proteins have been identified [34] but gradually with the increasing identification of new PR proteins, presently, 17 families of PR proteins are recognized based on their amino acid sequence similarities, enzymatic activities, or other biological properties and numbered in the order in which they were discovered (Table 1) [29, 35, 36]. In spite of their common name, these proteins display a great diversity in species specificity and in the mechanism of action and do not share any structural relationship among themselves.
Table 1.
Different PR-protein families and allergens identified.
| Family | Proteins | Functions | Allergens identified with source and allergenic symptoms |
|---|---|---|---|
| PR-1 | PR-1 a, PR-1 b, and PR-1 c | Antifungal | Cuc m 3 (muskmelon)—oral allergy syndrome |
| PR-2 | β-1,3-Glucanases | Cleaves β-1,3-glucans | Hev b 2 (latex)—contact dermatitis Ole e 9 (olive)—respiratory allergy Mus a 5 (banana)—oral allergy syndrome |
| PR-3 | Chitinase types I, II, IV, V, VI, and VII | Endochitinase | Pers a 1 (avocado)—itchy eyes or nose, asthma, swelling, and so forth. Mus a 2 (banana)—food allergy like swelling of lips, anaphylaxis, and so forth |
| PR-4 | Chitinase types I and II | Antifungal and chitinase | Hev b 6.01, Hev b 6.02, and Hev b 6.03 (latex)—contact dermatitis |
| PR-5 | Thaumatin-like proteins | Antifungal | Jun a 3 (mountain cedar), Cry j 1 (Japanese cedar), and Cup a 3 (Arizona cypress)—rhinitis, conjunctivitis, and asthma Pru av 2 (cherry), Mal d 2 (apple), Cap a 1 (bell pepper), Act d 2 (kiwi), and Mus a 4 (banana)—oral allergy syndrome |
| PR-6 | Tomato proteinase inhibitor I | Proteinase inhibitor | — |
| PR-7 | Tomato endoproteinase P | Endoproteinase | — |
| PR-8 | Cucumber chitinase | Chitinase III | Hevamine (latex)—contact dermatitis. Ziz m 1 (Indian jujube)—oral allergy syndrome Cof a 1 (coffee)—eye and airway allergy |
| PR-9 | Tobacco lignin-forming peroxidase | Peroxidase | — |
| PR-10 | Parsley “PR-1” Bet v 1, Mal d 1, Api g 1, and Dau c 1 |
Ribonuclease-like | Bet v 1 (birch pollen)— allergic rhinoconjunctivitis and asthma Pru av 1 (cherry), Mal d 1 (apple), Api g 1 (celery), and Dau c 1 (carrot)—oral allergy syndrome Gly m 4 (soy), Vig r 1 (mung bean), Cor a 1 (hazelnut), and Cas s 1 (chestnut)—oral allergy syndrome |
| PR-11 | Tobacco chitinase type V | Chitinase | — |
| PR-12 | Radish Rs-AFP3 | Defensin | — |
| PR-13 | Arabidopsis THI2.1 | Thionin | — |
| PR-14 | Lipid transfer proteins | Shuttling of phospholipids and fatty acids | Par j 1 (weed)—rhinitis and asthma Pru p 3 (peach), Mal d 3 (apple), Pru av 3 (cherry), Pru ar 3 (apricot), Cor a 8 (hazelnut), Cas s 8 (chestnut), and Zea m 14 (maize)—oral allergy syndrome |
| PR-15 | Barley OxOa | Oxalate oxidase | — |
| PR-16 | Barley OxOLP | Oxalate-like oxidase | — |
| PR-17 | Tobacco PRp27 | Unknown | — |
PR proteins exhibit multiple functions within the plant. Most PRs exhibit antifungal activity [37, 38] though antibacterial, insecticidal, nematicidal, and antiviral activity of some of the PR proteins have also been reported [39, 40]. Some of the PR proteins have enzymatic functions like β-1,3-glucanase [41] or chitinase activities [42]; some including defensins [43] and lipid transfer proteins [44] have membrane permeabilizing effect. PR proteins thus have crucial function in disease resistance, seed germination, and plants facilitation to adapt to the environmental stress.
Apart from PR proteins, plants under pathogen attack produce other families of proteins having defensive action like PR proteins. Two α-amylases are found to be induced in tobacco upon TMV infection [45]. Polygalacturonase inhibitor proteins (PGIPs) are produced by pathogen infection and stress-related signals in plants that can inhibit fungal endopolygalacturonases [46]. Others include cell wall hydroxyproline-rich glycoproteins [47] and lipoxygenases [48]. Certain plant storage proteins, like 2S-albumins, lectins, vicilins, glycine-rich proteins, and so forth, accumulate in storage vacuoles inside plant cells and perform essential roles as antimicrobial agents in response to pathogen attack [49, 50]. Ribosome-inactivating proteins (RIPs), cysteine-rich peptides, and so forth are also expressed by plants as antimicrobial molecules but are not induced by any pathogen attack [51, 52].
3. Plant PR Proteins and Allergenicity
In the recent years, a variety of PR proteins and their homologues causing allergenicity in humans have been isolated and characterized [53–56]. The size, stability, and resistance to proteases along with hydrolytic and membrane-permeabilizing ability in some make these proteins excellent candidates to elicit allergenic response [53]. Moreover, PR proteins are mostly associated with high degree of cross-reactivity because of structural similarity among some of the major proteins. Many patients allergic to one form of allergen like pollen also display allergic symptoms after ingesting some other allergens like certain fresh fruit, vegetable, or nut [57–59]. Thus, IgE antibodies originally produced in response to a particular allergen sensitization recognize comparable epitopes which are present on the surface of other plant proteins. Hence, reexposure to homologous plant allergens induces an allergic reaction in already sensitized individuals. Some of the common allergic syndromes like pollen-related food syndrome, latex-fruit syndrome, or the birch-mugwort-celery-spice syndrome are associated among the different PR proteins [60–62].
It has also been observed that plants growing under different conditions have varied levels of expression of the allergenic PR proteins and their homologues [63]. The expression of some of these classes of allergens also shows alterations due to environmental pollutants [55]. Based on sequence characteristics, a number of allergens classified as PR proteins are recognized from PR families 1, 2, 3, 4, 5, 8, 10, and 14 (Table 1) [29]. Thus, evaluating the structure and role of members of these different PR families in allergenicity will help to understand the allergenic cross-reactivity and will explain the differences in the frequency of sensitization and severity of allergenicity in sensitized individuals.
3.1. PR-1 Family Allergens
PR-1 proteins were first found to be expressed in tobacco in response to tobacco mosaic virus (TMV) infection having 14 to 17 kDa molecular weights [64]. Later, homologues of tobacco PR-1 proteins have been identified in barley, tomato, maize, rice, and so forth [65–68]. These widely distributed proteins of plant kingdom have antifungal activity at the micromolar level against a number of plant pathogenic fungi [66], but their mechanism of action is not known. No allergens were reported from PR-1 protein family till 2004. The first evidence of an allergen Cuc m 3 was reported from muskmelon which comprises many pollen allergens, thus delivering the involvement of this plant allergen family in food allergy [69]. Cuc m 3 shows more than 60% of sequence identity with PR-1 members from grape and cucumber.
3.2. PR-2 Family Allergens
PR-2 family of proteins are β-1,3-glucanases (glucan endo-1,3-β-glucosidases) which are monomeric enzymes having molecular weight around 20–23 kDa. These highly regulated enzymes catalyze the hydrolytic cleavage of β-1,3-glucans abundantly present in plant cell walls [70]. These enzymes function in response to pathogenic attack and are also involved in several physiological and developmental processes, for example, cell division [71], microsporogenesis [72], pollen germination [73], fertilization [74] and seed germination [75], and mobilisation of storage products in the endosperm of cereal grains [76]. These proteins were also induced in response to ozone and ultraviolet B light, mechanical injury, and freezing temperatures [77–79].
The PR-2 proteins are divided into three classes based on amino acid sequence identity, primary structure, cellular localization, and mode of expression [80]. The class I members with approximate size of 33 kDa are basic and localized in the cell vacuole and are found in tobacco, tomato, potato, and other plant species [81]. The class II and class III proteins are acidic proteins with average molecular weights around 34 to 36 kDa secreted into the extracellular space [82]. Antifungal activity has been observed only in class I β-glucanases. The proteins belonging to class I family have an additional C-terminal extension which is posttranslationally cleaved during intracellular transport and are likely to contain the vacuolar targeting signal [83].
Some of the allergens having sequence similarity to β-1,3-glucanases have been identified. Latex from Hevea brasiliensis contains several allergenic proteins that are involved in allergenicity resulting in symptoms like mild contact urticaria to asthma and anaphylactic reactions that frequently occur during surgical or endoscopic procedures [84, 85]. One of them is Hev b 2, a β-1,3-glucanase enzyme that is recognized by IgEs of latex-allergic patients. This protein shares 62.9% and 64.7% sequence identities with tobacco and tomato β-1,3-glucanases (Figure 1). This 36 kDa protein is present in different isoforms and with variable glycosylation content [86, 87]. Both its peptidic and carbohydrate moieties are known to possess allergenic determinants [88, 89].
Figure 1.

Multiple sequence alignment of β-1,3-glucanases from tobacco, tomato, banana, and latex (Hev b 2). The identical residues are highlighted in grey.
The structure of Hev b 2 adopts a TIM-barrel, (α/β)8 fold (PDB code: 3EM5). Several IgE binding epitopes have been recognized along the entire amino acid sequence of the major latex allergen Hev b 2 [90] (Figure 2(a)). The amino acid residues residing in the IgE binding epitopic regions are found to be mostly exposed on the surface and the epitopes usually correspond to charged regions on the molecular surface of the protein.
Figure 2.
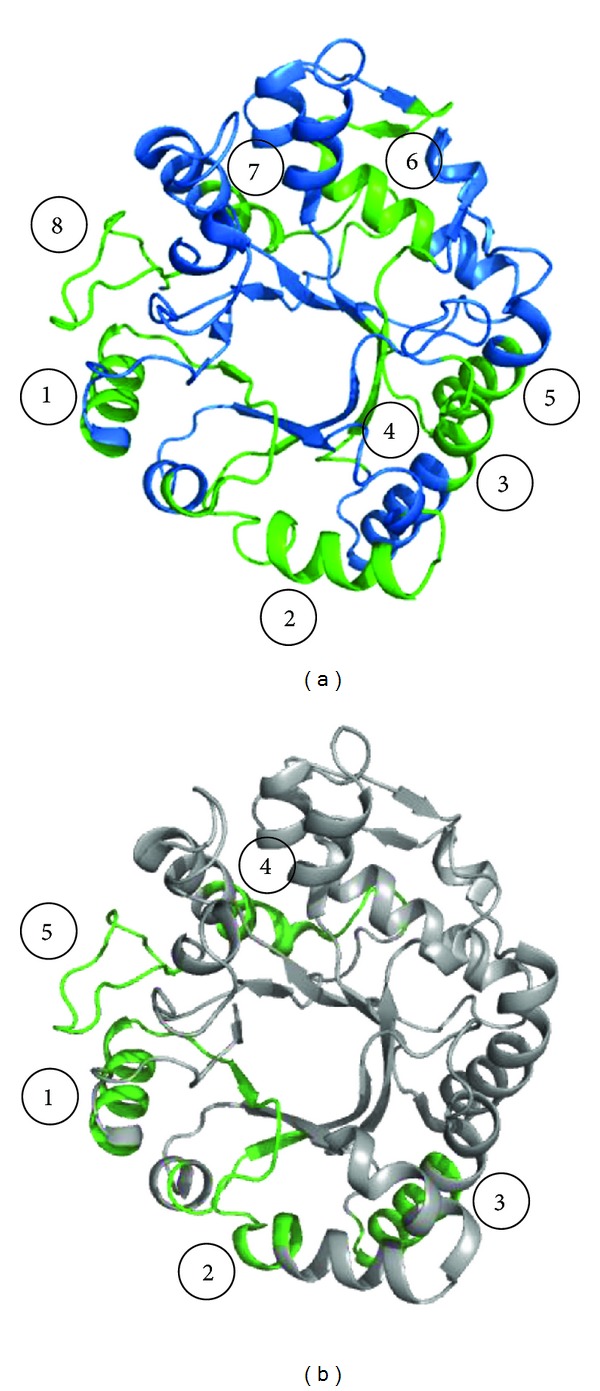
(a) Overall structure of Hev b 2 showing IgE binding epitopes (in green). (b) Overall structure of β-1,3-glucanase from banana showing IgE binding epitopes (in green).
Hevea latex allergy has been found to be associated with hypersensitivity to foods, especially avocado, banana, chestnut, fig, bell pepper, and kiwi, and is termed latex-fruit syndrome [91–93]. The reason for such cross-reactivity is that the proteins expressed in these fruits share very similar overall conformation and charge distribution to those of Hev b 2. Hev b 2 has about 60.8% sequence identity with banana β-1,3-glucanase, Mus a 5, the expression of which increases to a considerable amount during fruit ripening [94]. Five IgE binding epitopes similar to Hev b 2 have been identified in this protein (Figure 2(b)).
Another PR-2 protein, Ole e 9, has been characterized from olive pollen [95]. The protein is composed of two immunological independent domains: an N-terminal domain with β-1,3-glucanase activity and a C-terminal domain that binds 1,3-β-glucans. The overall structure of C-terminal domain of Ole e 9 has been found to comprise two parallel α-helices, a small antiparallel β-sheet with two short strands, and a 3–10 helix turn connected with each other by long coil segments (Figure 3). Two regions have been identified on the protein surface which are constituted of aromatic residues and have a possible role in sugar binding. Using epitope mapping four IgE epitopes have been characterized on the C-terminal domain of Ole e 9 which are mainly concentrated on the loops and few in secondary structural elements [96].
Figure 3.
