Abstract
Antibiotic resistance has prompted efforts to discover antibiotics with novel mechanisms of action. FtsZ is an essential protein for bacterial cell division, and has been viewed as an attractive target for the development of new antibiotics. Sanguinarine is a benzophenanthridine alkaloid that prevents cytokinesis in bacteria by inhibiting FtsZ self-assembly. In this study, a series of 5-methylbenzo[c]phenanthridinium derivatives were synthesized and evaluated for antibacterial activity against Staphylococcus aureus and Enterococcus faecalis. The data indicate that the presence of a 1- or 12-phenyl substituent on 2,3,8,9-tetramethoxy-5-methylbenzo[c]phenanthridinium chloride significantly enhances antibacterial activity relative to the parent compound or sanguinarine.
Keywords: Benzo[c]phenanthridine, FtsZ-targeting, Staphylococcus aureus, Enterococcus faecalis
Multidrug-resistant (MDR) bacterial pathogens like methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant enterococci (VRE) represent an increasing nosocomial health concern for both patients and healthcare professionals.1,2 The growing problem of resistance to current clinical antibiotics has created a critical need for novel therapeutic antibiotics with unique modes of action. FtsZ is a key protein involved in bacterial cell division (cytokinesis) and is highly conserved among bacterial pathogens. 3–5 The essential role that FtsZ plays in bacterial cell division makes this protein a promising therapeutic target. Interest in the development of small molecules that target FtsZ is reflected by several recent reviews on this topic.6–9
Sanguinarine 1 (Fig. 1) is a plant alkaloid that has been identified as a small molecule that alters bacterial FtsZ Z-ring formation and has antibacterial activity.10 Recent studies have also demonstrated that the structurally-related alkaloid, berberine also affects FtsZ self-polymerization.11,12 In the present study, the effect of varied substituents at the 1- and 12-positions of several 5-methylbenzo[c]phenanthridinium derivatives on antibacterial activity versus S. aureus and E. faecalis was evaluated.
Figure 1.
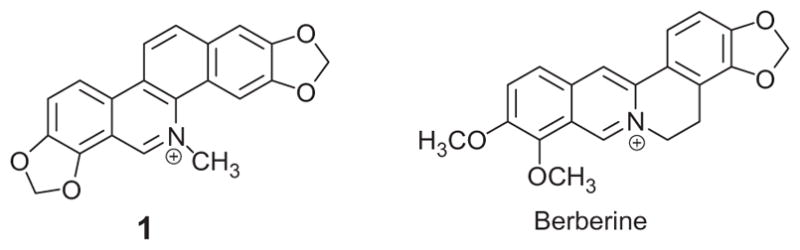
Structures of sanguinarine 1 and berberine.
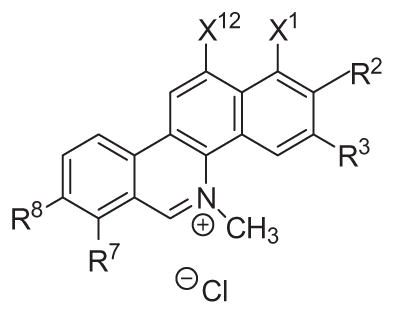
The 5-methylbenzo[c]phenanthridinium derivatives synthesized and evaluated for antibacterial activity are listed in Table 1. The methods used for preparation of 2 and 6 are summarized in Scheme 1. 2,3-Dihydroxynaphthalene was converted to its dimesylate and then treated with nitric acid in acetic anhydride to provide the 5-nitro derivative. Hydrolysis of the mesylates followed by treatment with methyl iodide provided 1-nitro-5,6-dimethoxynaphthalene, which was reduced to 5,6-dimethoxy-1-naphthylamine. Treatment of this naphthylamine with the acid chloride of either 6-bromo-2,3-methylenedioxybenzoic acid or 6-bromo-2,3-dimethoxybenzoic acid provided the benzamide derivatives, which were converted to their tertiary amides by treatment with NaH and then methyl iodide. These tertiary benzamide intermediates were converted to their respective 5-methylbenzo[c]phenanthridin-6-ones under Heck cyclization conditions. Subsequent treatment of these 5-methylbenzo[c]phenanthridin-6-ones with LAH, followed with acidification with HCl provided 2 and 6.
