Abstract
Objectives
1.) Expand the scope of neuroendocrine applications of functional neuroimaging techniques. 2.) Compare the covariance of amygdalar activity with that of the rest of the brain during pre- and post-menopausal levels of estrogen (E2). Based on the distribution of cortical E2 receptors and the neocortical regions where E2 has been shown to preferentially accumulate, we predict that E2 infusion will increase covariance of amygdalar activity with that of the temporal and frontal cortices.
Design
This basic physiology study employed a within-subject design. All participants were post-menopausal women (n =7). Analysis of covariance between whole brain and amygdalar regional cerebral glucose consumption (CMRglc) was conducted in a voxel-wise manner by means of the basic regression option in SPM2 and was applied to FDG-PET scans acquired at baseline and after a 24 hour graded E2 infusion.
Setting
an academic medical center; Massachusetts General Hospital, Boston, Massachusetts.
Results
E2 levels (mean ± sem) were significantly greater at 24 hours (257.9 pg/mL ± 29.7) than at 0 hours (28.1 pg/mL ± 3.4). Right amygdalar CMRglc showed a significant covariance with activity of three different regions of the temporal cortex during E2 infusion, but none at baseline. In addition, right amygdalar CMRglc covaried with that of the right medial and superior frontal gyri only during E2 infusion.
Conclusions
In addition to suggesting changes in amygdalar-cortical network connectivity as a result of short-term E2 exposure, these analyses provide evidence that basic neuroendocrine research may benefit from further use of FDG-PET and other functional neuroimaging modalities for network level analyses.
Keywords: estradiol, functional connectivity, temporal cortex, prefrontal cortex, amygdala, FDG-PET, functional neuroimaging, depression, working memory
Introduction
As a complement to research questions at the molecular and cellular level, basic neuroendocrine research is progressively addressing more questions at the systems or network level (Kawakami et al. 1979; DeVoogd, 1991; Ball et al. 2004; Campbell, 2007; Romano-Torres et al. 2007). While conventional neuroendocrine techniques for network level analyses offer certain methodological advantages, these techniques also frequently involve sacrificing the animal or lesioning a part of the brain. EEG circumvents these methodological issues, but does not provide the spatial resolution achieved by functional neuroimaging modalities such as PET and fMRI. Thus, PET and fMRI techniques have received increased application in the context of animal studies evaluating basic neuroendocrine physiology paradigms and probably even greater application in the context of behavioral psychoneuroendocrine paradigms in humans (Dreher et al. 2007; Goldstein et al. 2005; Ottowitz et al. 2004a; Ganguli et al. 2002; Shaywitz et al. 1999). Despite the increased purview of these applications, there has been very little use of functional neuroimaging techniques to evaluate basic neuroendocrine physiological paradigms in humans (Reiman et al. 1996; Ottowitz et al. 2004b).
In this regard, different lines of evidence point to the amygdala as a key component of cortical-subcortical circuits whose function may be substantially influenced by estrogen (E2). From an anatomical perspective, both the amygdala and cortex contain a significant density of E2 receptors (Osterlund et al. 2000; Shugrue et al. 1997; Taylor and Al-Azzawi, 2000) and extensive projections exist between the amygdala and cortex (Amaral and Price, 1984; Swanson and Petrovich, 1998). From a functional or physiological perspective, animal studies have shown that infusion of E2 into the amygdala affects activity of amygdalar projection sites, and conversely, that application of E2 to structures receiving amygdalar projections affects amygdalar activity (Lehmann and Erskine, 2005; Li et al. 1992; Dudley et al. 1990; Wong and Moss, 1992). Neuroimaging studies in humans have shown that activations of the amygdala and cortex change with E2 levels across the menstrual cycle (Dreher et al. 2007; Goldstein et al. 2005), providing evidence that E2 may also coordinate activity between the amygdala and cortex in humans.
In this study, we investigate the basic physiological capacity for an exogenous E2 challenge to alter network level interactions in humans by evaluating covariance of resting state activity (CMRglc: regional cerebral glucose consumption) between the amygdala and cortex during baseline post-menopausal E2 levels vs. levels typical of the follicular phase in pre-menopausal women.
Bixo et al. (1995) have shown the prefrontal cortex to be the neocortical region showing the greatest tendency to concentrate E2, whereas Osterlund and co-workers (2000) have shown that the most distinct collection of neocortical E2 receptors lie in the temporal lobe. Thus, we predicted that, relative to postmenopausal E2 levels, covariance of activity between the amygdala and frontal and temporal cortices would increase during E2 levels characteristic of the pre-menopausal follicular phase.
Material and Methods
Overview
This study was one component of a three-part basic physiology paradigm evaluating the effects of E2 on 1.) hypothalamic-pituitary regulatory processes 2.) prefrontal-hippocampal effective connectivity, and 3.) amygdalar-cortical network covariance (the current study). No component of our study involved evaluation of cognition, emotion, nor treatment effects of E2.