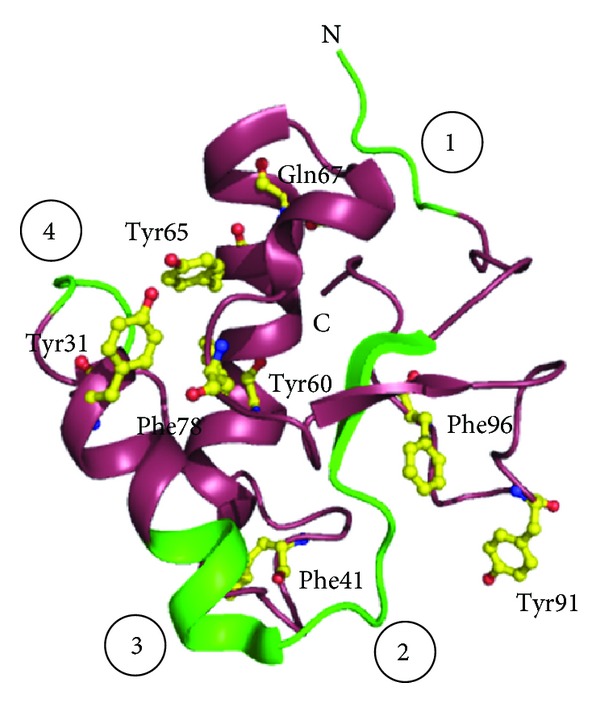
Structure of C-terminal domain of Ole e 9 from olive pollen. The four IgE binding epitopes (in green) marked from 1 to 4 are shown. The aromatic residues which form two distinct sugar binding faces are shown in yellow.
3.3. PR-3 Family Allergens
Among the seven different classes of chitinases, chitinases of classes I, II, and IV are grouped under PR-3 family proteins. Chitinases hydrolyze the glycosidic bonds in chitin, a component of the cell walls of fungi and exoskeletal elements of some animals [97]. Plant chitinases are monomeric proteins of 25–35 kDa molecular weights and are mostly endochitinases which break the chitin within the biopolymer. They produce 2–6 N-acetyl-glucosamine units and hydrolyze β-1,4-linkages between the N-acetylmuramic acid and the N-acetylglucosamine of lysozyme.
Some of the class I chitinases found in seed producing plants are basic proteins that are vacuolar and antifungal, whereas the acidic ones are extracellular with little antifungal activity. This class of chitinases contains a cysteine-rich 40-amino-acid domain at the N-terminus, the chitin binding hevein domain, a hypervariable domain (which is a proline-rich hinge region), and a catalytic domain [98]. The exact role of the hevein domain is not clear though it is required for chitin binding and for substrate affinity. It is speculated that the chitin-binding domain helps in increasing the efficiency of enzymatic cleavage of the polymer by attaching the catalytic domain onto the substrate. The intracellular localization depends on the presence of a C-terminal vacuolar targeting propeptide. A signal peptide is removed from the mature protein and a target sequence directing the protein to the vacuole is located at the C-terminus.
Class II chitinases having molecular weights of 27 to 28 kDa resemble class I proteins in terms of amino acid sequence, but they lack the N-terminal cysteine-rich hevein domain and the vacuolar target sequence. These enzymes are mainly acidic and extracellular and display 60–65% sequence similarities to class I chitinases. Some of these members induce antifungal activity in living plant cells rather than killing the invading fungus. Class IV proteins are similar to class I chitinases but are significantly smaller in size due to four major deletions [99].
Some of the major class I chitinase allergens of the PR-3 family have been identified from chestnut (Cas s 5) and avocado (Pers a 1) [100, 101]. Since these proteins are characterized by the presence of a conserved hevein-related structure, patients with previous exposure to latex prohevein or hevein are potential candidates for cross-reaction with these fruits and vegetables [102]. Pers a 1, a 32 kDa endochitinase, exhibits strong antifungal activity [101]. Class I chitinases are also recognized in banana (Mus a 2) with hevein-like domain having IgE binding properties. This protein shares about 74.0% amino acid sequence identity with Pers a 1 including the hevein-like domain present in both proteins (Figure 4). The stability of the proteins is maintained by the presence of cysteine residues. However, the three-dimensional structures of these proteins have not yet been reported [103].
Figure 4.
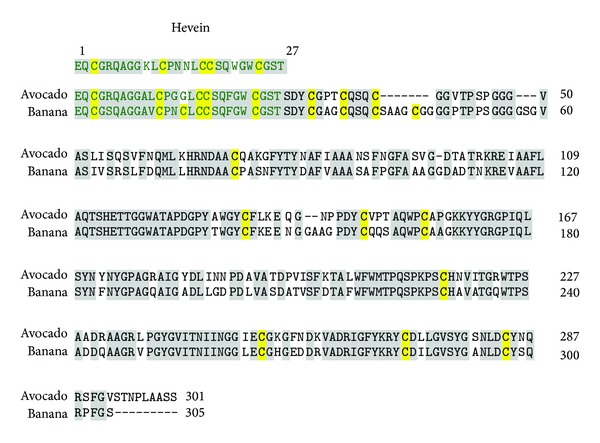
Sequence alignment of chitinase I from avocado (Pers a 1) and banana. The hevein-like domain (matching with sequence of hevein (1–27)) has also been marked in green. The identical residues and the cysteine residues are highlighted in grey and yellow, respectively.
3.4. PR-4 Family Allergens
PR-4 proteins are chitin-binding proteins, having molecular weights around 13 to 14.5 kDa. Some of the PR-4 proteins identified are tobacco protein CBP-20 and barley barwin [104, 105]. The common class I chitinases representing this family are prohevein and wound-inducible proteins. Prohevein, a cysteine-rich 20 kDa protein from Hevea brasiliensis, is designated as Hev b 6.01 and is one of the major IgE binding allergens in natural rubber latex allergy, especially common in health care workers [106]. It contains 14 cysteine residues that stabilize its tertiary conformation by forming multiple disulphide bridges. After posttranscriptional processing, prohevein generates the 4.7 kDa N-terminal Hev b 6.02 (hevein) and the 14 kDa C-terminal Hev b 6.03 (Figure 5(a)) both of which are allergenic. The former is involved in IgE binding and carries discontinuous B cell epitopes (Figure 5(b)), whereas Hev b 6.03 is responsible for proliferation response and contains human leucocyte antigen, HLA-DR4-binding motifs [107]. Hev b 6.03 shares more than 90% sequence similarity with wound-inducible proteins like potato stress proteins, WIN1 and WIN2 (Figure 6).
Figure 5.

(a) Amino acid sequence of prohevein showing two allergenic domains obtained after posttranslational cleavage: Hev b 6.02 domain (green) and Hev b 6.03 domain (blue). 14 cysteine residues are highlighted in yellow. (b) Amino acid sequence of hevein showing the IgE binding epitopes highlighted in cyan.
Figure 6.

Multiple sequence alignment of Hev b 6.03 with wound inducible proteins WIN 1 and WIN 2. The identical residues are highlighted in grey and the cysteine residues in yellow.
Hevein has significant sequence similarities (about 71.7%) with chitin-binding proteins of PR-3 and PR-4 families [106] and is one of the reasons of latex allergic patients resulting in food allergies. Hevein is the major cross-reacting allergen with avocado in subjects with latex allergy [108, 109]. The crystal structure of hevein is folded into a series of loops all linked together by four disulfide bonds (Figure 7). An aromatic patch is formed in the central part of the protein by 2 tryptophan and 1 tyrosine residues encircled by 4 glutamate side chains forming a carbohydrate-combining site constituting a conformational epitope [110]. Current studies have shown that hevein is an ideal target for application in latex immunotherapy. Hypoallergenic variants of prohevein have been obtained by site directed mutagenesis in hevein domain which showed attenuated B cell reactivity but retained human T lymphocyte reactivity [111].
Figure 7.
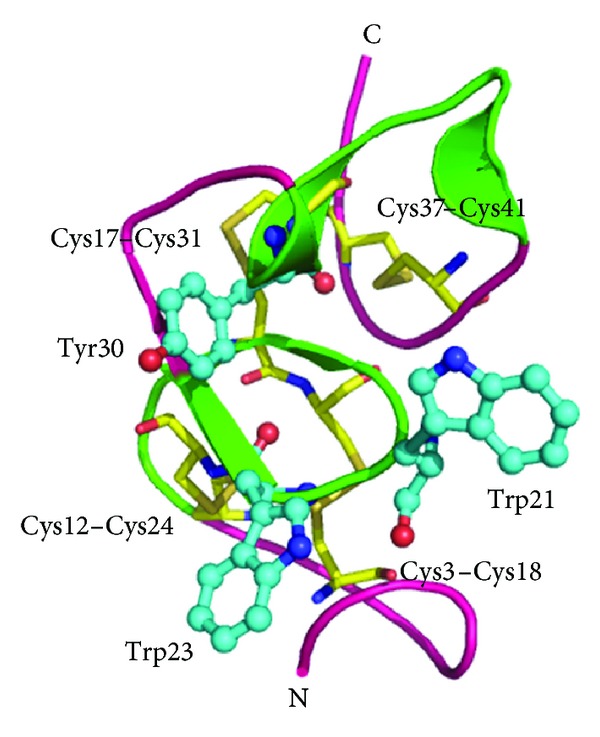
Overall structure of Hev b 6.02 (hevein) showing IgE binding epitopes (in green). Trp21, Trp23, and Tyr30 form an aromatic patch in the conformational epitope. The four disulphide linkages are shown in yellow.
The class II chitinases belonging to PR-4 proteins have been identified from tobacco and tomato with sequence similarity to win proteins, yet lacking the chitin-binding domain [112]. Whole or wounded turnips treated with salicylic acid, ethephon, or water resulted in the expression of an 18.7 kDa protein exhibiting appreciable allergenicity that was recognized by IgE of natural rubber latex allergic patients [113].
3.5. PR-5 Family Allergens
PR-5 proteins have high amino acid homology to sweet tasting protein, thaumatin, from the South African berry bush Thaumatococcus daniellii [114] and are known as thaumatin-like proteins (TLPs) though none of these proteins have been reported to have a sweet taste. These proteins were first identified in tobacco leaf extracts when the plant was infected with tobacco mosaic virus [115]. Though TLPs have been mostly observed in leaves of young plants, they are detected in high levels upon biotic or abiotic stress. Osmotin and NP24 proteins from tobacco and tomato, respectively, are homologous to thaumatin and accumulate after osmotic stress [116, 117]. TLPs are also developmentally expressed to a significant amount in flower buds of turnip and overripe fruits of cherries [118, 119].
Based on their molecular weight, proteins of this class are grouped into two types: the first class having molecular weights ranging from 22 to 26 kDa and the other class having molecular weights around 16 kDa due to an internal deletion of 58 amino acids. Generally, they are acidic, basic, or neutral TLPs. Some of these proteins exert antifungal activity possibly by directly inserting them into the fungal membrane forming a transmembrane pore, eventually resulting in influx of water followed by osmotic rupture. Zeamatin, an antifungal 22 kDa protein that acts by causing membrane permeabilization has been reported, corn seeds [120]. Similar proteins with considerable sequence homology and similar antifungal action are also reported from tobacco, oats, sorghum, and wheat [121].
The allergens of this group are mainly pollen or food derived allergens. Several pollen allergens like Jun a 3 (mountain cedar) [63], Cry j 1 (Japanese cedar) [122], Cup a 3 (Arizona cypress) [123], and Jun v 3 (Eastern red cedar) [124] have been reported. Some of these allergens like Jun a 3 showing variability of expression may contribute to variable allergenicity in different lots of pollen. Four IgE binding epitopes have been predicted in the sequence of Jun a 3: Ala120 to Lys131, Val132 to Lys144, Asn152 to Lys165, and Asn169 to Lys179. Jun a 3 has been found to exhibit cross-reactivity with Cry j 1, from Japanese cedar (Cryptomeria japonica) [63]. Cup a 3 from Arizona cypress (Cupressus arizonica) is homologous to Jun a 3 and shows increased allergenicity of pollen from industrialized areas [123].
Allergens of this group belonging to food allergens include Pru av 2 from cherries [125], Cap a 1 from bell pepper [62, 126], Mal d 2 from apple [127], Act d 2 from kiwi [128], and Pru p 2 from peach [129]. Pru av 2, the major allergen in cherries, is one of the main causes of oral allergy syndrome and shares considerable sequence identity with Jun a 3. Thaumatin-like protein, associated with baker's respiratory allergy, has been also identified in wheat (Triticum aestivum) [130]. A 24 kDa protein from grapes, homologous to Pru av 2, was reported as a minor allergen [131]. Cap a 1 identified from bell pepper shows an IgE-mediated contact allergy in patients with the mugwort-birch-celery-spice syndrome [62].
The crystal structure of allergenic TLP from banana (Mus a 4) has been characterized [132]. The protein has three distinct domains: the core domain constituted by a β-sandwich formed by two β-sheets, an extended α-helix domain, and a third domain with a hair-pin segment of two short β-strands connected to an extended loop. The structure is stabilized by eight disulphide bridges (Figure 8) which are conserved in other thaumatin-like molecules. A central cleft comprising acidic residues Glu83, Asp96, Asp101, and Asp181 imparts a strong electronegative character. Twelve highly exposed flexible linear epitopes for IgE binding have been speculated in banana TLP. Pru av 2 (PDB: 2AHN) and Mal d 2 (PDB: 3ZS3) have similar overall three-dimensional structure, conserved cysteine residues (Figure 9), and share about 72.9% sequence identity with each other.
Figure 8.
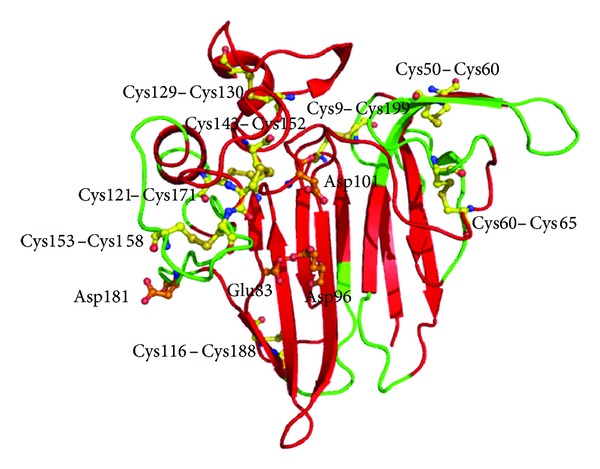
Three-dimensional structure of Banana TLP. Eight disulphide bridges and the acidic residues, Glu 83, Asp96, Asp101, and Asp111 are shown. Twelve amino acid stretches are marked in green.
