Abstract
Heterotaxy and situs abnormalities describe an abnormal arrangement of visceral organs in the thoracoabdominal cavity across the normal left–right axis of the body. It is associated with a high occurrence of congenital heart and abdominal defects, including anomalous pulmonary venous connections, systemic venous abnormalities, asplenia, and intestinal malrotation. Without proper diagnosis and surgical intervention, the prognosis of patients with heterotaxy syndrome and associated congenital defects is extremely poor. Complex intracardiac and extracardiac lesions are common in heterotaxy and can be difficult to assess by echocardiography. CT angiography (CTA) is a useful tool in this setting to accurately assess intracardiac and extracardiac abnormalities in this population for medical or surgical management. The intention of this pictorial essay is to review the most common cardiovascular defects involved with heterotaxy syndrome in addition to emphasizing the utility of CTA in the identification and classification of anomalies seen in these patients. This review briefly defines most common terminology used in situs abnormalities as well as presents CT images and 3-dimensional reconstructions of common anomalies associated with situs abnormalities. In summary, this review should prepare radiologists and pediatric cardiologists to describe heterotaxy and situs abnormalities in addition to recognizing the utility of CTA in these patients.
Keywords: Heterotaxy, Isomerism, Situs abnormalities, Situs inversus, Situs ambiguus, CT angiography
1. Introduction
Heterotaxy describes an abnormal arrangement of visceral organs in the thoracic and abdominal cavities across the normal left–right axis of the body.1,2 It is a nonspecific term that encompasses several different arrangements of cardiac chambers, great vessels, and extracardiac organs. Heterotaxy is often associated with a high prevalence of congenital heart defects.3 This article shows the most common forms of heterotaxy and related abnormal cardiac arrangements through the use of CT angiography (CTA).
2. Imaging technique
Scans are performed on a 64-slice single-source scanner (Somatom Sensation; Siemens Healthcare, Forchheim, Germany) or a first-generation dual-source scanner (Somatom Definition, Siemens). The following scan parameters are used: collimation of 0.6 mm, slice thickness of 0.75 mm, reconstruction interval of 0.3 mm, tube power of 80 kV, contrast volume of 2 mL/kg nonionic low-osmolar contrast material (300 mg I/mL iopromide; Ultravist; Bayer Healthcare, Berlin, Germany), and kernel of B20f. Automated tube current modulation is used (CAREDose; Siemens). Unless there is a suspicion for abnormal coronary origins/course, the scans are performed without electrocardiogram synchronization. Scans are performed in the arterial phase. However, because systemic venous abnormalities are common in these patients, our standard injection protocol is modified by dividing the contrast bolus into equal halves and adding a 30-second pause between the 2 portions. This allows opacification of the systemic veins returning to the heart without adding a second scan in the venous phase. Images are transferred to a separate workstation for image processing (Aquarius iNtuition; TeraRecon, San Mateo, CA).
3. Definitions
No single nomenclature system exists to describe heterotaxy, although systematic nomenclature has been proposed.4 Therefore, this review defines the most common terminology in an attempt to remain concise.
3.1. Situs
Situs refers to the position of visceral organs in the thoracic and abdominal cavities. Situs solitus describes the normal anatomic position of cardiac structures, as well as the remaining thoracoabdominal organs.5 Situs inversus, in its simplest form, refers to a left–right inversion of visceral and atrial positions within the thoracic and abdominal cavities, resulting in the mirror image of situs solitus.5,6 Situs ambiguus is a term that characterizes positions of both the atria and visceral organs in which lateralization across the left–right axis cannot be categorized as situs solitus or inversus.5 It is synonymous with left or right isomerism.6 Technically, any type of situs other than situs solitus (normal) can be described as heterotaxy, but the term heterotaxy is most commonly used in the context of situs ambiguus.
3.2. Isomerism
Isomerism describes the situation in which a person’s morphologically right structures or morphologically left structures are found on both sides of the body and, in morphologic terms, are symmetrical mirror images of each other.4
3.3. Right and left
When describing patients with abnormal situs, the words left and right can be confusing. It is imperative to describe both the side of the body the structure in question is on and the morphologic characteristics of that structure. For example, in situs solitus, the morphologic right atrium (RA) is located on the right and the morphologic left atrium (LA) is leftward. However, in situs inversus, the morphologic RA is located on the left and the morphologic LA is rightward. In situs ambiguus, both atria have morphologic characteristics of either a LA or RA.