In order to control for subject effects and the effects of endogenous E2, the same postmenopausal women were evaluated at E2 levels characteristic of the menopause vs. levels characteristic of the pre-menopausal follicular phase, i.e. as achieved by means of E2 infusion. E2 is known to enhance synaptic connectivity within 12–24 hours of administration (Gazzaley et al. 2002) and formation of dendritic spines is known to recruit basic metabolic processes (D'Ambrosi et al. 2001; Skladchikova et al. 1999), accordingly FDG-PET scans were acquired at the 0 hour baseline and after 24 hours of a continuous E2 infusion.
To identify neuroanatomical regions whose activity co-varied with that of the amygdala, voxel-wise whole brain covariate analyses were conducted by means of entering amygdalar ROI values into the ‘simple regression’ option of the statistical parametric mapping software program (SPM2, Department of Cognitive Neurology, London England). This was done separately for both the 0 and 24 hour scans. To enhance anatomical localization of boundaries for the amygdalar regions of interest (ROIs), structural MRI scans were also collected for each subject.
As a point of clarification, because the point of the study was to evaluate the effects of E2 on amygdalar-cortical network interactions, there is no dedicated analysis of the effects of E2 on regional brain activity. In other words, this study evaluated how different parts of the brain “talk to each other” under the influence of different E2 levels; this study does not evaluate the global effects of E2 on brain activity.
Subjects
Participants were recruited through the General Clinical Research Center of Massachusetts General Hospital (MGH). Only postmenopausal women were studied. The study was approved by the Federal Drug Administration and Partners Institutional Review Board and written, informed consent was provided prior to any study procedures. Admission to the General Clinical Research Center was preceded by general history and physical exam, and a laboratory evaluation of basic chemistry and complete blood count.
Hormone Preparation, Infusion, Collection, and Statistical Analysis
The E2 infusion was prepared as previously described (Taylor et al. 1995). Infusion rates were 0.1 ug/kg/hr for the initial 12 hours and 0.135 ug/kg/hr for the following 12 hours. Infusions were administered via a closed system, non-PVC fluid-path IV tubing (Accuset, IMED Corp., San Diego, CA). A Micro 965 Volumetric Infusion Pump was used to control the infusion rate (IMED Corp.).
Blood samples were collected for E2 at baseline and 24 hrs. E2 was measured using a two-site monoclonal non-isotopic system according to the manufacturer’s instructions (Axsym, Abbott Laboratories, Abbott Park, IL, USA). The inter-assay coefficients of variation for the E2 assay were 9.2, 5.4 and 9.6% at E2 concentrations of 85, 300, and 700 pg/mL (312.0, 1101, and 2570 pmol/L), respectively.
Matlab 7 was used to conduct paired t-tests evaluating for differences in E2 levels across the two scanning sessions.
PET Scanning and Image Reconstruction
[18F]fluorodeoxyglucose-PET (FDG-PET) scans were obtained on all subjects at baseline and after 24 hours of estrogen infusion. PET images were acquired using a Siemens HR+, 32-ring, 63-slice body tomograph. In both the transverse and axial directions, the intrinsic spatial resolution of the scanner is 4.5 mm full width half maximum (FWHM). The slice geometry includes 63 slices with 2.25 mm separation and a total axial field of 16.5 mm.
Scan acquisition began on the day of admission, at 15:00 hours. After having fasted for approximately 6 hours, subjects were injected with 5-6 millicuries of [18]FDG, while seated in a dimly lighted room. Forty-five minutes after radiopharmaceutical administration, subjects were positioned supine on the PET scanner bed. A vacuum formable reusable polystyrene bead-filled pillow was used to minimize potential head movements and to align each subject’s head so that slice acquisition occurred parallel to the canthomeatal line. After correct head positioning was achieved, a single 20 min emission measurement was acquired. Subsequently, after 24 hours of a graded E2 infusion conducted in accord with the aforementioned protocol, the second FDG-PET scan was collected.
Reconstruction of images was conducted by means of a conventional filtered back-projection algorithm to an in-plane resolution of 4.5 mm FWHM. Projection data were corrected for random coincidences and scattered radiation, non-uniformity of detector response, and dead time. Attenuation correction was conducted by means of transmission images acquired with a rotating pin source containing germanium 65.
MRI Acquisition and Coregistration with PET Images
To enhance anatomical accuracy for identification of amygdalar regions of interest, high-resolution T1-weighted 3D MRI scans were acquired for each subject. MRI sagittal scout images and subsequent contiguous axial three-dimensional T1-weighted spoiled gradient echo pulse sequences were acquired using a General Electric 1.5 Tesla Signa scanner (Milwaukee, WI). Acquisition parameters included a repetition time of 30 msec, echo time=9 msec, flip angle = 25∞, band width 15.63, number of scan locs = 124, field of view = 22, matrix = 256×192, number of excitations = 1, frequency direction = A/P, phase direction = L/R, and phase FOV 0.75. Water was used as the auto center frequency.
Coregistration of each subject’s PET and MRI scans was performed by means of the standard coregistration protocol included in SPM2 (Wellcome Department of Cognitive Neurology, London).
