Abstract
Berberine is a substituted dibenzo[a,g]quinolizin-7-ium derivative whose modest antibiotic activity is derived from its disruptive impact on the function of the essential bacterial cell division protein FtsZ. The present study reveals that the presence of a biphenyl substituent at either the 2- or 12-position of structurally-related dibenzo[a,g]quinolizin-7-ium derivatives significantly enhances antibacterial potency versus Staphylococcus aureus and Enterococcus faecalis. Studies with purified S. aureus FtsZ demonstrate that both 2- and 12-biphenyl dibenzo[a,g]quinolizin-7-ium derivatives act as enhancers of FtsZ self-polymerization.
Keywords: Antibacterial; Dibenzo[a,g]quinolizinium; FtsZ-targeting; Staphylococcus aureus; Enterococcus faecalis
Bacterial resistance to current clinical antibacterial drugs has created a critical need for new therapeutic antibacterial agents with novel targets and modes of action. Infections associated with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) represent an increasing nosocomial health concern for both patients and healthcare professionals.1,2 FtsZ is a key protein involved in bacterial cell division (cytokinesis).3,4 It is highly conserved among bacterial pathogens and, in several genetic studies, has been shown to be indispensable.5–8 Cell division in bacteria occurs at the site of formation of a cytokinetic Z-ring polymeric structure, which is comprised of FtsZ subunits.9 The essential role that FtsZ plays in bacterial cell division makes this protein a promising therapeutic target, and recent advances in the development of small molecules that target FtsZ have been the subject of several recent reviews.10–13 FtsZ-targeting antibacterial agents can exert their disruptive effects on the Z-ring by either enhancing or inhibiting FtsZ self-polymerization.14–21
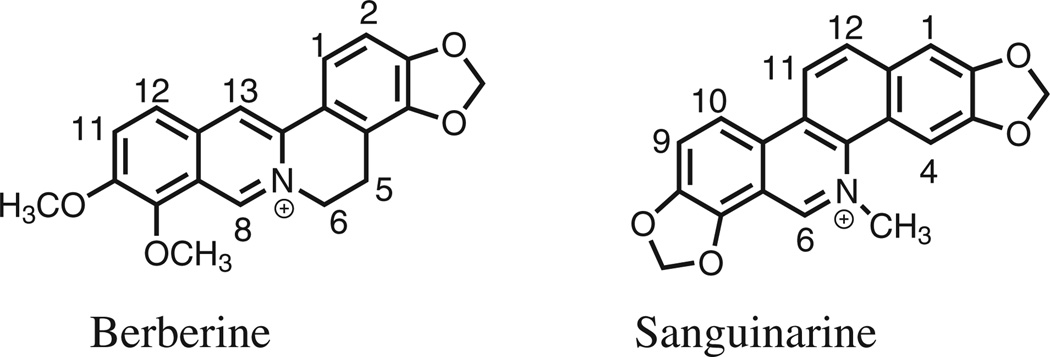
Berberine (Fig. 1) is a plant alkaloid that exhibits weak antibacterial activity, with MIC values typically on the order of 100–400 µg/mL versus Gram-positive bacteria and >500 µg/mL versus Gram-negative bacteria.22–24 Recent studies have suggested that the antibacterial activities of berberine and the structurally-related benzo[c]phenanthridine alkaloid sanguinarine are derived from the inhibitory effects of the compounds on FtsZ polymerization and Z-ring formation.22–24 We have recently demonstrated that the presence of phenyl or biphenyl substituents at the 1- or 12-position of substituted 5-methylbenzo[c]phenanthridinium derivatives structurally similar to sanguinarine enhanced relative antibacterial activity versus both S. aureus and E. faecalis (including MRSA and VRE strains). In this study, we explore the effect of aryl substituents at the 2- and the 12-position on the antistaphylo coccal and antienterococcal activities of a series of dibenzo[a,g] quinolizin-7-ium and 8-methyldibenzo[a,g]quinolizin-7-ium derivatives that are structurally-related to berberine.
Figure 1.
The structure and numbering of berberine and sanguinarine.