3.4. Atrial situs
The determination of atrial morphology in the setting of heterotaxy is based on the morphology of the atrial appendages. The morphologic RA is defined by a large pyramidal appendage with pectinate muscles that extend toward the atrioventricular valve. The morphologic LA is defined by a small finger-like appendage with pectinate muscles isolated to the body of the appendage.7 Identification of atrial appendage morphology can be especially difficult by echo-cardiography. CTA can be useful in the identification of atrial appendage morphology and, hence, in defining atrial situs. In addition, when a coronary sinus is present, it always runs inferior–posterior to the morphologic LA and empties into the morphologic RA7 (Fig. 1).
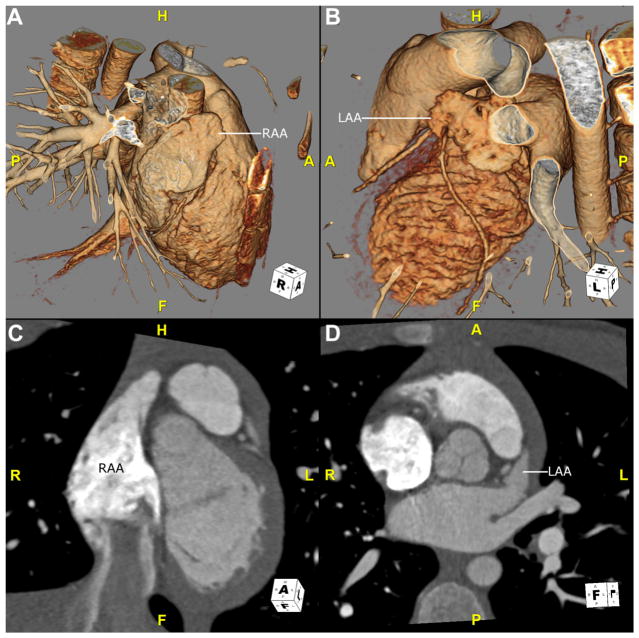
Fig. 1.
CTA images of atrial appendage anatomy in a healthy child with situs solitus. (A and B) Images are 3D volume-rendered reconstructions viewed from a right and left anterolateral position, respectively. (A) The right-sided morphologically RAA is shown, which is broad based and triangular. (B) A left-sided morphologically LAA is shown, which is narrow and finger-like. (C) This oblique coronal image shows a normal RAA. Note that the pectinate muscles extend into the body of the atrium. (D) This oblique transverse image shows a normal LAA, with narrow, smooth-walled neck. A, anterior, CTA, CT angiography; F, feet; H, head; L, left; LAA, left atrial appendage; P, posterior; R, right; RAA, right atrial appendage; 3D, 3-dimensional.
3.5. Pulmonary situs
In situs abnormalities, bronchopulmonary anatomy usually is concordant with atrial situs and can be useful in the evaluation of heterotaxy. Traditionally, bronchopulmonary situs is determined by the relationship of the pulmonary arteries to their ipsilateral bronchi.5 In the morphologic right lung, there is an eparterial bronchus; the bronchus branches superior to the first lobar division of the pulmonary artery. In the morphologic left lung, there is a hyparterial bronchus; the bronchus branches inferior to the first lobar division of the pulmonary artery (Fig. 2).8 However, this definition of pulmonary situs assumes that the pulmonary arteries are in the normal location, which can be problematic in patients with congenital heart disease. Consequently, one can also rely on the fact that the morphologic right main bronchus is shorter and more horizontal than the morphologic left main bronchus. Situs may also be evaluated by using lung anatomy; the morphologic right lung is tri-lobed, whereas the morphologic left lung is bi-lobed. The determination of bronchopulmonary situs is impossible with the use of echocardiography and difficult by chest radiograph. CTA can accurately delineate the bronchopulmonary anatomy, thereby overcoming the limitations of the above modalities (Fig. 3).