Figure 9.
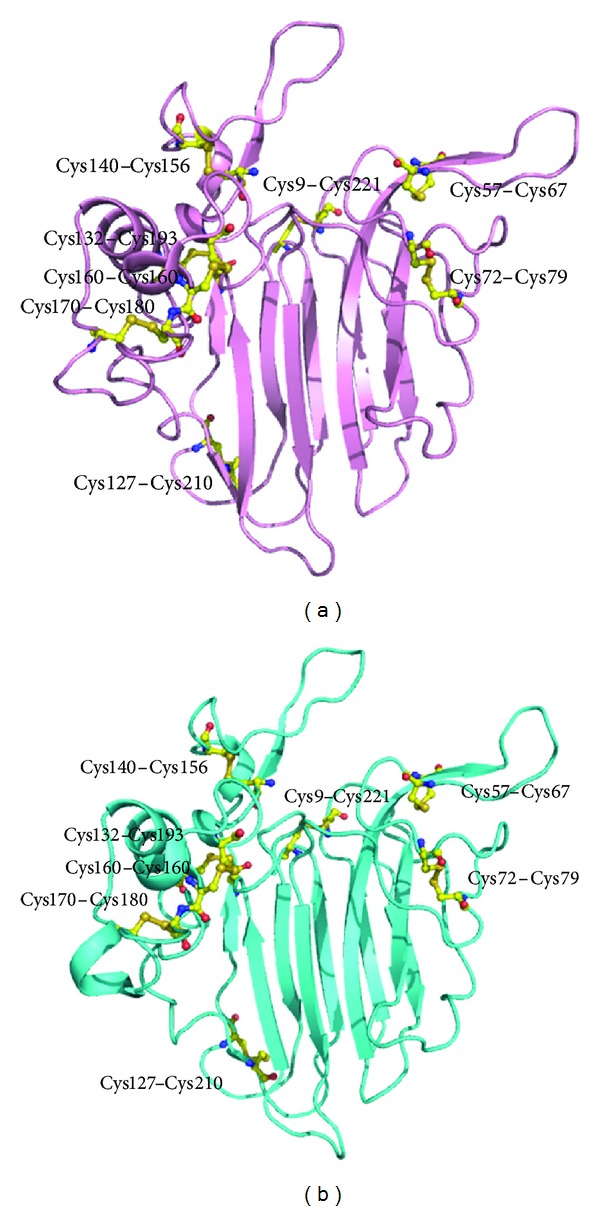
Three-dimensional structures of (a) Pru av 2 and (b) Mal d 2 showing the conserved cysteine residues.
Osmotin has been used in production of transgenic crops because of its ability in permeabilizing the plasma membrane and dissipating the membrane pH gradient of the fungal species [133]. However, osmotin was identified as a potential allergen and showed cross-reactivity with allergens from tomato and apple [134]. Three possible antibody recognition sites have been speculated in osmotin and validated by in vitro experiments [135].
3.6. PR-8 Family Allergens
PR-8 proteins comprise chitinases belonging to class III type having lysozyme activity [136]. One of the major latex proteins, representing this group, is hevamine (Hev b 14) which displays both lysozyme and chitinase activity. It is a 30 kDa basic chitinase from lutoid bodies of the latex of Hevea brasiliensis belonging to the family 18 glycosyl hydrolases and has been identified as an allergen present in latex products [137]. Hevamine plays an important role in the self-defense of the rubber tree against pathogenic fungi. However, unlike lysozyme, hevamine cleaves peptidoglycan between the C-1 of N-acetyl glucosamine and the C-4 of N-acetylmuramate.
The amino acid sequence of hevamine shows significant similarity to those of other chitinases/lysozymes from plants and fungi, while there is a lower similarity to chitinases from bacteria, insects, and viruses. The structure of hevamine has a single TIM-barrel (βα)8 fold with active site residues Asp125, Glu127, and Tyr183 and represents a new class of polysaccharide-hydrolyzing enzymes (Figure 10). The substrate specificity of this protein resides in the loops following the barrel strands that form the substrate binding site. The protein has the two family 18 consensus regions roughly corresponding to the third and fourth barrel strands [138]. Hevamine has been found to be one of the major latex allergens having IgE binding characteristics prevalent mainly in the health care workers of Taiwan [139, 140]. However, the details of antigenic determinants and their role in allergenicity are still unknown.
Figure 10.
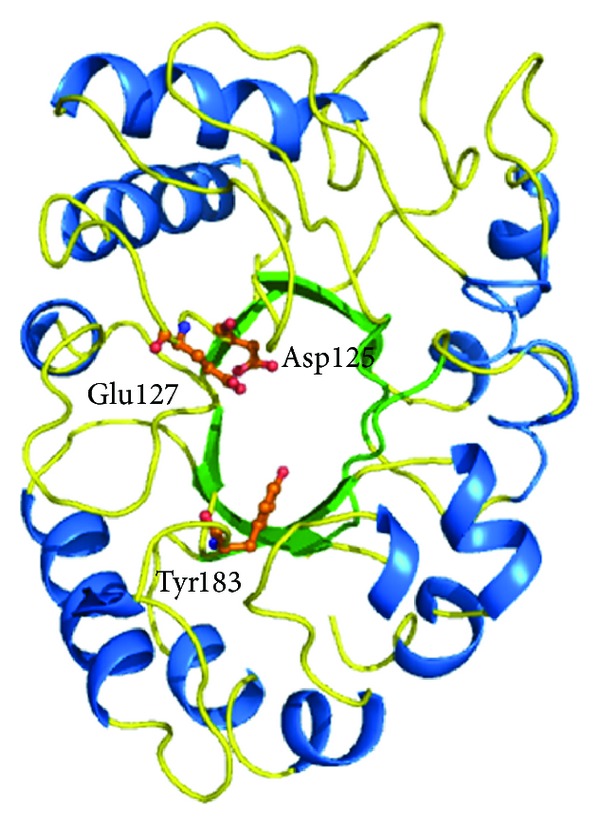
Overall structure of hevamine showing TIM barrel domain and the catalytic residues (in orange).
A similar class III chitinase, Ziz m 1, had been identified in Indian jujube (Ziziphus mauritiana) as one of the major allergens having IgE cross-reactivity with the latex allergen [141]. Two stretches of residues from Asn72 - Glu86 and from Val292 - Pro320 are the possible IgE binding epitopes characterized in Ziz m 1 [142]. Recent reports suggest that this chitinase can stimulate multiple cytokines, mainly interleukin-13, from peripheral blood mononuclear cells of latex fruit allergic patients [143]. Another chitinase III protein, Cof a 1 having allergenic potential, has been identified in coffee (Coffea arabica) [144].
3.7. PR-10 Family Allergens
PR-10 family proteins are intracellular proteins with unknown enzymatic function. Some proteins of PR-10 family are induced under various stress conditions and act as common allergens [145, 146]. However, few PR-10 proteins are also constitutively expressed, indicating a role of these proteins in plant development [147]. The members of this family have low molecular weight (around 15-16 kDa) and are slightly acidic, resistant to proteases, and mostly intracellular and cytosolic [148, 149]. PR-10 proteins are structurally not related to any other class of PR proteins. Apart from direct function in defense, these proteins are implicated in a general function during overall stress as well as during physiological changes in certain developmental stages [150].
Though this family of proteins is widely studied, the exact function of these proteins is still unclear. Some of these proteins are suggested to have a protective role because they are induced when plants undergo pathogenic or environmental stresses. However, some members are also constitutively expressed indicating a general biological role in plant development associated with these proteins. PR-10 proteins are encoded by multigene families which accounts for their multifunctional behavior [151]. Thus PR-10 proteins are not responsible for a particular function in the plant system but some of their conserved sequences and their wide spread existence suggest a crucial role of these proteins [152].
This class of proteins was first identified from parsley [153] followed by other common allergens found in birch pollen [154], celery [155], apple [156], and other fruits and vegetables. Of the different PR-10 proteins, birch-related pollen allergens are extensively studied. The 17.5 kDa protein Bet v 1 is mostly responsible for birch pollen allergy and patients allergenic to birch pollens have been found to develop specific IgE towards Bet v 1 [157]. Thirteen different isoforms of Bet v 1 were identified with the isoform Bet v 1a exhibiting the highest and Bet v 1l exhibiting the lowest allergenic activity [158].
Cross-reactivity between Bet v 1 and other food allergens leads to clinical oral allergy syndrome. Several of such allergens homologous to Bet v 1 have been characterized from apple (Mal d1) [156], sweet cherry (Pru av 1) [159], celery (Api g 1) [155], carrot (Dau c 1) [160], peach (Pru p 1) [161], and pear (Pyr c 1) [162]. A sequence comparison of some of these homologous allergens has been done showing a significant sequence similarity of Bet v 1 with the food allergens: about 52.5% with Mal d 1, 57.8% with Pru av 1, 39.8% with Api g 1, 35.9% with Dau c 1, and about 55.0% and 56.6% with Pru p 1 and Pyr c 1, respectively (Figure 11). Cross-reactivity is common when the IgE antibodies, produced originally in response to Bet v1 sensitization, recognize similar epitopes present on the surface of these food allergenic proteins [163]. PR-10 proteins responsible for allergenic reactions to legumes are also homologous to Bet v 1 and have been reported from soy (Gly m 4) [164], peanut (Ara h 8) [165], and mungbean seedlings (Vig r 1) [166]. Birch pollen allergenicity has been also associated with allergenicity from hazelnuts and chestnuts. Cor a 1 [167] and Cas s 1 [168] are the major allergens responsible for allergenicity from hazelnut and chestnut, respectively. Bet v 1 shares maximum sequence similarity (80.5%) with Cor a 1 (Figure 11).
Figure 11.
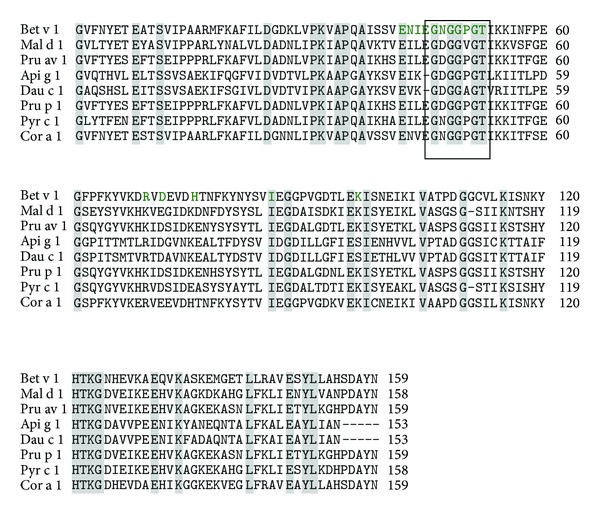
Multiple sequence alignment of birch pollen allergen Bet v 1 with food allergens: Mal d 1, Pru av 1, Api g 1, Dau c 1, Pru p 1, Pyr c 1, and Cor a 1. The identical sequences are highlighted in grey. The residues of Bet v 1 responsible for IgE binding are marked in green. The nearly conserved glycine rich loop is highlighted in box.
Bet v1 consists of seven stranded β-sheet structure wrapped around a 25-residue long C-terminal α-helix structure both of which are separated by two consecutive helices (Figure 12). A hydrophobic cavity is created by the hydrophobic residues clustering in the interior region with some polar residues pointing into the core [169]. The presence of such an internal cavity suggests a possible role in binding with hydrophobic ligands. A glycine rich loop connecting the 2 β-strands, β2 and β3 is nearly conserved among all the homologous PR-10 proteins. This might have a putative role in lipid binding [170].
Figure 12.
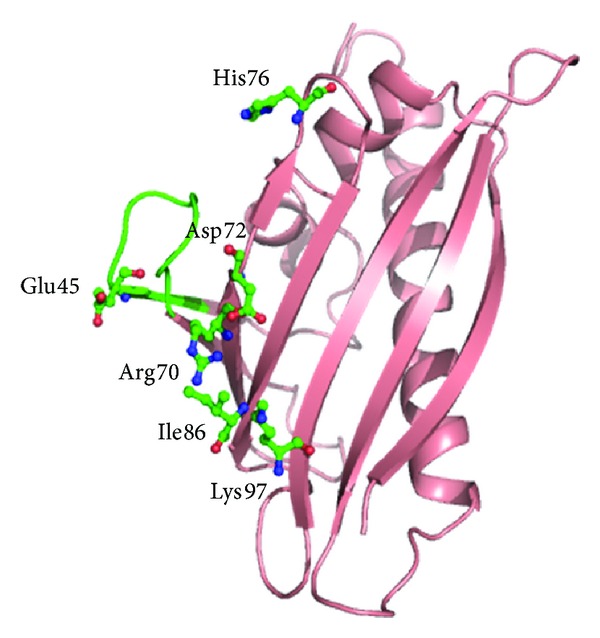
Overall structure of Bet v 1 protein showing the conformational epitope formed by amino acid residues from Glu42 to Thr52 (in green) and additional dispersed amino acids Arg70, Asp72, His76, Ile86, and Lys97 (in green) for Fab binding. The critical residue for antibody binding Glu45 is marked.
Some of the ligands binding to Bet v 1 with low micromolar affinity such as fatty acids, flavonoids, and cytokinins have been identified. The crystal structure of hypoallergenic isoform Bet v 1l in complex with deoxycholate suggests that Bet v 1 homologous proteins can act as a general plant steroid carrier [171]. It has been evaluated from the crystal structure of this birch pollen allergen in complex with the Fab fragment of a murine monoclonal IgG antibody (BV16), that the centrally located residue Glu45 is critical for antibody recognition and forms two hydrogen bonds with the heavy-chain variable region of the Fab fragment [172]. A conformational epitope containing amino acid residues from Glu42 to Thr52 (which also contains the glycine rich loop) and additional dispersed amino acids Arg70, Asp72, His76, Ile86, and Lys97 has been identified to be responsible for binding to antibody (Figure 12).