Amygdalar Regions of Interest; Definition of, and Data Acquisition
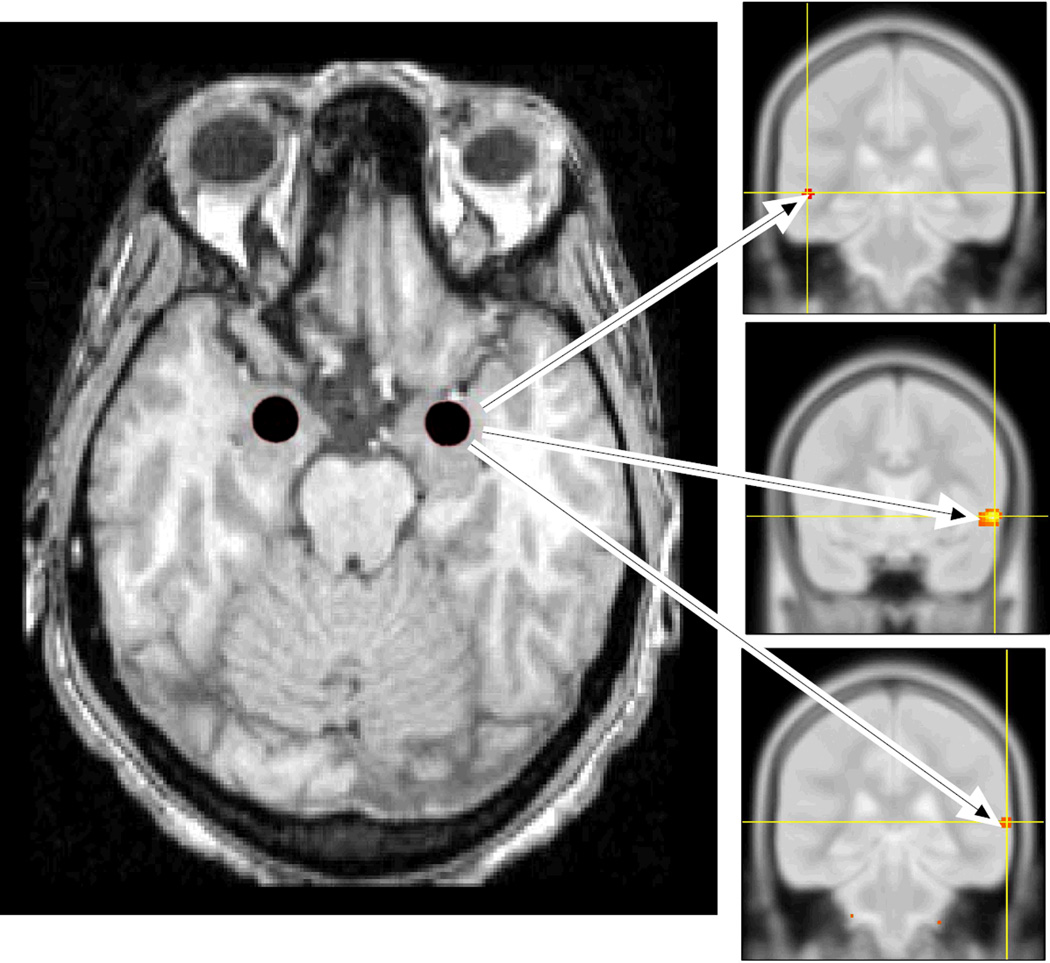
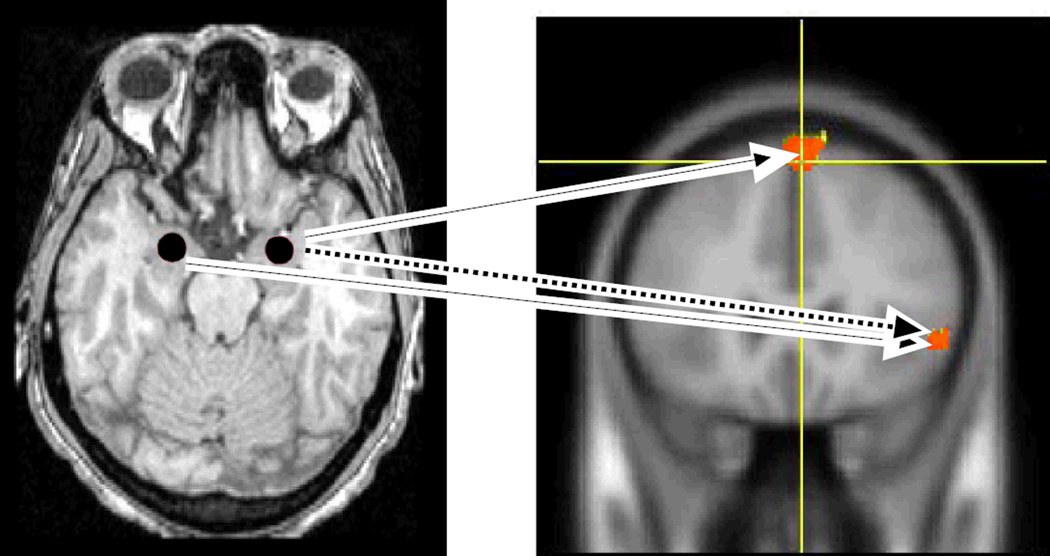
The MarsBaR toolbox (Brett et al. 2002) of the SPM2 software package (Wellcome Department of Cognitive Neurology, London) was used to define bilateral amygdalar regions of interest (ROIs) on each subject’s MRI scan. A central, spherical portion of the amygdala was sampled in accord with anatomical guidelines of Bronen and Cheung (1991). ROI dimensions were standardized at 6 mm diameter across all subjects in order to eliminate any variability in ROI counts that might be caused by variations in ROI volume (Figures 1 and 2, left sided panels).