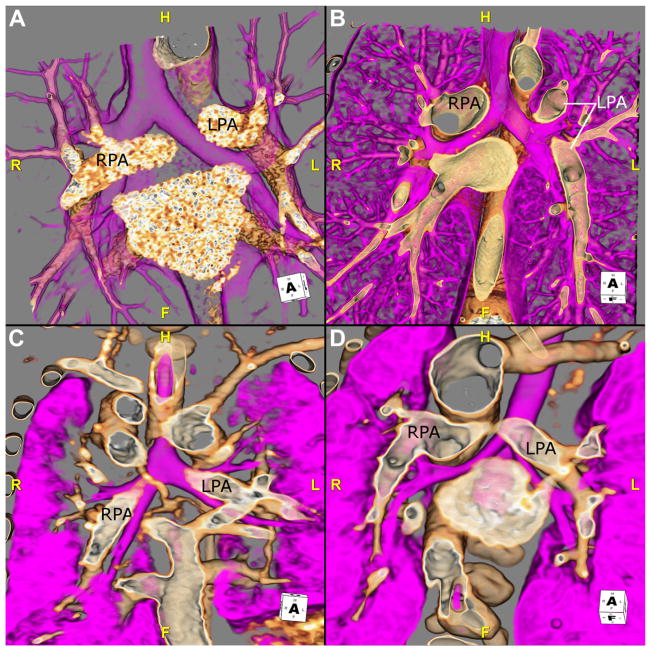
Fig. 2.
The relationship of the bronchus to its ipsilateral pulmonary artery can be useful in determining situs. These images are 3D volume-rendered CTA reconstructions in 4 different patients. Each image shows the airway (pink) and the RPA and LPA. (A) This image shows this relationship in a patient with situs solitus. The right bronchus is eparterial; the bronchus branches superior to the first lobar division of the pulmonary artery. The left bronchus is hyparterial; the bronchus branches inferior to the first lobar division of the pulmonary artery. (B) The relationship of the bronchi and the pulmonary arteries RPA and LPA of a patient with situs inversus is shown. The right-sided bronchus is hyparterial, consistent with a morphologic left bronchus, whereas the left-sided bronchus eparterial is consistent with a morphologic right bronchus. (C) This image shows a patient with right isomerism. Both bronchi are eparterial. (D) This image is from a patient with left isomerism, showing bilateral hyparterial bronchi. CTA, CT angiography; F, feet; H, head; L, left; LPA, left pulmonary artery; R, right; RPA, right pulmonary artery; 3D, 3-dimensional.
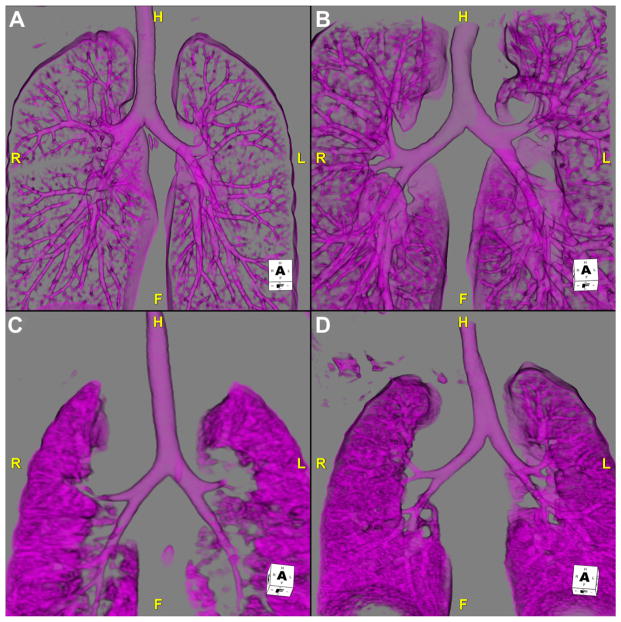
Fig. 3.
The tracheobronchial tree anatomy can also be valuable in determining situs. These images are 3D volume-rendered reconstruction of the airways in 4 different patients. (A) This image shows the airways of the patient with situs solitus. The right-sided bronchus is short and horizontal compared with the left-sided bronchus. (B) The tracheobronchial tree of a patient with situs inversus is shown. The right-sided bronchus is long and angled, consistent with a morphologic left bronchus, whereas the left-sided bronchus is shorter and less angled, consistent with a morphologic right bronchus. (C) This image shows a patient with right isomerism. Both bronchi are short and horizontal. This patient has tri-lobed lungs bilaterally. (D) This image, from a patient with left isomerism, shows long and angled bilateral bronchi. This patient has bi-lobed lungs bilaterally. F, feet; H, head; L, left; R, right; 3D, 3-dimensional.