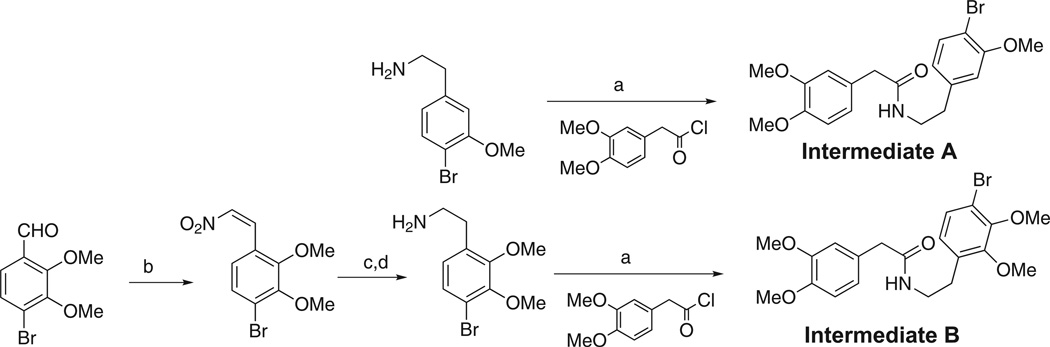
Two key intermediates were used for the preparation of the 1-substituted dibenzo[a,g]quinolizin-7-ium salts evaluated for antibacterial activity in this study, Table 1. Commercially available 4-bromo-3-methoxyphenethylamine was treated with 3, 4-dimethoxybenzoylchloride as outlined in Scheme 1 to form N- [2-[1-(4-bromo-3-methoxyphenyl)]ethyl]-3,4-dimethoxyphenylacetamide derivative, intermediate A. As outlined in Scheme 2, intermediate A was employed for the synthesis of 1–4 in Table 1. For the preparation of intermediate B, veratrole was treated with excess n-butyllithium and then reacted with bromine. The resulting 1,4-dibromo-2,3-dimethoxy-benzene was then treated with n-butyllithium and DMF to provide the intermediate 4-bromo-2,3,-dimethoxybenzaldehyde in Scheme 1. Condensation of this benzaldehyde with nitromethane provided the 2-nitroethylene intermediate, which was reduced with NaBH4 and then treated with Zn powder in acetic acid to yield 2-bromo-2,3-dimethoxyphenethylamine. Treatment of this phenethylamine with 3,4-dimethoxyphenylacetylchloride provided the N-[2-[1-(4-bromo-2, 3-dimethoxyphenyl)]ethyl]phenylacetamide derivative as intermediate B. This intermediate was used as outlined in Scheme 2 for the preparation of 6–13 in Table 1.
Table 1.
Dibenzo[a,g]quinolizin-7-ium salts synthesized with either a 2-aryl or a 12-aryl substituent
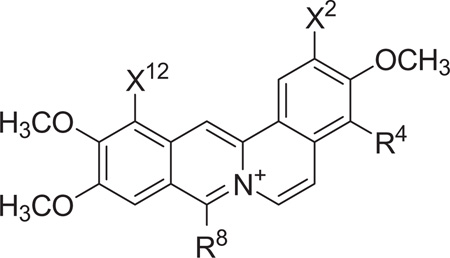 | ||||
|---|---|---|---|---|
| X2 | R4 | R8 | X12 | |
| 1 | H | H | H | |
| 2 | H | −CH3 | H | |
| 3 | H | H | H | |
| 4 | H | −CH3 | H | |
| 5 | −OCH3 | H | H | |
| 6 | −OCH3 | −CH3 | H | |
| 7 | −OCH3 | H | H | |
| 8 | −OCH3 | −CH3 | H | |
| 9 | −OCH3 | H | H | |
| 10 | −OCH3 | −CH3 | H | |
| 11 | −OCH3 | CH(CH3)2 | H | |
| 12 | 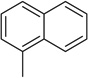 |
−OCH3 | H | H |
| 13 | 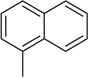 |
−OCH3 | −CH3 | H |
| 14 | H | −OCH3 | H | |
| 15 | H | −OCH3 | −CH3 | |
| 16 | −OCH3 | H | H | |
| 17 | −OCH3 | H | −CH3 | |
Scheme 1.
Preparation of intermediate A and intermediate B. Reagents and conditions: (a) 2M Na2CO3, CHCl3, 0 °C; (b) NH4OAc, CH3NO2, glacial acetic acid, 120 °C, 72%; (c) NaBH4, 1,4-dioxane/EtOH (2:1), 91%; (d) acetic acid/Zn powder, 57%.