Table 1.
1- and 12-Substituted 5-methylbenzo[c]phenanthridinium compounds synthesized and evaluated
| ||||||
|---|---|---|---|---|---|---|
| Compd | R2 | R3 | R7 | R8 | X1 | X12 |
| 1 | –O–CH2–O– | –O–CH2–O– | H | H | ||
| 2 | –OCH3 | –OCH3 | –O–CH2–O– | H | H | |
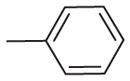
| 3 | –OCH3 | –OCH3 | –O–CH2–O– |
|
H | |
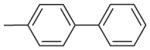
| 4 | –OCH3 | –OCH3 | –O–CH2–O– |
|
H | |
| 5 | –OCH3 | –OCH3 | –O–CH2–O– | H |
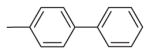
|
|
| 6 | –OCH3 | –OCH3 | –OCH3 | –OCH3 | H | H |
| 7 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |
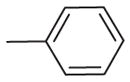
|
H |
| 8 | –OCH3 | –OCH3 | –OCH3 | –OCH3 | H |
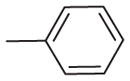
|
| 9 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |
|
H |
| 10 | –OCH3 | –OCH3 | –OCH3 | –OCH3 | H |
|
| 11 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |
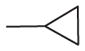
|
H |
| 12 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |
|
H |
| 13 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |

|
H |
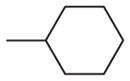
| 14 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |
|
H |
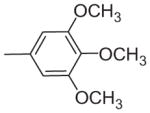
| 15 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |
|
H |
| 16 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |
|
H |
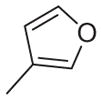
| 17 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |
|
H |
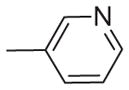
| 18 | –OCH3 | –OCH3 | –OCH3 | –OCH3 |
|
H |
Scheme 1.
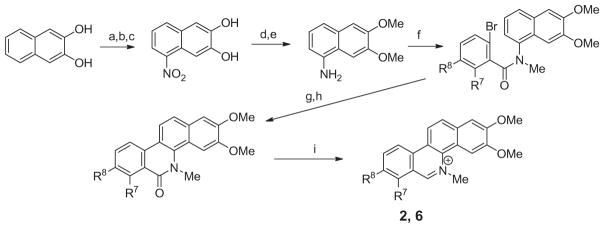
Preparation of 2,3-dimethoxy-7,8-methylenedioxy-5-methylbenzo[c]phenanthridinium chloride 2 and 2,3,7,8-tetramethoxy-5-methylbenzo[c]phenanthridinium chloride 6. Reagents and conditions: (a) MsCl, Et3N, CHCl3, 0 °C; (b) Ac2O, HNO3, 37–39 °C; (c) NaOH, H2O, 100 °C; (d) MeI, K2CO3, DMF, 50 °C; (e) hydrazine monohydrate, Pd/C (10%), EtOH, 85 °C; (f) (i) 5–bromobenzo[d][1,3]dioxole-4-carboxylic acid towards the preparation of 2 or 6-bromo-2,3-dimethoxybenzoic acid towards the preparation of 6, oxalyl chloride, CH2Cl2, 45 °C; (ii) 6,7-dimethoxyaniline, Et3N, CH2Cl2, rt; (g) MeI, NaH, DMF, rt; (h) Pd(OAc)2, p(o-tolyl)3, Ag2CO3, DMF, 155 °C; (i) LAH, THF; then HCl (10%).
The method used for the preparation of several 1-substituted 5-methylbenzo[c]phenanthridinium chlorides is outlined in Scheme 2. 1-Nitro-5,6-dimethoxynaphthalene was converted to 1-nitro-4-bromo-5,6-dimethoxynaphthalene, which was treated with various boronic acids under Suzuki coupling conditions to provide 1-susbtituted-2,3-dimethoxy-5-nitronaphthalene derivatives. Reduction of the nitro group and acylation using either 2,3-dimethoxy- or 2,3-methylenedioxy-6-bromobenzoyl chloride provided the benzamide derivatives. Formation of the tertiary N-methylbenzamides, followed by cyclization to their 5-methylbenzo[c]phenanthridin-6-ones, and reduction with LAH with subsequent treatment with HCl provided the desired 1-substituted-5-methylbenzo[c]phenanthridinium chlorides.