Figure 1. Sites of covariance of right amygdalar and temporal lobe CMRglc during estrogen infusion.
Left Panel: Transverse structural MRI section of the brain, imaged at the level of the midbrain and temporal lobe showing spherical amygdalar regions of interest (ROIs) shaded in red. To facilitate anatomical placement, ROIs were drawn on each subject’s high resolution MRI scan, which was co-registered to their PET scans; the right sided panels include examples of PET images. Panels on Right: Regions in the temporal lobe showing covariance with the right amygdala included the left middle temporal gyrus at MNI coordinates -52, -32, -2 (top right), and the anterior and posterior aspects of the right superior temporal gyrus at MNI coordinates 58, 8, -4, (middle right), and 68, -34, 8, (bottom right), respectively. These covariances were significant only during estrogen infusion. Both the MRI and PET images are positioned in neurological convention, i.e. the right hemisphere is located on the right side of the image. (CMRglc = regional cerebral glucose consumption).
Figure 2. Sites of covariance of CMRglc between each amygdala and the prefrontal cortex during estrogen infusion.
Left Panel: Transverse structural MRI section of the brain, imaged at the level of the midbrain and temporal lobe showing spherical amygdalar regions of interest (ROIs) shaded in red. Right Panel: Prefrontal sites of covariance with the right amygdala included the right superior frontal gyrus (MNI coordinates 8, 48, 48; identified by the yellow crosshairs) and the right ventrolateral prefrontal cortex (VLPFC), (MNI coordinates 54, 28, 0 at 0 hours, and 54, 22, 0 at 24 hours). The dashed blue arrow indicates a significant covariance that was present during both baseline scanning and scanning after 24 hours of estrogen infusion, whereas the solid red arrows indicate a significant covariance that was present only after 24 hours of estrogen infusion. The top red arrow indicates a significant covariance present between the right amygdala and right medial frontal gyrus/superior frontal gyrus (MNI coordinates 8, 48, 48), whereas the bottom red arrow indicates a significant covariance between the left amygdala and right VLPFC (MNI coordinates 54, 22, 0). Both the MRI and PET images are positioned in neurological convention, i.e. the right hemisphere is located on the right side of the image. (CMRglc = regional cerebral glucose consumption).
After definition of amygdalar ROIs, and co-registration of corresponding PET and MRI scans, the MarsBaR toolbox of SPM2 was used to extract amygdalar ROI values from the PET images (for each subject, for both imaging sessions).
Whole Brain-Amygdalar ROI Covariate Analyses
Amygdalar ROI values were used in a standard seed analysis (Cordes, et al. 2000; Della-Maggiore, et al., 2000; Dougherty et al 2004), where the covariance between the amygdalar CMRglc and all other regions of the brain is calculated. This allowed us to search the entire brain for regions that were positively (or negatively) correlated with activity of the amygdala. To provide rudimentary control for multiple comparisons, significance was thresholded at p=0.001 and a spatial extent of 5 contiguous voxels (Wager et al. 2007; Friston et al. 1994).
Results
Subjects
Participants ranged in age from 48-76 years and had a mean age of 64.2 years. All subjects were at least one year from menopause. All subjects lived independently and were free of active medical and psychiatric disorders. No subjects were receiving E2 at the time of the study, nor within at least the year prior to the study.
Steroid Hormone Levels
E2 levels [mean (sem)] increased from 28.1(3.4) pg/mL at baseline, to 257.9(29.7) pg/mL at 24 hours. E2 levels at 24 hours differed from baseline (p<0.05).
Sites of Amygdalar-Cortical Covariance
All brain regions whose CMRglc showed significant covariance with amygdalar CMRglc at time 0, and/or at time 24 hours, are listed in Table 1. In brief summary of the findings of relevance to our a priori hypothesis, it is notable that there were no sites of significant covariance between amygdalar CMRglc and CMRglc of the temporal cortex at baseline E2 levels, whereas three sites of significant covariance between the amygdala and temporal cortex were apparent during E2 infusion (Figure 1). Figure 2 highlights amygdalar sites of covariance with the prefrontal cortex that were present at 0 and 24 hours (the right amygdala and VLPFC) versus sites of covariance that were present only at 24 hours (the right amygdala and medial/ superior frontal gyri, and the left amygdala and right VLPFC).
Table 1.