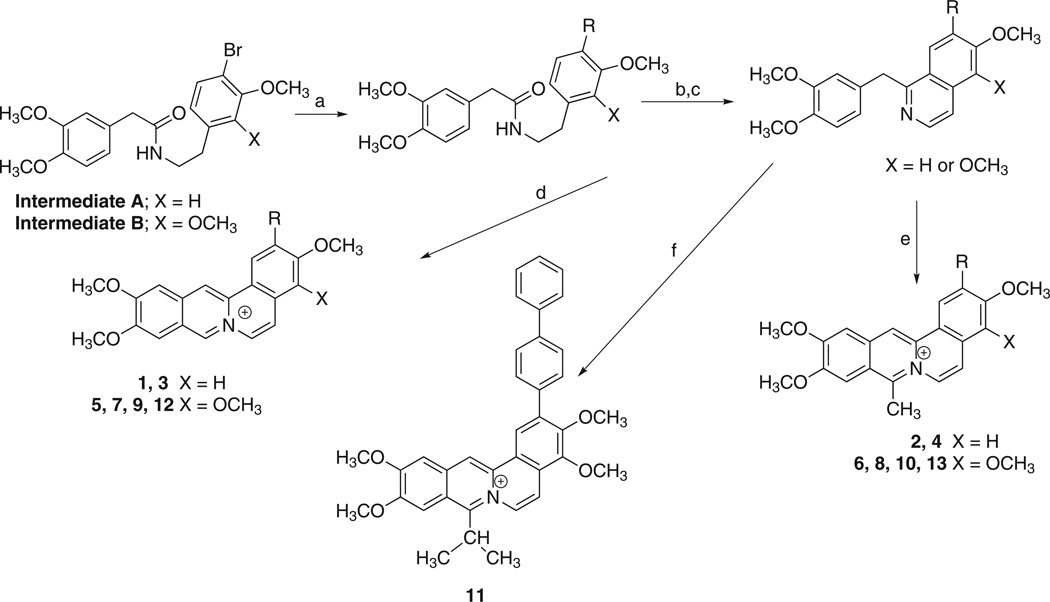
Scheme 2.
Methods used for the preparation of 2-substituted dibenzo[a,g]quinolizin-7-ium salts. Reagents and conditions: (a) R-B(OH)2, K2CO3, Pd (PPh3)4, dioxane:H2O (3:1), 100 °C; (b) POCl3, ACN, reflux, 1–2 h; (c) Pd/C, tetralin, 210–220 °C; (d) POCl3, DMF, HCl; (e) acetic anhydride, fuming H2SO4; (f) isobutyric anhydride, fuming H2SO4.
Both intermediate A and B were subject to Suzuki coupling conditions using various arylboronates (Scheme 2). In the presence of POCl3 in acetonitrile, these derivatives provided the requisite precursors for the formation of various 7-substituted 3,4-dihydro-1-(3,4-dimethoxybenzyl)isoquinolines. Oxidation in the presence of palladium on carbon in tetralin provided the aromatized 1-benzylisoquinoline derivatives. Treatment of these derivatives with POCl3 in dimethylformamide yielded the desired 2-substituted 3,4,10,11-tetramethoxydibenzo[a,g]quinolizin-7-ium chlorides. Alternatively, treatment of these 1-benzylisoquinoline derivatives with acetic anhydride in the presence of fuming (20%) H2SO4 gave 2-substituted 8-methyl-3,4,10,11-tetramethoxydibenzo[a,g]quinolizin-7-ium acetylsulfonates.
