Abstract
Reduced intensity conditioning (RIC) regimens have the potential to decrease transplant-related morbidity and mortality. However, engraftment failure has been prohibitively high after RIC unrelated umbilical cord blood transplantation (UCBT) in chemotherapy-naïve children with non-malignant diseases (NMD). Twenty-two children with a median age of 2.8 years, many with severe comorbidities and prior viral infections were enrolled in a novel RIC protocol consisting of hydroxyurea, alemtuzumab, fludarabine, melphalan and thiotepa followed by single UCBT. Patients were transplanted for inherited metabolic disorders (N=8), primary immunodeficiencies (N=9), hemoglobinopathies (N=4) and Diamond Blackfan anemia (N=1). Most UCB units were HLA-mismatched with median infused total nucleated cell dose of 7.9 × 107/kg. No serious organ toxicities were attributable to the regimen. The cumulative incidence of neutrophil engraftment was 86.4% (95% confidence interval [CI], 65%–100%) in a median of 20 days, with the majority sustaining >95% donor chimerism at 1 year. Cumulative incidence of acute graft-versus-host disease (GVHD) grades II–IV and III–IV by day 180 was 27.3% (95% CI, 8.7%–45.9%) and 13.6% (95 CI, 0%–27.6%), respectively. Cumulative incidence of extensive chronic GVHD was 9.1% (95% CI, 0%–20.8%). The primary causes of death were viral infections (N=3), acute GVHD (N=1) and transfusion reaction (N=1). One-year overall and event-free survivals were 77.3% (95% CI, 53.7%–89.8%) and 68.2% (95% CI, 44.6%–83.4%) with 31 months median follow-up. This is the first RIC protocol demonstrating durable UCB engraftment in children with NMD. Future risk-based modifications of this regimen could decrease the incidence of viral infections. (www.clinicaltrials.gov/NCT00744692)
Keywords: Umbilical cord blood transplantation, Reduced intensity conditioning, Pediatric disorders, Non-malignant diseases, Thalassemia, Hemophagocytic lymphohistiocytosis (HLH)
INTRODUCTION
Allogeneic hematopoietic stem cell transplantation (HSCT) can cure a variety of non-malignant diseases (NMD) in children, either by direct replacement of abnormal bone marrow precursors as in hemoglobinopathies and primary immunodeficiencies (PID) or indirectly, by providing cellular enzyme replacement to diseased areas such as liver and brain, in inherited metabolic disorders (IMD) (1–3). For patients lacking matched related donors, unrelated donor umbilical cord blood transplantation (UCBT) is increasingly being utilized because of easy availability and less restrictive human leukocyte antigen (HLA) matching requirements. Myeloablative conditioning (MAC) with UCBT has been used successfully for 2 decades to treat a variety of malignant and NMD (4–7), however, high transplant-related mortality (TRM), and the risks of long-term adverse effects, such as infertility and secondary malignancies have remained a barrier for children with hopefully decades of life expectancy (8–10).
Mixed donor chimerism leads to disease resolution in patients with hemoglobinopathies and PID, which can remain stable over time (11–14). These observations provide a rational basis to design reduced intensity conditioning (RIC) regimens aimed at decreasing non-hematopoietic toxicity without compromising the clinical benefits of persistent donor hematopoiesis. There is a spectrum of RIC regimens, ranging from minimally intensive/non-myeloablative to near-ablative with reduced toxicity (13–18). However, successful RIC trials were reported mainly after HSCT from living related or unrelated donors. The use of UCBT after RIC has been successful in heavily pre-treated adults transplanted for malignant conditions, but has been associated with high rates of primary graft failure or graft rejection and autologous reconstitution in chemotherapy-naïve children, exceeding 50% in patients undergoing single unit UCBT for hemoglobinopathies (19–22). In these cases, graft failure might result from the fact that lower numbers of infused progenitors may have been unable to outcompete recovering host hematopoiesis and lymphopoiesis. Therefore, we developed a novel immunoablative, nevertheless, RIC regimen, designed to establish durable donor cell engraftment, and report the results of this prospective, single arm, phase I pilot study in chemotherapy-naïve children with NMD undergoing single donor UCBT.
METHODS
Patients
From December 2008 to June 2012, 22 children with NMD lacking matched related donors scheduled to undergo a single UCBT were enrolled at Duke University, Durham, N.C. (N=19) and All Children’s Hospital, St. Petersburg, FL (N=3). The study was approved by the IRB of both institutions. Written assent or informed consent was obtained from all parents/caretakers or, when ≥18 years of age, patients, in accordance with the Declaration of Helsinki. Eligible patients had to be between 2 months and 21 years of age with a diagnosis of NMD (PID, IMD, hemoglobinopathies, or other transfusion dependent anemias). Patients with IMD exhibiting high performance status, severe aplastic anemia, and those who had undergone HSCT within the previous 6 months were excluded. (www.clinicaltrials.gov NCT00744692).
Donors
Grafts were selected based on HLA Class I (A, B) intermediate-resolution and HLA Class II (DRB1) allelic level typing. The pre-cryopreservation total nucleated cell count (TNCC) had to be >3 × 107/kg for 5/6 or 6/6 HLA-matched grafts and >5 × 107/kg for 4/6 HLA-matched grafts. Potential units were screened by international cord blood banking standards and for normal enzyme activity in IMD patients. Pre-cryopreservation graft characteristics were obtained from the supplying cord blood banks while the respective hospital laboratories that thawed, washed and tested the units provided post-thaw data.
Conditioning Regimen, Graft-versus-host disease (GVHD) prophylaxis, Transplantation and Supportive care
All patients were treated with alemtuzumab 1mg/kg/dose I.V. on three successive days -21 to -19, following a test dose of 0.2mg/kg on day -22; hydroxyurea 30mg/kg/day orally from day -22 until day -10; fludarabine (Flu) 30mg/m2/day I.V. from days -9 to -5; melphalan (Mel) 70mg/m2/day I.V. on days -4, -3; and thiotepa 200mg/m2 I.V. on day -2. Frequent baths (Q4 hours × 6) were prescribed after thiotepa administration. The thawed and washed UCB unit was infused intravenously on day 0 as previously described (23,24).
Starting day -3, all patients received GVHD prophylaxis with tacrolimus 0.03mg/kg/day I.V. and mycophenolate 15mg/kg/dose I.V. every 8 hours. Tacrolimus trough levels were maintained between 8–15ng/mL. In patients without active GVHD, mycophenolate was discontinued after 45 days and tacrolimus was tapered after 9 months post-transplant.