The secondary structure and the tertiary fold of Pru av 1, Api g 1, and Dau c 1 are identical to Bet v1 [173]. They have a large internal hydrophobic cavity that can interact with hydrophobic ligands and are important for physiological functions. The hydrophobic cavity is large enough to accommodate two such molecules. Binding studies of various phytosteroids to Pru av 1 suggest similar interactions like Bet v 1. Since both of these proteins have high level of sequence identity and similar overall backbone conformation, they share identical molecular surface in terms of shape and charge distribution. This explains the prevalence of cross-reactive IgE binding epitopes in these proteins [174]. The epitope for antibody binding in Bet v 1 has been found to be nearly conserved in Pru av 1 with Glu45 which may play a similar role in this molecule.
Some structural differences exist between Bet v 1 with Api g 1 and Dau c 1 although these allergens exhibit cross-reactivity among themselves [160, 175]. The latter two share about more than 80% sequence similarity with each other. Api g 1 lacks the negatively charged Glu45, in contrast to Bet v 1 and Pru av 1 [175]. The epitopes responsible for binding are found to be different from those elucidated by the structure of Bet v 1 in complex with Fab fragment of monoclonal BV16 antibody. Three conserved surface patches responsible for IgE binding are found to be common among Bet v 1, Pru av1, Api g 1, and Dau c 1 (Figure 13). It has also been observed that cross-reactivity between Bet v 1 and Mal d 1 occurs not only at the serologic level but also at the level of allergen specific T helper cells [176]. Eight cross-reacting T cell epitopes have been observed between the two allergens.
Figure 13.
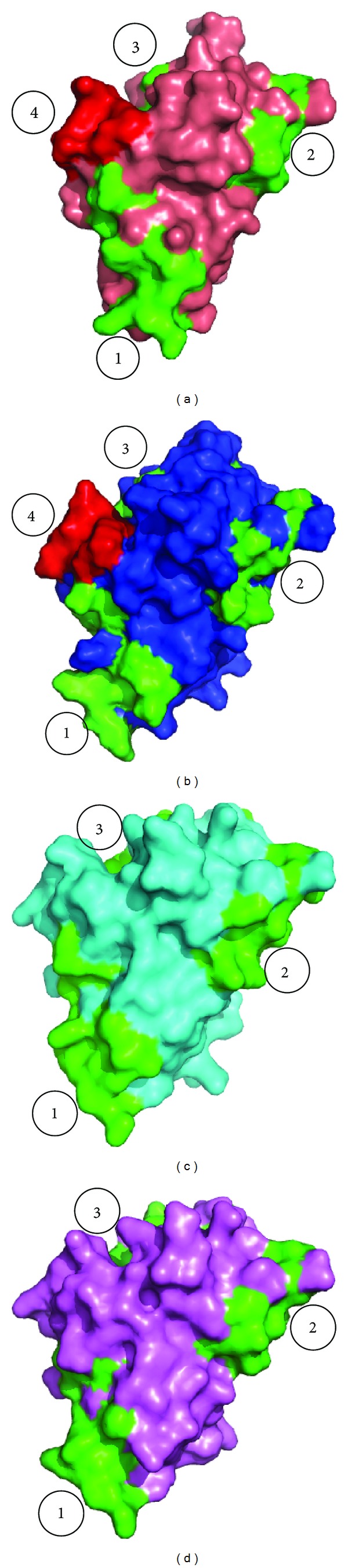
The three common surface epitopes (in green) recognized for IgE binding marked from 1 to 3 in (a) Bet v 1, (b) Pru av 1, (c) Api g 1, and (d) Dau c 1. The conformational epitope required for Fab binding (in red) in Bet v 1 (marked 4) is nearly conserved in Pru av 1 but absent in Api g 1 and Dau c 1 because of the absence of Glu45.
3.8. PR-14 Family Allergens
PR-14 proteins are identified as lipid transfer proteins (LTPs) originally named after their ability to transfer phospholipids and other fatty acid groups across cell membranes. They are highly conserved group of small proteins with molecular weights in the range of 9-10 kDa present in high amounts in higher plants and can also bind to acyl groups. These proteins are present in significant amounts in vascular tissue and in the outer cell layers of plants. They are involved in plant defense against bacterial and fungal pathogeneses as well as under different environmental stresses such as drought, heat, cold, or salt [177, 178]. There are evidences which suggest that LTPs are also involved in cutin formation, where they act as carriers of acyl monomers and in the process of cell wall extension [179]. They are divided into two types: those specific for certain classes of phospholipids and those that are able to accommodate several lipid classes, called nonspecific LTPs. Allergenic features of nonspecific LTPs (ns-LTPs) were reported in fruits, vegetables, nuts, pollen, and latex [180]. Due to their extreme proteolytic resistance, these allergens are able to traverse up to the gastrointestinal immune system, allowing sensitization and inducing specific IgE thereby eliciting severe clinical symptoms [181].
LTPs are the most important allergens of the Rosaceaefruits, such as peach (Pru p 3) [17], apple (Mal d 3) [182], apricot (Pru ar 3) [183], cherry (Pru av 3) [184], and plum (Pru d 3) [185], when no pollinosis is involved. Due to significant sequence identity (more than 81%) (Figure 14(a)) shared by ns-LTPs from Rosaceae fruits along with the considerable degree of immunological cross-reactivity, it has been suggested that they have comparable IgE binding epitopes [186]. Patients allergic to PR-14 proteins in fruits tend to have a higher rate of anaphylaxis (36%) than patients having fruit allergy by PR-10 proteins (18%).
Figure 14.
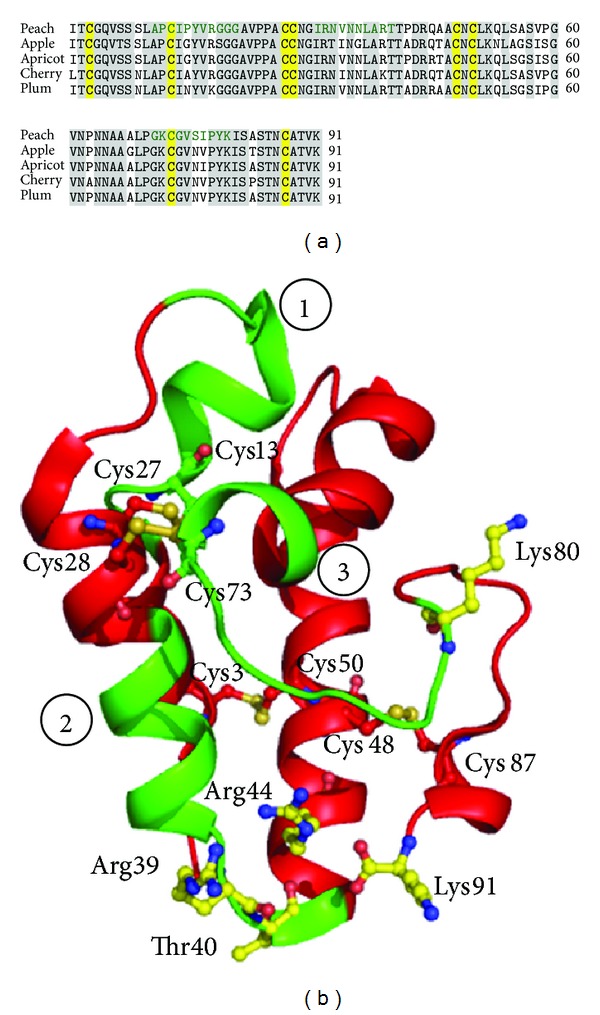
(a) Multiple sequence alignment of allergens of ns-LTPs from four Rosaceaefruits: peach, apple, apricot, and cherry. The identical sequences are highlighted in grey. Eight conserved cysteine residues are highlighted in yellow. The speculated three IgE binding epitopes of Pru p 3 from peach are marked in green. (b) Overall structure of ns-LTP, Pru p 3 showing the three possible IgE epitope binding regions (in green) marked from 1 to 3. The five charged residues in yellow having a possible role in epitope recognition and the eight cysteine residues forming four disulphide bridges are also marked.
The structure of the allergen belonging to this family, Pru p 3 from peach, has been extensively studied [187]. The main structural motif is represented by an α-helical compact domain, where four helices are connected by short loops (Figure 14(b)). Eight conserved cysteine residues were observed forming four disulfide bridges which makes it highly resistant to harsh temperature and pH changes. It had been speculated that five positively charged residues, Arg39, Thr40, Arg44, Lys80, and Lys91, are the possible candidates involved in epitope formation. Moreover, using a library of 10-mer synthetic peptides, which screened the whole protein sequence, three potential IgE-binding epitope regions have been identified [188] (Figure 14(b)), which are nearly conserved in other LTPs of Rosaceaefruits (Figure 14(a)).
Recent reports have also indicated that ns-LTPs from species other than Rosaceae, such as nuts, cereals, grapes, oranges, and vegetables, might also be involved for plant food allergies [189]. Severe reactions against hazelnut and chestnut are linked to sensitization to the LTPs, Cor a 8 [190], and Cas s 8 [191], respectively. The major allergens of maize (Zea m 14) [192] and barley (Hor v 14) [193] are also reported to be LTPs and are highly homologous with the peach LTP. LTPs of PR-14 family are also reported from pollens including Par j 1 from weeds of Parietaria judaica affecting 50% of allergic patients in the Mediterranean area [194].
4. PR-Proteins and Disease Resistant Genetically Modified Crops
PR proteins are of immense importance as preservative agents in food industry and for producing disease resistant plants by genetic engineering [195]. Various studies have revealed that transgenic plants overexpressing genes of the PR-1, PR-2, PR-3, and PR-5 families mediate host plant resistance to phytopathogenic fungi [196–198]. Coexpression of multiple antifungal protein genes in transgenic plants seems to be more effective than expression of single genes [199]. It is possible that such genetically modified (GM) plants with enhanced expression of PR proteins will also be associated with increased allergenicity and toxicity thus raising a serious question for their commercial acceptability. PR protein, osmotin used for developing transgenic crops, showed cross-reactivity with tomato and apple allergens [134].
Different strategies are adopted to monitor the transformed crops for their allergenicity and various guidelines are defined by the Food and Agriculture Organization (FAO), World Health Organization (WHO), and Codex Alimentarius Commission (Codex) to determine whether a new GM crop can be commercialized [200]. The present approach uses a combination of methodologies to evaluate the allergenicity assessment [201]. The allergenicity potential of modified food is primarily speculated on the basis of whether the source of the transformed protein is a plant known to produce allergen and also on the characteristic features of the introduced protein. The homology of the latter to known allergens along with IgE reactivity of the transformed protein with individuals with known allergies to the original source of the novel gene or related allergies is assessed. Moreover, the resistance of the novel protein to pepsin and the immunoreactivity of the novel protein in appropriate animal models are also evaluated [202]. Such step by step approach will provide valuable insights to estimate whether the transformed protein will be allergenic or not.
5. Conclusions
PR proteins and their homologues are responsible for the defense against various stresses including pathogen attacks, wounding, use of chemicals, and pollutants. Recent studies have suggested their immense importance in agricultural and food industry with the introduction of transgenic plants. However, many of these protective proteins of the plants have demonstrated allergenicity specially the PR proteins belonging to the families 1, 2, 3, 4, 5, 8, 10, and 14. Though there are not much similarities among the different families of PR proteins in terms of their sequence identities or structures, similarity in the amino acid sequences among allergens from diverse plants within the same family results in cross-reactivity. The allergenicity of the PR proteins is also guided by several environmental factors like the use of chemical inducers in agriculture and environmental pollutants. Exploring new PR proteins implicated in allergenicity and a complete understanding of their structures and IgE binding epitopes are necessary for their safe use in plant engineering. The knowledge of the localization of IgE epitopes on the allergen helps in the identification of cross-reactivity among homologous proteins and may also contribute to the design of effective immunotherapy strategies for certain allergy producing substances like latex, pollen, and so forth along with their respective related allergies.
