Abstract
Objectives
To determine whether a cognitive intervention delivered by lay health educators (LHEs) in senior centers was effective in improving cognition in obese older adults.
Methods
This cluster randomized trial was conducted in 16 senior centers from which 228 senior adults were recruited. The centers were randomized to either the cognitive intervention or a control, weight-loss intervention. The primary outcome variable, cognitive function, was measured using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS).
Results
Analyses of RBANS indices as continuous variables did not indicate significant differences between arms. However, after adjusting for baseline delayed memory, gender, and baseline body mass index, seniors in the cognitive intervention arm had a 2.7 times higher odds of a reliable improvement (clinically significant) in delayed memory from baseline as compared to those in the control intervention (95% CI, 1.3-5.6, p=.011). The intervention effect was not significant for the proportion showing reliable improvement in immediate memory or in attention. Attendance at the 12-session program was high with an average of 83% (67-92%) sessions attended and 87% of participants in the cognitive arm indicating they would recommend the program.
Discussion
Cognitive interventions can be effectively delivered in the community by LHEs.
Keywords: Memory training, volunteer delivered program, senior centers
Objectives
With the increasing number of elderly individuals in the US (Administration on Aging, 2005) the numbers with cognitive impairment and dementia are also expected to increase from the current 5 million to 15 million by 2050 (Alzheimer's Association, 2012). Cognitive decline, even among those without dementia, is associated with risk for functional decline in activities of daily living (Stuck et al., 1999) and increased health care costs (Plassman et al., 2008). In addition, concerns about cognitive abilities are common among older adults (SeniorJournal.com, 2003) and appear to be positively associated with symptoms of depression (Crane, Bogner, Brown, & Gallo, 2007) and negatively associated with quality of life (Mol et al., 2007).
In addition, there is an epidemic of obesity and growing evidence of the relationship between body mass index (BMI) and cognitive decline. (WHO, 1998). Several studies have found that being overweight or obese were independent risk factors for cognitive decline (Doruk, Naharci, Bozoglu, Isik, & Kilic, 2010; Lee et al., 2010; Naderali, Ratcliffe, & Dale, 2009; Nilsson & Nilsson, 2009). In fact, obese individuals show smaller whole brain and total gray matter volume than normal and overweight individuals (Gunstad et al., 2008).
Studies in the area of cognitive rehabilitation have demonstrated that age associated memory loss and the memory loss of dementia can be delayed with cognitive interventions (Loewenstein, Acevedo, Czaja, & Duara, 2004; Talassi et al., 2007). These studies attest to the neuroplasticity of the brain, that is, the brain's ability to change in response to aging, development or the environment (including learning). Cognitive interventions have also been used with community-dwelling adults who did not meet formal eligibility criteria for cognitive impairment, including SeniorWISE (Wisdom is Simply Exploration) (McDougall et al., 2010) ACTIVE (Advanced Cognitive Training for Independent and Vital Elderly) (Ball et al., 2002) and IMPACT (Improvement in Memory with Plasticity-based Adaptive Cognitive Training (Smith et al., 2009). These interventions have been found to be efficacious in improving various objective measures of cognition (Ball et al., 2002; McDougall et al., 2010; Smith et al., 2009) and memory complaints (McDougall, 2002), thus providing evidence that meaningful improvements in cognitive performance can be achieved.
Senior centers are a practical venue for the translation of evidence-based cognitive interventions because they have an established participant base (Beisgen & Kraitchman, 2002) and potential for integrating evidence-based interventions into existing program infrastructures. Since senior centers are often well-integrated in the community, there may be a great potential for the implementation of interventions by lay health educators (LHEs) also referred to as community health workers, lay health advisors, and other terms (HRSA Bureau of Health Professions, 2007).
LHE-lead programs have successfully addressed chronic diseases including cardiovascular disease (Brownstein et al., 2005) and diabetes (Norris et al., 2006). However, previous evaluations of cognitive training for community-dwelling senior adults have not explored the potential of using LHEs to deliver evidence-based cognitive interventions to underserved, at-risk populations such as those living in rural areas and have not focused on obese elders. This paper reports on the delivery by LHEs of an adaptation of the SeniorWISE cognitive program with obese elders in senior centers across a rural state.
Methods
Overview
This cluster randomized trial (NCT-01377506) was conducted in senior centers across the rural state of Arkansas. The main purpose of the trial was to test the effectiveness of a weight-loss intervention with obese elders, with the evidence-based cognitive training serving as an attention control intervention. The original trial of the weight-loss intervention is described in detail in a separate publication (West et al., 2011). To participate, senior centers had to agree to be randomized. One of the challenges in conducting randomized trials in community settings is that concerns are frequently raised about randomizing participants to a control group that receives no treatment (Israel et al., 2008). To enhance community acceptability, senior centers were randomized to two different evidence-based active interventions: a cognitive intervention or a weight-loss intervention (the control arm for the cognitive intervention). The interventions were matched in structure and contact time. However, the weight-loss intervention made no mention of cognitive functioning and provided none of the cognitive improvement strategies offered in the cognitive intervention. This manuscript reports the effectiveness of the cognitive intervention with the weight-loss intervention serving as the control. The program was given the title of Counseling Older Adults in Cognition and Health Eating Strategies (COACHES) to reflect the fact that participants could be assigned to either intervention. This title also reflected that the LHEs served as “coaches” to the participants. All study procedures followed a written protocol, and all participants provided written consent. The study was approved by the University of Arkansas for Medical Sciences Institutional Review Board (IRB).
Senior Centers
Senior centers were recruited by mail, phone and personal contact by study investigators at meetings attended by center administrators, and through referral by a community advisory board. In addition to being willing to accept randomization, senior centers were asked to identify two to three individuals (i.e., community volunteers or employees from the senior center) who would be willing to be trained and serve as LHEs to implement the program. Senior centers also had to be willing to provide space for the groups to meet and space for private data collection visits with research staff. Senior centers were not paid for participation; however all intervention materials were provided. A total of 16 senior centers were recruited and randomized between June 2008 and February 2010.
