Abstract
There is great interest in the potential of the human endocrine pancreas for regeneration by β-cell replication or neogenesis. Our aim was to explore this potential in adult human pancreases and in both islet and exocrine tissue transplanted into mice. The design was to examine pancreases obtained from cadaver donors, autopsies and fresh surgical specimens and compare these findings with those obtained from islet and duct tissue grafted into the kidney.
Islets and exocrine tissue were transplanted into normoglycemic ICR/SCID mice and studied 4 and 14 wk later. β-cell replication as assessed by double staining for insulin and Ki67 was 0.22 ± 0.03 % at 4 wk and 0.13 ± 0.03 % at 14 wk. In contrast, no evidence of β-cell replication could be found in 11 cadaver donor and 10 autopsy pancreases. However, Ki67 staining of β-cells in frozen sections obtained at surgery was comparable to that found in transplanted islets.
Evidence for neogenesis in transplanted pancreatic exocrine tissue was supported by finding β-cells within the duct epithelium, and the presence of cells double stained for insulin and cytokeratin 19 (CK19). However, β-cells within the ducts never constituted more than 1% of the CK19 positive cells. With confocal microscopy, 7 of 12 examined cells expressed both markers, consistent with a neogeneic process. Mice with grafts containing islet or exocrine tissue were treated with various combinations exendin-4, gastrin and epidermal growth factor; none increased β-cell replication or stimulated neogenesis.
In summary, human β-cells replicate at a low level in islets transplanted into mice and in surgical pancreatic frozen sections but rarely in cadaver donor or autopsy pancreases. The absence of β-cell replication in many adult cadaver or autopsy pancreases could, in part, be an artifact of the postmortem state. Thus, it appears that adult human β-cells maintain a low level of turnover through replication and neogenesis.
Keywords: diabetes, turnover, β-cell neogenesis, β-cell replication
Introduction
β-cell deficiency is fundamental to the pathogenesis of both type 1 and type 2 diabetes. In type 1 diabetes β-cells are decimated by autoimmunity and in type 2 diabetes β-cell mass is reduced by 40–60 % (4), but normoglycemia can be restored in both by β-cell transplantation. This important proof-of-principle has been most clearly shown with islet transplantation (24) and for type 2 diabetes with pancreas transplantation (15). The major obstacles preventing widespread clinical application are in controlling immune destruction and finding an adequate supply of β-cells.
Impressive progress has been made in directing human embryonic stem cells to mature functional β-cells (12). Considerable attention has also been focused on determining the capacity of the adult human pancreas for β-cell regeneration either by stimulating β-cell replication or by forming new β-cells from a precursor population through neogenesis. In rodents, β-cell replication can be active enough for substantial regeneration (16), and neogenesis from duct precursor cells has the potential to generate new islets (1). In humans, β-cell replication in most adult pancreas appears to be negligible (4,6,8,17) and there is evidence for some neogenesis (1,3). A major question is whether meaningful regeneration of β-cells can be stimulated in human pancreas. This is a particularly important issue because agents, such as glucagon-like peptide-1 (GLP-1) receptor agonists, gastrin, epidermal growth factor (EGF), and islet neogenesis-associated peptide (INGAP), used as single agents or in combination, have been shown to stimulate regeneration in rodents (1). Unfortunately, it is not possible to measure β-cell mass in the pancreas of living human subjects; additionally assessment of β-cell function with measurements of insulin secretion does not accurately reflect β-cell mass.
A model for determining the potential of human β-cell replication and neogenesis is to study human islet and duct tissue transplanted under the kidney capsule of immunocompromised mice. Studies using this model have provided evidence that there is potential for regeneration through β-cell replication and neogenesis (13,26,27,29). The findings of Suarez-Pinzon et al are of particular interest because they found that human pancreatic duct cells in grafts had a large number of cells that were double stained for cytokeratin 19 (CK19) and insulin, and this number increased markedly to over 50% of the CK19 population after stimulation with gastrin and glucagon-like peptide 1 (GLP-1) (26). These findings with double-stained cells provide evidence that neogenesis was active in these grafts and could be stimulated. The aim of the current study is to use the same model to extend these findings.
Methods
Human pancreases from cadaver donors, autopsies and fresh pancreatic tissue obtained at surgery (Table 1)
Table 1.
Sources of pancreatic tissue used for study.
| Pancreas tissue Source | Subject numbers | Use | Tissue preparation and immunostaining |
|---|---|---|---|
| Cadaver Organ Donors | 13 | Islets and exocrine tissue - transplantation into mice | Removal of kidney – PFA and paraffin Staining for insulin, glucagon, cytokeratin, Ki67 and BrdU |
| Cadaver Organ Donors | 11 | Histology of pancreas | PFA and paraffin Staining for insulin, glucagon, Ki67 and BrdU |
| Autopsy Non- diabetic – within 4 hr of death | 7 | Histology of pancreas | PFA and paraffin Staining for insulin and Ki67 |
| Autopsy Diabetic | 3 | Histology of pancreas | PFA and Paraffin Staining for insulin and Ki67 |
| Surgical - Fresh | 10 | Histology of pancreas | Frozen sections Staining for insulin, glucagon and Ki67 |
Human cadaveric pancreases were obtained from brain-dead donors by the New England Organ Bank after obtaining informed consent from donor relatives. Medical records of 13 donors from whom islets were obtained were reviewed for the absence of diabetes, and other parameters including: age, height, and weight. Donor information is shown in Table 2. Additional cadaver donor pancreases obtained from 11 subjects without diabetes were used for immunohistological examination (ages 15–58, mean 42). The cold ischemic time for both groups of cadaver pancreases ranged from 4–16 hr. Additional tissue was evaluated from 10 other pancreases (7 non-diabetic and 3 diabetic) from Sweden, which were obtained for autopsy within four hours of death (see Results). Finally, frozen sections of fresh pancreas were obtained from patients in Sweden undergoing pancreatic surgery. Tissue from 10 patients (ages 33–84, mean 61) were examined for Ki67 staining. Of the 10 patients, none had diabetes, 9 had adenocarcinoma with almost all having Whipple surgery, and one had a questionable insulinoma. The sections studied were all distant from the tumors.
Table 2.