Acknowledgments
The authors acknowledge the financial support from the Indian Council of Medical Research (ICMR), New Delhi. Tej P. Singh thanks the Department of Biotechnology (DBT), for the award of Distinguished Biotechnology Research Professorship. Mau Sinha and Rashmi Prabha Singh thank the Department of Science and Technology (DST), Ministry of Science and Technology, New Delhi, and the Council of Scientific and Industrial Research (CSIR), respectively, for the award of fellowships. Sanket Kaushik thanks ICMR, New Delhi, and Avinash Singh thanks DBT for their fellowship awards.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Johansson SG. IgE in allergic diseases. Proceedings of the Royal Society of Medicine. 1969;62(9):975–976. doi: 10.1177/003591576906200947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marsh DG, Lichtenstein LM, Norman PS. Induction of IgE-mediated immediate hypersensitivity to group I rye grass pollen allergen and allergoids in non-allergic man. Immunology. 1972;22(6):1013–1028. [PMC free article] [PubMed] [Google Scholar]
- 3.Taketomi EA, Sopelete MC, Moreira PFDS, Vieira FDAM. Pollen allergic disease: pollens and its major allergens. Brazilian Journal of Otorhinolaryngology. 2006;72(4):562–567. doi: 10.1016/S1808-8694(15)31005-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennedy P. Acute reaction to apple-eating. British Medical Journal. 1978;2(6150):1501–1502. doi: 10.1136/bmj.2.6150.1501-d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burrall BA. Plant-related allergic contact dermatitis. Clinical Reviews in Allergy. 1989;7(4):417–439. [PubMed] [Google Scholar]
- 6.Buisseret PD. Seasonal allergic symptoms due to fungal spores. British Medical Journal. 1976;2(6034):507–508. doi: 10.1136/bmj.2.6034.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spiegelberg HL, Simon RA. Increase of lymphocytes with Fc receptors for IgE in patients with allergic rhinitis during the grass pollen season. Journal of Clinical Investigation. 1981;68(4):845–852. doi: 10.1172/JCI110339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mayoral M, Calderón H, Cano R, Lombardero M. Allergic rhinoconjunctivitis caused by Cannabis sativa pollen. Journal of Investigational Allergology and Clinical Immunology. 2008;18(1):73–74. [PubMed] [Google Scholar]
- 9.Martin JA, Compaired JA, de la Hoz B, et al. Bronchial asthma induced by chick pea and lentil. Allergy. 1992;47(2, part 2):185–187. doi: 10.1111/j.1398-9995.1992.tb00962.x. [DOI] [PubMed] [Google Scholar]
- 10.Valdivieso R, Subiza J, Varela-Losada S, et al. Bronchial asthma, rhinoconjunctivitis, and contact dermatitis caused by onion. Journal of Allergy and Clinical Immunology. 1994;94(5):928–930. doi: 10.1016/0091-6749(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 11.Chandra RK. Food hypersensitivity and allergic diseases. European Journal of Clinical Nutrition. 2002;56(3):S54–S56. doi: 10.1038/sj.ejcn.1601487. [DOI] [PubMed] [Google Scholar]
- 12.Rougé P, Culerrier R, Thibau F, Didier A, Barre A. A case of severe anaphylaxis to kidney bean: Phaseolin (vicilin) and PHA (lectin) identified as putative allergens. Allergy. 2011;66(2):301–302. doi: 10.1111/j.1398-9995.2010.02466.x. [DOI] [PubMed] [Google Scholar]
- 13.Gette MT, Marks JE., Jr. Tulip fingers. Archives of Dermatology. 1990;126(2):203–205. [PubMed] [Google Scholar]
- 14.Goncalo S, Freitas JD, Sousa I. Contact dermatitis and respiratory symptoms from Narcissus pseudonarcissus . Contact Dermatitis. 1987;16(2):115–116. doi: 10.1111/j.1600-0536.1987.tb01400.x. [DOI] [PubMed] [Google Scholar]
- 15.Mills ENC, Jenkins J, Marigheto N, Belton PS, Gunning AP, Morris VJ. Allergens of the cupin superfamily. Biochemical Society Transactions. 2002;30(part 6):925–929. doi: 10.1042/bst0300925. [DOI] [PubMed] [Google Scholar]
- 16.Vrtala S, Fischer S, Grote M, et al. Molecular, immunological, and structural characterization of Phl p 6, a major allergen and P-particle-associated protein from timothy grass (Phleum pratense) pollen. Journal of Immunology. 1999;163(10):5489–5496. [PubMed] [Google Scholar]
- 17.Duffort OA, Polo F, Lombardero M, et al. Immunoassay to quantify the major peach allergen Pru p 3 in foodstuffs. Differential allergen release and stability under physiological conditions. Journal of Agricultural and Food Chemistry. 2002;50(26):7738–7741. doi: 10.1021/jf0258398. [DOI] [PubMed] [Google Scholar]
- 18.Huby RDJ, Dearman RJ, Kimber I. Why are some proteins allergens? Toxicological Sciences. 2000;55(2):235–246. doi: 10.1093/toxsci/55.2.235. [DOI] [PubMed] [Google Scholar]
- 19.Lehrer SB, Horner WE, Reese G. Why are some proteins allergenic? Implications for biotechnology. Critical Reviews in Food Science and Nutrition. 1996;36(6):553–564. doi: 10.1080/10408399609527739. [DOI] [PubMed] [Google Scholar]
- 20.van Loon LC. Pathogenesis-related proteins. Plant Molecular Biology. 1985;4(2-3):111–116. doi: 10.1007/BF02418757. [DOI] [PubMed] [Google Scholar]
- 21.Rigden J, Coutts R. Pathogenesis-related proteins in plants. Trends in Genetics. 1988;4(4):87–89. doi: 10.1016/0168-9525(88)90091-1. [DOI] [PubMed] [Google Scholar]
- 22.Lamb CJ, Lawton MA, Dron M, Dixon RA. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- 23.Delaney TP, Uknes S, Vernooij B, et al. A central role of salicylic acid in plant disease resistance. Science. 1994;266(5188):1247–1250. doi: 10.1126/science.266.5188.1247. [DOI] [PubMed] [Google Scholar]
- 24.Xu Yi XY, Chang Pi Fang L, Liu Dong LD, et al. Plant defense genes are synergistically induced by ethylene and methyl jasmonate. Plant Cell. 1994;6(8):1077–1085. doi: 10.1105/tpc.6.8.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linthorst HJ, van Loon LC, van Rossum CM, et al. Analysis of acidic and basic chitinases from tobacco and petunia and their constitutive expression in transgenic tobacco. Molecular Plant-Microbe Interactions. 1990;3(4):252–258. doi: 10.1094/mpmi-3-252. [DOI] [PubMed] [Google Scholar]
- 26.Lotan T, Ori N, Fluhr R. Pathogenesis-related proteins are developmentally regulated in tobacco flowers. The Plant Cell. 1989;1(9):881–887. doi: 10.1105/tpc.1.9.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Loon LC, Pierpoint WS, Boller T, Conejero V. Recommendations for naming plant pathogenesis-related proteins. Plant Molecular Biology Reporter. 1994;12(3):245–264. [Google Scholar]
- 28.van Loon LC, van Kammen A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. “Samsun” and “Samsun NN”. II: changes in protein constitution after infection with tobacco mosaic virus. Virology. 1970;40(2):199–211. doi: 10.1016/0042-6822(70)90395-8. [DOI] [PubMed] [Google Scholar]
- 29.van Loon LC, van Strien EA. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiological and Molecular Plant Pathology. 1999;55(2):85–97. [Google Scholar]
- 30.Buchel AS, Linthorst HJM. PR-1: a group of plant proteins induced upon pathogen infection. In: Datta SK, Muthukrishnan S, editors. Pathogenesis-Related Proteins in Plants. Boca Raton, Fla, USA: CRC Press LLC; 1999. pp. 21–47. [Google Scholar]
- 31.Ohshima M, Itoh H, Matsuoka M, Murakami T, Ohashi Y. Analysis of stress-induced or salicylic acid-induced expression of the pathogenesis-related 1a protein gene in transgenic tobacco. Plant Cell. 1990;2(2):95–106. doi: 10.1105/tpc.2.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yalpani N, Silverman P, Wilson TMA, Kleier DA, Raskin I. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. Plant Cell. 1991;3(8):809–818. doi: 10.1105/tpc.3.8.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chamnongpol S, Willekens H, Moeder W, et al. Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(10):5818–5823. doi: 10.1073/pnas.95.10.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kitajima S, Sato F. Plant pathogenesis-related proteins: molecular mechanisms of gene expression and protein function. Journal of Biochemistry. 1999;125(1):1–8. doi: 10.1093/oxfordjournals.jbchem.a022244. [DOI] [PubMed] [Google Scholar]
- 35.Datta SK, Muthukrishnan S. Pathogenesis-Related Proteins in Plants. Boca Raton, Fla, USA: CRC Press; 1999. [Google Scholar]
- 36.van Loon LC, Rep M, Pieterse CMJ. Significance of inducible defense-related proteins in infected plants. Annual Review of Phytopathology. 2006;44:135–162. doi: 10.1146/annurev.phyto.44.070505.143425. [DOI] [PubMed] [Google Scholar]
- 37.Hejgaard J, Jacobsen S, Svendsen I. Two antifungal thaumatin-like proteins from barley grain. FEBS Letters. 1991;291(1):127–131. doi: 10.1016/0014-5793(91)81119-s. [DOI] [PubMed] [Google Scholar]
- 38.Caruso C, Caporale C, Chilosi G, et al. Structural and antifungal properties of a pathogenesis-related protein from wheat kernel. Journal of Protein Chemistry. 1996;15(1):35–44. doi: 10.1007/BF01886809. [DOI] [PubMed] [Google Scholar]
- 39.Cutt JR, Harpster MH, Dixon DC, Carr JP, Dunsmuir P, Klessig DF. Disease response to tobacco mosaic virus in transgenic tobacco plants that constitutively express the pathogenesis-related PR1b gene. Virology. 1989;173(1):89–97. doi: 10.1016/0042-6822(89)90224-9. [DOI] [PubMed] [Google Scholar]
- 40.Edreva A. Pathogenesis-related proteins: research progress in the last 15 years. General and Applied Plant Physiology. 2005;31(1-2):105–124. [Google Scholar]
- 41.Kombrink E, Schroder M, Hahlbrock K. Several ”pathogenesis-related“ proteins in potato are 1,3-β-glucanases and chitinases. Proceedings of the National Academy of Sciences of the United States of America. 1988;85(3):782–786. doi: 10.1073/pnas.85.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heitz T, Segond S, Kauffmann S, et al. Molecular characterization of a novel tobacco pathogenesis-related (PR) protein: a new plant chitinase/lysozyme. Molecular and General Genetics. 1994;245(2):246–254. doi: 10.1007/BF00283273. [DOI] [PubMed] [Google Scholar]
- 43.Lay FT, Anderson MA. Defensins—components of the innate immune system in plants. Current Protein and Peptide Science. 2005;6(1):85–101. doi: 10.2174/1389203053027575. [DOI] [PubMed] [Google Scholar]
- 44.Regente MC, Giudici AM, Villalaín J, de la Canal L. The cytotoxic properties of a plant lipid transfer protein involve membrane permeabilization of target cells. Letters in Applied Microbiology. 2005;40(3):183–189. doi: 10.1111/j.1472-765X.2004.01647.x. [DOI] [PubMed] [Google Scholar]
- 45.Heitz T, Geoffroy P, Fritig B, Legrand M. Two apoplastic α-amylases are induced in tobacco by virus infection. Plant Physiology. 1991;97(2):651–655. doi: 10.1104/pp.97.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Lorenzo G, D’Ovidio R, Cervone F. The role of polygalacturonase-inhibiting proteins (PGIPS) in defense against pathogenic fungi. Annual Review of Phytopathology. 2001;39:313–335. doi: 10.1146/annurev.phyto.39.1.313. [DOI] [PubMed] [Google Scholar]
- 47.Deepak S, Shailasree S, Kini RK, Hause B, Shetty SH, Mithöfer A. Role of hydroxyproline-rich glycoproteins in resistance of pearl millet against downy mildew pathogen Sclerospora graminicola. Planta. 2007;226(2):323–333. doi: 10.1007/s00425-007-0484-4. [DOI] [PubMed] [Google Scholar]
- 48.Akram A, Ongena M, Duby F, Dommes J, Thonart P. Systemic resistance and lipoxygenase-related defence response induced in tomato by Pseudomonas putida strain BTP1. BMC Plant Biology. 2008;8(article 113) doi: 10.1186/1471-2229-8-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cândido Ede S, Pinto MF, Pelegrini PB, et al. Plant storage proteins with antimicrobial activity: novel insights into plant defense mechanisms. FASEB Journal. 2011;25(10):3290–3305. doi: 10.1096/fj.11-184291. [DOI] [PubMed] [Google Scholar]
- 50.Peumans WJ, van Damme EJ. Lectins as plant defense proteins. Plant Physiology. 1995;109(2):347–352. doi: 10.1104/pp.109.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peumans WJ, Hao Q, van Damme EJM. Ribosome-inactivating proteins from plants: more than RNA N-glycosidases? FASEB Journal. 2001;15(9):1493–1506. doi: 10.1096/fj.00-0751rev. [DOI] [PubMed] [Google Scholar]
- 52.Broekaert WF, Terras FRG, Cammue BPA. Induced and preformed antimicrobial proteins. In: Slusarenko AJ, Fraser RS, van Loon LC, editors. Mechanisms of Resistance to Plant Diseases. Dordrecht, The Netherlands: Kluwer Academic Publishers; 2000. pp. 371–477. [Google Scholar]
- 53.Hoffmann-Sommergruber K. Plant allergens and pathogenesis-related proteins: what do they have in common? International Archives of Allergy and Immunology. 2000;122(3):155–166. doi: 10.1159/000024392. [DOI] [PubMed] [Google Scholar]
- 54.Ebner C, Hoffmann-Sommergruber K, Breiteneder H. Plant food allergens homologous to pathogenesis-related proteins. Allergy, Supplement. 2001;56(67):43–44. doi: 10.1034/j.1398-9995.2001.00913.x. [DOI] [PubMed] [Google Scholar]
- 55.Midoro-Horiuti T, Brooks EG, Goldblum RM. Pathogenesis-related proteins of plants as allergens. Annals of Allergy, Asthma and Immunology. 2001;87(4):261–271. doi: 10.1016/S1081-1206(10)62238-7. [DOI] [PubMed] [Google Scholar]