Alemtuzumab was administered in the outpatient clinic for most patients following intensive premedication with acetaminophen, diphenhydramine, ibuprofen and corticosteroids. All patients were hospitalized in the Pediatric Blood and Marrow Transplant unit of Duke University Medical Center or All Children’s Hospital prior to day -4 and through engraftment and stabilization post-transplant. Median length of stay for the initial hospitalization was 47 days (range 29–263). Fungal and antiviral prophylaxis was voriconazole, and acyclovir respectively. Pneumocystis jiroveci pneumonia, veno-occlusive disease (VOD) prophylaxis, nutrition, and transfusion support was as previously described(24). All patients received intravenous immunoglobulin (500mg/kg/dose) weekly until day +100, starting day-1. Filgrastim (Amgen, Thousand Oaks, CA) was started at 5 mcg/kg/day on day +1 and weaned post-engraftment. Cytomegalovirus (CMV) and adenovirus DNA were monitored weekly post- alemtuzumab. One patient received irradiated, G-CSF-mobilized, parental granulocyte transfusions until engraftment for history of Mycobacterium avium, Pseudomonas, and Candida infections. Four patients subsequently enrolled on the ADV HALT study (Randomized, Placebo-controlled, Phase 2 Study of preemptive CMX001 treatment for adenovirus disease prevention); two on a multicenter study of banked third-party virus-specific T-cells to treat CMV, Epstein-Barr virus (EBV), or adenovirus infections after HSCT (25), and two on expanded access study of human mesenchymal stem cell (Prochymal®) infusion for steroid-refractory acute GVHD. (Tables 3 and 5)
Table 3.
Treatment and outcomes of EBV, CMV, Adenovirus infections
| UPN | Infection | Treatment | Response | F/U (mo) | Outcome | Day died | Cause of death | ||
|---|---|---|---|---|---|---|---|---|---|
| 1 | EBV | Rituximab | Resolved | 52.9 | |||||
| 2 | 45.5 | ||||||||
| 3 | Adenoviremia | Cidofovir CTL |
Resolved | Death | 170 | Hemolytic transfusion reaction | |||
| 4 | CMV viremia | Foscarnet, GCV | Resolved | AR | |||||
| 5 | 40.4 | ||||||||
| 6 | 35.4 | ||||||||
| 7 | 34.8 | ||||||||
| 8 | Adenoviremia | CMX001 | Resolved | 33.6 | |||||
| 9 | CMV viremia | Foscarnet, GCV | Resolved | 31.3 | |||||
| 10 | 30.9 | ||||||||
| 11 | Adenoviremia | Cidofovir CMX001 |
Death | GF | Death | 25 | Disseminated adenoviral infection | ||
| 12 | 26.6 | ||||||||
| 13 | Adenovirus | Cidofovir | Resolved | 26.4 | |||||
| 14 | Adenovirus | Cidofovir CTL |
Death | Death | 24 | Adenoviremia, Parainfluenza 3 | |||
| 15 | AR | ||||||||
| 16 | EBV | Rituximab | Resolved | 16.4 | |||||
| 17 | Adenoviremia | CMX001 | Ongoing treatment due to chronic GVHD | 15.9 | |||||
| 18 | 15.5 | ||||||||
| 19 | 15.3 | ||||||||
| 20 | Adenoviremia | Cidofovir | 14.3 | ||||||
| 21 | CMV viremia | Foscarnet, GCV | Death | Death | 27 | CMV pneumonia | |||
| 22 | Adenoviremia CMV |
Cidofovir CMX001 |
Death | Death | 258 | Acute GVHD, adenoviremia, CMV | |||
F/U = follow-up; CTL = Trivirus (adenovirus, CMV, EBV) specific cytotoxic T lymphocytes
Table 5.
Details of acute and chronic GVHD
| UPN | Match Conventi onal A, B, DRB1 | Match 8 allele Hi Res A, B, C, DRB1 | AGVHD Max Skin stage | AGVHD Max Gut stage | AGVHD Max Liver stage | Acute GvHD Grade Consens us | Chronic GvHD | cGVHD Site | AIHA | Rituximab | MSC | Notes on outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4/6 | 6/8 | 2 | 0 | 0 | 1 | No | Yes | Yes (AIHA, EBV) | Resolved | Off IS | ||
| 2 | 6/6 | 8/8 | 1 | 0 | 0 | 1 | No | Resolved | Off IS | ||||
| 3 | 5/6 | 6/8 | 1 | 0 | 0 | 1 | No | Resolved | Died | ||||
| 4 | 6/6 | 7/8 | NE | NE | NE | NE | NE | ||||||
| 5 | 5/6 | 6/8 | 0 | 0 | 0 | 0 | No | Off IS | |||||
| 6 | 5/6 | 5/8 | 3 | 0 | 0 | 2 | Extensive | Skin | Yes | Yes (AIHA) | Ongoing | ||
| 7 | 5/6 | 5/8 | 0 | 0 | 0 | 0 | Limited | Skin | Resolved | Off IS | |||
| 8 | 4/6 | 4/8 | 0 | 0 | 0 | 0 | Limited | Skin | Resolved | Off IS | |||
| 9 | 5/6 | 6/8 | 1 | 0 | 0 | 1 | Limited | Skin | Resolved | Off IS | |||
| 10 | 5/6 | 6/8 | 0 | 1 | 0 | 2 | Limited | Skin | Resolved | Weaning IS | |||
| 11 | 4/6 | 5/8 | 0 | 0 | 0 | 0 | NE | Died | |||||
| 12 | 4/6 | 4/8 | 0 | 0 | 0 | 0 | Limited | Skin | Resolved | Off IS | |||
| 13 | 5/6 | 7/8 | 2 | 0 | 0 | 1 | Limited | Skin | Resolved | Off IS | |||
| 14 | 4/6 | 4/8 | NE | NE | NE | NE | NE | Died | |||||
| 15 | 4/6 | 3/8 | NE | NE | NE | NE | NE | ||||||
| 16 | 4/6 | 5/8 | 1 | 3 | 0 | 3 | No | Yes (EBV) | Resolved | Weaning IS | |||
| 17 | 5/6 | 6/8 | 3 | 3 | 0 | 3 | Extensive | Skin, gut | Yes | Ongoing | |||
| 18 | 5/6 | 6/8 | 1 | 0 | 0 | 1 | Limited | Skin | Resolved | Off IS | |||
| 19 | 6/6 | 6/8 | 3 | 0 | 0 | 2 | No | Yes | Yes (AIHA) | Resolved | Weaning IS | ||
| 20 | 5/6 | 7/8 | 0 | 0 | 0 | 0 | Limited | Skin | Resolved | Weaning IS | |||
| 21 | 6/6 | 8/8 | 0 | 0 | 0 | 0 | NE | Died | |||||
| 22 | 5/6 | 5/8 | 3 | 4 | 0 | 3 | No | Yes | Died |
MSC = Mesenchymal stem cells; NE = not evaluable; AIHA = Autoimmune hemolytic anemia; IS = immune suppression
Post-transplant assessments
Engraftment studies were performed by short tandem repeat methods in whole blood, CD15+ and CD3+ fractions on days 30, 60, 100, 180, 270, 1 year and annually thereafter. In parallel, immunophenotyping by 4-color FACS was performed to quantitate lymphocyte and dendritic cell subsets (26–28). Immunoglobulin levels were tested at regular intervals post-transplant. Organ function and disease-specific evaluations were performed per institutional practices.