Donor information for isolated islet and exocrine preparations.
| Donor | Age | Male/Female | BMI | % Islet purity |
|---|---|---|---|---|
| H-0623 | 45 | F | 37 | 95 |
| MGH-39 | 34 | M | 29 | 90 |
| MGH-40 | 70 | F | 31 | 80 |
| MGH-40b | 46 | M | 25.1 | 95 |
| MGH-41 | 53 | F | 22.7 | 90 |
| MGH-42 | 50 | M | 28.1 | 80 |
| MGH-44 | 55 | M | 22.9 | 70 |
| MGH-45 | 44 | F | 48.5 | 95 |
| MGH-49 | 60 | F | 24.8 | 95 |
| MGH-50 | 33 | F | 26.4 | 85 |
| MGH-60 | 42 | M | 31.9 | 90 |
| MGH-63 | 44 | M | 40.7 | 90 |
| MGH-68 | 34 | M | 35.4 | 85 |
Human islet isolation and mouse transplantation
Human islets and remaining exocrine tissue were isolated in the Human Islet Isolation Laboratory at Massachusetts General Hospital (MGH) using the standard collagenase/protease digestion method (20). The exocrine tissue immediately after isolation consists almost entirely of acinar and duct cells, but when either cultured or transplanted, the acinar cells in the preparation disappear. Male ICR/SCID mice (Taconic Farms, Inc., Hudson, NY) were anesthetized using ketamine/xylazine and placed on a heated plate for surgery. The left kidney was exposed through a lumbar incision. Then using a Hamilton syringe (Fisher, Pittsburg, PA) and the PE50 polyethylene tubing, 1000 human islet equivalents (IE) or one million cells of the exocrine fraction obtained from the islet isolation procedure, were placed, under the kidney capsule as previously described (5). Both islets and the exocrine fraction were transplanted immediately after the isolation. Mice were about 12 wks old when transplanted and weighed an average of 33 grams. Body weight (BW) and blood glucose (BG) were monitored once per wk at 9 am. BG was measured on blood obtained from the tip of the tail using OneTouch Ultra®2 blood glucose meter (Life Scan, Inc., Milpitas, CA). For almost all experiments, grafts were removed 4 wk after transplantation. However, a small number of grafts were removed at 14 wk to determine if β cell replication was maintained for this longer period of time. All animal experiments were approved by the Joslin Institutional Animal Care and Use Committee.
Streptozocin-treated mice
Diabetes in ICR-SCID mice was induced by a single intraperitoneal (i.p.) injection of streptozocin (STZ) (180 mg/dl STZ, Sigma-Aldrich Co. LLC, St. Louis, MO). Mice with fed blood sugar levels > 350 mg/dl at one wk after injection were considered diabetic and suitable for transplant.
Intraperitoneal glucose tolerance test
For intraperitoneal glucose tolerance test (IPGTT) mice fasted overnight were injected i.p. with glucose at 2 g/kg BW. Blood glucose from the tail was measured as above at 0, and 15, 30, 60 and 120 min after glucose injection.
Drug treatment of mice with grafts
There were 6 treatment groups using non-STZ treated mice (Table 3):
Table 3.
Drug treatment of non-diabetic mice carrying transplants of human pancreatic islets or exocrine tissue.
| Group # | Treatment | Graft | Donors/Recipients | Drug regimen | Time of graft collection |
|---|---|---|---|---|---|
| 1 | Gastrin & Exendin-4 | Islets | 2 donors, 4 recipients | Gastrin bid final 3 wk, Exendin qd final 1 wk. | 4 wk after TX |
| 2 | Gastrin | Islets | 2 donors, 5 recipients | Gastrin bid final 3 wk | 4 wk after TX |
| 3 | Exendin-4 | Islets | 3 donors, 10 recipients | Exendin qd final 1 wk | 4 wk after TX |
| 4 | Gastrin & EGF | Islets | 2 donors, 6 recipients | Gastrin and EGF, both qd for last 10 days | 4 wk after TX |
| 5 | Exendin-4 | Exocrine | 4 donors, 14 recipients | Exendin qd for final 1 wk. | 4 wk after TX |
| 6 | Gastrin & EGF | Exocrine | 2 donors, 6 recipients | Gastrin and EGF, both qd for last 10 days | 4 wk after TX |
Treatment once daily (qd), treatment twice daily (bid). See methods section for further details.
Gastrin and exendin-4 treatment (islet grafts). Mice with grafts of human islets (2 donors, 4 recipients) were treated with gastrin and exendin-4. Gastrin (i.p. at 150 μg/kg BW, (Leu15)-Gastrin I human, Sigma-Aldrich Co. LLC.) was injected twice a day (8 am and 6 pm) for 3 wk starting one wk after transplantation. Exendin-4 (1 nmol/kg BW, Eli Lilly and Company, Indianapolis, IN,) was injected i.p once a day (6 pm) for the final wk before sacrifice.
Gastrin treatment (islet grafts). Mice with grafts of human islets (2 donors, 5 recipients) were treated with gastrin alone as described above.
Exendin-4 treatment (islet grafts). Mice with grafts of human islets (3 donors, 10 recipients) were treated only with exendin-4 for the final wk of 4 wk as described in 1.
Gastrin and epidermal growth factor (EGF) treatment (islet grafts). Mice with grafts of human islets (2 donors, 6 recipients) were treated with gastrin (i.p. at 1 mg/kg) plus epidermal growth factor (EGF) (i.p. 30 μg/kg, Recombinant Human Epidermal Growth Factor, BD Biosciences, San Jose, CA); both were given once per day at 6 pm for the last 10 days of the 4 wk transplant period.
Exendin-4 treatment (exocrine cell grafts). Mice with grafts of pancreatic exocrine cells (4 donors, 14 recipients) were treated with exendin-4 for the final wk of 4 wk as described in 1.
Gastrin and epidermal growth factor (EGF) treatment (exocrine cell grafts). Mice with grafts of pancreatic exocrine cells (2 donors, 6 recipients) were treated with gastrin plus EGF as described in 4.
BrdU injection
Freshly made 5-bromo-2′-deoxyuridine (BrdU) (i.p. 100 mg/kg BW, Sigma-Aldrich Co. LLC) was injected into mice at 8 h intervals 24 h prior to sacrifice.
Tissue processing and immunostaining
Kidneys containing the grafts were excised from anesthetized mice and fixed in 4 % paraformaldehyde (PFA) for 2 hr and stored in PBS at 4 °C until paraffin embedding. Sections (5 μm) were immunostained for insulin, glucagon, Ki67, BrdU, and cytokeratin (CK) 19, and evaluated with the TUNEL assay. After hydration, sections were blocked with an avidin-biotin blocking kit (Vector Laboratories, Inc., Burlingame, CA) per manufacturer’s instructions and normal donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) 1:50 for 1 hr at RT. For Ki67, BrdU and CK19 immunostaining, sections were first soaked in 0.3 % Triton® X-100 (Sigma-Aldrich Co. LLC.) in PBS for 15 min., followed by 2 washes and microwaved for antigen retrieval in citrate buffer pH4.0. Before immunolabelling, fresh frozen sections were fixed in cold PFA for 10 min at −20 °C followed by 10 min at 4°C, and then by 20 min in PBS at room temperature (RT) and blocked with 0.2% glycerol in PBS for 5 min. Then the slides were treated with an avidin/biotin blocking kit and normal donkey serum as described above.
Primary antibodies were: rabbit anti-glucagon antibody (AB932, Millipore, Billerica, MA, 1:3000, overnight at 4 °C), mouse anti-human Ki67 (BD Pharmingen, 556003, San Jose, CA; 1:50 overnight at 4 °C or 1:200 for frozen sections), mouse anti-bromo-deoxyuridine (M0744, Dako North America, Inc., Carpinteria, CA; 1:50 overnight at 4 °C) and mouse anti-human CK19 (M0888, Dako North America, Inc., 1:50, overnight at 4 °C). Secondary antibodies used for 1 h at RT were: biotinylated-donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, Inc.; 1:400) or biotinylated-donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.; 1:200) followed by streptavidin-conjugated Alexa Fluor® 488 (Invitrogen, Carlsbad, CA; 1:400) or for CK19, Cy3-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories, Inc.; 1:200) for 1 h.