Senior Adult Participants
Participant recruitment procedures were identical for all senior centers. To be eligible to participate, senior adults were required to be non-institutionalized, 60 years of age or older, able to walk for exercise, and obese (BMI ≥ 30). The age criterion was chosen because this is the age at which senior adults are eligible to attend senior centers. The BMI inclusion and exercise criteria were included because the purpose of the funded study (West et al., 2011) was to test a weight- loss intervention for obese older adults. Senior adults had to have a Mini Mental Status Exam score ≥ 23 (Folstein, Folstein, & McHugh, 1975) and be available to participate for the duration of the study. Exclusion criteria also included self-report of heart attack or stroke in the previous 6 months, significant recent weight loss or concurrent weight- loss treatment, pharmacotherapy for memory complaints, self-report of a health condition likely to limit lifespan or prevent participation in a weight-loss program, or other conditions that might compromise full program participation. Recruitment efforts were led by the LHEs from the senior centers, supported by a recruitment toolkit provided by research staff. This toolkit included a brochure and slide presentation with an overview of the program, eligibility criteria, and program timeline. Senior adults were recruited from within the Senior Centers and from the community through public service announcements, local newsletters, churches, flyers and e-mails. Senior adults were offered small incentives upon completion of data collection (t-shirt, tote bag, etc).
The Cognitive Intervention
From the empirically validated cognitive programs available at the time the study began, we selected SeniorWISE because it had been implemented in community settings and was made available by the lead investigator at no cost to the research team.(McDougall et al., 2010). The COACHES cognitive intervention used concepts and materials from the SeniorWISE curriculum and adapted the material so that the intervention was similar in structure and length to the COACHES weight-loss intervention. Program materials were at the 5th to 7th grade reading level. The cognitive intervention consisted of 12 weekly group sessions of approximately 1 hour each completed over a 3-4 month period.
Using a standardized administration protocol, LHEs taught participants basic information about how the brain functions, how memory processes operate, how aging and other factors affect these processes, and how to enhance memory functioning. Table 1 lists the topics for each of the 12 sessions. Each group session presented a selected cognitive strategy in an interactive lecture and discussion format, provided opportunities for participants to practice the strategy in the session through worksheets and group exercises, and provided a written homework assignment that encouraged independent practice. Participants were encouraged to discover through their homework assignment which strategies worked best for them in the specific situations they experienced as challenging and to set and work incrementally to achieve personally- important goals. The overall program was skills-based and organized around the fundamental goal of building self-confidence and providing tools to enhance cognition.
Table 1. Cognitive Intervention Program Session Topics.
| Session | Topic/Brief Description - |
|---|---|
| 1 | Welcome to the Memory Training Program - first relaxation practice, group guidelines, reasons for enrolling, program overview, common beliefs about memory and aging, preview of lesson topics. |
| 2 | Memory Processes - How the brain stores and retrieves information, sensory, short-term and long-term memory processes of the brain |
| 3 | Remembering Names—review of memory processes, remembering names by using attention and association strategies |
| 4 | Physical Factors that Affect Memory - chronic diseases, medications, hearing, vision, alcohol and fatigue |
| 5 | Social/Behavioral Factors that Affect Memory - disorganization, low expectations, mental inactivity, isolation, physical inactivity, and foods you eat. |
| 6 | Memory and Goal Setting - long-term goals and short-term objectives, key aspects of helpful goals, and appropriate rewards for meeting goals |
| 7 | Memory and Emotions - — emotional factors that have a negative impact of memory, and positive actions that can be taken to reduce the negative impacts |
| 8 | Self Talk and External Strategies - situation to use self-talk and helpful external reminders |
| 9 | Strategies for Remembering Several Items - Chunking, stories, and first letter cue strategies |
| 10 | Active Observation - developing active observation skills and their importance to memory |
| 11 | Problem Solving—learning the steps to figure out how to solve problems |
| 12 | Visualization/Retracing - creating a visual image in your mind |
Lack of confidence in memory ability and anxiety about memory loss can interfere with memory performance (Payne et al., 2012). Therefore, in addition to traditional memory enhancement strategies, the cognitive intervention, like the SeniorWISE program, provided instruction to participants in relaxation methods to reduce anxiety as well as exercises to enhance participants' confidence in their ability to remember, by giving them opportunities to experience success. Each session began with a relaxation exercise. A total of 12 different relaxation strategies were introduced and rehearsed during the sessions. Homework included practice of the relaxation technique during the week.
Training of Cognitive LHEs
Individuals recruited to be LHEs received initial training of approximately 32 hours of in-person interactive seminars offered at the senior centers to certify LHEs in the intervention to which their center had been randomized. Training was provided by project staff with degrees in psychology, social work or public health with specialty area training in health behavior and health education and a mean of 8 years of training and experience in conducting behavior change interventions. These project staff were trained and supervised by doctoral level investigators with substantial clinical and research expertise in clinical psychology, neuropsychology, dementia and memory disorders.
Initial training for the cognitive intervention included the rationale for the intervention, an overview of the COACHES study, review of the SeniorWISE program and its adaption for COACHES, methods for ensuring confidentiality in research, review of the 12 weekly sessions, and review of handouts and homework materials for the sessions. The training was skills-based; LHEs were provided opportunities to role play leading groups and practice cognitive improvement strategies, including relaxation techniques and homework assignments. Strategies for delivering interventions and managing groups, as well as ways to provide constructive, reinforcing feedback on homework assignments, were also included. Finally, during training, LHEs were also required to complete the institutional online human subjects' research certification training.
LHEs received ongoing training in weekly phone calls following each intervention session which sought to reinforce the knowledge and skills gained during the initial training and address unanticipated problems or questions experienced by the LHEs in delivery of the intervention. In weekly phone sessions between the LHEs from a center and a research staff member designated as that center's resource person, each session was reviewed to identify and resolve any issues or questions that arose in that session. LHEs were also invited to contact their assigned resource person on an “as needed” basis. LHEs were observed conducting an intervention sessions to assure intervention fidelity and provide constructive feedback using an Observation Checklist. All LHEs' performance was satisfactory and similar across centers.
Measures
Demographic characteristics were obtained using a self-report questionnaire at baseline. All other assessments were conducted at baseline and post-treatment, unless otherwise noted. All measures were administered by research assistants trained by a neuropsychologist. The Mini Mental Status Exam (Folstein et al., 1975) is a quick and simple way to quantify cognitive function, and was used to screen participants for cognitive loss at baseline only. Body weight was measured in street clothes with shoes removed using a calibrated digital scale (Tanita BWB 800). Height was measured using a stadiometer (Seca Corporation, Hanover, MD). Body mass index was calculated as weight (kg)/ height (m2).