Scheme 2.
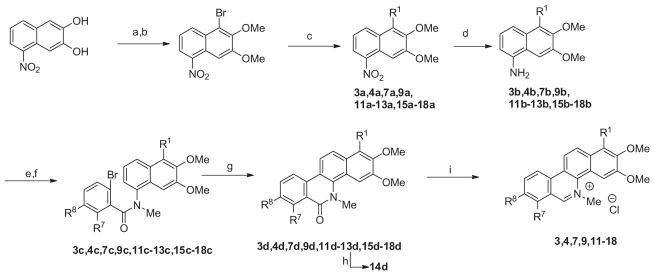
Methods used for the preparation of 1-substituted-5-methylbenzo[c]phenanthridinium chlorides. Reagents and conditions: (a) Br2, CH2Cl2, 0 °C; (b) MeI, K2CO3, DMF, 50 °C; (c) R1–B(OH)2, Pd(PPh3)4, Na2CO3 toluene/H2O, 90 °C; for 11a,13a PdOAc, PCy3, K2PO4 toluene/H2O, 90 °C; for 12a vinyltributyltin, Pd(PPh3)4, THF, reflux (d) hydrazine monohydrate, Pd/C (10%), EtOH, 85 °C; for 12b SnCl2, EtOH; for 13b H2 (g) Pd/C (10%); (e) (i) 6-bromo-2,3-dimethoxybenzoic acid, oxalyl chloride, CH2Cl2, 45 °C; for 3,4 6-bromo-2,3-methylenedioxybenzoic; oxalyl chloride, CH2Cl2, 45 °C (ii) the naphthylamine in CH2Cl2 was added to the appropriate acid chloride, Et3N, rt; (f) MeI, NaH, DMF, rt; (g) Pd(OAc)2, p(o-tolyl)3, Ag2CO3, DMF, 155 °C; (h) H2 (g), Pd/C (10%), EtOH (i) LAH, THF, 0 °C; then HCl (10%).
The synthesis of 12-susbtituted 5-methylbenzo[c]phenanthridinium chlorides was accomplished as illustrated in Scheme 3. We prepared 1-amino-5,6-dimethoxynaphthalene from 1-nitro-5,6-dihydroxynaphthalene by initially forming the 5,6-dimethoxy derivative, followed by reduction of the nitro substituent. Treatment of this 1-naphthylamine with iodine in pyridine initially at 0 °C and allowing it to warm to room temperature provided 4-iodo-5,6-dimethoxy-1-naphthylamine. Suzuki coupling with various organoboronates was performed with this iodo derivative. Conversion of the resulting 1-naphthylamine to its secondary bromobenzamide and then to its N-methyl tertiary benzamide, followed by Heck cyclization provided the desired 12-substituted 5-methylbenzo[c]phenanthridin-6-ones. Reduction of these intermediates with LAH followed by treatment with HCl provided 5, 8, and 10.
Scheme 3.
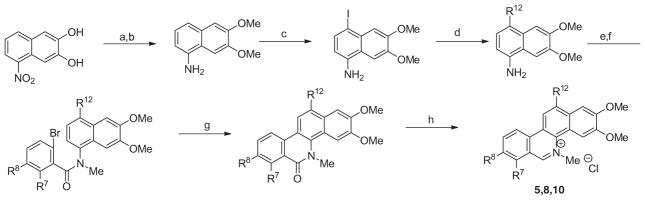
Methods used for the preparation of 12-substituted 5-methylbenzo[c]phenanthridinium chlorides. Reagents and conditions: (a) MeI, K2CO3, DMF, 50 °C; (b) hydrazine monohydrate, Pd/C (10%), EtOH, 85 °C; (c) I2, pyridine, dioxane, 0 °C to rt; (d) R1–B(OH)2, Pd(PPh3)4, Na2 CO3, toluene/H2O, 90 °C; (e) (i) 6-bromo-2,3-methylenedioxybenzoic acid towards the preparation of 5 or 2,3-dimethoxybenzoic acid towards the preparation of 8 and 11, oxalyl chloride, CH2Cl2, 45 °C; (ii) Requisite 6,7-dimethoxyaniline, Et3N, CH2Cl2, rt; (f) MeI, NaH, DMF, rt; (g) Pd(OAc)2, p(o-tolyl)3, Ag2CO3, DMF, 155 °C; (h) (i) LAH, THF, 0 °C; (ii) HCl (10%).