Assessment of functional T-cell responses to microbial antigens
Multiplex cytokine measurements using a cytokine bead array (CBA) and lymphoproliferative assays as internal controls were set up to test in parallel for the presence and magnitude of virus-specific T-cell response. Mononuclear cell preps were made and 105 mononuclear cells were seeded/well of 96 well plates in complete medium composed of RPMI 1640, 10% PHS, 100x antibiotic/antimycotic, L-glutamine, Hepes and β-mercaptoethanol, (Life Technologies Corp, USA). Viral antigens (Meridian Life Sciences, Memphis, TN) derived from cytomegalovirus (CMV, R9A104), herpes simplex virus (HSV, R9A002), and varicella zoster virus (VZV, R02030) were diluted from frozen stocks and added for a total 200ul. Negative control wells received no antigen; while positive control wells received 105 ClinExVivo® beads (Life Technologies, Carlsbad, CA) as described. Duplicate wells were incubated for 5 days at 37°C, and then 75ul medium was removed and frozen for batched CBA analysis. Secreted cytokines (IFNγ, TNFα, IL-10, GM-CSF, IL-2 and IL-4) were quantitated using the BioPlex® (Biorad, Hercules, CA) CBA system. The wells were pulsed with 25μl of medium containing 1μCi of 3H-Thymidine (Perkin Elmer, Waltham, MA, USA) and incubated for 6–8 hours at 37°C. Results were expressed numerically as the mean counts per minutes of duplicates as described (29,30).
T cell receptor excision circle (TREC) measurements
Peripheral blood mononuclear cells were prospectively cryopreserved at defined intervals before and after UCBT. Thymopoiesis was assessed by amplification and quantification of delta-deletion TRECs as previously described (31). Primers for the TREC sequence were 5′-CCC TTT CAA CCA TGC TGA CAC-3′ (forward) and 5′-GGG TGC AGG TGC CTA TGC-3′ (reverse), which produced an 80-bp fragment detected with the probe 5′-FAM-TCT GGT TTT TGT AAA GGT GCC CAC TCC TG-BHQ-1-3′. TREC quantification was normalized for cell input by parallel quantification of the human β-globin gene. The primers for human β-globin were 5′-GAA GAG CCA AGG ACA GGT ACG-3′ (forward) and 5′-CCT GGG AGT AGA TTG GCC AA-3′ (reverse), which produced an 85-bp fragment detected by the probe 5′-FAM-CTG TCA TCA CTT AGACCT CAC CCT GTG-BHQ-1-3′.
Statistical Methods
The study was designed as a prospective, single arm, safety and efficacy pilot study to evaluate the RIC regimen for single UCBT. The primary hypothesis/objective was that the RIC regimen would result in adequate engraftment defined as the presence of >25% donor chimerism at 6-months post-UCBT. Simon’s (optimum) two-stage design was employed to test the null hypothesis that the donor engraftment rate is ≤0.30 (Ho: p≤0.30) versus the alternative that it is ≥0.65 (Ha: p≥0.65). Primary graft failure was defined as the failure to reach ANC>500/mm3 by day 42 or absence of donor hematopoiesis ≥10% in whole blood cells by day 100. The day 100 and 180 stopping rules for graft failure were not crossed. Cumulative incidence estimates of neutrophil recovery, platelet engraftment, acute GVHD grades II–IV, and III–IV, and chronic GVHD were assessed using standard competing risk analyses with death as a competing risk.(32) Neutrophil recovery was defined as the first day of an absolute neutrophil count (ANC) >0.5 × 109/L for 3 consecutive days not secondary to granulocyte infusions; and platelet engraftment as first day of platelet counts >20 × 109/L or >50 × 109/L for 7 consecutive days without transfusions as previously described (24). Acute and chronic GVHD were graded per established criteria (33,34). The cumulative incidence of infection (any, bacterial, viral or fungal) was estimated from the start of the RIC regimen using death as a competing risk. The Kaplan-Meier method was used to describe overall survival (OS) and event-free survival (EFS) (35). OS considered a patient death as the event and censored on the last date of contact while EFS considered death, graft failure or rejection as the events. Descriptive statistics of immune reconstitution were provided at 3, 6, 9, 12 and 24 months post-UCBT. Analyses were conducted using the SAS System version 9.3 (Cary, NC).
RESULTS
Patient characteristics
Patient demographics (N=22) are shown in Table 1. The median age was 2.8 years (range 0.3–8.0) and median weight was 13.7 kg (range 5.3–26.9) on the day of transplant. Most patients were males (59%) and Caucasians (68%), while, 23% were African-American and 9% were Asian. Patients were diagnosed with immunodeficiency disorders (n=9), IMD (n=8), hemoglobinopathies (n=4) and Diamond Blackfan anemia (n=1). Twenty one patients received their first HSCT, while one patient with thalassemia major was enrolled 15 months after rejecting a matched sibling bone marrow transplant (BMT) after MAC regimen.
Table 1.
Baseline patient and graft characteristics
| UPIN | Diagnosis | Age (yrs) | Sex | Minority (Y/N) | Pre-transplant Co-morbidities | Wt (kg) on day 0 | HLA Match 6 allele A, B, DRB1 | HLA Match 8 allele Hi Res A, B, C, DRB1 | CMV Pt | TNCC (×10e7/kg reinfused | CD34+ (×10e5/kg reinfused) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Hunter | 2.03 | M | Y | Cardiomyopathy, obstructive sleep apnea | 25.3 | 4/6 | 6/8 | Neg | 7.91 | 3.70 |