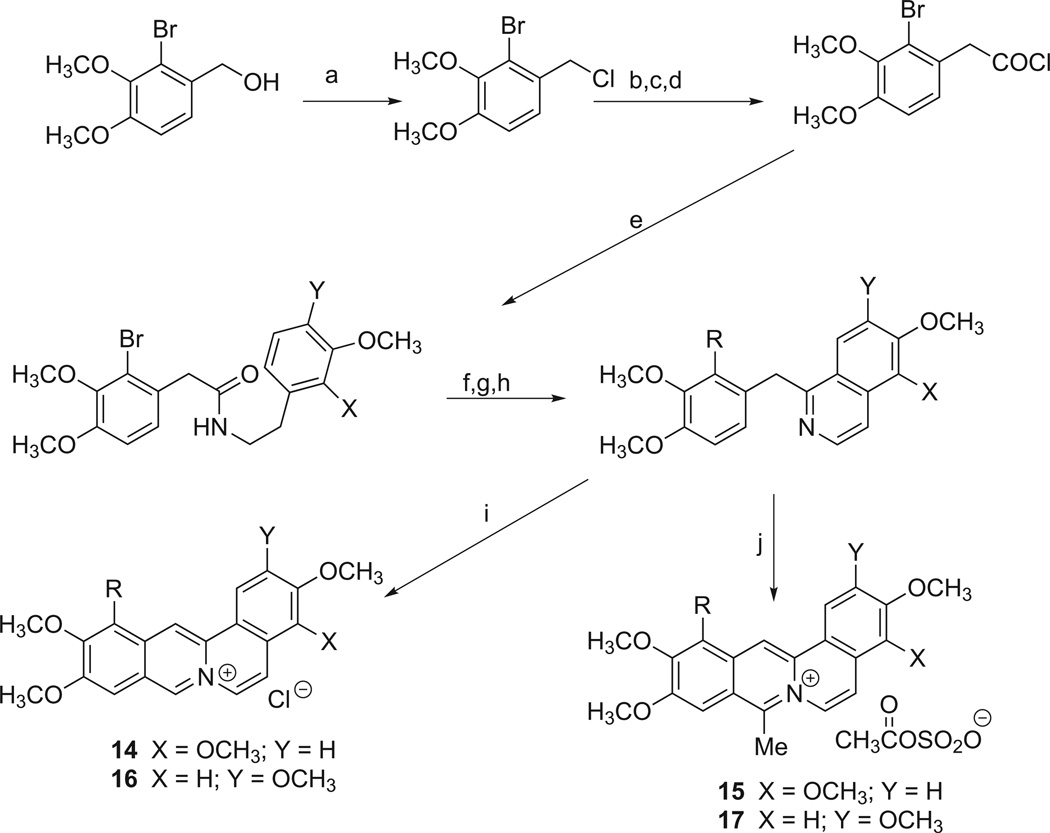
The synthesis of 12-aryl substituted dibenzo[a,g]quinolizin-7-ium salts was accomplished as outlined in Scheme 3. Treatment of either 2,3-dimethoxyphenethylamine or 3,4-dimethoxyphenethylamine with the acid chloride derivative of 2-bromo-3,4-dimethoxyphenylacetic acid provide the N-[2-[1-(2,3-dimethoxyphenyl)]- ethyl]2-bromo-3,4-dimethoxyphenylacetamide or the N-[2-[1-(3,4-dimethoxyphenyl)]ethyl]-2-bromo-3,4-dimethoxyphenylacetamide, respectively. These bromo derivatives were reacted under Suzuki coupling conditions with several arylboronates, cyclized in the presence of POCl3 in acetonitrile and oxidized to their respective 1-benzylisoquinoline derivatives. Treatment of these derivatives with POCl3 in dimethylformamide yielded the desired 12-substituted dibenzo [a,g]quinolizin-7-ium chlorides, 14 and 16. Alternatively, treatment of these 1-benzylisoquinoline derivatives with acetic anhydride in the presence of fuming (20%) H2SO4 gave the 12-substituted 8-methyldibenzo[a,g]quinolizin-7-ium acetylsulfonates, 15 and 17.
Scheme 3.
Methods used for the preparation of 12-substituted dibenzo[a,g]quinolizin-7-ium salts. Reagents and conditions: (a) 12N HCl, toluene, 88%; (b) KCN, Me2SO, 90%; (c) HOAC, H2O, conc. H2SO4, quant.; (d) (COCl)2, DCM, 2 h; (e) 2M Na2CO3, CHCl3, 0 °C; (f) R-B(OH)2, K2CO3, Pd (PPh3)4, dioxane:H2O (3:1), 100 °C; (g) POCl3, ACN, reflux, 1–2 h; (h) Pd/C, tetralin, 210–220 °C; (i) POCl3, DMF, HCl; (j) acetic anhydride, fuming H2SO4
These dibenzo[a,g]quinolizin-7-ium derivatives were evaluated for their antibacterial activity against methicillin-sensitive Staphylococcus aureus (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) as well as vancomycin-sensitive Enterococcus faecalis (VSE) and vancomycin-resistant Enterococcus faecalis (VRE). Their relative antibacterial activities are listed in Table 2. Berberine did not exhibit appreciable antibiotic activity when evaluated against the strains of S. aureus or E. faecalis used in this study. Compounds 1–4 exhibit significant antibacterial activity against MSSA. The 2-(4-toluyl) derivatives 3 and 4 are more active than the 2-phenyl derivatives 1 and 2 against both S. aureus strains and VSE. The presence or absence of an 8-methyl substituent has a modest effect on antibacterial activity among these trimethoxy derivatives, with this effect being variable and typically reflected by a two-fold difference in MIC values.