3.6. Abdominal situs
In most cases, atrial arrangement in situs abnormalities parallels abdominal visceral arrangement.9 Position of the abdominal aorta, inferior vena cava (IVC), stomach, spleen, and liver are normal in situs solitus and reversed in situs inversus. In situs ambiguus, these relationships are more complicated and are described below.
4. Situs inversus
The most common version of situs inversus is situs inversus totalis in which there is a complete mirror-image arrangement with normal atrioventricular and ventriculoarterial connections that result in normal circulation.5 All contents of the thoracic and abdominal cavity are reversed over the left–right axis. The morphologic LA is on the right and the morphologic RA is on the left.4 The left-sided lung is tri-lobed with an eparterial bronchus, whereas the right-sided lung is bi-lobed with a hyparterial bronchus.8 The larger lobe of the liver is located left of the midline, whereas the stomach and spleen are on the right. The IVC ascends to the morphologic RA on the left.4 Situs inversus totalis rarely has associated congenital heart defects, with an incidence range from 2% to 5%10 (Fig. 4).
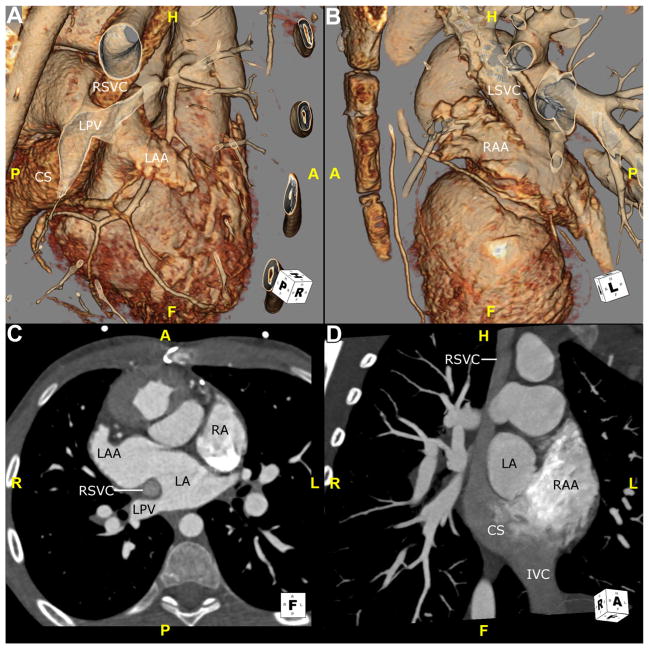
Fig. 4.
This CTA demonstrates the anatomy in a child with situs inversus, along with congenitally corrected transposition. (A and B) These 3D volume-rendered reconstructions are viewed from a right and left anterolateral position, respectively. In contrast to Figure 1, image A shows a narrow, finger-like morphologically LAA on the right side of the thorax. A RSVC is seen coursing anterior to the LPVs and entering the CS. (B) Abroad based, triangular morphologically RAA lateralized to the left is shown. A LSVC is seen entering the left-sided atrium. (C) This transverse image shows a morphologically LAA on the right side of the heart. Note that the pectinate muscles are confined to the tip of the appendage. The LPV is seen entering the morphologic left atrium. (D) This maximum-intensity projection image is viewed in an oblique coronal plane. It shows a RAA, with pectinate muscles extending into the body of the atrium, on the left. The RSVC is seen entering the CS, which courses inferior–posterior to the morphologic LA to enter the morphologic RA. A left-sided IVC also enters the morphologic RA. CS, coronary sinus; CTA, CT angiography; F, feet; H, head; IVC, inferior vena cava; L, left; LA, left atrium; LAA, left atrial appendage; LPV, left pulmonary vein; LSVC, left superior vena cava; P, posterior; R, right; RA, right atrium; RAA, right atrial appendage; RSVC, right superior vena cava; 3D, 3-dimensional.
5. Isomerism
5.1. Right isomerism
Right isomerism is a condition in which structures are bilateral within the thoracoabdominal cavity with morphologic right characteristics.11 The main characteristic of right isomerism is atria with bilateral right atrial appendages. Bilateral tri-lobed lungs with epiarterial bronchi are seen. It is often associated with asplenia, which occurs in 42% of patients with right isomerism.12 Other anomalies include bilateral superior vena cavae, symmetrical liver, pulmonary venous abnormalities, and intestinal malrotation1,13,14 (Figs. 5 and 6).