| 2 | Sanfilippo B Syndrome | 3.35 | M | N | 15.1 | 6/6 | 8/8 | Neg | 5.18 | 3.78 | |
| 3 | Sanfilippo B Syndrome | 3.32 | F | N | 15.8 | 5/6 | 6/8 | Neg | 4.14 | 0.87 | |
| 4 | Cartilage Hair Hypoplasia | 1.30 | M | N | CMV viremia, transfusion dependent anemia, chronic neutropenia | 8.2 | 6/6 | 7/8 | Pos | 13.90 | 4.31 |
| 5 | MLD | 6.76 | M | N | 20.4 | 5/6 | 6/8 | Neg | 4.58 | 2.43 | |
| 6 | Zap 70 deficiency | 3.32 | F | Y | Resp: MAI, Pseudomonas, Parainfluenza 1, Candida, bronchiectasis; Enteroviral meningitis, hydrocephalus, VP shunt | 11.2 | 5/6 | 5/8 | Neg | 6.60 | 2.6 |
| 7 | HLH | 0.45 | F | N | 7.9 | 5/6 | 5/8 | Neg | 15.3 | 3.67 | |
| 8 | HLH | 0.58 | F | Y | Adenoviremia, adenovirus in stool and urine | 6.20 | 4/6 | 4/8 | Neg | 11.80 | 2.48 |
| 9 | β thalassemia major | 1.56 | M | Y | 9.5 | 5/6 | 6/8 | Pos | 8.17 | 5.10 | |
| 10 | CID | 1.14 | F | N | ITP, HHV-6 pneumonitis, Enterovirus encephalitis, colitis | 10.1 | 5/6 | 6/8 | Neg | 7.80 | 2.73 |
| 11 | Krabbe | 3.09 | F | Y | Blindness | 12.2 | 4/6 | 5/8 | Neg | 14.30 | 4.58 |
| 12 | Krabbe | 3.25 | F | N | Ataxia | 19.1 | 4/6 | 4/8 | Neg | 7.36 | 1.55 |
| 13 | DBA | 2.56 | M | N | Iron overload | 15.9 | 5/6 | 7/8 | Neg | 6.22 | 1.50 |
| 14 | Hurler | 1.71 | F | Y | Obstructive sleep apnea. Stool adenovirus +, h/o Parainfluenza 3 infection | 11.8 | 4/6 | 4/8 | Neg | 10.8 | 4.86 |
| 15 | ALD | 8.02 | M | Y | 26.9 | 4/6 | 3/8 | Neg | 6.99 | 3.7 | |
| 16 | βThalassemia major | 3.65 | M | Y | 14.2 | 4/6 | 5/8 | Neg | 7.07 | 1.63 | |
| 17 | β thalassemia major | 5.44 | F | Y | Prior myeloablative transplant; h/o severe hemorrhagic cystitis | 18 | 5/6 | 6/8 | Pos | 11 | 3.5 |
| 18 | Omenn syndrome | 0.45 | M | Y | 5.26 | 5/6 | 6/8 | Pos | 22 | 7.72 | |
| 19 | βThalassemia major | 2.58 | M | Y | 13.2 | 6/6 | 6/8 | Neg | 5.04 | 4.58 | |
| 20 | CD40L deficiency | 3.38 | M | N | 14.1 | 5/6 | 7/8 | Neg | 9.46 | 3 | |
| 21 | Omenn syndrome | 0.31 | M | N | Erythroderma, FTT, CMV | 6 | 6/6 | 8/8 | Pos | 15.8 | 2.4 |
| 22 | PNP deficiency | 4.27 | M | N | FTT, food intolerance, chronic enteritis, CMV urine, adenovirus in stool | 16.6 | 5/6 | 5/8 | Pos | 5.58 | 1.3 |
ALD = adrenoleukodystrophy; CID = combined immunodeficiency; CMV = cytomegalovirus; DBA = Diamond Blackfan anemia; F = female; FTT = failure to thrive; HHV-6 = human herpes virus 6; Hi res = high resolution; HLH = Hemophagocytic lymphohistiocytosis; h/o = history of; ITP = immune thrombocytopenic purpura; kg = kilograms; M = male; MAI = Mycobacterium avium; MLD = metachromatic leukodystrophy; Neg = negative; PNP = purine nucleoside phosphorylase; Pos = positive; Pt = patient; UPIN = patient number; VP = ventriculoperitoneal; Wt = weight; Y/N = Yes/No; yrs = years; ZAP 70 =zeta chain associated protein kinase 70;
Inherited metabolic disorders (IMD) = Hunter, Hurler, Krabbe, Sanfilippo B, Metachromatic leukodystrophy (MLD), adrenoleukodystrophy (ALD);
Primary immunodeficiency diseases (PID) = Cartilage Hair Hypoplasia, Zap 70 deficiency, HLH, CID, Omenn syndrome, CD40Ligand deficiency, PNP deficiency)
Graft characteristics
The median pre-cryopreservation TNCC and CD34+ doses were 11.1 × 107/kg (range 5.9–31.3) and 4.35 × 105/kg (range 1.71–8.1), respectively. Median infused TNCC and CD34+ doses were 7.9 × 107/kg (range 4.1–22) and 3.3 × 105/kg (range 0.9–7.7), respectively. Using conventional HLA matching criteria to select UCB grafts, donor-recipient matching was 4/6 in 7 (31.8%), 5/6 in 11 (50%), and 6/6 in 4 (18.2%) patients. Review of HLA typing at allelic level including HLA-C revealed matches of 3/8 in 1 (4.5%), 4/8 in 3 (13.6%), 5/8 in 5 (22.7%), 6/8 in 8 (36.4%), 7/8 in 3 (13.6%) and 8/8 in 2 (9.1%). Thus, approximately 40% of recipients were mismatched for 3 or more loci at the allelic level. Donors were matched for gender in 12 (55%) and ABO blood type in 10 (45.5%) recipients. Major and/or bidirectional ABO mismatch was noted in 7 patient/donor pairs (31.8%).
Neutrophil and Platelet Engraftment
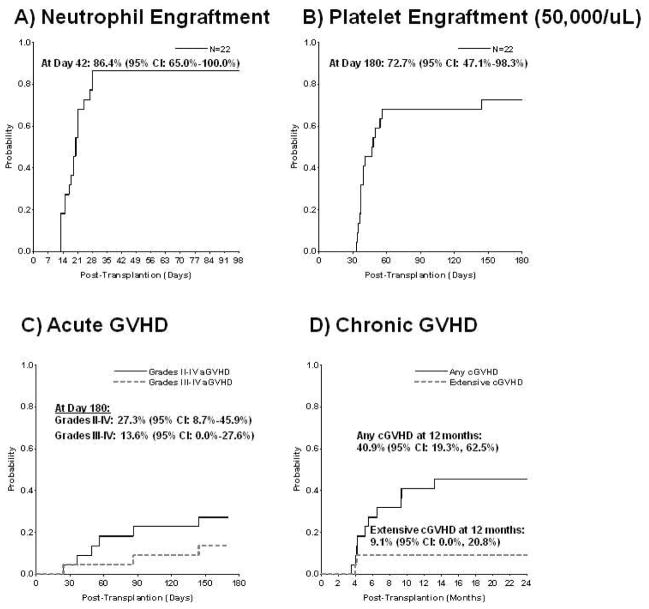
The cumulative incidence of neutrophil engraftment with donor cells by day 42 was 86.4% (95% confidence interval [CI], 65%–100%; Figure 1A). Nineteen patients engrafted at a median of 20 days (range 12–28). Three patients experienced graft failure: primary graft failure (n-=1) who died on day 24 (UPIN 14) with 100% donor cells before engrafting, and autologous reconstitution (n=2). The latter two patients with autologous reconstitution (UPIN 15, UPIN 4) successfully underwent second transplants, 3.5 and 5 months later; with double UCBT after myeloablative Busulfan (Bu)/Cyclophosphamide (Cy)/ATG, and with matched unrelated donor BMT after thiotepa/ATG conditioning, respectively. Both are alive with donor engraftment. There have been no secondary graft failures. The cumulative incidence of platelet engraftment (50,000/uL) by day 180 was 72.7% (95% CI, 47.1%–98.3%) occurring in a median of 48 days (range 33–144; Figure 1B).
Figure 1. ENGRAFTMENT.