The primary outcome variable, cognitive function, was measured using the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph, Tierney, Mohr, & Chase, 1998). This measure was selected because it is a brief (approximately 30 minutes) yet broad measure of cognitive and memory functioning, has available alternate forms for repeat administration, and can be compared to other similar trials. The RBANS comprises 12 subscales and 5 indices: Immediate Memory, Visuospatial/Construction, Language, Attention, and Delayed Memory. RBANS scores range from 40 to 160 and have a mean of 100 with a standard deviation (SD) of 15. Normative data are based on age and thus provide a standardized score of cognitive function relative to other individuals of a similar age. There are two parallel forms of the RBANS which allow for repeat assessment over short periods of time with minimization of practice effects (Randolph et al., 1998). The RBANS was scored by a blinded licensed neuropsychologist.
In the present study, the RBANS Immediate Memory, Delayed Memory and Attention indices were the outcomes of interest since they most closely parallel the cognitive constructs targeted in the intervention. The Immediate Memory index includes a verbal list learning task and a contextual (i.e., story) learning task. The Delayed Memory index includes a delayed recall challenge for the list learning task with associated recognition, delayed recall for the contextual (i.e., story) task, and delayed recall of a geometric figure previously copied. The Attention index is composed of auditory attention (i.e., digit span) as well as a visuomotor coding task. Achieving at least a 10 point increase in RBANS Delayed Memory or Attention or a 9 point increase in RBANS Immediate Memory was considered a clinically significant improvement as defined by statistically reliable change for a given participant as calculated from a 90% confidence limit for improvement using the standard error of measurement (SEM) (Jacobson & Truax, 1991). The average reported SEM for our participants' age range was 5.3 for immediate memory, 6.0 for delayed memory, and 5.8 for attention (Randolph et al., 1998).
Measures of treatment receipt or process measures included: 1) senior adult attendance at group sessions, recorded by LHEs; 2) submission of homework as noted on intervention session process logs which were faxed to the research team following each session; 3) physical activity estimated by the CHAMPS Physical Activity Questionnaire for Older Adults (Stewart et al., 2001); and 4) a 29-item COACHES investigator-developed Behavioral Inventory. Twelve items on this latter measure were related to the strategies recommended in the cognitive intervention (e.g., “Did new activities to keep my mind sharp”), 15 items were related to the control intervention (e.g., “Weighed myself daily”), and 2 items were relevant to both programs (e.g., “Requested support for my health behaviors”). Participants rated how well each of the statements on a 5-point scale described their behavior ranging from not-at-all to definitely. Responses on the 12 memory strategies items were viewed as a measure of adherence to treatment recommendations; therefore a memory strategies score was computed using the 12 memory-related items. The CHAMPS was included because physical activity was one of the recommended memory improvement strategies (Erickson et al., 2011; Kramer, Erickson, & Colcombe, 2006; Snowden et al., 2011; Tseng, Gau, & Lou, 2011). Participants also completed an evaluation of the program in which they rated its usefulness, how much they enjoyed it and if they would recommend it to a friend.
Statistical Analysis
An overall treatment arm comparison between cognitive intervention and control senior centers was conducted using the three primary RBANS indices (Immediate Memory, Delayed Memory, and Attention) in a MANOVA-like model using a general linear mixed model to analyze post-treatment change from baseline. The model included independent variables for intervention, baseline RBANS index score, and baseline RBANS index type. The model also included gender and baseline BMI since the two arms differed in these sociodemographic characteristics. The covariance structure accounted for variability between senior centers and participants due to clustering of participants within centers and RBANS indices within participants. Following this analysis, individual indices were tested separately in similar mixed models. A Bonferroni-adjustment was applied, and the intervention effect was considered significant for a given index when the p-value was less than 0.05/3, or 0.017. A similar generalized linear mixed model approach for binary outcomes was used to test the equality of proportions achieving a statistically reliable improvement in index scores i.e. 90% confidence limit for improvement based on SEM (Jacobson & Truax, 1991). Comparisons of baseline characteristics and secondary RBANS indices according to arm or according to lost to follow-up or retained were also made using a mixed model approach that accounted for clustering. A similar approach was used to model the relationships between change in cognitive variables and participant demographic characteristics and process measures (attendance, homework and the Behavioral Inventory) within the cognitive intervention arm only. All statistical analyses were performed using SAS Version 9.2 (SAS Institute, Cary, NC).
Results
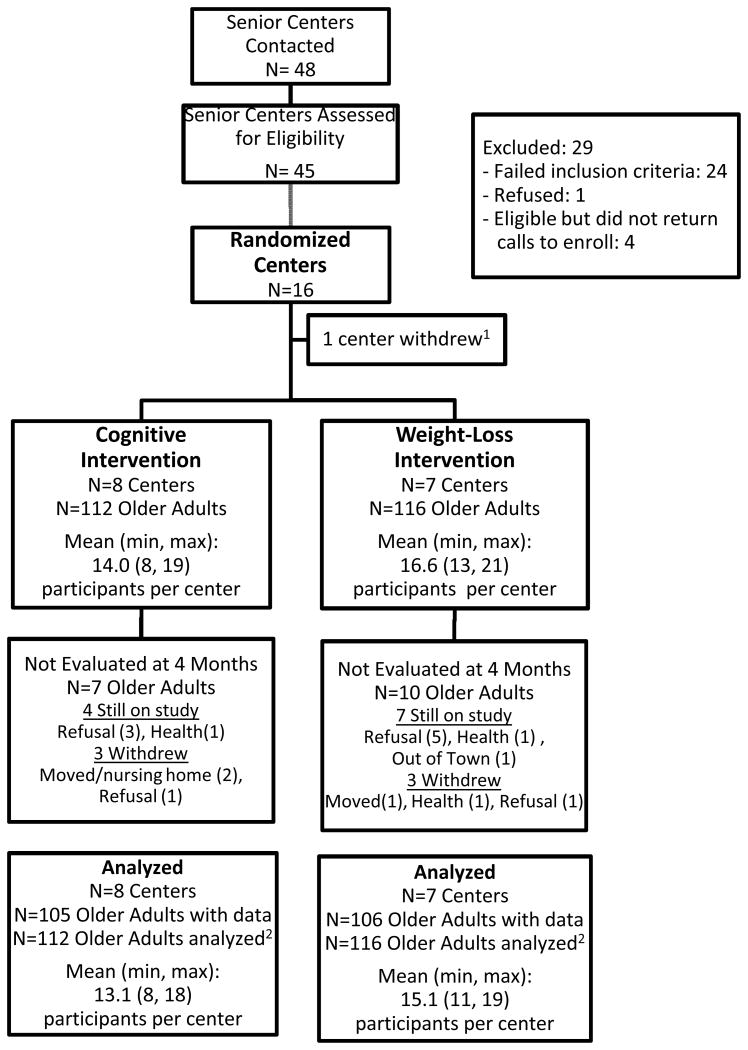
Sixteen senior centers were recruited. Participating senior centers were in counties with average populations of 105,000, with approximately 21,000 adults 60 years or older. Centers had a similar mix of services and programs including meal programs, exercise rooms, and transportation services. One center had to withdraw prior to randomization because one of the LHEs developed a significant medical condition and no other LHE could be identified to replace her. The 15 participating centers provided an average of 2.7 LHEs with 6 centers providing 2 LHEs, 8 centers providing 3, and 1 center providing 4. Sixty percent of the LHEs were senior center staff and the remainder were community volunteers. The distribution of senior center staff and community volunteers was similar in the cognitive intervention and control arms. The majority (90%) of LHEs were women and Caucasian (95%) with an average age of 59 ± 12 years. Overall attrition among the LHEs was low with all but 2 remaining as LHEs for the duration of the study; 1 moved to another community and the other withdrew due to a change in employment.