Sanguinarine, 1, exhibited significant activity against both the methicillin-sensitive and methicillin-resistant strains of S. aureus (MSSA and MRSA, respectively) used in this study. It also had modest activity against both the vancomycin-sensitive and vancomycin- resistant strains of E. faecalis (VSE and VRE, respectively). Compound 2, which resembles berberine with regard to the relative location of its methylenedioxy and methoxyl substituents, was significantly less active against the MSSA and MRSA strains, and did not exhibit appreciable activity against the VSE and VRE strains. The addition to 2 of a phenyl substituent at the 1-position or a biphenyl substituent at either the 1- or 12-position (3–5) dramatically increased antibacterial activity against all the S. aureus and E. faecalis strains examined. The tetramethoxy analog of these alkaloids 6 was also prepared and evaluated for antibacterial activity. These analogs were viewed as attractive, as they would be less likely to cause adverse effects in mammalian cells by possible intercalation into DNA. While 6 is similar to berberine, having only weak antibacterial activity (MIC >64 μg/ml), the antibacterial activity could be substantially increased by the addition of a phenyl substituent (7,8) or a biphenyl substituent (9,10) at either its 1- or 12-position. The 1-biphenyl derivative 9 was particularly active against these four bacterial strains. Several additional 1-substituted derivatives of 6 were evaluated for antibacterial activity. The cyclohexen-1-yl (13), 3,4,5-trimethoxyphenyl (15), and 3-pyridinyl (18) derivatives were all less potent than 7. The presence at the 1-position of a cyclopropyl, vinyl, p-(dimethylamino) phenyl, or 3-furanyl substituent was associated with comparable, but not improved, antibacterial activity relative to 7. These data indicate that several 1- and 12-substituted benzo[c] phenanthridines could be developed with potent activity against both MSSA and MRSA. With regard to MRSA, several of these derivatives had MICs comparable to or lower than that of vancomycin or the other clinical antibiotics listed in Table 2. In each instance, the MICs for these 1- and 12-substituted benzo[c]phenanthridines against E. faecalis were somewhat higher than those against S. aureus. Significantly, however, many of the 1- and 12-substituted benzo[c]phenanthridines had greater activities versus VRE than any of the listed clinical antibiotics (Table 2).
Table 2.
Antibacterial activities of various 1- and 12-substituted benzo[c]phenanthridinium derivatives against S. aureus and E. faecalis
| a MIC (lg/mL) | ||||
|---|---|---|---|---|
| S. aureus 8325-4 (MSSA) | S. aureus ATCC 33591 (MRSA) | E. faecalis ATCC 19433 (VSE) | E. faecalis ATCC 51575 (VRE) | |
| 1 | 2 | 2 | 8 | 16 |
| 2 | 32 | 32 | 64 | >64 |
| 3 | 1 | 2 | 8 | 8 |
| 4 | 2 | 2 | 8 | 8 |
| 5 | 2 | 2 | 4 | 8 |
| 6 | 32 | 32 | >64 | >64 |
| 7 | 1 | 1 | 4 | 4 |
| 8 | 1 | 2 | 8 | 16 |
| 9 | 0.5 | 0.5 | 4 | 8 |
| 10 | 0.5 | 0.5 | 16 | 16 |
| 11 | 1 | 2 | 8 | 8 |
| 12 | 1 | 2 | 8 | 8 |
| 13 | 2 | 4 | 16 | 32 |
| 14 | 1 | 2 | 8 | 8 |
| 15 | 4 | 4 | 32 | 32 |
| 16 | 1 | 1 | 8 | 8 |
| 17 | 1 | 1 | 16 | 16 |
| 18 | 8 | 32 | 32 | 32 |
| Oxacillin | 0.06 | >64 | 8 | >64 |
| Vancomycin | 1 | 2 | 1 | >64 |
| Erythromycin | 0.13 | >64 | 1 | >64 |
| Tetracycline | 0.06 | 64 | 0.5 | >64 |
| Clindamycin | 0.03 | >64 | 2 | >64 |
Minimum inhibitory concentration (MIC) assays were conducted in accordance with Clinical Laboratory Standards Institute (CLSI) guidelines for broth microdilution. 13 MIC is defined as the lowest compound concentration at which bacterial growth is ≥90% inhibited.