Then, to visualize the hormone-expressing cells, the sections were incubated for 90 min at RT with guinea pig anti-human insulin (AB2440, Millipore; 1:200 in PBS) or rabbit anti-glucagon antibody (AB932, Millipore; 1:200) followed by washes and incubation with Texas Red conjugated anti-species specific IgG (Jackson ImmunoResearch Laboratories, Inc.; 1:200) or Fluorescein (FITC)-conjugated donkey anti-species specific IgG (Jackson ImmunoResearch Laboratories, Inc.; 1:400) for 90 min at RT.
TUNEL-insulin
In situ Cell Death Detection Kit, Fluorescein (Roche Diagnostics, Indianapolis, IN) was used for TUNEL, following manufacturer’s instructions using microwave retrieval and then immunostaining for insulin as described above.
Image capturing and analysis
Images were taken using an Olympus BH2 scope connected with Olympus DP71 camera (Olympus America Inc., Center Valley, PA) using DP controller program. Pictures were opened with Adobe Photoshop and cells were counted manually. Alternatively, confocal images were obtained using LSM-710 confocal microscopy (Carl Zeiss Microscopy, LLC, Thornwood, NY) with optical Z-sections every 0.45 μm.
Statistics
Data are reported as mean ± standard error (SEM). Correlations were made with the Pearson product moment correlation coefficient. Statistical significance was assessed with the two-tailed Student’s t-test.
RESULTS
IPGTTs in ICR-SCID mice transplanted with human islets
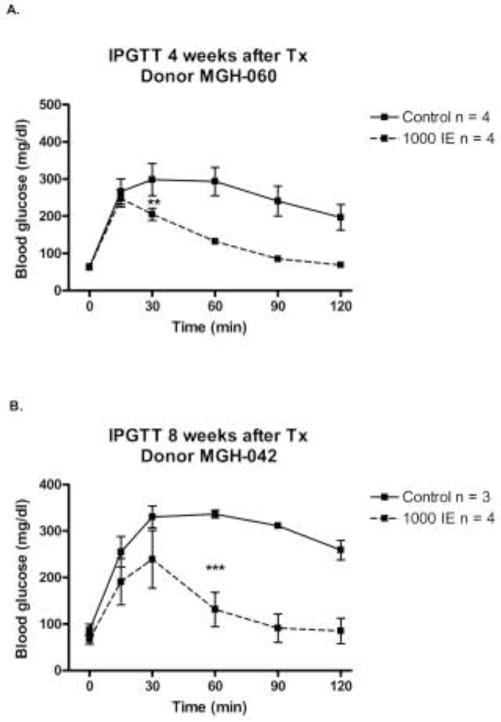
Intraperitoneal glucose tolerance tests (IPGTT) were performed in non-STZ treated ICR-SCID mice 4 and 8 wk after transplantation. In mice engrafted with 1000 IE human islets from a single islet isolation (MGH-060) for 4 wk (Figure 1a), glucose clearance after glucose injection was clearly more efficient than in control mice, being significant (p<0.01) at 30 min and after. Mice transplanted with another batch of human islets (MGH-042, 1000 IE) for 8 wk had similarly improved blood glucose clearance after the glucose challenge (Figure 1b). Fasting glucose levels did not differ significantly between these two groups (61 ± 5 in transplanted vs.74 ± 7 mg/dl in the non-transplanted controls, p = 0.14). Fed blood glucose levels measured simultaneously 24 times (using 8 transplanted and 7 control mice) were lower in the transplanted group compared to the control mice, 155 ± 3 and 169 ± 4 respectively, p = 0.008.
Figure 1.
Intraperitoneal glucose tolerance test (IPGTT) performed in non-STZ ICR-SCID mice transplanted with 1000 IE human islets and non-transplanted mice (controls). Glucose (2 g/kg BW) was injected at time 0. A. IPGTT after 4 wk transplantation (Tx) of human islets from donor MGH-060. Mice were 16 wk old at time of IPGTT. B. IPGTT after 8 wk Tx of human islets from donor MGH-042. Mice were 18 wk of age. Results are expressed as mean ± SEM. ** p < 0.01 and *** p < 0.001.
Insulin and glucagon ratio in grafts of ICR-SCID mice transplanted with human islets
The ratio of insulin-stained to glucagon-stained cells was determined in grafts of non-STZ treated mice transplanted with human islets (1000 IE) under the kidney capsule (Figure 2). Table 4 shows that the β/α-cell ratio ranged from 0.47 to 4.30 with a mean of 1.84 ± 0.33 in grafts removed 4 wk after transplantation and from 1.32 to 2.40 with a mean of 1.73 ± 0.24 in grafts removed 14 wk after transplantation. For two donors, grafts were assessed at both 4 and 14 wk and when compared, the β/α cell -ratios remained essentially the same. There was no correlation between the β/α-cell ratio and either BMI (r2 = 0.01, p = 0.75) or age (r2 = 0.11, p = 0.34) of the donors.
Figure 2.
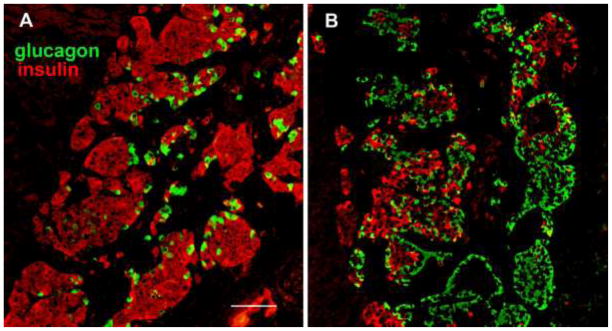
Human islets engrafted under the mouse kidney capsule immunostained for insulin (red) and glucagon (green). Islets from donor MGH-39 (A) and donor MGH-63 (B). Magnification bar = 100 μm.
Table 4.
Insulin and glucagon ratio (β and α cell ratio) from human islets transplanted under the kidney capsule of non-STZ normoglycemic ICR-SCID mice.
| Donor | Insulin/Glucagon 4 weeks | number grafts/Total insulin cells | Insulin/Glucagon 14 weeks | number grafts/Total insulin cells |
|---|---|---|---|---|
| MGH-39 | 4.30 ± 0.83 | 5/8654 | - | - |
| MGH-40 | 1.14 ± 0.21 | 7/7755 | - | - |
| MGH-40b | 1.95 ± 0.27 | 6/6082 | - | - |
| MGH-41 | 1.09 ± 0.20 | 4/2143 | - | - |
| MGH-42 | - | - | 1.32 ± 0.17 | 6/6970 |
| MGH-44 | 2.46 ± 0.81 | 3/1764 | - | - |
| MGH-45 | 2.40 ± 0.52 | 4/5153 | 2.40 ± 0.40 | 3/3832 |
| MGH-49 | - | - | 1.70 ± 0.60 | 6/7880 |
| MGH-50 | 1.70 ± 0.40 | 4/9758 | - | - |
| MGH-60 | 1.34 ± 0.11 | 3/2362 | 1.50 ± 0.20 | 2/1844 |
| MGH-63 | 0.47 ± 0.06 | 4/1181 | - | - |
| MGH-68 | 1.54 | 1/477 | - | - |
| Mean | 1.84 | 4/4533 | 1.73 | 4/5131 |
| SEM | 0.33 | 0.24 |
Number of grafts corresponds to the number of mice transplanted with islets from the same donor, stained for insulin and glucagon, and the total of insulin positive cells counted from all the grafts. Results are expressed as a mean ± SEM.