(a) Cumulative incidence of neutrophil engraftment > 500 cells/mm3 with donor cells by day 42
(b) Cumulative incidence of platelet engraftment > 50,000/mm3 by day 180 GVHD
(c) Cumulative incidence of acute GVHD, grades II–IV (solid line) and III–IV (dotted line)
(d) Cumulative incidence of chronic GVHD, overall (solid line) and extensive(dotted line)
Donor Chimerism
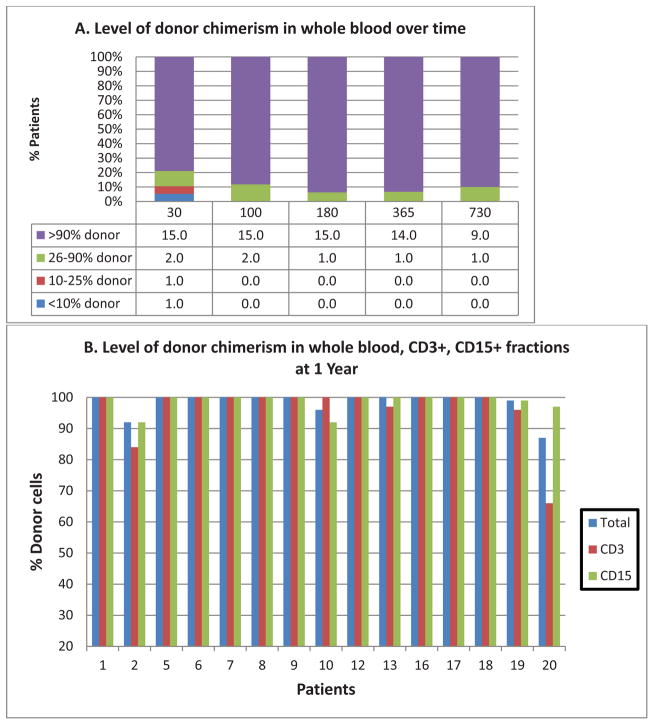
A primary objective of this study was to determine the proportion of patients engrafted with >25% donor cells 6 months post-transplant. Eighteen patients survived >6 months, 16 of whom achieved this endpoint. Specifically, whole blood donor chimerism >90% was demonstrated in 15/16 patients at 180 days, 14/15 patients at 1 year, and 9/10 patients followed at 2 years. Similar levels of donor cell chimerism were seen in CD15+ (15/16 at 180 days, 15/15 at 1 year, 9/10 at 2 years); and CD3+ fractions (13/16 at 180 days, 13/15 at 1 year, 9/10 at 2 years). (Figure 2 and Table 4)
Figure 2. DONOR CHIMERISM.
(a) Level of whole blood donor chimerism over time; X-axis = Days post-transplant; Y axis = % of evaluable patients
(b) Level of donor chimerism in whole blood, CD3+ & CD15+ fractions at 1 year (N=15)
Table 4.
Donor Chimerism(%)
| Day 30 | Day 100 | Day 180 | 1 year | 2 year | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UPN | T | L | M | T | L | M | T | L | M | T | L | M | T | L | M |
| 1 | >98 | 95 | >98 | >98 | 96 | 98 | >98 | 98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 |
| 2 | >98 | >98 | >98 | 95 | 78 | 90 | 92 | 72 | 89 | 92 | 84 | 92 | 88 | 88 | 86 |
| 3 | 10 | 88 | 10 | 63 | 91 | 58 | Died day 170 | ||||||||
| 4 | 0 | 0 | 0 | Autologous recovery day 30 | |||||||||||
| 5 | >98 | >98 | >98 | >98 | 86 | >98 | >98 | 86 | >98 | >98 | >98 | >98 | >98 | >98 | >98 |
| 6 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 |
| 7 | >98 | >98 | >98 | >98 | 90 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 |
| 8 | >98 | >98 | >98 | >98 | 83 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 |
| 9 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 |
| 10 | >98 | n/a | n/a | 94 | 86 | 92 | >98 | >98 | 96 | 96 | >98 | 92 | 91 | >98 | 92 |
| 11 | Engrafted day13, died day 25 | ||||||||||||||
| 12 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | 98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 |
| 13 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | 97 | >98 | >98 | >98 | >98 |
| 14 | Graft failure, died day 24 | ||||||||||||||
| 15 | 91* | 8* | 99* | Autologous recovery day 41 | |||||||||||
| 16 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | |||
| 17 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | |||
| 18 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | |||
| 19 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | >98 | 96 | >98 | |||
| 20 | 87 | 71 | 41 | 87 | 55 | 61 | 88 | 58 | 96 | 87 | 66 | 97 | |||
| 21 | Engrafted day13, died day 27 | ||||||||||||||
| 22 | 83 | >98 | 68 | >98 | >98 | >98 | 98 | >98 | 97 | Died day 258 | |||||
= 0% on day 41, thus conisdered autologous recovery
T = whole blood; L = CD3+ fraction; M = CD15+ fraction; donor chimerism %
Immune reconstitution
In engrafting patients, the median absolute lymphocyte and CD4+ T-cell counts at 3, 6 and 12 months were 1291 (382–3008) and 154 (0–678) (N=17); 1405 (556–4470) and 418 (1–1507)(N=16); and 2193 (333–5070) and 805 (6–2013)(N=15), respectively (Figure 3). These values are similar to other pediatric reports using ATG-based rejection prophylaxis after myeloablative regimens (36–39).
Figure 3. IMMUNE RECCONSTITUTION.
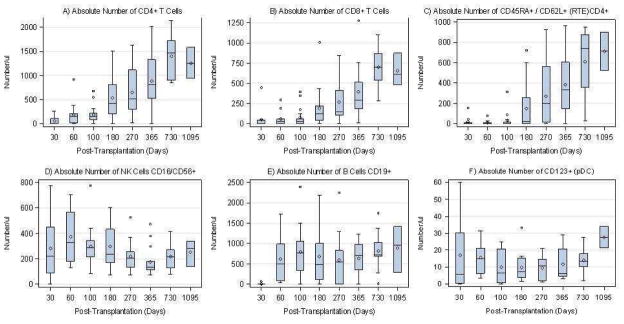
Absolute numbers of lymphocyte subsets: CD4+, CD8+, CD16,56+ (NK), CD19+, CD45RA+/62L+/CD4+ (RTE), CD123+(pDC)
Box plots are shown serially over time for above immune parameters. The diamond in the box represents the mean, while the horizontal line in the box represents the median. The lower box represents the 25th percentile and the upper box represents the 75th percentile. Upper and lower whiskers represent +/− 1.5 times the interquartile range
At 180 days, normal, age-adjusted NK, B, and CD4+ counts were found in 14/16 (87.5%), 11/16 (68.8%) and 5/16 (31.3%) patients respectively. At 1 year, normal, age-adjusted NK, B, and CD4+ counts were seen in 13/15 (86.7%), 12/15 (80%), and 12/15 (80%) patients, respectively.(40)
Nine of 15 engrafted and surviving patients with a minimum 1 year follow-up are off systemic immunosuppression, seven of whom have started immunizations. IgA levels were available for 14 of 15 patients followed at least 1 year; and were normal in 11 patients reflecting effective T-cell dependent isotype switching by B cells unaffected by possible IVIG supplementation; and low in 3 patients. Two of the patients with low IgA had received rituximab, and 1 had extensive chronic GVHD.