Table 2.
Antistaphylococcal and antienterococcal activities of ibenzo[a,g]quinolizin-7-ium salts with either 2- or 12-aryl substituents
| MICa (µg/mL) | ||||
|---|---|---|---|---|
|
S. aureus 8325-4 (MSSA) |
S. aureus ATCC 33591 (MRSA) |
E. faecalis ATCC 19433 (VSE) |
E. faecalis ATCC 51575 (VRE) |
|
| 1 | 8 | 32 | 32 | 64 |
| 2 | 8 | >64 | >64 | >64 |
| 3 | 2 | 8 | 16 | >64 |
| 4 | 4 | 8 | 8 | >64 |
| 5 | 4 | 8 | 16 | 32 |
| 6 | 2 | 8 | 8 | 16 |
| 7 | 4 | 4 | 4 | 8 |
| 8 | 1 | 2 | 4 | 4 |
| 9 | 2 | 2 | 8 | 4 |
| 10 | 1 | 2 | 4 | 8 |
| 11 | 0.5 | 0.5 | 2 | 2 |
| 12 | 4 | 2 | 8 | 8 |
| 13 | 1 | 2 | 4 | 8 |
| 14 | 2 | 8 | 8 | 16 |
| 15 | 1 | 4 | 16 | 16 |
| 16 | 2 | 4 | 8 | 32 |
| 17 | 1 | 4 | 4 | 32 |
| Berberine | >64 | >64 | >64 | >64 |
| Oxacillin | 0.06 | >64 | 8 | >64 |
| Vancomycin | 1 | 2 | 1 | >64 |
| Erythromycin | 0.13 | >64 | 1 | >64 |
| Tetracycline | 0.06 | 64 | 0.5 | >64 |
| Clindamycin | 0.03 | >64 | 2 | >64 |
Minimum inhibitory concentration (MIC) assays were conducted in accordance with Clinical Laboratory Standards Institute (CLSI) guidelines for broth microdilution.25 MIC is defined as the lowest compound concentration at which bacterial growth is ≥90% inhibited.
Antibacterial activities of the 2-aryl 3,4,10,11-tetramethoxydibenzo[a,g]quinolizin-7-ium derivatives 5–13 were also consistently associated with an increase in antibacterial activity against both S. aureus strains relative to 1 and 2. The effect of an 8-methyl substituent among these tetramethoxy derivatives on antibacterial activity is modest and, in general, tends toward only a slightly greater antibacterial effect. Only in the case of 9 when evaluated against VRE is a slightly greater antibiotic activity observed relative to its 8-methyl derivative, 10.
There was a notable difference between the 3,10,11-trimethoxy- and 3,4,10,11-tetramethoxydibenzo[a,g]quinolizin-7-ium derivatives with regard to activity against E. faecalis. None of the trimethoxy derivatives has an MIC value less than 64 µg/mL against the vancomycin-resistant strain. Interestingly, several of the 2-aryl-3,4,10,11-tetramethoxy derivatives exhibited enhanced activity toward both strains of E. faecalis. The 8-isopropyl derivative, 11, was the most potent dibenzo[a,g]quinolizin-7-ium analog evaluated in both strains of S. aureus and E. faecalis.
Comparison of the antibiotic activities of 9 and 10 with the 1-naphthyl derivatives (12,13) and the 12-biphenyl derivatives(14,15) reveals that these compounds exhibit similar activity. The relative antibiotic activities of 14 and 15 tend to be somewhat less than 9 and 10 against the resistant S. aureus and E. faecalis strains. A similar trend is observed in comparing the antibacterial activities of 12-biphenyl-2,3,10,11-trimethoxydibenzo[a,g]quinolizin-7-ium derivatives 16 and 17 with the 2-biphenyl-3,4,10,11-tetramethoxydibenzo[a,g]quinolizin-7-ium derivatives 9 and 10.