β-cell replication in grafts after 4 and 14 wk transplantation
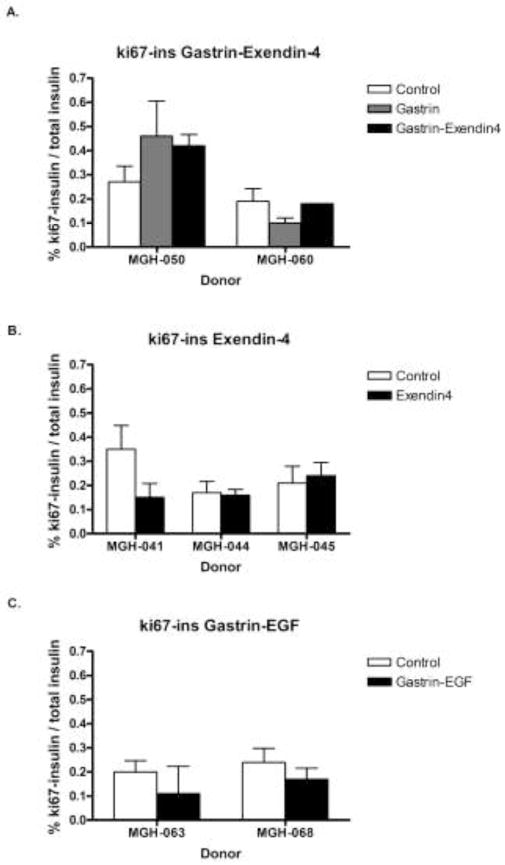
β-cells replicated at a low frequency in grafts 4 and 14 wk after transplantation into non-STZ treated recipients. Replication of β-cells was identified by double immunostaining for the replication marker Ki67 and insulin (Figure 3). The percentage of insulin positive cells replicating (Ki67 positive) in grafts ranged from 0.06 % to 0.42 % after 4 weeks transplantation, while being 0.08 % – 0.22 % after 14 weeks transplantation, as shown in Table 5. There is no correlation between the percentage on Ki67+insulin+ cells and either BMI (r2 = 0.007, p= 0.8) or age of the donors (r2 = 0.23, p= 0.15). β-cell replication was also assessed by BrdU incorporation 24 h after administration (Table 6). The percentage of BrdU+insulin+ cells 4 wk after transplantation in the control non-treated group and EGF-gastrin group was 0.17% ± 0.06 and 0.15% ± 0.04, respectively. Gastrin alone, gastrin and exendin-4, exendin-4 alone, and gastrin and EGF treatments did not increase β-cell replication in human islets engrafted into non-STZ mice, as shown in Figure 4.
Figure 3.
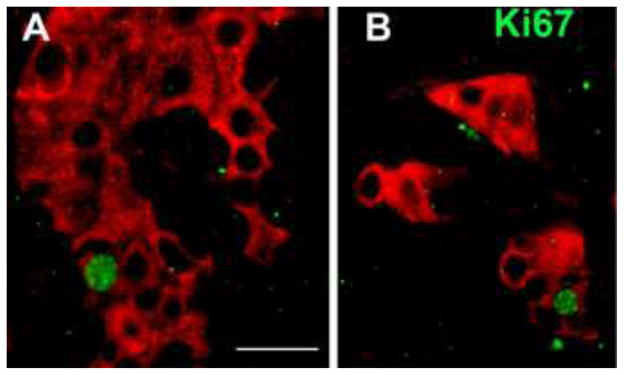
Human islets engrafted under the mouse kidney capsule immunostained for insulin (red) and Ki67 (green). Magnification bar = 20 μm.
Table 5.
Percentage of double positive cells for Ki67 and insulin in grafts from non-STZ ICR-SCID mice transplanted with human islets after 4 and 14 wk.
| Donor | % Ki67-Insulin 4 weeks | number grafts/Total insulin cells | % Ki67-Insulin 14 weeks | number grafts/Total insulin cells |
|---|---|---|---|---|
| MGH-39 | 0.42 ± 0.03 | 4/9598 | - | - |
| MGH-40 | 0.11 ± 0.03 | 7/15471 | - | - |
| MGH-40b | 0.06 ± 0.01 | 6/12764 | - | - |
| MGH-41 | 0.35 ± 0.10 | 3/5673 | - | - |
| MGH-42 | - | - | 0.10 ± 0.03 | 6/11167 |
| MGH-44 | 0.17 ± 0.05 | 3/7269 | - | - |
| MGH-45 | 0.21 ± 0.07 | 3/7433 | 0.10 ± 0.03 | 3/6792 |
| MGH-49 | - | - | 0.08 ± 0.01 | 5/14746 |
| MGH-50 | 0.27 ± 0.07 | 4/9738 | - | - |
| MGH-60 | 0.19 ± 0.05 | 3/6789 | 0.22 ± 0.03 | 2/5438 |
| MGH-63 | 0.20 ± 0.05 | 3/6568 | - | - |
| MGH-68 | 0.24 ± 0.06 | 3/6725 | - | - |
| Mean | 0.22 | 4/8803 | 0.13 | 4/9536 |
| SEM | 0.03 | 0.03 |
There are no significant differences between % Ki67-insulin at 4 vs. 14 wk after transplantation (p = 0.12, unpaired T-test). Results are expressed as mean ± SEM.
Table 6.
Percentage of double positive cells stained for BrdU and insulin in human islet grafts of non-STZ ICR-SCID mice.
| Donor | Control % BrdU-Insulin | number grafts/Total insulin cells | EGF-Gastrin % BrdU-Insulin | number grafts/Total insulin cells |
|---|---|---|---|---|
| MGH-63 | 0.11 ± 0.04 | 3/6928 | 0.11 ± 0.06 | 3/5242 |
|
| ||||
| MGH-68 | 0.22 ± 0.04 | 3/6725 | 0.19 ± 0.03 | 4/9446 |
|
| ||||
| Mean | 0.17 | 3/6827 | 0.15 | 3/7344 |
| SEM | 0.06 | 0.04 | ||
Grafts from non-STZ treated mice were injected with BrdU 24 prior to sacrifice in 8 hr intervals. The table shows the results from control and EGF-Gastrin treated mice and data are expressed as mean ± SEM.
Figure 4.