Seven of 10 patients followed >24 months maintain normal IgG levels without IVIG support.
Thymic output
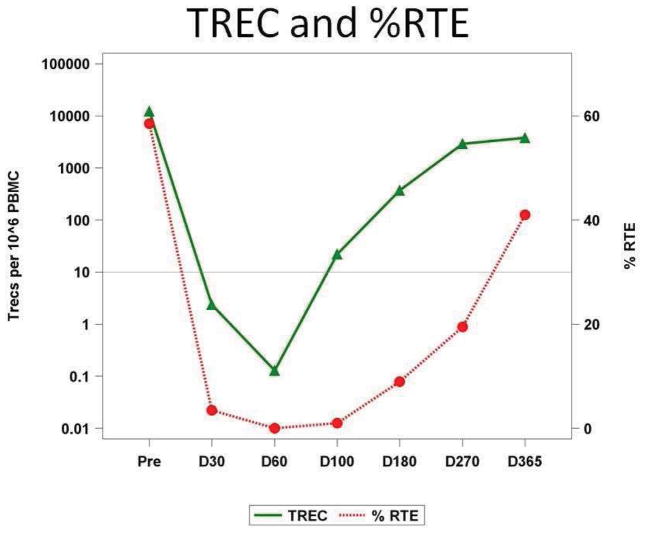
The appearance of recent thymic emigrants (RTE) is a powerful marker of thymopoiesis critical for acquiring a broad, naïve TCR repertoire. It was quantitated by qPCR for TREC and independently by flow cytometry monitoring CD45RA and CD62L co-expressing CD4+ cells. There was a notable paucity of RTE and TREC+ cells in most patients within the first 100 days (Figure 4) followed by a steep rise at day 180 reflecting the resumption of thymopoiesis. The exceptions were UPIN 22, who died on day +258 with GVHD complications associated with profound lymphopenia and recurrence of pre-transplant CMV and adenovirus infections; UPIN 16, who had grade III acute GVHD; and UPIN 17, who had extensive chronic GVHD and had failed a prior myeloablative transplant.
Figure 4. Thymopoiesis (TREC and RTE% reconstitution).
TREC = T-cell receptor excision circles; RTE = recent thymic emigrants (CD45RA+/CD62L+/CD4+); Median TREC (filled triangle) and %RTE (filled circle) are connected serially over time
Anti-viral immunity
Sensitive BioPlex assay monitored the Duke cohort (N=19) for virus-specific cytokines following in vitro challenge with herpes family of viruses: CMV, HSV and VZV. The young age of this study cohort explains the relative low incidence of pre-transplant exposure; CMV (n=5), HSV (n=1) and VZV (n=4). There were no VZV specific responses detected but only one seropositive patient was alive/studied beyond day 100. The one patient (UPIN 17) with positive pre-transplant HSV serology demonstrated specific cytokine response first at day 180 detectable by simultaneous IFNγ and GM-CSF secretion (93pg/ml and 210pg/ml) reflecting probable subclinical viral reactivation earlier.
There were 5 patients with pre-UCBT CMV exposure. Notably, two PID patients were viremic already pre-alemtuzumab, one died before day 30 due to CMV pneumonia (UPIN 21), the other had autologous reconstitution (UPIN 4). Of the other 3 patients, UPIN 22 died of adenovirus and CMV with practically no circulating T-cells (<10/ul) even at 6 months while UPIN 9 (107pg/ml) and 17 (77pg/ml) both demonstrated detectable virus-specific IFNγ secretion and proliferative responses by day 100. Neither experienced recurrent CMV viremia. CMV-specific GM-CSF, IL-13, and TNFα secretion was also detected but was discordant between these 2 patients. In sum, anti CMV, -HSV responses were detected only in seropositive patients and, when detected, there was no associated disease.
Graft-Versus-Host Disease
The cumulative incidence of grades II–IV and III–IV acute GVHD by day 180 was 27.3% (95% CI, 8.7%–45.9%) and 13.6% (95 CI, 0%–27.6%), respectively (Figure 1C). The cumulative incidence of any chronic GVHD at 1 year was 40.9% (95% CI, 19.3%–62.5%). However, the cumulative incidence of extensive chronic GVHD was only 9.1% (95% CI, 0%–20.8%) (Figure 1D). Of the 10 patients developing chronic GVHD, 8 had disease limited to skin and 2 had extensive (skin N= 1; skin, gut N= 1) disease. Autoimmune hemolytic anemia (AIHA) was noted in 3 patients (isolated AIHA, N=2; AIHA with chronic GVHD, N=1; cumulative incidence 9.1% [95% CI, 0%–20.8%]) and responded in all patients to steroid and rituximab therapy. Of these 12 patients (10 with classic chronic GVHD and 2 additional patients with isolated AIHA), 5 remain on chronic immunosuppression 14–35 months post-transplant. Two patients received mesenchymal stem cell treatment for steroid refractory GVHD. One (UPN 17) has had a partial response, whereas the other (UPN 22) died of acute GVHD despite MSC treatment. (Table 5)
Infections
Cumulative incidence of infectious events at 1 year was high at 90.9% (95% CI, 67.5%–100%) for any infection; 81.8% (95% CI, 57.9%–100%) for viral; 13.6% (95% CI, 0%–27.7%) for fungal; and 86.4% (95% CI, 63.1%–100%) for bacterial infections. CMV, adenovirus and EBV represented the majority of viruses encountered.
CMV
Six patients were at risk for CMV disease due to seropositivity and/or viremia (N=2). Systemic therapy was required for four of them pre-transplant (2 before and 2 after initiation of conditioning), two of whom (UPIN 21, 22), both with PID, developed CMV disease and died post-transplant.
Adenovirus
Three patients had adenovirus detected pre-transplant (2 in stool alone, 1 in stool, urine and blood), all of whom developed adenoviremia post-transplant, including 2 with adenoviral disease. Overall, adenoviremia developed in 8 of 22 patients (36%) post-transplant, all of whom received systemic anti-adenoviral therapy. Two patients with inadequate response to cidofovir received banked third party virus specific cytotoxic T lymphocytes. One of these patients cleared adenoviremia (UPN 3), whereas the other patient did not and died on day 24 (UPN 14). An investigational agent, CMX001 was used to treat 4 patients. One patient (UPN 8) had clearance of adenoviremia and is off CMX001 treatment for >2years. Treatment is ongoing for UPN 17 due to continued risk resulting from extensive chronic GVHD and ongoing severe lymphopenia. Two patients eventually died, one (UPN 11) with fulminant adenoviral disease on day 25, and another (UPN 22) with severe acute GVHD on day 258 with adenoviral and CMV infections. In all, three patients developed adenoviral disease that was associated with mortality. (Table 3)
EBV
No patient had EBV infection pre-transplant. Post-transplant, 2 patients developed EBV infection. EBV viremia with mesenteric lymphadenopathy was noted in one patient (UPN 1) on day +88. He responded to rituximab and is alive and well ≥4 years post-transplant. Transient increase in EBV copy numbers in another patient (UPN 16) responded to 2 doses of rituximab, and is alive >1 year post-transplant.