These data indicate that several 1- and 12-susbtituted 5-methylbenzo[c]phenanthridinium have significant antimicrobial activity against multidrug-resistant (MDR) strains of S. aureus and E. faecalis. Studies are in progress to further investigate the structure– activity of 5-methylbenzo[c]phenanthridinium derivatives and related compounds with regard to antimicrobial and FtsZ-targeting activity.
Acknowledgments
This study was supported by research agreements between TAXIS Pharmaceuticals, Inc. and both Rutgers, The State University of New Jersey (E.J.L.) and the University of Medicine and Dentistry of New Jersey (D.S.P). S. aureus 8325-4 was the generous gift of Dr. Glenn W. Kaatz (John D. Dingell VA Medical Center, Detroit, MI). The Brucker Avance III 400 MHz NMR spectrometer used in this study was purchased with funds from NCRR Grant No. 1S10RR23698-1A1. Mass spectrometry was provided by the Washington University Mass Spectrometry Resource with support from the NIH National Center for Research Resources Grant No. P41RR0954.
References and notes
- 1.Leavis HL, Willems RJL, Top J, Spalburg E, Mascini EM, Fluit AC, Hoepelman A, de Neeling AJ, Bonten MJM. Emerg Infect Dis. 2003;9:1108. doi: 10.3201/eid0909.020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. J Am Med Assoc. 2007;298:1763. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 3.Addinall SG, Holland B. J Mol Biol. 2002;318:219. doi: 10.1016/S0022-2836(02)00024-4. [DOI] [PubMed] [Google Scholar]
- 4.Margolin W. Nat Rev Mol Cell Biol. 2005;6:862. doi: 10.1038/nrm1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lock RL, Harry EJ. Nat Rev. 2008;7:324. doi: 10.1038/nrd2510. [DOI] [PubMed] [Google Scholar]
- 6.Kapoor S, Panda D. Expert Opin Ther Targets. 2009;1037:13. doi: 10.1517/14728220903173257. [DOI] [PubMed] [Google Scholar]
- 7.Foss MH, Eun YJ, Weibel DB. Biochemistry. 2011;50:7719. doi: 10.1021/bi200940d. [DOI] [PubMed] [Google Scholar]
- 8.Schaffner-Barbero C, Martin-Fontecha M, Chacon P, Andreu JM. ACS Chem Biol. 2011;7:269. doi: 10.1021/cb2003626. [DOI] [PubMed] [Google Scholar]
- 9.Margalit DN, Romberg L, Mets RB, Hebert AM, Mitchision TJ, Kirschner MW, RayChaudhuri D. Proc Natl Acad Sci USA. 2004;101:11821. doi: 10.1073/pnas.0404439101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beuria TK, Santra MK, Panda D. Biochemistry. 2005;44:16584. doi: 10.1021/bi050767+. [DOI] [PubMed] [Google Scholar]
- 11.Domadia PN, Bhunia A, Sivaraman J, Swarup S, Dasgupta D. Biochemistry. 2008;47:3225. doi: 10.1021/bi7018546. [DOI] [PubMed] [Google Scholar]
- 12.Boberek JM, Stach J, Good L. PLoS ONE. 2010;5:e13745. doi: 10.1371/journal.pone.0013745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CLSI Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard-Eighth Edition. Clinical and Laboratory Standards Institute; Wayne, PA: 2009. [Google Scholar]