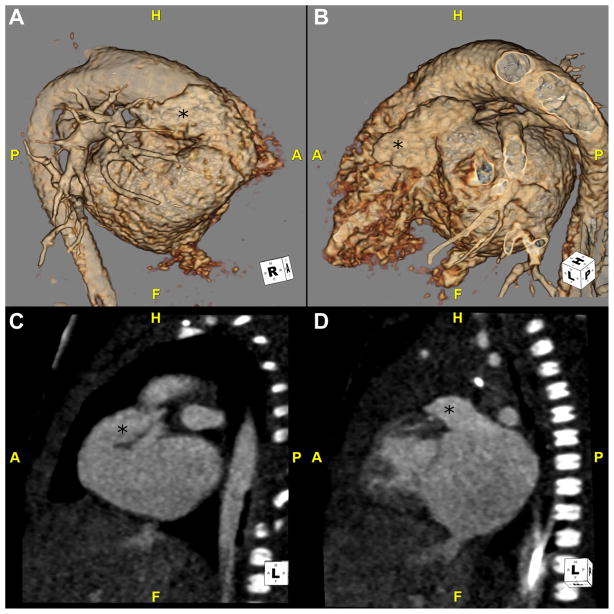
Fig. 5.
This is a CTA in a neonate with right isomerism. (A and B) These 3D volume-rendered reconstructions are viewed from a right and left anterolateral position, respectively. The atrial appendages (*) look similar and show characteristics of a morphologic right atrial appendage. They are both broad-based, triangular structures. This patient also has bilateral RSVC and LSVC. Patients with right isomerism will often have pulmonary venous abnormalities. (C) This maximum intensity projection image in the coronal plane shows the pulmonary veins in this patient connecting to a PVC behind the atria and draining via a VV that dives below the diaphragm. Note the large, central liver. (D) This oblique coronal plane shows 2 broad appendages (*) that wrap anteriorly on either side of the AO. AO, aorta; CTA, CT angiography; F, feet; H, head; LSVC, left superior vena cava; PVC, pulmonary venous confluence; RSVC, right superior vena cava; VV, vertical vein.
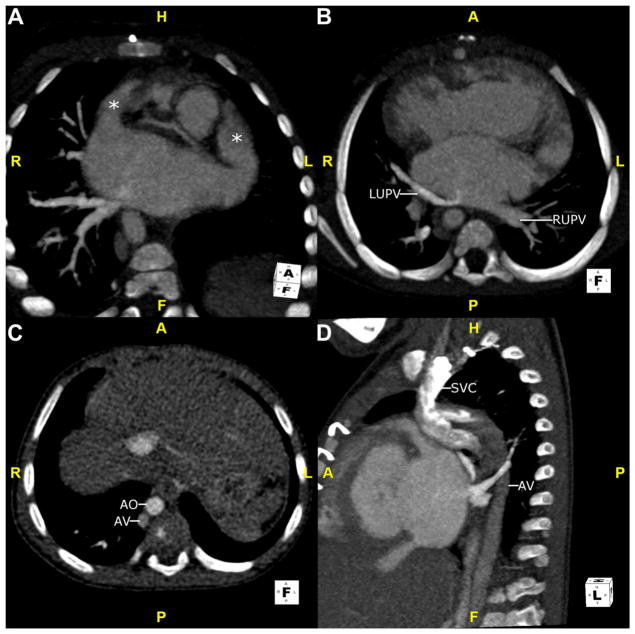
Fig. 6.
This is another neonate with right isomerism. (A) This 3D volume-rendered reconstruction is viewed from behind to show mixed, supracardiac totally anomalous pulmonary venous return. The RLPV (arrow) and LPV (arrows) drain into the LSVC. The RUPV (arrow) drains into the RSVC. (B) This maximum intensity projection image is viewed in an oblique coronal plane. The RSVC drains into the RSA. The LSVC drains into the LSA. F, feet; H, head; L, left; LPV, left pulmonary vein; LSA, left-sided atrium; LSVC, left superior vena cava; R, right; RLPV, right lower pulmonary vein; RSA, right-sided atrium; RSVC, right superior vena cava; RUPV, right upper pulmonary vein.