A total of 228 senior adults were enrolled in the study. On average, each senior center recruited 15.2 senior adults (SD=3.8, range = 8 - 21). The recruitment response rate did not differ between arms. The majority of participants were female (84%) and moderately to severely obese (mean BMI =36.1, SD=5.2), There were more men in the cognitive arm than in the control arm and participants had higher BMIs in the control arm (Table 2). Therefore, subsequent analyses controlled for these variables. No other differences in baseline sociodemographic variables were apparent between senior adults in the two study arms.
Table 2. Baseline Characteristics of Enrolled Senior Adults.
| Demographics | Total Sample (N=228) | Cognitive Intervention (N=116) | Weight-Loss Intervention (N=112) | P-value | |||
|---|---|---|---|---|---|---|---|
| Age, mean (sd) | 71.2 | (6.6) | 71.9 | (6.6) | 70.6 | (6.6) | 0.257 |
| Gender, % female | 84 | 77 | 91 | 0.004 | |||
| Race, % White | 92 | 92 | 92 | 0.878 | |||
| Completed High School, % | 89 | 88 | 91 | 0.741 | |||
| Married, % | 49 | 53 | 45 | 0.507 | |||
| Living with another adult in household, % | 55 | 56 | 53 | 0.790 | |||
| Children living in household with senior adult (1 or more), % | 4.4 | 7.1 | 1.7 | 0.083 | |||
| Current employment status, % | |||||||
| Currently employed (full, part, self) | 13 | 12 | 14 | 0.642 | |||
| Retired or disabled | 80 | 79 | 82 | ||||
| Other (Volunteer, homemaker) | 7 | 10 | 4 | ||||
| Weight and Activity | |||||||
| Body Mass Index, mean (sd) | 36.1 | (5.1) | 35.1 | (4.3) | 37.1 | (5.7) | 0.003 |
| CHAMPS caloric expenditure/week in all exercise-related activities (kcal) | 2953 | (2321) | 2906 | (2130) | 2999 | (2500) | .908 |
| Memory-Related | |||||||
| RBANS Intermediate Memory, mean (sd) | 96.1 | 15.5 | 96.3 | 15.1 | 95.9 | 16.0 | 0.977 |
| RBANS Delayed Memory, mean (sd) | 99.4 | 14.1 | 98.6 | 14.7 | 100.1 | 13.6 | 0.484 |
| RBANS Attention, mean (sd) | 93.3 | 15.0 | 93.8 | 15.8 | 92.8 | 14.3 | 0.922 |
| MMSE | 28.2 | (1.6) | 28.4 | (1.5) | 28.0 | (1.6) | 0.207 |
| Perceived Memory Problems Score | 12.0 | (3.3) | 12.7 | (3.2) | 11.3 | (3.2) | 0.002 |
A total of 112 participants were enrolled in senior centers randomized to the cognitive intervention. Their mean age was 71.9 (SD=6.6), years; 76.8% were female and 92% were White. The majority had a high school education or higher (88.4%) and were retired (78.6%). Almost all (91%) had attended the senior center before enrolling in the cognitive intervention.
Baseline cognitive function, as measured by the RBANS indices of interest and the MMSE, did not significantly differ by study arm (Table 2). Mean (SD) baseline RBANS scores for all participants were 96.1 (15.5) for Immediate Memory, 99.4 (14.1) for Delayed Memory, and 93.3 (15.0) for Attention, indicating that our sample was comparable to a normative sample in Delayed Memory, but they scored lower on Immediate Memory and Attention than age-matched individuals in the normative sample.
There were no differences between the interventions in attendance. The cognitive participants and control participants both attended a median interquartile range (IQR) of 10 (8-11) of the 12 sessions or 83% (67-92%) of the sessions offered. Seventy-six percent of seniors in the cognitive intervention completed 50% or more of the 10 possible homework assignments.
Assessments were conducted with 211 (93%) participants at follow-up, i.e. at the end of the 12-session program (cognitive arm n=105, 93%; control arm n=106, 91%) (Figure 1). Participants in the cognitive arm were no more likely to be missing from the follow-up assessment than controls. The baseline characteristics of those who provided follow-up data were similar to those who did not with respect to age (mean, 71 vs. 70 years), gender (84% vs. 88% women), baseline BMI (mean, 36.0 vs. 36.7), and level of education (89% vs. 94% completed high school), respectively. There were no significant differences in baseline RBANS according to those who had follow-up data and those who did not (supporting the assumption of data missing at random that was inherent in our analyses), although there is a trend for lower immediate memory for those who did not provide follow-up data. Additionally, the lack of significant difference on baseline characteristics between those who failed to return and those who did return supported the assumption of missing at random that was inherent in our analyses.
Figure 1. Study Flow Diagram.