Covariance of Whole Brain vs. Amygdalar CMRglc at Baseline and During Estrogen Infusion
| ESTROGEN DOSING and Amygdalar Lateralization |
Neuroanatomical Region |
MNI Coordinates | Z score |
|---|---|---|---|
|
BASELINE, POSTMENOPAUSAL ESTROGEN LEVELS |
|||
| Left Amygdala: | L precuneus | −20, −62, 44 | (+) 4.36 |
| R cuneus | 4, −62, 4 | (−) 3.44 | |
| L pulvinar | −20, −26, −2 | (+) 3.37 | |
| Right Amygdala: | R precentral gyrus | 58, −4, 32 | (−) 3.11 |
| R VLPFC | 54, 28, 0 | (−) 2.93 | |
| 24 HOURS of ESTROGEN INFUSION | |||
| Left Amygdala: | R VLPFC | 58, 28, −2 | (−) 4.44 |
| R lingual gyrus | 12, −86, −2 | (−) 4.15 | |
| R cuneus | 2, −68, 12 | (−) 3.80 | |
| Right Amygdala: | R inferior occipital gyrus | 38, −82, −10 | (+) 4.79 |
| R superior temporal gyrus | 58, 8, −4 | (−) 4.11 | |
| R VLPFC | 54, 22, 0 | (−) 3.25 | |
| L middle temporal gyrus | −52, −32, −2 | (+) 3.24 | |
| L precentral gyrus | −28, −6, 72 | (−) 3.01 | |
| R MedFG/SFG | 8, 48, 48 | (−) 2.97 | |
| R superior temporal gyrus | 68, −34, 8 | (−) 2.97 | |
CMRglc: regional cerebral glucose consumption. MNI: Montreal Neurological Institute. R: right. L: left. VLPFC: ventrolateral prefrontal cortex. MedFG/SFG: interface of the medial frontal gyrus and superior frontal gyrus. Note that a (−) z-score corresponds to negative covariance between CMRglc of the identified anatomical site and the amygdala; a (+) z-score corresponds to positive covariance between CMRglc of the identified anatomical site and the amygdala.
Discussion
To the best of our knowledge, there have been no previous neuroimaging studies evaluating the effects of a hormonal challenge on amygdalar-cortical network interactions. In this regard, our study was primarily oriented toward highlighting an integration of methodologies that may hold general significance for a range of basic neuroendocrine research questions. For example, the amygdala is a putative site of neuroendocrine significance, and may serve as a neuroendocrine effector site for many parts of the brain. Thus, in terms of potential application to animal studies, in vivo methodologies that enable investigation of the effects of hormones on the amygdala in the context of the whole brain may provide important advantages in comparison to techniques that isolate specific brain regions and lose the capacity to evaluate the comprehensive interconnectedness of the brain in its native state.
In terms of increased application in the setting of human research, our study has highlighted the potential for functional neuroimaging techniques to evaluate not just behavioral psychoneuroendocrine paradigms (e.g. the effects of hormones on cognition and emotion), but also basic neuroendocrine paradigms evaluating the effects of hormones on physiological parameters such as network connectivity. Our study prioritized this agenda by evaluating subjects in the absence of active cognitive or emotional challenge, i.e. subjects were evaluated in the resting state. While theories involving the brain’s resting state are still somewhat rudimentary (Fox and Raichle, 2007), some consensus exists that, in the absence of active cognitive or emotional challenge, a default network of coordinated activations and deactivations occurs across specific brain regions (Raichle et al. 2001; Damoiseaux et al. 2006). A meta-analysis of the cerebral “resting state” as derived from PET studies has suggested that the angular gyrus (bilaterally), the left anterior precuneus, posterior cingulate, left medial frontal and anterior cingulate cortex, left superior and medial frontal sulcus, and left inferior frontal cortex are active at rest, whereas the cerebellum is deactivated (Mazoyer et al. 2001). Although competing models are still evolving, functional MRI (fMRI) studies have shown some overlap with the above, with one of the most notable differences being that models derived from fMRI studies have generally shown resting state activations and deactivations to occur bilaterally (DeLucca et al. 2006; Damoiseaux et al. 2007).
Our findings show that E2 may affect the degree to which certain structures coordinate their activity during the resting state. For example, our findings show that at postmenopausal E2 levels (baseline), there were five brain regions showing significant covariance of resting state CMRglc with amygdalar CMRglc, whereas during E2 infusion, there were ten cortical sites showing significant covariance with the amygdala. Consistent with our a priori hypothesis, the frontal and temporal cortical regions showed a substantial increase in covariance with amygdalar activity. For example, at baseline E2 levels, there were no temporal regions showing significant covariance with the amygdala, whereas during E2 infusion, there were three sites. Similarly, there was only one prefrontal site of covariance with the amygdala at 0 hours, but two at 24 hours. In complement to the relevance of these findings to resting state networks, the induction of covariance of activity within amygdalar-temporal circuits holds relevance to behavioral states that are necessarily present even when the brain is at rest. For example, the amygdala and temporal pole are both putative sites of relevance to mood (Beauregard et al. 2006) and in contrast to the relatively fleeting nature of an individual’s affect or transient emotional state, mood is an emotional state persisting across time (Moran, 2003). Thus, mood must (at least in part) rely on the resting state activity of structures such as the amygdala. Indeed, FDG-PET has been used to show that resting state metabolism of the amygdala is elevated in abnormal mood states such as depression (Drevets et al. 2002). Of further relevance to our paradigm, it is especially notable that a depressed mood in women has been shown to be associated with reduced E2 levels (Young et al. 2000), and that the transition to menopause (with its associated fluctations in E2 levels), is accompanied by a notable increase in depressive symptoms (Hay et al. 1994). Because our findings have shown that E2 levels typical of the pre-menopause (the levels attained during 24 hour scanning) induce a covariance between activity of the amygdala and temporal cortex that is not present during levels typical of the menopause (our baseline scanning E2 levels), it can be speculated that significant reductions in E2 levels may contribute to depressive disorders by means of predisposing for dampened or aberrant amygdalar-cortical network interactions within the temporal lobe.