AFB bacteremia was noted in 2 patients, one with PID (UPN 10) on day +123 and one with Krabbe disease (UPN 12) on day +134. Both patients cleared their infections with multidrug treatment, and are well >2 years post-transplant and off therapy.
Organ toxicity
This regimen was well tolerated by patients, including those with significant pre-transplant comorbidities (Table 1). All patients developed some mucositis, which required parenteral nutrition and analgesia, but VOD, idiopathic pneumonitis, pericardial effusion, hemorrhagic cystitis, or neurologic events attributable to the regimen were not seen in anyone. One patient who had developed severe hemorrhagic cystitis during prior BMT needing approximately 3 months of platelet support, prostaglandin and hyperbaric oxygen therapy, was enrolled on this study for a second transplant and tolerated the RIC regimen well without recurrence of cystitis. Several other patients had pre-transplant comorbidities such as severe bronchiectasis, chronic pulmonary disease, enteroviral meningitis, and obstructive sleep apnea and tolerated the regimen well (Table 1).
Survival
Median follow up of surviving patients is 30.89 months (range 14.31–52.93). Overall and event-free survival at 1 year post-transplant is 77.3% (95% CI, 53.7%–89.8%) and 68.2% (95% CI, 44.6%–83.4%), respectively (Figure 5). Five patients died (Table 2), of whom three died before day +28 associated with viral infections. One immunodeficient patient died on day +258 from severe acute GVHD with concomitant adenoviral and CMV disease. Pre-transplant T-cell deficiency with adenovirus and CMV detection in stool and urine, respectively, increased risk for reactivation post-transplant. One patient, a mixed chimera on day +100 (total chimerism 63%, 91% lymphoid, 58% myeloid) died on day +170 of a hemolytic transfusion reaction after empirically receiving donor type blood.
Figure 5. SURVIVAL.
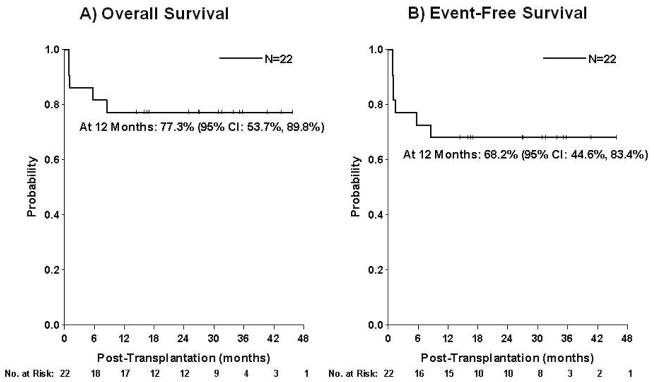
Kaplan-Meier estimate of (a) Overall survival (b) Event-free survival
Table 2.
Causes of death
| UPIN | Day post-transplant | Diagnosis | Cause of death |
|---|---|---|---|
| 3 | 170 | Sanfilippo B | Hemolytic transfusion reaction |
| 11 | 25 | Krabbe | Disseminated adenovirus |
| 14 | 24 | Hurler | Adenoviremia, Parainfluenza 3 |
| 21 | 27 | Omenn syndrome | CMV pneumonia |
| 22 | 258 | PNP deficiency | Acute GVHD, adenoviremia, CMV |
CMV = cytomegalovirus; GVHD = graft-versus-host disease; PNP = purine nucleoside phosphorylase; UPIN = patient number
DISCUSSION
We report the outcomes of a prospective, pilot study evaluating a novel RIC regimen consisting of alemtuzumab, hydroxyurea, fludarabine, melphalan and thiotepa in a pediatric cohort of 22 patients with NMD undergoing single unrelated donor UCBT. The majority of patients (19/22) were chemotherapy-naïve and 9/22 were transplanted for PID, most of whom arrived to transplant with pre-existent viral infections (n=7) reflecting their underlying T-cell defects. The regimen was designed to decrease exposure to alkylating agents, while strengthening the immunosuppressive component (fludarabine and alemtuzumab) with the overall goal of facilitating donor engraftment while reducing non-hematopoietic organ toxicity. The cumulative incidence of neutrophil engraftment was 86%, comparable to myeloablative UCBT, and significantly higher than that reported after RIC UCBT in pediatric NMD (37.5–70.6%) (19,22,24,41–45). Consistent donor engraftment was noted in patients belonging to diverse diagnostic groups, including those at a higher risk of engraftment failure.
Most patients achieved high levels of durable donor chimerism over the period of observation (median >24 months). High level (>90%) whole blood donor chimerism was noted in 14/15 patients at 1 year, and 9/10 patients at 2 years post-transplant. Whole blood chimerism remains >80% and T-cell chimerism remains >65% in all engrafted surviving patients (n=15). The durability of donor engraftment will be monitored long-term. We are optimistic that donor chimerism will persist given the high (>50%) level of T-cell chimerism in patients thus far. This level of chimerism is similar to our previously reported cohort of 159 patients with IMD undergoing myeloablative single UCBT (24). All 5 patients with inherited transfusion dependent anemias and both patients with hemophagocytic lymphohistiocytosis (HLH) maintain donor chimerism >90% at the time of this report.
Engraftment after UCBT has been a challenge in hemoglobinopathies even with MAC regimens (19–21,46,47) due to factors such as compensatory marrow hyperactivity, alloimmunization from frequent transfusions, and the presence of an immunocompetent, chemotherapy-naïve host (48). Retrospective registry analysis of mostly myeloablative UCBT in hemoglobinopathies showed primary graft failure as the predominant cause of failure, occurring in 27 of 51 patients with 2-year DFS of 45% despite an optimal cell dose (20). With RIC regimens, engraftment is even more challenging. High graft failure rate with a 2-year EFS of 50% was recently reported with Bu/Flu/alemtuzumab (21). Similarly, in a multicenter study of sickle cell disease patients undergoing UCBT after a RIC regimen of alemtuzumab/Flu/Mel, 5/8 patients had autologous recovery (1-year EFS of 37.5%) (19). In these patients, stringent criteria were used for HLA matching (≥5/6) and cell dose (median pre-cryopreservation TNCC 6.4×107/kg, range 3.1–7.6), and thus did not appear to impact the outcome, suggesting that perhaps the regimen needed to be intensified to ensure consistent engraftment. In the current study, the alemtuzumab/Flu/Mel backbone (18, 19), which was reported with increased primary graft failure after single UCBT, was augmented with thiotepa and hydroxyurea, promoting engraftment. Thiotepa is a potent immunosuppressive and myelosuppressive drug and has been successfully used with melphalan (49–51). It has promoted engraftment in murine transplant models (52, 53) as well as in clinical studies of T cell depleted HSCT and UCBT (54–56). Hydroxyurea can induce cycling of the stem cell pool potentially sensitizing them to melphalan and thiotepa (57). Alemtuzumab was administered distal to UCBT to maximize host immunoablation while reducing its impact on infused donor lymphocytes.