Percentage of double positive Ki67 and insulin cells in human islet grafts transplanted under the kidney capsule of non-STZ ICR-SCID mice for 4 wk and treated with: A. Gastrin alone,150 μg/kg, twice a day (8 am and 6 pm) for 3 wk or gastrin (same dosage) + exendin-4 1 nmol/kg once a day (6 pm) for 1 wk; B. Exendin-4, 1 nmol/kg, once a day (6 pm) for 1 wk; C. EGF, 30 μg/kg, and gastrin, 1 mg/kg, once a day for 10 days. Control bars represent mice transplanted with human islets but no drug treatment. Results are expressed as mean ± SEM. D. Number of grafts and insulin positive cells counted in tabular form.
We also assessed β-cell death by TUNEL-insulin double-staining from non-treated controls at 4 wk and 14 wk, and groups treated with exendin-4, gastrin, gastrin + exendin-4, gastrin + EGF at 4 wk. The percentage results of TUNEL+insulin+ cells in these groups were 0.14 ± 0.02, 0.12 ± 0.02, 0.10 ± 0.03, 0.21 ± 0.05, 0.14 (n=1), 0.07 ± 0.05, respectively. For each of these conditions between 2137 and 6286 insulin positive cells were examined. All of these mice were non-STZ-treated.
Examination of human cadaveric and autopsy pancreases, and frozen pancreatic biopsies for β-cell replication
When formalin-fixed paraffin sections of 11 cadaveric pancreases (subject ages 15–58, mean 42) were stained for Ki67 and insulin, no Ki67+insulin+ cells were found after analyzing a total of 596 islets with an average of 40 β-cells per islet. Indeed, no ki67 positive cells were found in any of the sections.
A second group of 7 pancreases from non-diabetes individuals (67–81, mean age 74) obtained within 4 hours after death were available from the archive at Uppsala University and immunolabelled with Ki-67 antibody diluted 1:50 and 1:100, (SP6 rabbit monoclonal, Abcam, Cambridge, UK). We also used microwave - heat-treatment in 0.01M Sodium citrate (20 minutes) for antigen retrieval. This antibody works well on formalin fixed frozen sections (positive control). In addition 3 cases with diabetes were included (65–80, mean age 76). No positive nuclei could be detected in any of the cases.
However, when frozen pancreatic biopsies were similarly double-stained for Ki67 and insulin, 12 Ki67+insulin+ of a total 6630 insulin cells were found (0.18 % Ki67+insulin+). Moreover, all pancreatic frozen biopsies had Ki67 positive cells other than insulin positive cells, which were non-β islet cells, exocrine cells or non-identified cells.
Human islets transplanted into diabetic STZ-treated mice
In a separate experiment, human islets (1000 IE, donor H0623B) were similarly transplanted under the kidney capsule of normoglycemic non-STZ treated and STZ-treated diabetic ICR-SCID mice. One of 6 diabetic mice became normoglycemic one wk after transplantation, remaining normal for 4 wk. The β/α-cell ratio was significantly lower in islets engrafted in diabetic mice than in the normoglycemic recipients (n=6 ; 1.40 ± 0.08 vs 2.94 ± 0.36, p= 0.007). However, no difference was found in β-cell replication as assessed by counting cells double-stained for Ki67 and insulin (Table 7).
Table 7.
Insulin/glucagon ratio and % Ki67-insulin positive cells of human islet grafts from diabetic and non-diabetic ICR-SCID mice.
| Insulin/Glucagon | number grafts/Total insulin cells | % Ki67-Insulin | number grafts/Total insulin cells | |
|---|---|---|---|---|
| Diabetics | 1.40 ± 0.08 | 5/1247 | 0.89 ± 0.25 | 5/2445 |
| Cured | 2.22 | 1/901 | 0.42 | 1/1388 |
| Controls | 2.94 ± 0.36* | 6/3578 | 0.63 ± 0.17 | 5/7856 |
Insulin/glucagon ratio and % Ki67-insulin positive cells in human islet grafts (donor H-0623) transplanted into STZ-treated hyperglycemic mice (Diabetics) and non-STZ treated normoglycemic mice (Controls). Cured refers to a STZ-treated hyperglycemic mouse that became normoglycemic after transplantation. Results expressed as mean ± SEM.
p = 0.007.
β-cell neogenesis from human duct cells
Human exocrine tissue was transplanted under the kidney capsule of normoglycemic non-STZ treated ICR-SCID mice, the grafts were retrieved after 4 wk, and double-staining was performed for cytokeratin (CK) 19 and either insulin or glucagon (Figure 5). By 4 wk no acinar cells could be found and the graft consisted almost entirely of duct cells. Table 8 shows the number of insulin and glucagon cells found within or outside of duct structures as identified by CK19 positive cells. These cells were few in number and there was no evidence that any of the drug treatments increased the frequency of insulin or glucagon positive cells in either location. To look further for evidence of neogenesis, 12 insulin positive cells within the duct epithelium were analyzed to see if they expressed the duct marker CK19 as well. By doing confocal optical z-stacks we found that 7 of these 12 cells expressed both CK19 and insulin (Figure 6).
Figure 5.
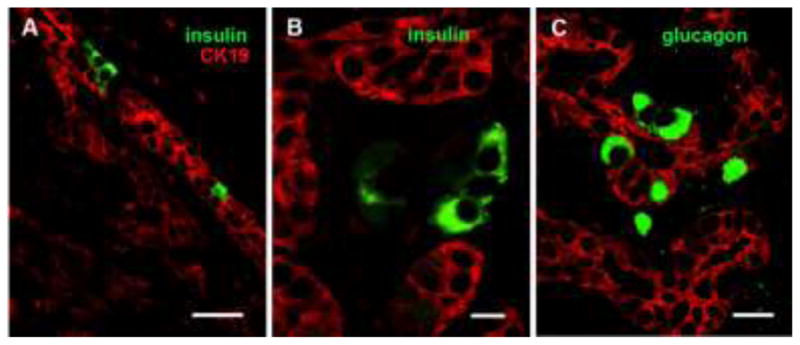
Human exocrine tissue engrafted under kidney capsule had hormone positive cells within the ductal structures. Immunostaining for CK19 (red) for ducts and insulin (green) (A, B) and glucagon (green) (C). In B the Insulin positive cells (green) are outside of the duct (red). Magnification bars = 50 μm (A), 10 μm (B) and 25μm (C)
Table 8.