5.2. Left isomerism
Left isomerism is a condition in which structures are bilateral within the thoracoabdominal cavity with morphologic left characteristics.11 The main characteristic of left isomerism is bilateral left atrial appendages.14 Bronchopulmonary characteristics include 2 bi-lobed lungs with their corresponding main hyparterial bronchi. Abdominal characteristics include an interrupted IVC with azygous continuation and a midline liver. Polysplenia is present in 55% of patients with left isomerism.15 Because the sinus and atrioventricular nodes are right atrial structures, these patients often have sinus node dysfunction or congenital heart block. Patients may also have anomalous pulmonary venous return, although pulmonary venous abnormalities are more common with right isomerism1,13,14 (Figs. 7 and 8).
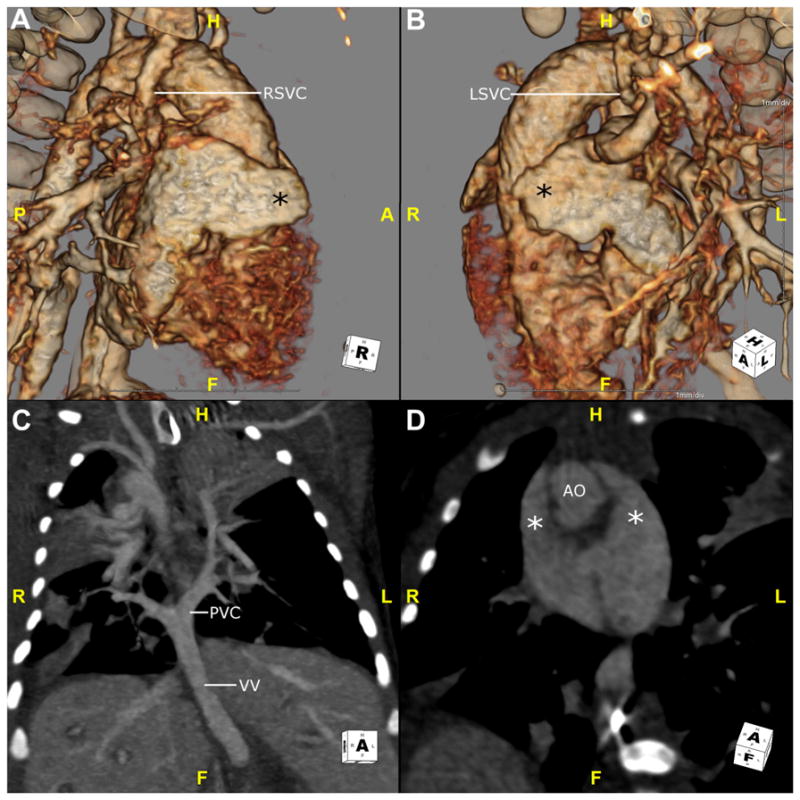
Fig. 7.
This is a CTA in a neonate with left isomerism. (A and B) These 3D volume-rendered reconstructions are viewed from a right and left anterolateral position, respectively. (C and D) These sagittal images are through the right-sided and left-sided atrial appendages, respectively. The appendages (*) look similar and show characteristics of a morphologic left atrial appendage. They are narrow, finger-like structures. A, anterior; CTA, CT angiography; F, feet; H, head; P, posterior.
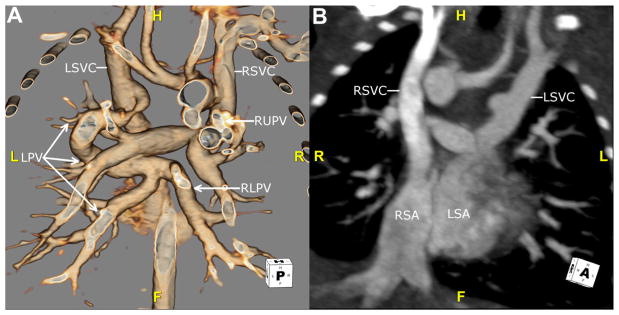
Fig. 8.