- 56.Hoffmann-Sommergruber K. Pathogenesis-related (PR)-proteins identified as allergens. Biochemical Society Transactions. 2002;30(6):930–935. doi: 10.1042/bst0300930. [DOI] [PubMed] [Google Scholar]
- 57.Halmepuro L, Vuontela K, Kalimo K, Bjorksten F. Cross-reactivity of IgE antibodies with allergens in birch pollen, fruits and vegetables. International Archives of Allergy and Applied Immunology. 1984;74(3):235–240. doi: 10.1159/000233550. [DOI] [PubMed] [Google Scholar]
- 58.Blanco C, Carrillo T, Castillo R, Quiralte J, Cuevas M. Latex allergy: clinical features and cross-reactivity with fruits. Annals of Allergy. 1994;73(4):309–314. [PubMed] [Google Scholar]
- 59.Asero R. Lipid transfer protein cross-reactivity assessed in vivo and in vitro in the office: pros and cons. Journal of Investigational Allergology and Clinical Immunology. 2011;21(2):129–136. [PubMed] [Google Scholar]
- 60.Rossi RE, Monasterolo G, Operti D, Corsi M. Evaluation of recombinant allergens Bet v 1 and Bet v 2 (profilin) by Pharmacia CAP System in patients with pollen-related allergy to birch and apple. Allergy. 1996;51(12):940–945. [PubMed] [Google Scholar]
- 61.Wagner S, Breiteneder H. The latex-fruit syndrome. Biochemical Society Transactions. 2002;30(part 6):935–940. doi: 10.1042/bst0300935. [DOI] [PubMed] [Google Scholar]
- 62.Leitner A, Jensen-Jarolim E, Grimm R, et al. Allergens in pepper and paprika: immunologic investigation of the celery-birch-mugwort-spice syndrome. Allergy. 1998;53(1):36–41. doi: 10.1111/j.1398-9995.1998.tb03771.x. [DOI] [PubMed] [Google Scholar]
- 63.Midoro-Horiuti T, Goldblum RM, Kurosky A, Wood TG, Brooks EG. Variable expression of pathogenesis-related protein allergen in mountain cedar (Juniperus ashei) pollen. Journal of Immunology. 2000;164(4):2188–2192. doi: 10.4049/jimmunol.164.4.2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cornelissen BJ, Hooft van Huijsduijnen RA, van Loon LC, Bol JF. Molecular characterization of messenger RNAs for “pathogenesis related” proteins la, lb and lc, induced by TMV infection of tobacco. The EMBO Journal. 1986;5(1):37–40. doi: 10.1002/j.1460-2075.1986.tb04174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Joosten MHAJ, Bergmans CJB, Meulenhoff EJS, Cornelissen BJC, De Wit PJGM. Purification and serological characterization of three basic 15-kilodalton pathogenesis-related proteins from tomato. Plant Physiology. 1990;94(2):585–591. doi: 10.1104/pp.94.2.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Niderman T, Genetet I, Bruyere T, et al. Pathogenesis-related PR-1 proteins are antifungal. Isolation and characterization of three 14-kilodalton proteins of tomato and of a basic PR-1 of tobacco with inhibitory activity against Phytophthora infestans. Plant Physiology. 1995;108(1):17–27. doi: 10.1104/pp.108.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gillikin JW, Burkhart W, Graham JS. Complete amino acid sequence of a polypeptide from Zea mays similar to the pathogenesis-related-1 family. Plant Physiology. 1991;96(4):1372–1375. doi: 10.1104/pp.96.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu Q, Xue Q. Computational identification of novel PR-1-type genes in Oryza sativa . Journal of Genetics. 2006;85(3):193–198. doi: 10.1007/BF02935330. [DOI] [PubMed] [Google Scholar]
- 69.Asensio T, Crespo JF, Sanchez-Monge R, et al. Novel plant pathogenesis-related protein family involved in food allergy. Journal of Allergy and Clinical Immunology. 2004;114(4):896–899. doi: 10.1016/j.jaci.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 70.Hoj PB, Fincher GB. Molecular evolution of plant beta-glucan endohydrolases. Plant Journal. 1995;7(3):367–379. doi: 10.1046/j.1365-313x.1995.7030367.x. [DOI] [PubMed] [Google Scholar]
- 71.Fulcher RG, Mc Cully ME, Setterfield G, Sutherland J. β-1,3-glucans may be associated with cell plate formation during cytokinesis. Canadian Journal Botany. 1976;54:459–542. [Google Scholar]
- 72.Bucciaglia PA, Smith AG. Cloning and characterization of Tag 1, a tobacco anther beta-1,3-glucanase expressed during tetrad dissolution. Plant Molecular Biology. 1994;24(6):903–914. doi: 10.1007/BF00014444. [DOI] [PubMed] [Google Scholar]
- 73.Meikle PJ, Bonig I, Hoogenraad NJ, Clarke AE, Stone BA. The location of (1→3)-β-glucans in the walls of pollen tubes of Nicotiana alata using a (1→3)-β-glucan-specific monoclonal antibody. Planta. 1991;185(1):1–8. doi: 10.1007/BF00194507. [DOI] [PubMed] [Google Scholar]
- 74.Helleboid S, Bauw G, Belingheri L, Vasseur J, Fulbert JL. Extracellular β-1,3-glucanases are induced during early somatic embryogenesis in Cichorium. Planta. 1998;205(1):56–63. doi: 10.1007/s004250050296. [DOI] [PubMed] [Google Scholar]
- 75.Leubner-Metzger G, Frundt C, Vogeli-Lange R, Meins Jnr F. Class I beta-1,3-glucanases in the endosperm of tobacco during germination. Plant Physiology. 1995;109(3):751–759. doi: 10.1104/pp.109.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fincher GB, Stone BA. Physiology and biochemistry of germination in barley. In: MacGregor AW, Bhatty RS, editors. Barley: Chemistry and Technology. St. Paul, American Association of Cereal Chemists; 1993. pp. 247–295. [Google Scholar]
- 77.Thalmair M, Bauw G, Thiel S, Döhring T, Langebartels C, Sandermann H., Jr. Ozone and ultraviolet B effects on the defense-related proteins β-1,3-glucanase and chitinase in Tobacco. Journal of Plant Physiology. 1996;148(1-2):222–228. [Google Scholar]
- 78.Hincha DK, Meins F, Jr., Schmitt JM. β-1,3-glucanase is cryoprotective in vitro and is accumulated in leaves during cold acclimation. Plant Physiology. 1997;114(3):1077–1083. doi: 10.1104/pp.114.3.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brederode FT, Linthorst HJM, Bol JF. Differential induction of acquired resistance and PR gene expression in tobacco by virus infection, ethephon treatment, UV light and wounding. Plant Molecular Biology. 1991;17(6):1117–1125. doi: 10.1007/BF00028729. [DOI] [PubMed] [Google Scholar]
- 80.Payne G, Ward E, Gaffney T, et al. Evidence for a third structural class of β-1,3-glucanase in tobacco. Plant Molecular Biology. 1990;15(6):797–808. doi: 10.1007/BF00039420. [DOI] [PubMed] [Google Scholar]
- 81.Bulcke MV, Bauw G, Castresana C, van Montagu M, Vandekerckhove J. Characterization of vacuolar and extracellular β-(1,3)-glucanases of tobacco: evidence for a strictly compartmentalized plant defense system. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(8):2673–2677. doi: 10.1073/pnas.86.8.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Simmons CR. The physiology and molecular biology of plant 1,3-β-D-glucanases and 1,3;1,4-β-D-glucanases. Critical Reviews in Plant Sciences. 1994;13(4):325–387. [Google Scholar]
- 83.Sticher L, Hinz U, Meyer AD, Meins F., Jr. Intracellular transport and processing of a tobacco vacuolar β-1,3-glucanase. Planta. 1992;188(4):559–565. doi: 10.1007/BF00197049. [DOI] [PubMed] [Google Scholar]
- 84.Hirshman CA. Latex anaphylaxis. Anesthesiology. 1992;77(2):223–225. [PubMed] [Google Scholar]
- 85.De Zotti R, Larese F, Fiorito A. Asthma and contact urticaria from latex gloves in a hospital nurse. British Journal of Industrial Medicine. 1992;49(8):596–598. doi: 10.1136/oem.49.8.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chye ML, Cheung KY. β-1,3-glucanase is highly-expressed in laticifers of Hevea brasiliensis . Plant Molecular Biology. 1995;29(2):397–402. doi: 10.1007/BF00043663. [DOI] [PubMed] [Google Scholar]
- 87.Sunderasan E, Hamzah S, Hamid S, et al. Latex B-serum β-1,3-glucanase (Hev b 2) and a component of the microhelix (Hev b 4) are major latex allergens. Journal of Natural Rubber Research. 1996;10:82–99. [Google Scholar]
- 88.Kurup VP, Yeang HY, Sussman GL, et al. Detection of immunoglobulin antibodies in the sera of patients using purified latex allergens. Clinical and Experimental Allergy. 2000;30(3):359–369. doi: 10.1046/j.1365-2222.2000.00748.x. [DOI] [PubMed] [Google Scholar]
- 89.Kurup VP, Sussman GL, Yeang HY, et al. Specific IgE response to purified and recombinant allergens in latex allergy. Clinical and Molecular Allergy. 2005;3(article 11) doi: 10.1186/1476-7961-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Barre A, Culerrier R, Granier C, et al. Mapping of IgE-binding epitopes on the major latex allergen Hev b 2 and the cross-reacting 1,3-β-glucanase fruit allergens as a molecular basis for the latex-fruit syndrome. Molecular Immunology. 2009;46(8-9):1595–1604. doi: 10.1016/j.molimm.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 91.Blanco C, Carrillo T, Castillo R, Quiralte J, Cuevas M. Avocado hypersensitivity. Allergy. 1994;49(6):454–459. doi: 10.1111/j.1398-9995.1994.tb00839.x. [DOI] [PubMed] [Google Scholar]
- 92.Brehler R, Theissen U, Mohr C, Luger T. ‘Latex-fruit syndrome’: frequency of cross-reacting IgE antibodies. Allergy. 1997;52(4):404–410. doi: 10.1111/j.1398-9995.1997.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 93.Wagner S, Radauer C, Hafner C, et al. Characterization of cross-reactive bell pepper allergens involved in the latex-fruit syndrome. Clinical and Experimental Allergy. 2004;34(11):1739–1746. doi: 10.1111/j.1365-2222.2004.02103.x. [DOI] [PubMed] [Google Scholar]
- 94.Clendennen SK, May GD. Differential gene expression in ripening banana fruit. Plant Physiology. 1997;115(2):463–469. doi: 10.1104/pp.115.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Huecas S, Villalba M, Rodríguez R. Ole e 9, a major olive pollen allergen is a 1,3-β-glucanase: isolation, characterization, amino acid sequence, and tissue specificity. Journal of Biological Chemistry. 2001;276(30):27959–27966. doi: 10.1074/jbc.M103041200. [DOI] [PubMed] [Google Scholar]
- 96.Treviño MÁ, Palomares O, Castrillo I, et al. Solution structure of the C-terminal domain of Ole e 9, a major allergen of olive pollen. Protein Science. 2008;17(2):371–376. doi: 10.1110/ps.073230008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jollès P, Muzzarelli RAA. Chitin and Chitinases. Basel, Switzerland: Birkhauser; 1999. [Google Scholar]
- 98.Neuhaus JM, Sticher L, Meins F, Jr., Boller T. A short C-terminal sequence is necessary and sufficient for the targeting of chitinases to the plant vacuole. Proceedings of the National Academy of Sciences of the United States of America. 1991;88(22):10362–10366. doi: 10.1073/pnas.88.22.10362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Graham LS, Sticklen MB. Plant chitinases. Canadian Journal of Botany. 1994;72(8):1057–1083. [Google Scholar]
- 100.Diaz-Perales A, Collada C, Blanco C, et al. Class I chitinases with hevein-like domain, but not class II enzymes, are relevant chestnut and avocado allergens. Journal of Allergy and Clinical Immunology. 1998;102(1):127–133. doi: 10.1016/s0091-6749(98)70063-6. [DOI] [PubMed] [Google Scholar]
- 101.Sowka S, Hsieh LS, Krebitz M, et al. Identification and cloning of Prs a 1, a 32-kDa endochitinase and major allergen of avocado, and its expression in the yeast Pichia pastoris . Journal of Biological Chemistry. 1998;273(43):28091–28097. doi: 10.1074/jbc.273.43.28091. [DOI] [PubMed] [Google Scholar]
- 102.Posch A, Wheeler CH, Chen Z, et al. Class I endochitinase containing a hevein domain is the causative allergen in latex-associated avocado allergy. Clinical and Experimental Allergy. 1999;29(5):667–672. doi: 10.1046/j.1365-2222.1999.00502.x. [DOI] [PubMed] [Google Scholar]
- 103.Sanchez-Monge R, Blanco C, Dïaz-Perales A, et al. Isolation and characterization of major banana allergens: identification as fruit class I chitinases. Clinical and Experimental Allergy. 1999;29(5):673–680. doi: 10.1046/j.1365-2222.1999.00526.x. [DOI] [PubMed] [Google Scholar]
- 104.Ponstein AS, Bres-Vloemans SA, Sela-Buurlage MB, van den Elzen PJM, Melchers LS, Cornelissen BJC. A novel pathogen- and wound-inducible tobacco (Nicotiana tabacum) protein with antifungal activity. Plant Physiology. 1994;104(1):109–118. doi: 10.1104/pp.104.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ludvigsen S, Poulsen FM. Three-dimensional structure in solution of barwin, a protein from barley seed. Biochemistry. 1992;31(37):8783–8789. doi: 10.1021/bi00152a014. [DOI] [PubMed] [Google Scholar]
- 106.Alenius H, Kalkkinen N, Lukka M, et al. Prohevein from the rubber tree (Hevea brasiliensis) is a major latex allergen. Clinical and Experimental Allergy. 1995;25(7):659–665. doi: 10.1111/j.1365-2222.1995.tb01114.x. [DOI] [PubMed] [Google Scholar]
- 107.Raulf-Heimsoth M, Rozynek P, Brüning T, Rihs HP. Characterization of B- and T-cell responses and HLA-DR4 binding motifs of the latex allergen Hev b 6.01 (prohevein) and its post-transcriptionally formed proteins Hev b 6.02 and Hev b 6.03. Allergy. 2004;59(7):724–733. doi: 10.1111/j.1398-9995.2004.00475.x. [DOI] [PubMed] [Google Scholar]