In addition to the enhanced covariance of activity between the amygdala and temporal cortex during E2 infusion, it is notable that our E2 infusion was associated with an increased covariance between the amygdala and prefrontal cortex. Specifically, at baseline postmenopausal E2 levels, although covariance of CMRglc was noted between the right amygdala and right ventrolateral PFC (VLPFC), only during E2 infusion was significant covariance noted between both the left and right amygdala and right VLPFC. In addition, only during E2 infusion did activity of the right amygdala covary with that of the right medial and superior frontal gyri (Rmed/SFG) (Table 1 and Figure 2). The right superior frontal gyrus and right VLPFC have both been shown to mediate working memory (Petrides, 2005; Shaywitz et al. 1999), a neuropsychological domain repeatedly shown to be enhanced by E2 (Maki, 2005; Lacreuse et al. 2004). Several studies have shown that the amygdala may contribute to working memory (Pegna et al. 2002; Barros et al. 2002); thus, our findings suggest that the capacity for the amygdala to do so may be enhanced by E2.
Different limitations of our study deserve mention. For example, our definition of the amygdalar ROIs was conducted in a rather straightforward manner, e.g. simple placement of a sphere in the approximate center of the amygdala. Because different regions of the amygdala have different projection sites, studies employing more detailed ROI placement criteria may identify different sites of covariance. Our paradigm, did however, use the high resolution MRI co-registration techniques available at the time our study was conducted. As high-resolution MRI techniques are developed further, future protocols will presumably allow for greater anatomical precision. In addition to issues of anatomical resolution, the generalizability of our findings are limited by our small sample size; thus, our preliminary findings certainly require replication.
Nonetheless, despite the aforementioned limitations, our study describes one potential manner in which functional neuroimaging techniques can serve as an important adjunct to evaluate the basic physiological effects of hormones on neural networks. Furthermore, our study provides preliminary evidence that E2 may have a significant impact on amygdalar-cortical network interactions, especially those characterizing the resting state. Modifications and extensions of our paradigm, including use of fMRI technology, may benefit a myriad of basic neuroendocrine research enquiries such as the investigation of hypothalamic-pituitary-ovarian and adrenal (i.e. HPO and HPA) regulatory processes, the effects of hormones on functional and effective connectivity, and the relevance of hormones to resting state networks.
Acknowledgments
The authors gratefully acknowledge support from NIH Grants R01 AG13241, K24HD01290 and M01 RR01066. The authors also thank Mariko Jameson for her technical assistance.
Abbreviations & Units
- CMRglc
regional cerebral metabolic glucose consumption
- EEG
electroencephalography
- E2
estradiol
- FDG-PET
fluoro-deoxy-glucose positron emission tomography
- fMRI
functional magnetic resonance imaging
- FWHM
full width half maximum
- msec
milliseconds
- PMW
postmenopausal women
- pg/mL
picograms per milliliter
- ROIs
regions of interest
- sem
standard error of the mean
- SPM
statistical parametric mapping
- ug/kg/hr
microgram/kilogram/hour
- VLPFC
ventrolateral prefrontal cortex
Bibliography
- 1.Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) J Comp Neurol. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- 2.Ball GF, Auger CJ, Bernard DJ, Chalier TD, Sartor JJ, Riters LV, et al. Seasonal Plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann NY Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- 3.Barros DM, Pereira P, Medina JH, Izquierdo I. Modulation of working memory and of long- but not short-term memory by cholinergic mechanisms in the basolateral amygdala. Behav Pharmacol. 2002;13:163–167. doi: 10.1097/00008877-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Beauregard M, Paquette V, Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–846. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- 5.Bixo M, Backstrom T, Winblad B, Andersson A. Estradiol and testosterone in specific regions of the human female brain in different endocrine states. J Steroid Biochem Mol Bio. 1995;55:297–303. doi: 10.1016/0960-0760(95)00179-4. [DOI] [PubMed] [Google Scholar]
- 6.Brett M, Anton JL, Valabregue R, Poline BP. Region of interest analysis using an SPM toolbox. Abstract presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai Japan. 2002. [Google Scholar]
- 7.Bronen RA, Cheung G. Relationship of hippocampus and amygdala to coronal MRI landmarks. Magn Reson Imaging. 1991;9:449–457. doi: 10.1016/0730-725x(91)90434-n. [DOI] [PubMed] [Google Scholar]