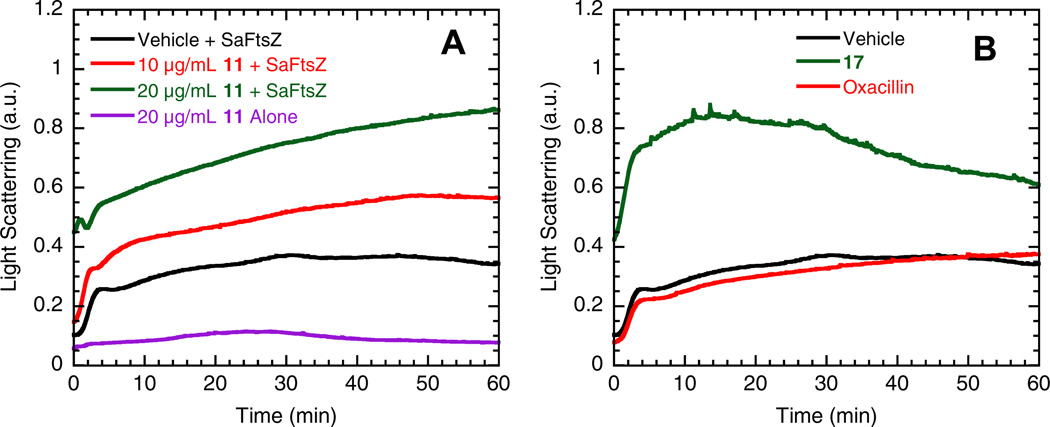
A 90°-angle light scattering assay was used to monitor the impact, if any, of select dibenzo[a,g]quinolizin-7-ium compounds on the dynamics of self-polymerization by purified S. aureus FtsZ (SaFtsZ). In this assay, FtsZ polymerization is detected in solution by a time-dependent increase in light scattering. As an illustrative example for a dibenzo[a,g]quinolizin-7-ium compound with an aryl (biphenyl) substitution at the 2-position, Figure 2A shows the time-dependent light scattering profiles of SaFtsZ in the absence and presence of 11 at concentrations of 10 µg/mL and 20 µg/mL. The time-dependent increase in light scattering observed in the presence of vehicle only is reflective of FtsZ self-polymerization in the absence of test compound. Significantly, the increase in light scattering observed in the presence of 11 is greater than that in the absence of compound, with the extent of this light scattering enhancement being dependent on compound concentration. The lack of light scattering change associated with 20 µg/mL 11 in the absence of FtsZ (Fig. 2A) confirms that the enhanced light scattering induced by 11 in the presence of FtsZ reflects a corresponding stimulation of FtsZ self-polymerization and not simply compound aggregation or precipitation.
Figure 2.
Impact of select dibenzo[a,g]quinolizin-7-ium compounds on the self-polymerization of purified S. aureus FtsZ (SaFtsZ), as determined by monitoring time-dependent changes in 90°-angle light scattering. (A) Light scattering profiles of SaFtsZ (8.3 µM) in the presence of DMSO vehicle (black) or 11 at a concentration of either 10 (red) or 20 (green) µg/mL. For comparative purposes, the corresponding light scattering profile of 20 µg/mL 11 alone (violet) is also included as a no-protein control. (B) Light scattering profiles of SaFtsZ (8.3 µM) in the presence of DMSO vehicle (black) or 20 µg/mL of either 17 (green) or the comparator antibiotic oxacillin (red). Experiments were conducted at 25 °C in solution containing 50 mM Tris•HCl (pH 7.4), 50 mM KCl, 2 mM magnesium acetate, 1 mM CaCl2, and 1 mM GTP. GTP was combined with vehicle, test compound, or control drug, and the reactions were initiated by addition of the protein. The reactions (150 µL total volume) were continuously monitored in quartz ultramicro cells (pathlength of 10 mm in the excitation direction and 2 mm in the emission direction) using an AVIV ATF 105 spectrofluorimeter, with the excitation and emission wavelengths set at 470 nm (at which the dibenzo[a,g]quinolizin-7-ium compounds absorb little or no light) and the corresponding excitation and emission bandwidths set at 2 nm. Readings were acquired in 5-second intervals with a corresponding time constant of 1 second.