Cognitive Improvement
Analyses of RBANS indices as continuous variables did not indicate significant differences between arms (overall test of differences across the three continuous RBANS indices (p = 0.59)). Averaging over all three RBANS indices, there was a significant improvement in the cognitive arm of 4.3 points (95% CI, 2.0-6.6, p=0.002) and a significant improvement in the control arm of 3.4 points (95% CI, 1.0-5.9, p=0.011). In individual mixed models, participants in the cognitive arm did not show significantly more improvement from baseline to 4 months in the RBANS Immediate Memory index (3.7, 95% CI, 0.9-6.5) than those in the control arm (2.5, 95% CI, -0.5-5.6) after adjusting for baseline Immediate Memory, gender, and BMI, ((p=0.50). Also, the improvement in RBANS Delayed Memory index did not differ significantly for seniors in the cognitive arm (2.7, 95% CI, 0.4-5.4) as compared to the control arm (2.2, 95% CI -0.7-5.1) (p=0.78). Likewise, the improvement in RBANS Attention index did not differ significantly for those in cognitive arm (4.3, 95% CI, 1.4-7.2) as compared to the control arm (3.2, 95% CI, -0.1-6.4) (p=0.55). Both arms showed declines in the Visuospatial and Language indices, which were secondary endpoints not expected to be impacted by the intervention, and there were no significant group differences (p = 0.77 and p = 0.98, respectively, see Table 3). The random center effect was small and not significantly different from 0 for any of the RBANS indices, indicating that there was very little center-to-center variability.
Table 3. Improvement from Baseline According to Arm.
| RBANS Index | Arm | Baseline Mean (SD) | Improvement at 4 months Mean (SD) | Model-Based 4-Month Change from Baseline Mean (95% CI) | P-value* |
|---|---|---|---|---|---|
| Primary Endpoints | |||||
| Immediate Memory | Memory | 96.3 (15.1) | 5.2 (12.6) | 3.7 (0.9-6.5) | 0.012 |
| Weight Loss | 95.9 (16.0) | 4.4 (13.2) | 2.5 (-0.5-5.6) | 0.101 | |
| Delayed Memory | Memory | 98.6 (14.7) | 3.9 (11.3) | 2.7 (0.0-5.4) | 0.047 |
| Weight Loss | 100.1 (13.6) | 2.7 (10.4) | 2.2 (-0.7-5.1) | 0.122 | |
| Attention | Memory | 93.8 (15.8) | 4.3 (11.2) | 4.3 (1.4-7.2) | 0.007 |
| Weight Loss | 92.8 (14.3) | 3.1 (11.8) | 3.2 (-0.1-6.4) | 0.054 | |
| Secondary Endpoints | |||||
| Visuospatial | Memory | 99.6 (17.8) | -4.2 (16.0) | -3.1 (-6.9-0.7) | 0.104 |
| Weight Loss | 97.3 (18.6) | -4.3 (15.7) | -3.8 (-8.0-0.4) | 0.070 | |
| Language | Memory | 97.3 (11.4) | -1.5 (11.5) | -0.6 (-2.5-1.4) | 0.561 |
| Weight Loss | 96.0 (11.4) | -1.6 (11.7) | -0.5 (-2.7-1.7) | 0.627 |
Testing the hypothesis that the improvement in RBANS subscale was significantly different from 0 within each arm after adjusting for baseline RBANS subscale, gender, and baseline BMI in a mixed model ANOVA.
Note: At 4 months N=105 for memory, n=106 for weight loss.
Analyses also examined whether changes in RBANS Immediate Memory, Delayed Memory, and Attention indices from baseline to post-training were of a clinically-significant magnitude (binary outcome); specifically, did RBANS scores increase by at least 10 points for Delayed Memory or Attention or 9 points for Immediate Memory. Among senior adults in the cognitive arm, 35% (37/105) demonstrated a clinically significant improvement in Delayed Memory as compared to 19% (20/106) of those in the control arm. After adjusting for baseline Delayed Memory, gender and baseline BMI, seniors in the cognitive arm had a 2.7 times higher odds of a reliable improvement in Delayed Memory from baseline as compared to those in the control arm (95% CI, 1.3-5.6, p=0.011). The intervention effect was not significant for the proportion showing reliable improvement in Intermediate Memory (40% vs. 46%) and Attention (33% vs. 27%) for cognitive vs. control arms, respectively.
Baseline demographic characteristics were examined to determine what factors might be associated with change in RBANS scores in the cognitive arm. Age, gender, marital status (married vs. unmarried), education (high school vs. <high school), employment status (employed vs. retired/disabled vs. other), and baseline BMI were all considered. None of these factors was associated with a change in RBANS scores, except for gender with Immediate Memory. Female seniors improved by 5.7 points on this subscale as compared to -0.1 points for males (p=0.048).
There were no significant associations of homework or attendance (continuous or binary) with any of the 3 primary indices. Pearson's correlation coefficients for Immediate Memory, Delayed Memory, and Attention were respectively -0.06, -0.01, and -0.06 with homework and were -0.04, -0.09, and -0.09 with attendance. Spearman's correlation coefficients for Immediate Memory, Delayed Memory, and Attention were respectively -0.01, -0.06, and -0.01 with homework and were 0.04, -0.12, and 0 with attendance.
Multivariate models were used to examine the associations of attendance (50% or more vs. less), change in CHAMPS, and the 12-item Behavioral Inventory score with change in RBANS scores in the cognitive arm. Homework (50% or more vs. less) was not included since there was 91% agreement between homework and attendance. No process variables were associated with a change in the RBANS indices. To further explore which, if any, of the behavioral strategies that participants reported using were associated with improved cognition, associations between the 12 individual items on the Behavior Inventory and the RBANS indices were examined. Three of the memory-related strategies, were associated with improved Delayed Memory. Specifically, higher self-reported utilization of stress management/relaxation (b=1.9, p=0.026), use of visualization and mnemonic strategies (b=1.7, p=0.033), and name strategies (b=1.7, p=0.047) were each significantly correlated with improved Delayed Memory.
In addition, as indicated in the Behavioral Inventory, more participants in the cognitive arm engaged in stress management/relaxation (p<0.001) and visualization and mnemonic strategies than those in the control arm (p<0.001); and more participants in the cognitive arm reported that they “reduced worry about memory functioning” than did those in the control arm (p <0.001).
Seventy percent of participants rated the program as extremely useful, 82% rated it as extremely enjoyable and 87% said they would recommend it to a friend. There were no differences across centers in the proportion rating the program favorably.