- 8.Campbell RE. Defining the gonadotrophin-releasing hormone neuronal network: transgenic approaches to understanding neurocircuitry. J Neuroendocrinol. 2007;19:561–573. doi: 10.1111/j.1365-2826.2007.01561.x. [DOI] [PubMed] [Google Scholar]
- 9.Cordes D, Haughton VM, Arfanakis K, Wendt GJ, Turski PA, Moritz CH, et al. Mapping functionally related regions of brain with functional connectivity MR imaging. Am J Neuroradiol. 2000;21(9):1636–1644. [PMC free article] [PubMed] [Google Scholar]
- 10.D'Ambrosi N, Murra B, Cavaliere F, Amadio S, Bernardi G, Burnstock G, et al. Interaction between ATP and nerve growth factor signalling in the survival and neuritic outgrowth from PC12 cells. Neuroscience. 2001;108(3):527–534. doi: 10.1016/s0306-4522(01)00431-6. [DOI] [PubMed] [Google Scholar]
- 11.Della-Maggiore V, Sekuler AB, Grady CL, Bennett PJ, Sekuler R, McIntosh AR, et al. Corticolimbic interactions associated with performance on a short-term memory task are modified by age. J Neurosci. 2000;20(22):8410–8416. doi: 10.1523/JNEUROSCI.20-22-08410.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Luca M, Beckmann CF, De Stefano N, Matthews PM, Smith SM. fMRI resting state networks define distinct modes of long distance interactions in the human brain. NeuroImage. 2006;29:1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 13.Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, et al. Consistent resting-state networks across healthy subjects. Proc Natl Acad Sci U S A. 2006;103(37):13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, et al. Reduced resting-state brain activity in the 'default network' in normal aging. Cereb Cortex. 2007 doi: 10.1093/cercor/bhm207. in press. [DOI] [PubMed] [Google Scholar]
- 15.DeVoogd TJ. Endocrine modulation of the development and adult function of the avian song system. Psychoneuroendocrinology. 1991;16:41–66. doi: 10.1016/0306-4530(91)90070-a. [DOI] [PubMed] [Google Scholar]
- 16.Dougherty DD, Rauch SL, Deckersbach T, Marci C, Loh R, Shin LM, et al. Ventromedial prefrontal cortex and amygdala dysfunction during an anger induction positron emission tomography study in patients with major depressive disorder with anger attacks. Arch Gen Psychiatry. 2004;61:795–804. doi: 10.1001/archpsyc.61.8.795. [DOI] [PubMed] [Google Scholar]
- 17.Dreher JC, Schmidt PJ, Kohn P, Furman D, Rubinow D, Berman KF. Menstrual cycle phase modulates reward-related neural function in women. Proc Natl Acad Sci. 2007;104:2465–2470. doi: 10.1073/pnas.0605569104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drevets WC, Price JL, Bardgett ME, Reich T, Todd RD, Raichle ME. Glucose metabolism in the amygdala in depression: relationship to diagnostic subtype and plasma cortisol levels. Pharmacol Biochem Behav. 2002;71(3):431–447. doi: 10.1016/s0091-3057(01)00687-6. [DOI] [PubMed] [Google Scholar]
- 19.Dudley CA, Lee Y, Moss RL. Electrophysiological identification of a pathway from the septal area to the medial amygdala: sensitivity to estrogen and luteinizing hormone-releasing hormone. Synapse. 1990;6:161–168. doi: 10.1002/syn.890060207. [DOI] [PubMed] [Google Scholar]
- 20.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 21.Friston KJ, Worsley KJ, Frackowiak RSJ, Mazziotta JC, Evans AC. Assessing the significance of focal activations using their spatial extent. Hum Brain Mapp. 1994;1:210–220. doi: 10.1002/hbm.460010306. [DOI] [PubMed] [Google Scholar]
- 22.Ganguli H, Singh A, Brar J, Carter C, Mintun M. Hydrocortisone induced regional cerebral activity changes in schizophrenia: a PET scan study. Schizophrenia Research. 2002;56:241–247. doi: 10.1016/s0920-9964(01)00219-5. [DOI] [PubMed] [Google Scholar]
- 23.Gazzaley A, Kay S, Benson DL. Dendritic spine plasticity in hippocampus. Neuroscience. 2002;111:853–862. doi: 10.1016/s0306-4522(02)00021-0. [DOI] [PubMed] [Google Scholar]
- 24.Goldstein JM, Jerram M, Poldrack R, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. J Neurosci. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hay AG, Bancroft J, Johnstone EC. Affective symptoms in women attending a menopause clinic. Br J Psychiatry. 1994;164(4):513–516. doi: 10.1192/bjp.164.4.513. [DOI] [PubMed] [Google Scholar]
- 26.Kawakami M, Akema T, Ando S. Electrophysiological studies on the neural networks among estrogen and progesterone effective brain areas on lordosis behavior of the rat. Brain Res. 1979;169:287–301. doi: 10.1016/0006-8993(79)91031-x. [DOI] [PubMed] [Google Scholar]