As an illustrative example for the impact on SaFtsZ polymerization of a dibenzo[a,g]quinolizin-7-ium compound with an aryl (biphenyl) substitution at the 12-position, Figure 2B shows the time-dependent light scattering profiles of SaFtsZ in the absence and presence of 17 at a concentration of 20 µg/mL. Oxacillin is also included (at 20 µg/mL) as a control non-FtsZ-targeting antibiotic. Note that oxacillin exerts a negligible impact on SaFtsZ polymerization, an expected result given that the antibacterial target of oxacillin is the bacterial cell wall rather than FtsZ. A comparison of the light scattering profiles of 11 and 17 at 20 µg/mL (the green profiles in Fig. 2A and B) reveals the following two differential features: (i) 17 stimulates FtsZ polymerization more rapidly than 11, as reflected by the light scattering profile of 17 reaching a plateau earlier than that of 11. (ii) After reaching a plateau, the light scattering profile of 17 also undergoes a time-dependent decrease, suggestive of a corresponding time-dependent dissociation from larger to smaller FtsZ polymers or polymeric bundles. These two differential features may contribute, at least in part, to the reduced activities of 17 relative to 11 against both the MSSA and MRSA strains tested.
Stimulation of FtsZ polymerization has been shown to be the antibacterial mechanism of action for agents such as the substituted benzamides (e.g., PC190723).14,17,18 In addition, FtsZ has been implicated as the antibacterial target of the naturally occurring dibenzoquinolizinium berberine, although the antibacterial potency of berberine is substantively weaker than that of our substituted dibenzoquinolizinium compounds.23 Viewed as a whole, the light scattering results point to the stimulation of FtsZ polymerization dynamics as underlying the antibacterial activity of the 2- and 12-substituted dibenzo[a,g]quinolizin-7-ium compounds.
In summary, the presence of an aryl substituent at either the 2- or 12-position of tetramethoxy dibenzoquinolizinium significantly increases FtsZ-targeted antistaphylococcal and antienterococcal activity relative to berberine, with the compounds having notable antibacterial activity against multidrug-resistant strains of both MRSA and VRE. Against VRE, all of the biphenyl-substituted derivatives have MIC values lower than those of the clinical antibiotics used as controls in this study. Studies are in progress to develop suitable formulations for assessment of their relative antibacterial activity in vivo using appropriate animal models of infection.
Supplementary Material
Acknowledgments
This study was supported by research agreements between TAXIS Pharmaceuticals, Inc. and both Rutgers, The State University of New Jersey (E.J.L.) and the University of Medicine and Dentistry of New Jersey (D.S.P). We would like to thank Drs. Steve Tuske and Eddy Arnold (Center for Advanced Biotechnology and Medicine, Rutgers University) for their assistance with the expression and purification of the S. aureus FtsZ protein. S. aureus 8325-4 was the generous gift of Dr. Glenn W. Kaatz (John D. Dingell VA Medical Center, Detroit, MI). The Brucker Avance III 400 MHz NMR spectrometer used in this study was purchased with funds from NCRR Grant No. 1S10RR23698-1A1. Mass spectrometry was provided by the Washington University Mass Spectrometry Resource with support from the NIH National Center for Research Resources Grant No. P41RR0954.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2012.08.123.