Discussion
Translation research provides a much needed bridge between efficacy trials and public health practice (Narayan et al., 2000) and a call has been made for its broader implementation (Selker, 2010). With the aging of America and increasing burdens of rising obesity and declining health (Centers for Disease Control and Prevention & The Merck Company Foundation, 2007), translation of evidence-based behavioral interventions is critical to augment traditional medical care paradigms with health promotion activities in community settings (Beilenson, 2005; Rohrbach, Grana, Sussman, & Valente, 2006) particularly in communities with substantial representation of older adults. Thus, data on the translation of previously tested interventions by LHEs is important. The current study has demonstrated that community-based LHEs can successfully deliver a well-received cognitive intervention to older adults attending senior centers. The large majority of enrollees attended most of the intervention sessions, indicating the level of interest in the topic area and the acceptability of the intervention approach. The fact that more participants in the cognitive arm reported reduced worry about memory functioning indicates that the cognitive intervention was effective in addressing perceptions of the targeted behaviors despite the lack of significant between-group differences in RBANS scores. However, these older adults in the cognitive arm achieved a clinically significant improvement in Delayed Memory in 4 months. This is an exciting finding and suggests the potential for some protection from future cognitive risk (Ballard, Khan, Clack, & Corbett, 2011). A recent study (Hampstead, Stringer, Stilla, Giddens, & Sathian, 2012) demonstrated via functional neuroimaging that cognitive training through use of mnemonic strategies resulted in hippocampal changes, suggesting that such cognitive strategies can successfully impact memory functioning per se.
Since participation in the cognitive intervention was limited to obese individuals because of the eligibility requirements of the initial trial, the present findings can only be generalized to obese elders. However, obese older adults are at high risk for cognitive decline and obesity is likely to be more normative in future cohorts of elders. Moreover, since the cognitive intervention was not designed specifically for obese older adults, there is every reason to think it would be appropriate for elders across the weight spectrum, although further evaluation would be required to determine whether a similar magnitude of improvement is produced among leaner senior adults.
It is difficult to compare the outcomes from the COACHES cognitive intervention with other cognitive training intervention programs for community-dwelling elders due to different measurement approaches used in the different studies; however, some common metrics were used and can offer points of comparison. For example, the age and MMSE scores of participants in the COACHES cognitive intervention arm were similar to those of participants in other studies of cognitive improvement programs for community-dwelling elders including SeniorWISE, ACTIVE and IMPACT. Further, the memory training group in SeniorWISE reported a greater reduction in memory complaints than did controls (McDougall et al., 2010). Similarly, in the COACHES study participants in the cognitive arm reported reduced worry about memory functioning relative to individuals in the control arm. In the ACTIVE trial, 26% of participants showed reliable improvement on memory measures (Ball et al., 2002), defined as performance on a measure that exceeded baseline performance by 1 SEM (Dudek, 1979). Using the same metric, 50% of the COACHES cognitive participants showed reliable improvement on Immediate Memory, 45% on Delayed Memory, 45% on Attention, and 43% on these three indices combined. IMPACT used four RBANS indices and found an average of 3.9 point improvement (range = 2.7-5.1) across the indices in the intervention group. This is similar to the average 4.3 point improvement for the three RBANS indices in the COACHES cognitive intervention. Although we did not find significant between- group differences with two very different but active behavioral interventions, the fact that over a third of our participants achieved a reliable improvement in all of the primary outcome measures indicates the importance of reporting both statistical and clinical significance.
That we did not observe statistically significant between-group differences in RBANS indices may indicate that the intervention dose was insufficient and the intervention needs to be lengthened. The ideal intensity and duration of cognitive interventions to produce targeted cognitive improvements have yet to be determined and need further consideration. Dissemination of health promotion programs into real world settings requires attention to the burden on participants and therefore program design must balance treatment duration with desired impact. A 12-hour intervention may be too brief to see improvements across a broader range of cognitive functioning which includes immediate memory and attention in addition to delayed memory. There is also the possibility that although participants did not meet formal eligibility criteria for cognitive impairment as assessed by the MMSE, they could still have had some cognitive impairment and therefore been less likely to benefit from an intervention that focused primarily on memory, as was found in the ACTIVE trial (Unverzagt et al., 2007). A further possibility is that since the participants were obese, they could have been less responsive to the intervention because of the loss of cognitive reserve from smaller brain volume (Gunstad et al., 2008).
Moreover, it is also possible that the weight-loss control arm participants had improved cognition resulting from weight loss and associated lifestyle changes (West et al., 2011) thus lessening the ability to detect differences between the two arms. Although a higher proportion of seniors showed clinically significant improvement in the cognitive arm, significant improvement in continuous RBANS scores were not seen. This inconsistency may be reflective of seniors in the control arm improving somewhat in their average scores, but not enough to cross the higher threshold of clinically-significant change in large enough numbers to match the experience of individuals in the cognitive intervention arm. Research published after initiation of the present study has demonstrated relationships between improved cognitive functioning and both weight loss and increased physical activity (Erickson et al., 2011; Snowden et al., 2011; Tseng et al., 2011) In light of this new research, active control conditions for future investigations of interventions to improve cognitive functioning should not include weight loss or physical activity components. Additionally, it is not known whether obese elders have brain plasticity comparable to non-obese elders. It is also recognized that the use of the RBANS as our primary measure of cognitive outcome may have had limited sensitivity to detect subtle cognitive changes in this population.
Although establishing the effectiveness of LHEs for delivery of community-based cognitive interventions provides a major step forward in the translation literature, the sample was limited with respect to ethnic diversity and the sample as a whole was fairly well educated. In addition, there remain many other questions to be examined in future studies. For example, next generation studies might benefit from including measures of depression and anxiety to capture a broader range of potential favorable outcomes associated with cognitive interventions. At least one study has shown an improvement on these dimensions among elders completing a cognitive training program (McDougall et al., 2010).
In conclusion, this was the first known study to evaluate the effectiveness of LHEs in delivering a cognitive intervention in senior centers. LHEs were recruited, trained and retained to deliver the 12-week cognitive intervention. Further, senior adults in the cognitive arm demonstrated a significantly greater likelihood of achieving a clinically-meaningful improvement in Delayed Memory than was observed in the control arm. Moreover, the treatment program also successfully trained participants in relaxation techniques and reduced worry regarding memory performance. The observed improvements in cognitive function were comparable to those reported in other cognitive intervention studies, despite the fact that participants in the current study were obese and potentially less able to benefit. The positive outcomes of the COACHES intervention indicate that LHEs can deliver an effective cognitive intervention and offer great potential for wide dissemination of evidence-based cognitive interventions in heretofore unlikely locations, particularly rural and underserved areas which may not have easy access to health care facilities and which may be markedly constrained in health care resources.