Exocrine tissue transplanted under the kidney capsule of non-STZ normoglycemic ICR-SCID mice.
| a) Number of insulin positive cells in and out of the duct epithelium.
| |||||||
|---|---|---|---|---|---|---|---|
| Donor | Conditions | Insulin out ducts | Insulin in ducts | n (grafts) | ck19 | Insulin out ducts/ck19% | Insulin in ducts/ck19% |
| MGH- 040 | Control | 25 | 1 | 3 | 2127 | 1.18 | 0.05 |
| exendin-4 | 52 | 1 | 4 | 4032 | 1.29 | 0.02 | |
|
| |||||||
| MGH-040B | Control | 0 | 3 | 4 | 4397 | 0 | 0.07 |
| exendin-4 | 0 | 2 | 2 | 959 | 0 | 0.21 | |
|
| |||||||
| MGH-041 | Control | 0 | 0 | 2 | 1089 | 0 | 0 |
| exendin-4 | 1 | 2 | 4 | 2228 | 0.04 | 0.09 | |
|
| |||||||
| MGH-042 | Control | 7 | 23 | 3 | 4052 | 0.17 | 0.57 |
| exendin-4 | 4 | 12 | 4 | 5154 | 0.08 | 0.23 | |
|
| |||||||
| MGH-063 | Control | 0 | 2 | 1 | 587 | 0 | 0.34 |
| gastrin-EGF | 3 | 5 | 3 | 5158 | 0.06 | 0.10 | |
|
| |||||||
| MGH-068 | Control | 1 | 5 | 3 | 4760 | 0.02 | 0.11 |
| gastrin-EGF | 9 | 9 | 3 | 5066 | 0.18 | 0.18 | |
| b) Number of glucagon positive cells in and out of the duct epithelium.
| |||||||
|---|---|---|---|---|---|---|---|
| Donor | Conditions | Glucagon out ducts | Glucagon in ducts | n (grafts) | ck19 | Glucagon out ducts/ck19% | Glucagon in ducts/ck19% |
| MGH-042 | Control | 1 | 3 | 3 | 2450 | 0.04 | 0.12 |
| exendin-4 | 0 | 10 | 4 | 3619 | 0 | 0.28 | |
|
| |||||||
| MGH-063 | Control | 0 | 5 | 1 | 587 | 0 | 0.85 |
| gastrin-EGF | 2 | 11 | 3 | 5158 | 0.04 | 0.21 | |
|
| |||||||
| MGH-068 | Control | 0 | 2 | 3 | 4760 | 0 | 0.04 |
| gastrin-EGF | 0 | 1 | 3 | 5066 | 0 | 0.02 | |
Figure 6.
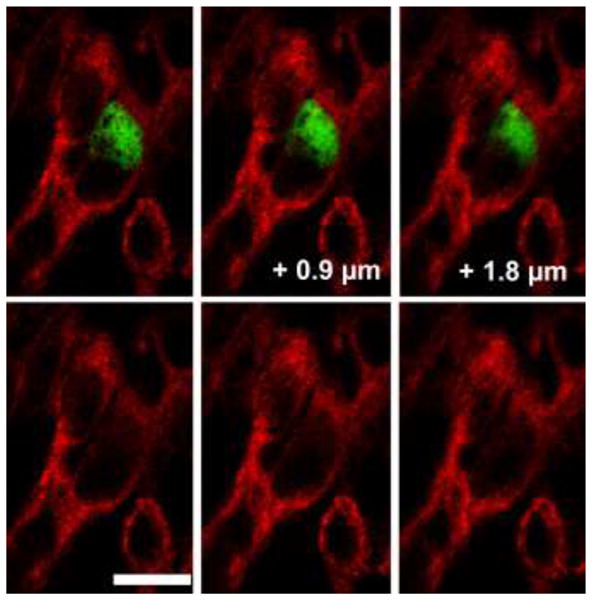
Human exocrine tissue engrafted under kidney capsule had some insulin positive cells within the ductal structures that co-expressed cytokeratin 19, the marker of duct cells, suggesting an on-going process of differentiation. CK19 (red); Insulin (green). Three optical sections of a confocal Z stack separated by 0.9 μm are shown in the upper panel. Bottom panel only shows the red channel to visualize the CK19 immunostaining. Magnification bar = 10 μm.
Discussion
The present study examines grafts of human islets transplanted under the kidney capsule of immunocompromised ICR-SCID mice with regard to cell composition and the regenerative potential of β-cells by replication and neogenesis.
There has been a surprising amount of disagreement about β-cells as a percent of total islet cells in the adult human pancreas with estimates varying from 50 to over 80%, as has been recently reviewed (18). Our analysis using electron microscopic determination of islet cell type on preparations of isolated human islets obtained a value of 73.6 % (18). In our present study the ratio of insulin to glucagon stained cells in human islet grafts ranged from 0.47 – 4.3 with a mean of 1.84 (table 2). Assuming that about 5 – 10 % of islet cells are neither α- or β-cells, our data indicate that about 60 % of the islet cells in the grafts were β-cells, which being lower than 74 % suggests that β-cells were preferentially lost. This is not very surprising because it is generally thought that β-cells are more fragile than other cell types. For example, streptozotocin kills β-cells but not hepatocytes that have a similar GLUT2 glucose transport mechanism. As noted above, the β-/α-cell ratios varied substantially (ranging from 4.30 to 0.47). The paucity of β-cells in some grafts could be due to a pathological process in the donor, such as type 2 diabetes that was not recognized during donor selection leading to β-cell reduction, or perhaps resulting from loss of β-cells during the trauma of isolation. It can be noted that a study of α-cell mass in adult human pancreases found surprising variability (7). Interestingly, the survival of human α-cells in the grafts is very different from our findings with transplanted rat islets, in which there was a marked preferential loss of α-cells in the grafts over a 12 wk period (9).
β-cell replication, albeit at a low level, was found in all of our grafts containing human islets, and the percent of β-cells with Ki67 staining averaged 0.22 %, which is in general agreement with other studies (13,29). In some of our grafts the Ki67 staining of β-cells was over 0.6%. Ki67 appears to be a valid way to mark cells in cell cycle and provides results in general agreement with results obtained with BrdU in our study and proliferating cell nuclear antigen (PCNA) and mini-chromosome maintenance protein (MCM-7) by others (10). The presence of β-cells replicating in all the grafts is in sharp contrast to the complete lack of replication found in the donor cadaveric and autopsy pancreases we examined. The remarkable rarity of dividing β-cells in adult human pancreases has been reported in other studies (4,8,17,21). In a study using cadaver pancreases, β-cell replication as determined with Ki67 was more prominent in younger donors, but only a minority of pancreases from older donors had measureable replication (8). In contrast, a frequency of Ki67 staining similar to what we see in our grafts was reported in most pancreases in some studies (11,19). It is notable that in a study examining tissue obtained after partial pancreatectomy surgery in adults, the Ki67 positivity was in the range of 0.5%. Perhaps the values were so high because the tissue was fixed a very short time after surgery (14).
The lack of replication of β-cells and even acinar cells in our donor cadaveric and autopsy pancreases raised questions about the complexities of Ki67 staining as has been discussed elsewhere (2,23). However, we could find Ki67 in lymph nodes as a positive control, and others have found Ki67 positive β-cells in pancreases of younger cadaver donors prepared in the same way as the older donors that had no evidence of β-cell replication (8). Interestingly, all our frozen sections from human pancreatic biopsies had islet and non-islet ki67 positive cells, and β-cells with Ki67 staining averaged 0.18 %, similar to what we found in our grafts (0.22 %). This raises the important question as to whether cadaveric donor and autopsy pancreases lose their Ki67 staining because of events surrounding illness and death, and/or because of the way pancreases are handled and preserved before being processed for histology. Thus, it is possible that after a person’s death β cells and other cells can remain alive for a period of time but are stressed enough for replication to slow. Then, if rescued by being placed into an in vivo transplant environment, the cells regain their health and can again enter the cell cycle. Therefore, β-cell turnover from replication in adult pancreases may be higher than previously appreciated and high enough to have an impact.