References and notes
- 1.Leavis HL, Willems RJL, Top J, Spalburg E, Mascini EM, Fluit AC, Hoepelman A, de Neeling AJ, Bonten MJM. Emerg. Infect. Dis. 2003;9:1108. doi: 10.3201/eid0909.020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SKJ. Am. Med. Assoc. 2007;298:1763. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 3.Addinall SG, Holland B. J. Mol. Biol. 2002;318:219. doi: 10.1016/S0022-2836(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 4.Margolin W. Nat. Rev. Mol. Cell Biol. 2005;6:862. doi: 10.1038/nrm1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Addinall SG, Bi E, Lutkenhaus J. J. Bacteriol. 1996;178:3877. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinho MG, Errington J. Mol. Microbiol. 2003;50:871. doi: 10.1046/j.1365-2958.2003.03719.x. [DOI] [PubMed] [Google Scholar]
- 7.Lutkenhaus J, Addinall SG. Annu. Rev. Biochem. 1997;66:93. doi: 10.1146/annurev.biochem.66.1.93. [DOI] [PubMed] [Google Scholar]
- 8.Lowe J, van den Entm F, Amos LA. Annu. Rev. Biophys. Biomol. Struct. 2004;33:177. doi: 10.1146/annurev.biophys.33.110502.132647. [DOI] [PubMed] [Google Scholar]
- 9.Lock RL, Harry EJ. Nat. Rev. 2008;7:324. doi: 10.1038/nrd2510. [DOI] [PubMed] [Google Scholar]
- 10.Kapoor S, Panda D. Expert Opin. Ther. Targets. 2009;13:1037. doi: 10.1517/14728220903173257. [DOI] [PubMed] [Google Scholar]
- 11.Foss MH, Eun Y-J, Weibel DB. Biochemistry. 2011;50:7719. doi: 10.1021/bi200940d. [DOI] [PubMed] [Google Scholar]
- 12.Schaffner-Barbero C, Martin-Fontecha M, Chacon P, Andreu JM. ACS Chem. Biol. 2012;7:269. doi: 10.1021/cb2003626. [DOI] [PubMed] [Google Scholar]
- 13.Margalit DN, Romberg L, Mets RB, Hebert AM, Mitchision TJ, Kirschner MW, RayChaudhuri D. Proc. Natl. Acad. Sci. U.S.A. 2004;101:11821. doi: 10.1073/pnas.0404439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andreu JM, Schaffner-Barbero C, Huecas S, Alonso D, Lopez-Rodriguez ML, Ruiz-Avila LB, Núñez-Ramírez R, Llorca O, Martín-Galiano AJ. J. Biol. Chem. 2010;285:14239. doi: 10.1074/jbc.M109.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beuria TK, Santra MK, Panda D. Biochemistry. 2005;44:16584. doi: 10.1021/bi050767+. [DOI] [PubMed] [Google Scholar]
- 16.Domadia PN, Bhunia A, Sivaraman J, Swarup S, Dasgupta D. Biochemistry. 2008;47:3225. doi: 10.1021/bi7018546. [DOI] [PubMed] [Google Scholar]
- 17.Haydon DJ, Bennett JM, Brown D, Collins I, Galbraith G, Lancett P, Macdonald R, Stokes NR, Chauhan PK, Sutariya JK, Nayal N, Srivastava A, Beanland J, Hall R, Henstock V, Noula C, Rockley C, Czaplewski L. J. Med. Chem. 2010;53:3927. doi: 10.1021/jm9016366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haydon DJ, Stokes NR, Ure R, Galbraith G, Bennett JM, Brown DR, Baker PJ, Barynin VV, Rice DW, Sedelnikova SE, Heal JR, Sheridan JM, Aiwale ST, Chauhan PK, Srivastava A, Taneja A, Collins I, Errington J, Czaplewski LG. Science. 2008;321:1673. doi: 10.1126/science.1159961. [DOI] [PubMed] [Google Scholar]
- 19.Kumar K, Awasthi D, Lee SY, Zanardi I, Ruzsicska B, Knudson S, Tonge PJ, Slayden RA, Ojima I. J. Med. Chem. 2011;54:374. doi: 10.1021/jm1012006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Läppchen T, Hartog AF, Pinas VA, Koomen GJ, den Blaauwen T. Biochemistry. 2005;44:7879. doi: 10.1021/bi047297o. [DOI] [PubMed] [Google Scholar]
- 21.Urgaonkar S, La Pierre HS, Meir I, Lund H, RayChaudhuri D, Shaw JT. Org. Lett. 2005;7:5609. doi: 10.1021/ol052269z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball AR, Casadei G, Samosorn S, Bremner JB, Ausubel FM, Moy TI, Lewis K. ACS Chem. Biol. 2006;1:594. doi: 10.1021/cb600238x. [DOI] [PubMed] [Google Scholar]
- 23.Boberek JM, Stach J, Good L. PLoS One. 2010;5:e13745. doi: 10.1371/journal.pone.0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwasa K, Kamigauchi M, Ueki M, Taniguchi M. Eur. J. Med. Chem. 1996;31:469. [Google Scholar]
- 25.CLSI Methods for dilution antimicrobial sucseptibility tests for bacteria that grow aerobically approved standard-eighth edition. Wayne PA: Clinical and Laboratory Standards Institute; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.