Acknowledgments
Funding: This research was supported in part by a research grant from the Centers for Disease Prevention and Control (R18 DP001145). The study was also supported by 1UL1RR029884 from the National Center for Research Resources. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Centers for Disease Prevention and Control, the National Center For Research Resources or the National Institutes of Health.
Contributor Information
Cornelia Beck, Email: beckcornelia@uams.edu, College of Medicine - Department of Geriatrics, Louise Hearne Chair in Dementia & Long-Term Care, University of Arkansas for Medical Sciences, 4301 West Markham St. #808 - Little Rock, AR 72205, Phone: 501-526-5750.
Jennifer Kleiner Fausett, Email: JSKleiner@uams.edu, College of Medicine - University of Arkansas for Medical Sciences, 4301 West Markham St. #568 - Little Rock, AR 72205, Phone: 501-526-8200.
Rebecca A. Krukowski, Email: RAKrukowski@uams.edu, College of Public Health - University of Arkansas for Medical Sciences, 4301 West Markham St. #820 - Little Rock, AR 72205, Phone: 501-526-8366.
Carol E. Cornell, Email: CCornell@uams.edu, College of Public Health - University of Arkansas for Medical Sciences, 4301 West Markham St. #820 - Little Rock, AR 72205, Phone: 501-526-6708.
T. Elaine Prewitt, Email: TPrewitt@uams.edu, College of Public Health - University of Arkansas for Medical Sciences, 4301 West Markham St. #820 - Little Rock, AR 72205, Phone: 501-526-6637.
Shelly Lensing, Email: SYLensing@uams.edu, College of Public Health - University of Arkansas for Medical Sciences, 4301 West Markham St. #781 - Little Rock, AR 72205, Phone: 501-686-8203.
Zoran Bursac, Email: BursacZoran@uams.edu, College of Public Health - University of Arkansas for Medical Sciences, 4301 West Markham St. #781 - Little Rock, AR 72205, Phone: 501-526-6723.
Holly C. Felix, Email: FelixHolly@uams.edu, College of Public Health - University of Arkansas for Medical Sciences, 4301 West Markham St. #820 - Little Rock, AR 72205, Phone: 501-526-6626.
ShaRhonda Love, Email: SJLove@uams.edu, College of Public Health - University of Arkansas for Medical Sciences, 4301 West Markham St. #820 - Little Rock, AR 72205, Phone: 501-686-5466.
Graham McDougall, Email: gmcdougall@mail.utexas.edu, School of Nursing - The University of Texas at Austin, 1700 Red River St - Austin, TX 78701-1499 Phone: 512-471-7936.
Delia Smith West, Email: WestDelia@uams.edu, College of Public Health - University of Arkansas for Medical Sciences, 4301 West Markham St. #820 - Little Rock, AR 72205 Phone: 501-526-6623.
References
- Administration on Aging. A Profile of Older Americans: 2005. 2005 http://www.aoa.gov/AoAroot/Aging_Statistics/Profile/2005/2005profile.pdf.
- Alzheimer's Association. Alzheimer's disease facts and figures. Alzheimers Dement. 2012;8(2):131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Ball K, Berch DB, Helmers KF, Jobe JB, Leveck MD, Marsiske M Vital Elderly Study, Group. Effects of cognitive training interventions with older adults: a randomized controlled trial. JAMA. 2002;288(18):2271–2281. doi: 10.1001/jama.288.18.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Khan Z, Clack H, Corbett A. Nonpharmacological treatment of Alzheimer disease. Can J Psychiatry. 2011;56(10):589–595. doi: 10.1177/070674371105601004. [DOI] [PubMed] [Google Scholar]
- Beilenson J. Diffusion of Innovations - How NCOA is Developing a Better Way to Disseminate Evidence-Based Health Programs. Innovations: The publicatons of the National Council on the Aging, Spring. 2005:1–4. [Google Scholar]
- Beisgen B, Kraitchman M. Senior Centers: Opportunities for successful Aging. 1st. New York City, NY: Springer Publishing Company; 2002. [Google Scholar]
- Brownstein J, Bone L, Dennison C, Hill M, Kim M, Levine D. Community health workers as interventionists in the prevention and control of heart disease and stroke. American Journal of Preventive Medicine. 2005;29(5):128–133. doi: 10.1016/j.amepre.2005.07.024. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention & The Merck Company Foundation. The State of Aging and Health in America 2007. Whitehouse Station, NJ.: 2007. [Google Scholar]
- Crane M, Bogner H, Brown G, Gallo J. The link between depressive symptoms, negative cognitive bias and memory complaints in older adults. Aging & Mental Health. 2007;11(6):708–715. doi: 10.1080/13607860701368497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doruk H, Naharci MI, Bozoglu E, Isik AT, Kilic S. The relationship between body mass index and incidental mild cognitive impairment, Alzheimer's disease and vascular dementia in elderly. J Nutr Health Aging. 2010;14(10):834–838. doi: 10.1007/s12603-010-0113-y. [DOI] [PubMed] [Google Scholar]
- Dudek F. Continuing misinterpretation of the standard error of measurement. Psychological Bulletin. 1979;86(2):335–337. [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108(7):3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Grieve S, Gordon E. Relationship between body mass index and brain volume in healthy adults. Int J Neurosci. 2008;118(11):1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- Hampstead BM, Stringer AY, Stilla RF, Giddens M, Sathian K. Mnemonic strategy training partially restores hippocampal activity in patients with mild cognitive impairment. Hippocampus. 2012;22(8):1652–1658. doi: 10.1002/hipo.22006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HRSA Bureau of Health Professions. Community Health Worker National Workforce Study. Washington, DC: US Department of Health and Human Services; 2007. p. 285. [Google Scholar]
- Israel B, Schulz E, Parker E, Becker A, Allen A, Guzman J. Critical Issues In Developing and Following CBPR Principles. In: Minkler M, Wallerstein N, editors. Community Based Participatory Research for Health From Process to Outcomes. Second. Jossey-Bass; 2008. pp. 47–66. [Google Scholar]
- Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol. 1991;59(1):12–19. doi: 10.1037//0022-006x.59.1.12. [DOI] [PubMed] [Google Scholar]