β-cells in the grafts are exposed to glucose levels higher than found in normoglycemic humans because mice normally have higher glucose levels than humans. This is related to the interesting finding that in the IPGTTs glucose is cleared more rapidly in the mice carrying transplanted human β-cells than controls, which presumably results from the human β-cells having a lower set-point than mouse β-cells. Thus, there is glycemic stimulation of these cells, which leads to continued insulin secretion that may be linked to increased replication, similar to that seen in a mouse model employing glucose infusions (13). To determine if the observed replication was dependent on the early 4 wk time point, we examined grafts 14 wk after transplantation and found replication at levels only modestly lower than at 4 wk in all grafts examined.
We also sought to determine if replication could be stimulated by a combination of exendin-4 and gastrin or of gastrin and EGF. These regimens were chosen after consideration of the literature and our own experience. Exendin-4 has been shown to stimulate β-cell replication in rats (30). None of these treatments could be found to stimulate replication in our human β-cells. Because the treatments were given from 10 days to 3 wk, it is possible we missed an earlier effect. Nonetheless, although we did not measure β-cell mass, we are concerned that prospects for increasing β-cell mass using these treatments in human islet transplantation are not encouraging.
We also asked if neogenesis from duct cells to islet cells was present and could be stimulated. We have previously shown that purified human duct cells could produce a small number of insulin-containing cells when transplanted under the kidney capsule of mice (31). There are now several studies showing that various agents can stimulate neogenesis of islet cells from human and rodent pancreatic tissue. Exendin-4, an analog of glucagon-like peptide 1 (GLP-1) has been shown to increase both β-cell replication and neogenesis in diabetic and control rats (30). Stimulation of neogenesis in mice by combination treatment with gastrin and epidermal growth factor (EGF) has also been reported (22). Even gastrin alone has been found to increases β-cell mass, by increasing both β-cell replication and neogenesis in rats after 95 % pancreatectomy (28). With regard to human pancreatic tissue transplanted under the kidney capsule of immunocompromised mice, combinations of gastrin plus EGF and of gastrin plus GLP-1 have been reported to stimulate neogenesis from pancreatic duct cells (25,26). Strikingly, after 5 wk of treatment with gastrin and GLP 1, 54 % of the CK19 positive cells in grafts were double stained for insulin as compared with only 7 % in the vehicle-treated mice (26).
In our study of neogenesis we transplanted tissue that was predominantly exocrine but not purified and treated the mice with exendin-4 for one wk prior to sacrifice at 4 weeks or with gastrin plus EGF for 10 days prior to sacrifice. We characterized insulin-positive cells outside the duct structures as most likely being β-cells that were introduced at the time of transplantation. Insulin positive cells within duct structures were considered better candidates for having been generated by neogenesis. Consistent with a neogeneic process, we found more glucagon positive cells within the duct structures than outside. We were particularly interested in finding cells that were double stained for CK19 and insulin, as these could be markers for neogenesis. Of 12 insulin positive cells in ducts that were carefully examined with optical sectioning by confocal microscopy, 7 were double positive for insulin and CK19. We interpret this as evidence of a slow process of β-cell neogenesis from duct cells in a transplant site. Our results are very different than those of Suarez-Pinzon et al (26). We found less than 1 % of all insulin positive cells associated with ducts and only about half of these were double stained for insulin and CK19, which is far less than the 7 % seen in their control vehicle-treated grafts. Our stimulated values for CK19+insulin+ cells were far lower than theirs; the shorter duration of our treatments seems like only a partial explanation for this major discrepancy.
In conclusion, our grafts of human pancreatic tissue studied 4 and 14 wk after transplantation into normoglycemic mice show evidence of low levels of β-cell replication and neogenesis, the latter suggested by cells double stained for insulin and CK19. However, neither process could be stimulated by short-term treatment with agents that included combinations of exendin-4, gastrin and EGF. Continued generation of β-cells probably counters what appears to be a slow constant rate of β-cell death as evidenced by TUNEL staining results. This study supports the concept that human β-cells have a low level of turnover and the possibility that regeneration could be stimulated with a therapeutic intervention.
Acknowledgments
The authors thank Alevtina Pinkhasov and Chris Cahill for expert technical assistance. This study was supported by grants from the Juvenile Diabetes Research Foundation (GCW) and the National Institutes of Health NIH R01 DK 66056 and DK 93909 (SBW), P30 DK36836 Joslin Diabetes Research Center (DRC) Advanced Microscopy Core, as well as the Diabetes Research and Wellness Foundation, and a fellowship from Plan Nacional I-D+I 2008-2011 FECYT-Ministerio de Ciencia e Innovación, Spain (FCG). Swedish Research Council (GTW5343), the Swedish Diabetes Association and the Family Ernfors Fund.
Footnotes
Disclosure of potential conflicts of interest: The authors have no conflicts of interest.