- 27.Lacreuse A, Chhabra RK, Hall MJ, Herndon JG. Executive function is less sensitive to estradiol than spatial memory: performance on an analog of the card sorting test in ovariectomized aged rhesus monkeys. Behav Processes. 2004;67:313–319. doi: 10.1016/j.beproc.2004.05.004. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann ML, Erskine MS. Glutamatergic stimulation of the medial amygdala induces steroid dependent c-fos expression within forebrain nuclei responsive to mating stimulation. Neuroscience. 2005;136:55–64. doi: 10.1016/j.neuroscience.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 29.Li CS, Kaba H, Saito H, Seto K. Oestrogen infusions into the amygdala potentiate excitatory transmission from the accessory olfactory bulb to tuberoinfundibular arcuate neurones in the mouse. Neurosci Lett. 1992;143:48–50. doi: 10.1016/0304-3940(92)90230-5. [DOI] [PubMed] [Google Scholar]
- 30.Maki PM. Estrogen effects on the hippocampus and frontal lobes. Int J Fertil Womens Med. 2005;50:67–71. [PubMed] [Google Scholar]
- 31.Mazoyer B, Zago L, Mellet E, Bricogne S, Etard O, Houdé O, et al. Cortical networks for working memory and executive functions sustain the conscious resting state in man. Brain Res Bull. 2001;54(3):287–298. doi: 10.1016/s0361-9230(00)00437-8. [DOI] [PubMed] [Google Scholar]
- 32.Moran M. Distinction Between Mood, Affect Eludes Many Residents. Psychiatric News. 2003;38:10. [Google Scholar]
- 33.Osterlund MK, Keller E, Hurd YL. The human forebrain has discrete estrogen receptor alpha messenger RNA expression: high levels in the amygdaloid complex. Neuroscience. 2000;95:333–342. doi: 10.1016/s0306-4522(99)00443-1. [DOI] [PubMed] [Google Scholar]
- 34.Ottowitz WE, Dougherty DD, Sirota A, Niaura R, Rauch SL, Brown WA. Neural and endocrine correlates of sadness in women: implications for neural network regulation of HPA activity. J Neuropsychiatry Clin Neurosci. 2004a;16:446–455. doi: 10.1176/jnp.16.4.446. [DOI] [PubMed] [Google Scholar]
- 35.Ottowitz WE, Dougherty DD, Fischman AF, Hall JE. PET studies in postmenopausal women reveal a decrease in cerebral metabolic rate in the hypothalamic area associated with estrogen negative feedback. The Endocrine Society Program and Abstracts; Proceedings of the 86th Annual Endocrine Society; June 16–19, 2004; New Orleans, Louisiana. 2004b. p. 139. [Google Scholar]
- 36.Pegna AJ, Caldara-Schnetzer AS, Perrig SH, Lazeyras F, Khatib A, Mayer E, et al. Is the right amygdala involved in visuospatial memory? Evidence from MRI volumetric measures. European Neurology. 2002;47:148–155. doi: 10.1159/000047973. [DOI] [PubMed] [Google Scholar]
- 37.Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Phil Trans R Soc B. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reiman EM, Armstrong SM, Matt KS, Mattox JH. The application of positron emission tomography to the study of the normal menstrual cycle. Hum Reprod. 1996;11:2799–2805. doi: 10.1093/oxfordjournals.humrep.a019214. [DOI] [PubMed] [Google Scholar]
- 40.Romano-Torres M, Phillips-Farfan BV, Chavira R, Rodriguez-Manzo G, Fernandez-Guasti A. Relationship between sexual satiety and brain androgen receptors. Neuroendocrinology. 2007;85:16–26. doi: 10.1159/000099250. [DOI] [PubMed] [Google Scholar]
- 41.Shaywitz SE, Shaywitz BA, Pugh KR, Fulbright RK, Skudlarski P, Mencl WE, et al. Effect of estrogen on brain activation patterns in postmenopausal women during working memory tasks. JAMA. 1999;281:1197–1202. doi: 10.1001/jama.281.13.1197. [DOI] [PubMed] [Google Scholar]
- 42.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 43.Skladchikova G, Ronn LC, Berezin V, Bock E. Extracellular adenosine triphosphate affects neural cell adhesion molecule (NCAM)-mediated cell adhesion and neurite outgrowth. J Neurosci Res. 1999;57(2):207–218. doi: 10.1002/(SICI)1097-4547(19990715)57:2<207::AID-JNR6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 44.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 45.Taylor AE, Whitney H, Hall JE, Martin K, Crowley WF. Midcycle levels of sex steroids are sufficient to recreate the follicle stimulating hormone but not the leutenizing hormone mid-cycle surge: evidence for the contribution of other ovarian factors to the surge in normal women. J Clin Endocrinol Metab. 1995;80:1541–1547. doi: 10.1210/jcem.80.5.7744998. [DOI] [PubMed] [Google Scholar]
- 46.Taylor AH, Al-Azzawi F. Immunolocalisation of oestrogen receptor beta in human tissues. J Mol Endocrinol. 2000;24:145–155. doi: 10.1677/jme.0.0240145. [DOI] [PubMed] [Google Scholar]
- 47.Wager TD, Hernandez L, Jonides J, Lindquist M. Elements of functional neuroimaging. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. 4th ed. Cambridge: Cambridge University Press; 2007. pp. 19–55. [Google Scholar]
- 48.Wong M, Moss RL. Modulation of single-unit activity in the rat medial amygdala by neurotransmitters, estrogen priming, and synaptic inputs from the hypothalamus and midbrain. Synapse. 1992;10:94–102. doi: 10.1002/syn.890100203. [DOI] [PubMed] [Google Scholar]
- 49.Young EA, Midgley AR, Carlson NE, Brown MB. Alteration in the hypothalamic-pituitary-ovarian axis in depressed women. Arch Gen Psychiatry. 2000;57(12):1157–1162. doi: 10.1001/archpsyc.57.12.1157. [DOI] [PubMed] [Google Scholar]