- Kramer AF, Erickson KI, Colcombe SJ. Exercise, cognition, and the aging brain. J Appl Physiol. 2006;101(4):1237–1242. doi: 10.1152/japplphysiol.00500.2006. [DOI] [PubMed] [Google Scholar]
- Lee Y, Back JH, Kim J, Kim SH, Na DL, Cheong HK, Kim YG. Systematic review of health behavioral risks and cognitive health in older adults. Int Psychogeriatr. 2010;22(2):174–187. doi: 10.1017/S1041610209991189. [DOI] [PubMed] [Google Scholar]
- Loewenstein D, Acevedo A, Czaja S, Duara R. Cognitive rehabilitation of mildly impaired Alzheimer disease patients on cholinesterase inhibitors. American Journal of Geriatric Psychiatry. 2004;12(4):395–402. doi: 10.1176/appi.ajgp.12.4.395. [DOI] [PubMed] [Google Scholar]
- McDougall G. Memory improvement in octogenarians. Applied Nursing Research. 2002;15(1):2–10. doi: 10.1053/apnr.2002.29518. [DOI] [PubMed] [Google Scholar]
- McDougall G, Becker H, Pituch K, Acee TW, Vaughan PW, Delville CL. The SeniorWISE study: improving everyday memory in older adults. Arch Psychiatr Nurs. 2010;24(5):291–306. doi: 10.1016/j.apnu.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mol M, Carpay M, Ramakers I, Rozendaal N, Verhey F, Jolles J. The effect of perceived forgetfulness on quality of life in older adults; A qualitative review. International Journal of Geriatric Psychiatry. 2007;22(5):393–400. doi: 10.1002/gps.1686. [DOI] [PubMed] [Google Scholar]
- Naderali EK, Ratcliffe SH, Dale MC. Obesity and Alzheimer's disease: a link between body weight and cognitive function in old age. Am J Alzheimers Dis Other Demen. 2009;24(6):445–449. doi: 10.1177/1533317509348208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayan K, Gregg E, Engelgau M, Moore B, Thompson T, Williamson D, Vinicor F. Translation research for chronic disease - The case of diabetes. Diabetes Care. 2000;23(12):1794–1798. doi: 10.2337/diacare.23.12.1794. [DOI] [PubMed] [Google Scholar]
- Nilsson LG, Nilsson E. Overweight and cognition. Scand J Psychol. 2009;50(6):660–667. doi: 10.1111/j.1467-9450.2009.00777.x. [DOI] [PubMed] [Google Scholar]
- Norris S, Chowdhury F, Van Le K, Horsley T, Brownstein J, Zhang X, Satterfield D. Effectiveness of community health workers in the care of persons with diabetes. Diabetic Medicine. 2006;23(5):544–556. doi: 10.1111/j.1464-5491.2006.01845.x. [DOI] [PubMed] [Google Scholar]
- Payne BR, Jackson JJ, Hill PL, Gao X, Roberts BW, Stine-Morrow EA. Memory self-efficacy predicts responsiveness to inductive reasoning training in older adults. J Gerontol B Psychol Sci Soc Sci. 2012;67(1):27–35. doi: 10.1093/geronb/gbr073. doi: gbr073 [pii] 10.1093/geronb/gbr073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plassman B, Langa K, Fisher G, Heeringa S, Weir D, Ofstedal M, Wallace R. Prevalence of cognitive impairment without dementia in the United States. Annals of Internal Medicine. 2008;148(6):427–434. doi: 10.7326/0003-4819-148-6-200803180-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Rohrbach LA, Grana R, Sussman S, Valente TW. Type II translation: transporting prevention interventions from research to real-world settings. Eval Health Prof. 2006;29(3):302–333. doi: 10.1177/0163278706290408. [DOI] [PubMed] [Google Scholar]
- Selker H. Beyond Translational Research from T1 to T4: Beyond “Separate but Equal” to Integration (Ti) CTS-Clinical and Translational Science. 2010;3(6):270–271. doi: 10.1111/j.1752-8062.2010.00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SeniorJournal.com. Seniors more worried about personal well-being than global threats. SeniorJournal.com. 2003 NewTechMedia.com.
- Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, Zelinski EM. A cognitive training program based on principles of brain plasticity: results from the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) study. J Am Geriatr Soc. 2009;57(4):594–603. doi: 10.1111/j.1532-5415.2008.02167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowden M, Steinman L, Mochan K, Grodstein F, Prohaska TR, Thurman DJ, Anderson LA. Effect of exercise on cognitive performance in community-dwelling older adults: review of intervention trials and recommendations for public health practice and research. J Am Geriatr Soc. 2011;59(4):704–716. doi: 10.1111/j.1532-5415.2011.03323.x. [DOI] [PubMed] [Google Scholar]
- Stewart A, Mills K, King A, Haskell W, Gillis D, Ritter P. CHAMPS physical activity questionnaire for older adults: outcomes for interventions. Medicine & Science in Sports & Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Stuck A, Walthert J, Nikolaus T, Bula C, Hohmann C, Beck J. Risk factors for functional status decline in community-living elderly people: A systematic literature review. Social Science & Medicine. 1999;48(4):445–469. doi: 10.1016/s0277-9536(98)00370-0. [DOI] [PubMed] [Google Scholar]
- Talassi E, Guerreschi M, Feriani M, Fedi V, Bianchetti A, Trabucchi M. Effectiveness of a cognitive rehabilitation program in mild dementia (MD) and mild cognitive impairment (MCI): A case control study. Archives of Gerontology and Geriatrics. 2007;44:391–399. doi: 10.1016/j.archger.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Tseng CN, Gau BS, Lou MF. The effectiveness of exercise on improving cognitive function in older people: a systematic review. J Nurs Res. 2011;19(2):119–131. doi: 10.1097/JNR.0b013e3182198837. [DOI] [PubMed] [Google Scholar]
- Unverzagt FW, Kasten L, Johnson KE, Rebok GW, Marsiske M, Koepke KM, Tennstedt SL. Effect of memory impairment on training outcomes in ACTIVE. J Int Neuropsychol Soc. 2007;13(6):953–960. doi: 10.1017/S1355617707071512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West DS, Bursac Z, Cornell CE, Felix HC, Fausett JK, Krukowski RA, Beck C. Lay Health Educators Translating an Evidence-based Weight Loss Intervention to Obese Older Adults in Rural Senior Centers. American Journal of Preventive Medicine. 2011;41(4):385–391. doi: 10.1016/j.amepre.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. The World Health Report 1998 Life in the 21st Century - A vision for all. Geneva: 1998. [Google Scholar]