- 108.Karisola P, Kotovuori A, Poikonen S, et al. Isolated hevein-like domains, but not 31-kd endochitinases, are responsible for IgE-mediated in vitro and in vivo reactions in latex-fruit syndrome. Journal of Allergy and Clinical Immunology. 2005;115(3):598–605. doi: 10.1016/j.jaci.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 109.Chen Z, Posch A, Cremer R, Raulf-Heimsoth M, Baur X. Identification of hevein (Hev b 6.02) in Hevea latex as a major cross-reacting allergen with avocado fruit in patients with latex allergy. Journal of Allergy and Clinical Immunology. 1998;102(3):476–481. doi: 10.1016/s0091-6749(98)70138-1. [DOI] [PubMed] [Google Scholar]
- 110.Reyes-López CA, Hernández-Santoyo A, Pedraza-Escalona M, Mendoza G, Hernández-Arana A, Rodríguez-Romero A. Insights into a conformational epitope of Hev b 6.02 (hevein) Biochemical and Biophysical Research Communications. 2004;314(1):123–130. doi: 10.1016/j.bbrc.2003.12.068. [DOI] [PubMed] [Google Scholar]
- 111.Drew AC, Eusebius NP, Kenins L, et al. Hypoallergenic variants of the major latex allergen Hev b 6.01 retaining human T lymphocyte reactivity. Journal of Immunology. 2004;173(9):5872–5879. doi: 10.4049/jimmunol.173.9.5872. [DOI] [PubMed] [Google Scholar]
- 112.Linthorst HJ, Danhash N, Brederode FT, van Kan JA, De Wit PJ, Bol JF. Tobacco and tomato PR proteins homologous to win and pro-hevein lack the “hevein” domain. Molecular Plant-Microbe Interactions. 1991;4(6):586–592. doi: 10.1094/mpmi-4-586. [DOI] [PubMed] [Google Scholar]
- 113.Hänninen AR, Mikkola JH, Kalkkinen N, et al. Increased allergen production in turnip (Brassica rapa) by treatments activating defense mechanisms. Journal of Allergy and Clinical Immunology. 1999;104(1):194–201. doi: 10.1016/s0091-6749(99)70135-1. [DOI] [PubMed] [Google Scholar]
- 114.Daniell WF. On the Synsepalum dulcificum, Decand, or miraculous berry of Western Africa. Pharmaceutical Journal. 1852;11:445–448. [Google Scholar]
- 115.Cornelissen BJC, Hooft van Huijsduijen RAM, Bol JF. A tobacco mosaic virus-induced tobacco protein is homologous to the sweet-tasting protein thaumatin. Nature. 1986;321(6069):531–532. doi: 10.1038/321531a0. [DOI] [PubMed] [Google Scholar]
- 116.Singh NK, Bracker CA, Hasegawa PM, et al. Characterization of osmotin: a thaumatin-like protein associated with osmotic adaptation in plant cells. Plant Physiology. 1987;85(2):529–536. doi: 10.1104/pp.85.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Pressey R. Two isoforms of NP24: a thaumatin-like protein in tomato fruit. Phytochemistry. 1997;44(7):1241–1245. doi: 10.1016/s0031-9422(96)00667-x. [DOI] [PubMed] [Google Scholar]
- 118.Cheong NE, Choi YO, Kim WY, Kim SC, Cho MJ, Lee SY. Purification of an antifungal PR-5 protein from flower buds of Brassica campestris and cloning of its gene. Physiologia Plantarum. 1997;101(3):583–590. [Google Scholar]
- 119.Fils-Lycaon BR, Wiersma PA, Eastwell KC, Sautiere P. A cherry protein and its gene, abundantly expressed in ripening fruit, have been identified as thaumatin-like. Plant Physiology. 1996;111(1):269–273. doi: 10.1104/pp.111.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Roberts WK, Selitrennikoff CP. Zeamatin, an antifungal protein from maize with membrane-permeabilizing activity. Journal of General Microbiology. 1990;136(9):1771–1778. [Google Scholar]
- 121.Vigers AJ, Roberts WK, Selitrennikoff CP. A new family of plant antifungal proteins. Molecular Plant-Microbe Interactions. 1991;4(4):315–323. doi: 10.1094/mpmi-4-315. [DOI] [PubMed] [Google Scholar]
- 122.Futamura N, Mukai Y, Sakaguchi M, et al. Isolation and characterization of cDNAs that encode homologs of a pathogenesis-related protein allergen from Cryptomeria japonica . Bioscience, Biotechnology and Biochemistry. 2002;66(11):2495–2500. doi: 10.1271/bbb.66.2495. [DOI] [PubMed] [Google Scholar]
- 123.Cortegano I, Civantos E, Aceituno E, et al. Cloning and expression of a major allergen from Cupressus arizonica pollen, Cup a 3, a PR-5 protein expressed under polluted environment. Allergy. 2004;59(5):485–490. doi: 10.1046/j.1398-9995.2003.00363.x. [DOI] [PubMed] [Google Scholar]
- 124.Midoro-Horiuti T, Goldblum RM, Brooks EG. Identification of mutations in the genes for the pollen allergens of eastern red cedar (Juniperus virginiana) Clinical and Experimental Allergy. 2001;31(5):771–778. doi: 10.1046/j.1365-2222.2001.01079.x. [DOI] [PubMed] [Google Scholar]
- 125.Fuchs HC, Bohle B, Dall’Antonia Y, et al. Natural and recombinant molecules of the cherry allergen Pru av 2 show diverse structural and B cell characteristics but similar T cell reactivity. Clinical and Experimental Allergy. 2006;36(3):359–368. doi: 10.1111/j.1365-2222.2006.02439.x. [DOI] [PubMed] [Google Scholar]
- 126.Anliker MD, Borelli S, Wüthrich B. Occupational protein contact dermatitis from spices in a butcher: a new presentation of the mugwort-spice syndrome. Contact Dermatitis. 2002;46(2):72–74. doi: 10.1034/j.1600-0536.2002.460202.x. [DOI] [PubMed] [Google Scholar]
- 127.Krebitz M, Wagner B, Ferreira F, et al. Plant-based heterologous expression of Mal d 2, a thaumatin-like protein and allergen of apple (Malus domestica), and its characterization as an antifungal protein. Journal of Molecular Biology. 2003;329(4):721–730. doi: 10.1016/s0022-2836(03)00403-0. [DOI] [PubMed] [Google Scholar]
- 128.Palacin A, Rodriguez J, Blanco C, et al. Immunoglobulin e recognition patterns to purified kiwifruit (Actinidinia deliciosa) allergens in patients sensitized to kiwi with different clinical symptoms. Clinical and Experimental Allergy. 2008;38(7):1220–1228. doi: 10.1111/j.1365-2222.2007.02927.x. [DOI] [PubMed] [Google Scholar]
- 129.Palacín A, Tordesillas L, Gamboa P, et al. Characterization of peach thaumatin-like proteins and their identification as major peach allergens. Clinical and Experimental Allergy. 2010;40(9):1422–1430. doi: 10.1111/j.1365-2222.2010.03578.x. [DOI] [PubMed] [Google Scholar]
- 130.Salcedo G, Quirce S, Diaz-Perales A. Wheat allergens associated with Baker’s asthma. Journal of Investigational Allergology and Clinical Immunology. 2011;21(2):81–92. [PubMed] [Google Scholar]
- 131.Pastorello EA, Farioli L, Pravettoni V, et al. Identification of grape and wine allergens as an endochitinase 4, a lipid-transfer protein, and a thaumatin. Journal of Allergy and Clinical Immunology. 2003;111(2):350–359. doi: 10.1067/mai.2003.35. [DOI] [PubMed] [Google Scholar]
- 132.Leone P, Menu-Bouaouiche L, Peumans WJ, et al. Resolution of the structure of the allergenic and antifungal banana fruit thaumatin-like protein at 1.7-Å. Biochimie. 2006;88(1):45–52. doi: 10.1016/j.biochi.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 133.Barthakur S, Babu V, Bansal KC. Over-expression of osmotin induces proline accumulation and confers tolerance to osmotic stress in transgenic tobacco. Journal of Plant Biochemistry and Biotechnology. 2001;10(1):31–37. [Google Scholar]
- 134.Sharma P, Singh AK, Singh BP, Gaur SN, Arora N. Allergenicity assessment of osmotin, a pathogenesis-related protein, used for transgenic crops. Journal of Agricultural and Food Chemistry. 2011;59(18):9990–9995. doi: 10.1021/jf202265d. [DOI] [PubMed] [Google Scholar]
- 135.Sharma P, Gaur SN, Arora N. In silico identification of IgE-binding epitopes of osmotin protein. PLoS ONE. 2013;8(1) doi: 10.1371/journal.pone.0054755.e54755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bernasconi P, Locher R, Pilet PE, Jollès J, Jollès P. Purification and N-terminal amino-acid sequence of a basic lysozyme from Parthenocissus quinquifolia cultured in vitro. Biochimica et Biophysica Acta. 1987;915(2):254–260. [Google Scholar]
- 137.Terwisscha van Scheltinga AC, Hennig M, Dijkstra BW. The 1.8 Å resolution structure of hevamine, a plant chitinase/lysozyme, and analysis of the conserved sequence and structure motifs of glycosyl hydrolase family 18. Journal of Molecular Biology. 1996;262(2):243–257. doi: 10.1006/jmbi.1996.0510. [DOI] [PubMed] [Google Scholar]
- 138.Perrakis A, Tews I, Dauter Z, et al. Crystal structure of a bacterial chitinase at 2.3 Å resolution. Structure. 1994;2(12):1169–1180. doi: 10.1016/s0969-2126(94)00119-7. [DOI] [PubMed] [Google Scholar]
- 139.Lee MF, Chen YH, Lin HC, Wang HL, Hwang GY, Wu CH. Identification of hevamine and Hev b 1 as major latex allergens in Taiwan. International Archives of Allergy and Immunology. 2005;139(1):38–44. doi: 10.1159/000089521. [DOI] [PubMed] [Google Scholar]
- 140.Lee MF, Wang NM, Han JL, Lin SJ, Tsai JJ, Chen YH. Estimating allergenicity of latex gloves using Hev b 1 and hevamine. Journal of Investigational Allergology and Clinical Immunology. 2010;20(6):499–505. [PubMed] [Google Scholar]
- 141.Lee MF, Hwang GY, Chen YH, Lin HC, Wu CH. Molecular cloning of Indian jujube (Zizyphus mauritiana) allergen Ziz m 1 with sequence similarity to plant class III chitinases. Molecular Immunology. 2006;43(8):1144–1151. doi: 10.1016/j.molimm.2005.07.021. [DOI] [PubMed] [Google Scholar]
- 142.Lee MF, Tsai JJ, Hwang GY, Lin SJ, Chen YH. Identification of immunoglobulin E (IgE)-binding epitopes and recombinant IgE reactivities of a latex cross-reacting Indian jujube Ziz m 1 allergen. Clinical and Experimental Immunology. 2008;152(3):464–471. doi: 10.1111/j.1365-2249.2008.03661.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lee MF, Lin SJ, Wang NM, Wu HJ, Chen YH. Plant chitinase III Ziz m 1 stimulates multiple cytokines, most predominantly interleukin-13, from peripheral blood mononuclear cells of latex-fruit allergic patients. Annals of Allergy, Asthma and Immunology. 2012;108(2):113–116. doi: 10.1016/j.anai.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 144.Manavski N, Peters U, Brettschneider R, Oldenburg M, Baur X, Bittner C. Cof a 1: identification, expression and immunoreactivity of the first coffee allergen. International Archives of Allergy and Immunology. 2012;159(3):235–242. doi: 10.1159/000337461. [DOI] [PubMed] [Google Scholar]
- 145.Warner SAJ, Scott R, Draper J. Characterisation of a wound-induced transcript from the monocot asparagus that shares similarity with a class of intracellular pathogenesis-related (PR) proteins. Plant Molecular Biology. 1992;19(4):555–561. doi: 10.1007/BF00026782. [DOI] [PubMed] [Google Scholar]
- 146.Walter MH, Liu JW, Grand C, Lamb CJ, Hess D. Bean pathogenesis-related (PR) proteins deduced from elicitor-induced transcripts are members of a ubiquitous new class of conserved PR proteins including pollen allergens. Molecular and General Genetics. 1990;222(2-3):353–360. doi: 10.1007/BF00633840. [DOI] [PubMed] [Google Scholar]
- 147.Breda C, Sallaud C, El-Turk J, et al. Defense reaction in Medicago sativa: a gene encoding a class 10 PR protein is expressed in vascular bundles. Molecular Plant-Microbe Interactions. 1996;9(8):713–719. doi: 10.1094/mpmi-9-0713. [DOI] [PubMed] [Google Scholar]
- 148.Somssich IE, Schmelzer E, Kawalleck P, Hahlbrock K. Gene structure and in situ transcript localization of pathogenesis-related protein 1 in parsley. MGG Molecular & General Genetics. 1988;213(1):93–98. doi: 10.1007/BF00333403. [DOI] [PubMed] [Google Scholar]
- 149.Ekramoddoullah AKM. Physiology and molecular biology of a family of pathogenesis-related PR-10 proteins in conifers. Journal of Crop Improvement. 2004;10(1-2):261–280. [Google Scholar]
- 150.Fernandes H, Michalska K, Sikorski M, Jaskolski M. Structural and functional aspects of PR-10 proteins. FEBS Journal. 2013;280(5):1169–1199. doi: 10.1111/febs.12114. [DOI] [PubMed] [Google Scholar]
- 151.Tokuriki N, Tawfik DS. Protein dynamism and evolvability. Science. 2009;324(5924):203–207. doi: 10.1126/science.1169375. [DOI] [PubMed] [Google Scholar]
- 152.Mogensen JE, Wimmer R, Larsen JN, Spangfort MD, Otzen DE. The major birch allergen, Bet v 1, shows affinity for a broad spectrum of physiological ligands. Journal of Biological Chemistry. 2002;277(26):23684–23692. doi: 10.1074/jbc.M202065200. [DOI] [PubMed] [Google Scholar]
- 153.Breiteneder H, Pettenburger K, Bito A, et al. The gene coding for the major birch pollen allergen BetvI, is highly homologous to a pea disease resistance response gene. EMBO Journal. 1989;8(7):1935–1938. doi: 10.1002/j.1460-2075.1989.tb03597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schenk MF, Cordewener JH, America AH, Van’T Westende WP, Smulders MJ, Gilissen LJ. Characterization of PR-10 genes from eight betula species and detection of Bet v 1 isoforms in birch pollen. BMC Plant Biology. 2009;9(article 24) doi: 10.1186/1471-2229-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Breiteneder H, Hoffmann-Sommergruber K, O’Riordain G, et al. Molecular characterization of Api g 1, the major allergen of celery (Apium graveolens), and its immunological and structural relationships to a group of 17-kDa tree pollen allergens. European Journal of Biochemistry. 1995;233(2):484–489. doi: 10.1111/j.1432-1033.1995.484_2.x. [DOI] [PubMed] [Google Scholar]