These images are from another child with left atrial isomerism. (A) This image is an oblique coronal plane. Similar to the prior figure, the appendages (*) are narrow and finger-like. (B) This image is a maximum intensity projection image in a transverse plane. It shows a common atrium with a pulmonary venous anomaly frequently present in left isomerism: the RUPV and LUPV drain separately to the ipsilateral side of the common atrium. (C) This image is a transverse plane. An enlarged AV is seen posterior and rightward to the AO, consistent with an interrupted inferior vena cava. (D) This maximum intensity projection image in a sagittal plane shows the enlarged AV that drains into the SVC. The right pulmonary artery has been connected to the SVC, consistent with the history of a Glenn procedure. A, anterior; AO, aorta; AV, azygous vein; CTA, CT angiography; F, feet; H, head; L, left; LUPV, left upper pulmonary vein; P, posterior; R, right; RUPV, right upper pulmonary vein; SVC, superior vena cava.
5.3. Associated anomalies
Patients with left and right isomerisms are at risk for a wide variety of additional congenital heart defects. Abnormalities can be found in the atrioventricular, ventriculoarterial, and venoatrial connections; the ventricular topology; the arrangements of the arterial trunks; the position of the heart; and the orientation of its apex. Extracardiac lesions are common and can be difficult to assess by echocardiography. CTA is a useful tool in this setting to accurately assess intracardiac and extracardiac abnormalities in this population for medical or surgical planning or both.
6. Segmental approach to imaging
The segmental approach to analyzing congenital heart defects was first introduced >30 years ago and has subsequently been modified to a 3-step approach that is generally accepted.9,16–18 The first step is to define the visceroatrial situs as defined above: situs solitus, situs inversus, or situs ambiguus (isomerism).
Second, the orientation of the ventricles is defined. Ventricular morphology is determined by the texture and location of trabeculae. The morphologic right ventricle (RV) has course trabeculae with a moderator band, whereas the left ventricle (LV) has fine trabeculae with a smooth septal surface. The orientation of the ventricles is commonly referred to as “ventricular looping,” in reference to the embryonic rotation of the cardiac tube during development. Rightward rotation is called D-loop and brings the future morphologic RV to its normal position rightward to the LV. A left rotation (L-loop) brings the morphologic RV to the left of the LV.19 Next, one should describe the atrioventricular connections as concordant (RA to RV and LA to LV), discordant (RA to LV and LA to RV), double inlet (RA and LA to LV or RV), ambiguous (in cases of isomerism), or absent right or left (in cases of atrioventricular valve atresia).
Finally, the connections and orientation of the great vessels are defined. The 3 most common ventriculoarterial connections are normal, transposition, and double outlet RV. Normally, the pulmonary artery arises from the RV, and the aorta from the LV. With transposition, the aorta arises from the morphologic RV, and the pulmonary artery arises from the LV. In double outlet RV, both great vessels arise from the RV. The orientation of the great vessels is defined by the location of the aortic valve in relation to the pulmonary valve. Normally, the aortic valve is posterior and rightward, but in situs inversus it is often leftward and posterior. In transposition, the aorta is anterior and rightward (D-transposition). However, when the morphologic RV is leftward, the aorta will be anterior and leftward (L-transposition). L-transposition generally refers to the situation in which discordant atrioventricular and ventriculoarterial connections are found and can also be called congenitally corrected transposition.
7. Conclusion
The spectrum of anomalies in patients with situs abnormalities is diverse and can be difficult to fully delineate by conventional means. A detailed description of the anatomic features in heterotaxy is needed to properly plan for medical and surgical management. CT angiography overcomes the limitations of echocardiography and is useful in identifying and classifying anomalies in these patients.
Footnotes
Conflict of interest: U.J.S. is a consultant for and receives research support from Bayer, Bracco, GE, Medrad, and Siemens. The remaining authors report no conflict of interest.