References
- 1.Bonner-Weir S, Li WC, Ouziel-Yahalom L, Guo L, Weir GC, Sharma A. Beta-cell growth and regeneration: replication is only part of the story. Diabetes. 2010;59(10):2340–2348. doi: 10.2337/db10-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bullwinkel J, Baron-Luhr B, Ludemann A, Wohlenberg C, Gerdes J, Scholzen T. Ki-67 protein is associated with ribosomal RNA transcription in quiescent and proliferating cells. J Cell Physiol. 2006;206(3):624–635. doi: 10.1002/jcp.20494. [DOI] [PubMed] [Google Scholar]
- 3.Butler AE, Cao-Minh L, Galasso R, Rizza RA, Corradin A, Cobelli C, Butler PC. Adaptive changes in pancreatic beta cell fractional area and beta cell turnover in human pregnancy. Diabetologia. 2010 doi: 10.1007/s00125-010-1809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003;52(1):102–110. doi: 10.2337/diabetes.52.1.102. [DOI] [PubMed] [Google Scholar]
- 5.Davalli AM, Ogawa Y, Scaglia L, Wu YJ, Hollister J, Bonner-Weir S, Weir GC. Function, mass, and replication of porcine and rat islets transplanted into diabetic nude mice. Diabetes. 1995;44(1):104–111. doi: 10.2337/diab.44.1.104. [DOI] [PubMed] [Google Scholar]
- 6.Gregg BE, Moore PC, Demozay D, Hall BA, Li M, Husain A, Wright AJ, Atkinson MA, Rhodes CJ. Formation of a Human beta-Cell Population within Pancreatic Islets Is Set Early in Life. J Clin Endocrinol Metab. 2012;97(9):3197–3206. doi: 10.1210/jc.2012-1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henquin JC, Rahier J. Pancreatic alpha cell mass in European subjects with type 2 diabetes. Diabetologia. 2011;54(7):1720–1725. doi: 10.1007/s00125-011-2118-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.In’t Veld P, De Munck N, Van Belle K, Buelens N, Ling Z, Weets I, Haentjens P, Pipeleers-Marichal M, Gorus F, Pipeleers D. Beta-cell replication is increased in donor organs from young patients after prolonged life support. Diabetes. 2010;59(7):1702–1708. doi: 10.2337/db09-1698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.King AJ, Fernandes JR, Hollister-Lock J, Nienaber CE, Bonner-Weir S, Weir GC. Normal relationship of beta- and non-beta-cells not needed for successful islet transplantation. Diabetes. 2007;56(9):2312–2318. doi: 10.2337/db07-0191. [DOI] [PubMed] [Google Scholar]
- 10.Kohler CU, Kreuter A, Rozynkowski MC, Rahmel T, Uhl W, Tannapfel A, Schmidt WE, Meier JJ. Validation of different replication markers for the detection of beta-cell proliferation in human pancreatic tissue. Regul Pept. 2010;162(1–3):115–121. doi: 10.1016/j.regpep.2009.12.021. [DOI] [PubMed] [Google Scholar]
- 11.Kohler CU, Olewinski M, Tannapfel A, Schmidt WE, Fritsch H, Meier JJ. Cell cycle control of beta-cell replication in the prenatal and postnatal human pancreas. Am J Physiol Endocrinol Metab. 2011;300(1):E221–230. doi: 10.1152/ajpendo.00496.2010. [DOI] [PubMed] [Google Scholar]
- 12.Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D’Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 13.Levitt HE, Cyphert TJ, Pascoe JL, Hollern DA, Abraham N, Lundell RJ, Rosa T, Romano LC, Zou B, O’Donnell CP, Stewart AF, Garcia-Ocana A, Alonso LC. Glucose stimulates human beta cell replication in vivo in islets transplanted into NOD-severe combined immunodeficiency (SCID) mice. Diabetologia. 2011;54(3):572–582. doi: 10.1007/s00125-010-1919-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menge BA, Tannapfel A, Belyaev O, Drescher R, Muller C, Uhl W, Schmidt WE, Meier JJ. Partial pancreatectomy in adult humans does not provoke beta-cell regeneration. Diabetes. 2008;57(1):142–149. doi: 10.2337/db07-1294. [DOI] [PubMed] [Google Scholar]
- 15.Nath DS, Gruessner AC, Kandaswamy R, Gruessner RW, Sutherland DE, Humar A. Outcomes of pancreas transplants for patients with type 2 diabetes mellitus. Clin Transplant. 2005;19(6):792–797. doi: 10.1111/j.1399-0012.2005.00423.x. [DOI] [PubMed] [Google Scholar]
- 16.Nir T, Melton DA, Dor Y. Recovery from diabetes in mice by beta cell regeneration. J Clin Invest. 2007;117(9):2553–2561. doi: 10.1172/JCI32959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perl S, Kushner JA, Buchholz BA, Meeker AK, Stein GM, Hsieh M, Kirby M, Pechhold S, Liu EH, Harlan DM, Tisdale JF. Significant human beta-cell turnover is limited to the first three decades of life as determined by in vivo thymidine analog incorporation and radiocarbon dating. J Clin Endocrinol Metab. 2010;95(10):E234–239. doi: 10.1210/jc.2010-0932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pisania A, Weir GC, O’Neil JJ, Omer A, Tchipashvili V, Lei J, Colton CK, Bonner-Weir S. Quantitative analysis of cell composition and purity of human pancreatic islet preparations. Lab Invest. 2010;90(11):1661–1675. doi: 10.1038/labinvest.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reers C, Erbel S, Esposito I, Schmied B, Buchler MW, Nawroth PP, Ritzel RA. Impaired islet turnover in human donor pancreata with aging. Eur J Endocrinol. 2009;160(2):185–191. doi: 10.1530/EJE-08-0596. [DOI] [PubMed] [Google Scholar]
- 20.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 21.Ritzel RA, Butler AE, Rizza RA, Veldhuis JD, Butler PC. Relationship between beta-cell mass and fasting blood glucose concentration in humans. Diabetes Care. 2006;29(3):717–718. doi: 10.2337/diacare.29.03.06.dc05-1538. [DOI] [PubMed] [Google Scholar]
- 22.Rooman I, Bouwens L. Combined gastrin and epidermal growth factor treatment induces islet regeneration and restores normoglycaemia in C57Bl6/J mice treated with alloxan. Diabetologia. 2004;47(2):259–265. doi: 10.1007/s00125-003-1287-1. [DOI] [PubMed] [Google Scholar]
- 23.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, Secchi A, Brendel MD, Berney T, Brennan DC, Cagliero E, Alejandro R, Ryan EA, DiMercurio B, Morel P, Polonsky KS, Reems JA, Bretzel RG, Bertuzzi F, Froud T, Kandaswamy R, Sutherland DE, Eisenbarth G, Segal M, Preiksaitis J, Korbutt GS, Barton FB, Viviano L, Seyfert-Margolis V, Bluestone J, Lakey JR. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355(13):1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 25.Suarez-Pinzon WL, Lakey JR, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin induces neogenesis of human islet {beta}-cells from pancreatic duct cells and an increase in functional {beta}-cell mass. J Clin Endocrinol Metab. 2005;90(6):3401–3409. doi: 10.1210/jc.2004-0761. [DOI] [PubMed] [Google Scholar]
- 26.Suarez-Pinzon WL, Lakey JR, Rabinovitch A. Combination therapy with glucagon-like peptide-1 and gastrin induces beta-cell neogenesis from pancreatic duct cells in human islets transplanted in immunodeficient diabetic mice. Cell Transplant. 2008;17(6):631–640. doi: 10.3727/096368908786092775. [DOI] [PubMed] [Google Scholar]
- 27.Suarez-Pinzon WL, Yan Y, Power R, Brand SJ, Rabinovitch A. Combination therapy with epidermal growth factor and gastrin increases beta-cell mass and reverses hyperglycemia in diabetic NOD mice. Diabetes. 2005;54(9):2596–2601. doi: 10.2337/diabetes.54.9.2596. [DOI] [PubMed] [Google Scholar]
- 28.Tellez N, Joanny G, Escoriza J, Vilaseca M, Montanya E. Gastrin treatment stimulates beta-cell regeneration and improves glucose tolerance in 95% pancreatectomized rats. Endocrinology. 2011;152(7):2580–2588. doi: 10.1210/en.2011-0066. [DOI] [PubMed] [Google Scholar]
- 29.Tyrberg B, Ustinov J, Otonkoski T, Andersson A. Stimulated endocrine cell proliferation and differentiation in transplanted human pancreatic islets: effects of the ob gene and compensatory growth of the implantation organ. Diabetes. 2001;50(2):301–307. doi: 10.2337/diabetes.50.2.301. [DOI] [PubMed] [Google Scholar]
- 30.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased b-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 31.Yatoh S, Dodge R, Akashi T, Omer A, Sharma A, Weir GC, Bonner-Weir S. Differentiation of affinity-purified human pancreatic duct cells to beta-cells. Diabetes. 2007;56(7):1802–1809. doi: 10.2337/db06-1670. [DOI] [PubMed] [Google Scholar]