- 156.Vanek-Krebitz M, Hoffmann-Sommergruber K, Laimer da Camara Machado M, et al. Cloning and sequencing of Mal d 1, the major allergen from apple (Malus domestica), and its immunological relationship to Bet v 1, the major birch pollen allergen. Biochemical and Biophysical Research Communications. 1995;214(2):538–551. doi: 10.1006/bbrc.1995.2320. [DOI] [PubMed] [Google Scholar]
- 157.Jarolim E, Tejkl M, Rohac M, et al. Monoclonal antibodies against birch pollen allergens: characterization by immunoblotting and use for single-step affinity purification of the major allergen Bet v I. International Archives of Allergy and Applied Immunology. 1989;90(1):54–60. doi: 10.1159/000235000. [DOI] [PubMed] [Google Scholar]
- 158.Swoboda I, Jilek A, Ferreira F, et al. Isoforms of Bet v 1, the major birch pollen allergen, analyzed by liquid chromatography, mass spectrometry, and cDNA cloning. Journal of Biological Chemistry. 1995;270(6):2607–2613. doi: 10.1074/jbc.270.6.2607. [DOI] [PubMed] [Google Scholar]
- 159.Neudecker P, Schweimer K, Nerkamp J, et al. Allergic cross-reactivity made visible. Solution structure of the major cherry allergen Pru av 1. Journal of Biological Chemistry. 2001;276(25):22756–22763. doi: 10.1074/jbc.M101657200. [DOI] [PubMed] [Google Scholar]
- 160.Marković-Housley Z, Basle A, Padavattan S, Maderegger B, Schirmer T, Hoffmann-Sommergruber K. Structure of the major carrot allergen Dau c 1. Acta Crystallographica D. 2009;65(part 11):1206–1212. doi: 10.1107/S0907444909034854. [DOI] [PubMed] [Google Scholar]
- 161.Botton A, Andreotti C, Costa G, Ramina A. Peach (Prunus persica L. Batsch) allergen-encoding genes are developmentally regulated and affected by fruit load and light radiation. Journal of Agricultural and Food Chemistry. 2009;57(2):724–734. doi: 10.1021/jf802709k. [DOI] [PubMed] [Google Scholar]
- 162.Karamloo F, Scheurer S, Wangorsch A, May S, Haustein D, Vieths S. Pyr c 1, the major allergen from pear (Pyrus communis), is a new member of the Bet v 1 allergen family. Journal of Chromatography B. 2001;756(1-2):281–293. doi: 10.1016/s0378-4347(01)00087-1. [DOI] [PubMed] [Google Scholar]
- 163.Mirza O, Henriksen A, Ipsen H, et al. Dominant epitopes and allergic cross-reactivity: complex formation between a Fab fragment of a monoclonal murine IgG antibody and the major allergen from birch pollen bet v 1. Journal of Immunology. 2000;165(1):331–338. doi: 10.4049/jimmunol.165.1.331. [DOI] [PubMed] [Google Scholar]
- 164.Berneder M, Bublin M, Hoffmann-Sommergruber K, Hawranek T, Lang R. Allergen chip diagnosis for soy-allergic patients: Gly m 4 as a marker for severe food-allergic reactions to soy. International Archives of Allergy and Immunology. 2013;161(3):229–233. doi: 10.1159/000345970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Riecken S, Lindner B, Petersen A, Jappe U, Becker WM. Purification and characterization of natural Ara h 8, the Bet v 1 homologous allergen from peanut, provides a novel isoform. Biological Chemistry. 2008;389(4):415–423. doi: 10.1515/BC.2008.038. [DOI] [PubMed] [Google Scholar]
- 166.Mittag D, Vieths S, Vogel L, et al. Birch pollen-related food allergy to legumes: identification and characterization of the Bet v 1 homologue in mungbean (Vigna radiata), Vig r 1. Clinical and Experimental Allergy. 2005;35(8):1049–1055. doi: 10.1111/j.1365-2222.2005.02309.x. [DOI] [PubMed] [Google Scholar]
- 167.Hirschwehr R, Valenta R, Ebner C, et al. Identification of common allergenic structures in hazel pollen and hazelnuts: a possible explanation for sensitivity to hazelnuts in patients allergic to tree pollen. Journal of Allergy and Clinical Immunology. 1992;90(6, part 1):927–936. doi: 10.1016/0091-6749(92)90465-e. [DOI] [PubMed] [Google Scholar]
- 168.Hirschwehr R, Jäger S, Horak F, et al. Allergenss from birch pollen and pollen of the European chestnut share common epitopes. Clinical and Experimental Allergy. 1993;23(9):755–761. doi: 10.1111/j.1365-2222.1993.tb00363.x. [DOI] [PubMed] [Google Scholar]
- 169.Gajhede M, Osmark P, Poulsen FM, et al. X-ray and NMR structure of bet v 1, the origin of birch pollen allergy. Nature Structural Biology. 1996;3(12):1040–1045. doi: 10.1038/nsb1296-1040. [DOI] [PubMed] [Google Scholar]
- 170.Marković-Housley Z, Degano M, Lamba D, et al. Crystal structure of a hypoallergenic isoform of the major birch pollen allergen Bet v 1 and its likely biological function as a plant steroid carrier. Journal of Molecular Biology. 2003;325(1):123–133. doi: 10.1016/s0022-2836(02)01197-x. [DOI] [PubMed] [Google Scholar]
- 171.Kofler S, Asam C, Eckhard U, Wallner M, Ferreira F, Brandstetter H. Crystallographically mapped ligand binding differs in high and low IgE binding isoforms of birch pollen allergen Bet v 1. Journal of Molecular Biology. 2012;422(1):109–123. doi: 10.1016/j.jmb.2012.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Spangfort MD, Mirza O, Ipsen H, van Neerven RJJ, Gajhede M, Larsen JN. Dominating IgE-binding epitope of Bet v 1, the major allergen of birch pollen, characterized by x-ray crystallography and site-directed mutagenesis. Journal of Immunology. 2003;171(6):3084–3090. doi: 10.4049/jimmunol.171.6.3084. [DOI] [PubMed] [Google Scholar]
- 173.Neudecker P, Lehmann K, Nerkamp J, et al. Mutational epitope analysis of Pru av 1 and Api g 1, the major allergens of cherry (Prunus avium) and celery (Apium graveolens): correlating IgE reactivity with three-dimensional structure. Biochemical Journal. 2003;376(part 1):97–107. doi: 10.1042/BJ20031057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Crameri R. Correlating IgE reactivity with three-dimensional structure. The Biochemical Journal. 2003;376(part 1):e1–e2. doi: 10.1042/BJ20031363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Schirmer T, Hoffimann-Sommergrube K, Susani M, Breiteneder H, Marković-Housley Z. Crystal structure of the major celery allergen Api g 1: molecular analysis of cross-reactivity. Journal of Molecular Biology. 2005;351(5):1101–1109. doi: 10.1016/j.jmb.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 176.Fritsch R, Bohle B, Vollmann U, et al. Bet v 1, the major birch pollen allergen, and Mal d 1, the major apple allergen, cross-react at the level of allergen-specific T helper cells. Journal of Allergy and Clinical Immunology. 1998;102(4, part 1):679–686. doi: 10.1016/s0091-6749(98)70287-8. [DOI] [PubMed] [Google Scholar]
- 177.Zilversmit DB. Lipid transfer proteins. Journal of Lipid Research. 1984;25(13):1563–1569. [PubMed] [Google Scholar]
- 178.Kader JC. Lipid-transfer proteins in plants. Annual Review of Plant Physiology and Plant Molecular Biology. 1996;47(1):627–654. doi: 10.1146/annurev.arplant.47.1.627. [DOI] [PubMed] [Google Scholar]
- 179.Nieuwland J, Feron R, Huisman BAH, et al. Lipid transfer proteins enhance cell wall extension in tobacco. Plant Cell. 2005;17(7):2009–2019. doi: 10.1105/tpc.105.032094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Salcedo G, Sánchez-Monge R, Barber D, Díaz-Perales A. Plant non-specific lipid transfer proteins: an interface between plant defence and human allergy. Biochimica et Biophysica Acta. 2007;1771(6):781–791. doi: 10.1016/j.bbalip.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 181.van Ree R. Clinical importance of non-specific lipid transfer proteins as food allergens. Biochemical Society Transactions. 2002;30(part 6):910–913. doi: 10.1042/bst0300910. [DOI] [PubMed] [Google Scholar]
- 182.Pastorello EA, Pravettoni V, Farioli L, et al. Clinical role of a lipid transfer protein that acts as a new apple-specific allergen. Journal of Allergy and Clinical Immunology. 1999;104:1099–1106. doi: 10.1016/s0091-6749(99)70095-3. [DOI] [PubMed] [Google Scholar]
- 183.Pastorello EA, D’Ambrosio FP, Pravettoni V, et al. Evidence for a lipid transfer protein as the major allergen of apricot. Journal of Allergy and Clinical Immunology. 2000;105(2, part 1):371–377. doi: 10.1016/s0091-6749(00)90090-3. [DOI] [PubMed] [Google Scholar]
- 184.Scheurer S, Lauer I, Foetisch K, et al. Strong allergenicity of Pru av 3, the lipid transfer protein from cherry, is related to high stability against thermal processing and digestion. Journal of Allergy and Clinical Immunology. 2004;114(4):900–907. doi: 10.1016/j.jaci.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 185.Pastorello EA, Farioli L, Pravettoni V, et al. Characterization of the major allergen of plum as a lipid transfer protein. Journal of Chromatography B. 2001;756(1-2):95–103. doi: 10.1016/s0378-4347(01)00074-3. [DOI] [PubMed] [Google Scholar]
- 186.Fernández-Rivas M, van Ree R, Cuevas M. Allergy to Rosaceae fruits without related pollinosis. Journal of Allergy and Clinical Immunology. 1997;100(6, part 1):728–733. doi: 10.1016/s0091-6749(97)70265-3. [DOI] [PubMed] [Google Scholar]
- 187.Pasquato N, Berni R, Folli C, et al. Crystal structure of peach Pru p 3, the prototypic member of the family of plant non-specific lipid transfer protein pan-allergens. Journal of Molecular Biology. 2006;356(3):684–694. doi: 10.1016/j.jmb.2005.11.063. [DOI] [PubMed] [Google Scholar]
- 188.García-Casado G, Pacios LF, Díaz-Perales A, et al. Identification of IgE-binding epitopes of the major peach allergen Pru p 3. Journal of Allergy and Clinical Immunology. 2003;112(3):599–605. doi: 10.1016/s0091-6749(03)01605-1. [DOI] [PubMed] [Google Scholar]
- 189.Egger M, Hauser M, Mari A, Ferreira F, Gadermaier G. The role of lipid transfer proteins in allergic diseases. Current Allergy and Asthma Reports. 2010;10(5):326–335. doi: 10.1007/s11882-010-0128-9. [DOI] [PubMed] [Google Scholar]
- 190.Schocker F, Lüttkopf D, Scheurer S, et al. Recombinant lipid transfer protein Cor a 8 from hazelnut: a new tool for in vitro diagnosis of potentially severe hazelnut allergy. Journal of Allergy and Clinical Immunology. 2004;113(1):141–147. doi: 10.1016/j.jaci.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 191.Roux KH, Teuber SS, Sathe SK. Tree nut allergens. International Archives of Allergy and Immunology. 2003;131(4):234–244. doi: 10.1159/000072135. [DOI] [PubMed] [Google Scholar]
- 192.Pastorello EA, Farioli L, Pravettoni V, et al. The maize major allergen, which is responsible for food-induced allergic reactions, is a lipid transfer protein. Journal of Allergy and Clinical Immunology. 2000;106(4):744–751. doi: 10.1067/mai.2000.108712. [DOI] [PubMed] [Google Scholar]
- 193.Nemni A, Borges JP, Rougé P, Barre A, Just J. Barley's lipid transfer protein: a new emerging allergen in pediatric anaphylaxis. Pediatric Allergy and Immunology. 2013;24(4):410–411. doi: 10.1111/pai.12062. [DOI] [PubMed] [Google Scholar]
- 194.Colombo P, Kennedy D, Ramsdale T, et al. Identification of an immunodominant IgE epitope of the Parietaria judaica major allergen. Journal of Immunology. 1998;160(6):2780–2785. [PubMed] [Google Scholar]
- 195.Dempsey DA, Silva H, Klessig DF. Engineering disease and pest resistance in plants. Trends in Microbiology. 1998;6(2):54–61. doi: 10.1016/S0966-842X(97)01186-4. [DOI] [PubMed] [Google Scholar]
- 196.Liu D, Raghothama KG, Hasegawa PM, Bressan RA. Osmotin overexpression in potato delays development of disease symptoms. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(5):1888–1892. doi: 10.1073/pnas.91.5.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Alexander D, Goodman RM, Gut-Rella M, et al. Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(15):7327–7331. doi: 10.1073/pnas.90.15.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Balasubramanian V, Vashisht D, Cletus J, Sakthivel N. Plant β-1,3-glucanases: their biological functions and transgenic expression against phytopathogenic fungi. Biotechnology Letters. 2012;34(11):1983–1990. doi: 10.1007/s10529-012-1012-6. [DOI] [PubMed] [Google Scholar]
- 199.Anand A, Zhou T, Trick HN, Gill BS, Bockus WW, Muthukrishnan S. Greenhouse and field testing of transgenic wheat plants stably expressing genes for thaumatin-like protein, chitinase and glucanase against Fusarium graminearum . Journal of Experimental Botany. 2003;54(384):1101–1111. doi: 10.1093/jxb/erg110. [DOI] [PubMed] [Google Scholar]
- 200.Young GJ, Zhang S, Mirsky HP, et al. Assessment of possible allergenicity of hypothetical ORFs in common food crops using current bioinformatic guidelines and its implications for the safety assessment of GM crops. Food and Chemical Toxicology. 2012;50(10):3741–3751. doi: 10.1016/j.fct.2012.07.044. [DOI] [PubMed] [Google Scholar]
- 201.Metcalfe DD. Genetically modified crops and allergenicity. Nature Immunology. 2005;6(9):857–860. doi: 10.1038/ni0905-857. [DOI] [PubMed] [Google Scholar]
- 202.Taylor SL. Protein allergenicity assessment of foods produced through agricultural biotechnology. Annual Review of Pharmacology and Toxicology. 2002;42:99–112. doi: 10.1146/annurev.pharmtox.42.082401.130208. [DOI] [PubMed] [Google Scholar]