References
- 1.Balan A, Lazoura O, Padley SP, Rubens M, Nicol ED. Atrial isomerism: a pictorial review. J Cardiovasc Comput Tomogr. 2012;6:127–136. doi: 10.1016/j.jcct.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 2.Kim SJ. Heterotaxy syndrome. Korean Circ J. 2011;41:227–232. doi: 10.4070/kcj.2011.41.5.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tonkin IL, Tonkin AK. Visceroatrial situs abnormalities: sonographic and computed tomographic appearance. AJR Am J Roentgenol. 1982;138:509–515. doi: 10.2214/ajr.138.3.509. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs JP, Anderson RH, Weinberg PM, et al. The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiol Young. 2007;17(suppl 2):1–28. doi: 10.1017/S1047951107001138. [DOI] [PubMed] [Google Scholar]
- 5.Hagler DJ, O’Leary PW. Cardiac malpositions and abnormalities of atrial and visceral situs. In: Emmanouilides GC, Riemenschneider TA, Allen HD, Gutgesell HP, editors. Moss and Adams’ Heart Disease in Infants, Children, and Adolescents: Including the Fetus and Young Adult. Vol. 2. Baltimore, MD: Williams & Wilkins; 1995. pp. 1307–1336. [Google Scholar]
- 6.Ghosh S, Yarmish G, Godelman A, Haramati LB, Spindola-Franco H. Anomalies of visceroatrial situs. AJR Am J Roentgenol. 2009;193:1107–1117. doi: 10.2214/AJR.09.2411. [DOI] [PubMed] [Google Scholar]
- 7.Anderson RH. Isomerism of the atrial appendages. Am J Cardiol. 1999;83:818–819. [PubMed] [Google Scholar]
- 8.Maldjian PD, Saric M. Approach to dextrocardia in adults: review. AJR Am J Roentgenol. 2007;188:S39–S49. doi: 10.2214/AJR.06.1179. quiz S35–S38. [DOI] [PubMed] [Google Scholar]
- 9.Van Praagh R. The importance of segmental situs in the diagnosis of congenital heart disease. Semin Roentgenol. 1985;20:254–271. doi: 10.1016/0037-198x(85)90009-4. [DOI] [PubMed] [Google Scholar]
- 10.Ellis K, Fleming RJ, Griffiths SP, Jameson AG. New concepts in dextrocardia. Angiocardiographic considerations. Am J Roentgenol Radium Ther Nucl Med. 1966;97:295–313. doi: 10.2214/ajr.97.2.295. [DOI] [PubMed] [Google Scholar]
- 11.Perloff JK. The cardiac malpositions. Am J Cardiol. 2011;108:1352–1361. doi: 10.1016/j.amjcard.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 12.Van Praagh S, Santini F, Sanders SP. Cardiac malpositions with special emphasis on visceral heterotaxy (asplenia and polysplenia syndromes) In: Fyler D, editor. Nadas’ Pediatric Cardiology. Philadelphia, PA: Hanley & Belfus Inc. Mosby; 1992. pp. 589–608. [Google Scholar]
- 13.Bartram U, Wirbelauer J, Speer CP. Heterotaxy syndrome – asplenia and polysplenia as indicators of visceral malposition and complex congenital heart disease. Biol Neonate. 2005;88:278–290. doi: 10.1159/000087625. [DOI] [PubMed] [Google Scholar]
- 14.Shiraishi I, Ichikawa H. Human heterotaxy syndrome - from molecular genetics to clinical features, management, and prognosis. Circ J. 2012;76:2066–2075. doi: 10.1253/circj.cj-12-0957. [DOI] [PubMed] [Google Scholar]
- 15.Peoples WM, Moller JH, Edwards JE. Polysplenia: a review of 146 cases. Pediatr Cardiol. 1983;4:129–137. doi: 10.1007/BF02076338. [DOI] [PubMed] [Google Scholar]
- 16.Arciniegas JG, Soto B, Coghlan HC, Bargeron LM., Jr Congenital heart malformations: sequential angiographic analysis. AJR Am J Roentgenol. 1981;137:673–681. doi: 10.2214/ajr.137.4.673. [DOI] [PubMed] [Google Scholar]
- 17.Shinebourne EA, Macartney FJ, Anderson RH. Sequential chamber localization–logical approach to diagnosis in congenital heart disease. Br Heart J. 1976;38:327–340. doi: 10.1136/hrt.38.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Praagh R. The segmental approach to diagnosis in congenital heart disease. In: Bergsma D, editor. Birth Defects: Original Article Series. Vol. 3. Baltimore, MD: Williams and Wilkins; 1972. pp. 4–23. [Google Scholar]
- 19.Lapierre C, Déry J, Guérin R, Viremouneix L, Dubois J, Garel L. Segmental approach to imaging of congenital heart disease. Radiographics. 2010;30:397–411. doi: 10.1148/rg.302095112. [DOI] [PubMed] [Google Scholar]