Of the two patients with autologous recovery, cord blood unit of one with cartilage hair hypoplasia lacked in vitro growth in colony forming unit (CFU) assay at the time of thaw; the other with adrenoleukodystrophy received a unit with 3/8 allelic HLA matching that showed transient full donor engraftment before being replaced by host cells. Poor graft potency and immune rejection respectively may have contributed to graft failure (58,59). Interestingly, both patients reconstituted host granulopoiesis within 4–6 weeks post-transplant, consistent with the notion that the preparative regimen is not fully myeloablative (60). Both patients underwent successful second transplants and are long-term survivors.
The two HLH patients are surviving with >90% donor chimerism for >2 years. TRM is a major barrier for success of conventional myeloablative regimens in HLH. In a CIBMTR retrospective HLH study (N=91), mostly with matched unrelated donor BMT and Bu/Cy/VP16+/−ATG, day 100 TRM was 35% (61). Alemtuzumab/fludarabine/melphalan has been demonstrated to decrease mortality in children with HLH receiving BMT with a 3-year survival of 92% compared to 43% for myeloablative Bu/Cy/ATG (14). However, several patients needed donor lymphocyte infusions to sustain mixed chimerism in the RIC group. Excellent engraftment and survival was also reported for BMT from Europe (62), but data with UCBT is limited (63).
This regimen was well tolerated in terms of organ toxicity as there was no cardiac, pulmonary, CNS, renal or bladder toxicity recorded. Pericardial effusion, hemorrhagic cystitis, VOD, and multiorgan failure have been associated with Bu/Cy regimen (64). Long-term follow-up will determine the impact of this regimen on late effects.
The major obstacle associated with this regimen is the high incidence of viral infections. Several factors are likely contributory. Clearly, patients with underlying T-cell immunodeficiencies and pre-transplant viral disease were extremely high risk for recurrent infections. Nevertheless, the alemtuzumab dose was relatively high compared to some other pediatric studies (14,18,62) and post-UCBT exposure may have depleted most of the T-cells accompanying the grafts leading to delayed T-cell reconstitution until thymic recovery (65). Alemtuzumab dose de-escalation could be explored in the future to allow faster lymphocyte reconstitution; however, insufficient host immunoablation could jeopardize engraftment. Patients with pre-existing viral burdens should either have their transplants delayed where feasible, or use graft sources that would allow transfer of virus-specific memory. To further enable the use of cord blood donor grafts, patients could pre-emptively receive aggressive antiviral or adoptive immunotherapy post-transplant.
Taken together, this conditioning regimen is the first RIC regimen to our knowledge to result in consistent and durable donor engraftment in children undergoing UCBT for a variety of diseases, including patients with intact immunity and high risk for graft failure. It was well tolerated even in patients with significant pre-transplant co- morbidities. Engraftment, survival, acute GVHD, and extensive chronic GVHD were not inferior to those previously reported after traditional myeloablative chemotherapy. Immune reconstitution and thymopoiesis was initially slow, but robust after 6 months in most patients. This regimen warrants further study and optimization for patients with non-malignant conditions undergoing UCBT.
Acknowledgments
Part of this works was supported by NHLBI R01HL091749 (Multiple PIs. Komanduri, Szabolcs).
The authors thank Jennifer Baker (Data Manager, Duke University), Michael Gates (Data Manager, All Children’s Hospital, St. Petersburg, FL), the staffs of both Pediatric Blood and Marrow Transplant Programs for providing excellent care to these patients; and the patients and their families.
Footnotes
CONFLICT OF INTEREST DISCLOSURE:
The authors report no potential conflict of interest.
AUTHORSHIP CONTRIBUTIONS:
SP contributed to study design, managed the clinical trial, collected and interpreted the data, and wrote the manuscript; PS designed research, obtained funding, interpreted the data, and wrote the manuscript; JK contributed to study design, interpreted the data, critically reviewed and edited the manuscript; DN contributed to study design; AM performed statistical analysis; CLB, KK, JA performed immunological studies and helped analyze that data; AP, GH, TAD, PLM, KP, KF, JM collected data and provided clinical input.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tolar J, Mehta PA, Walters MC. Hematopoietic Cell Transplantation for Nonmalignant Disorders. Biology of Blood and Marrow Transplantation. 2012;18:S166–S171. doi: 10.1016/j.bbmt.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 2.Szabolcs P, Cavazzana-Calvo M, Fischer A, Veys P. Bone Marrow Transplantation for Primary Immunodeficiency Diseases. Pediatr Clin North Am. 2010;57:207–237. doi: 10.1016/j.pcl.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Boelens JJ, Prasad VK, Tolar J, Wynn RF, Peters C. Current international perspectives on hematopoietic stem cell transplantation for inherited metabolic disorders. Pediatr Clin North Am. 2010;57:123–145. doi: 10.1016/j.pcl.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Kurtzberg J, Laughlin M, Graham ML, et al. Placental Blood as a Source of Hematopoietic Stem Cells for Transplantation into Unrelated Recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 5.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 6.Prasad VK, Kurtzberg J. Umbilical cord blood transplantation for non-malignant diseases. Bone Marrow Transplant. 2009;44:643–651. doi: 10.1038/bmt.2009.290. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman E. Milestones in umbilical cord blood transplantation. Blood Reviews. 2011;25:255–259. doi: 10.1016/j.blre.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 8.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders JE. Growth and development after hematopoietic cell transplant in children. Bone Marrow Transplant. 2007;41:223–227. doi: 10.1038/sj.bmt.1705875. [DOI] [PubMed] [Google Scholar]
- 10.Eapen M, Ahn KW, Orchard PJ, et al. Long-Term Survival and Late Deaths after Hematopoietic Cell Transplantation for Primary Immunodeficiency Diseases and Inborn Errors of Metabolism. Biology of Blood and Marrow Transplantation. 2012;18:1438–1445. doi: 10.1016/j.bbmt.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andreani M, Nesci S, Lucarelli G, et al. Long-term survival of ex-thalassemic patients with persistent mixed chimerism after bone marrow transplantation. Bone Marrow Transplant. 2000;25:401–404. doi: 10.1038/sj.bmt.1702151. [DOI] [PubMed] [Google Scholar]
- 12.Walters MC, Patience M, Leisenring W, et al. Stable mixed hematopoietic chimerism after bone marrow transplantation for sickle cell anemia. Biol Blood Marrow Transplant. 2001;7:665–673. doi: 10.1053/bbmt.2001.v7.pm11787529. [DOI] [PubMed] [Google Scholar]
- 13.Veys P. Reduced Intensity Transplantation for Primary Immunodeficiency Disorders. Immunology and Allergy Clinics of North America. 2010;30:103–124. doi: 10.1016/j.iac.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Marsh RA, Vaughn G, Kim M-O, et al. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010;116:5824–5831. doi: 10.1182/blood-2010-04-282392. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh MM, Kang EM, Fitzhugh CD, et al. Allogeneic Hematopoietic Stem-Cell Transplantation for Sickle Cell Disease. New England Journal of Medicine. 2009;361:2309–2317. doi: 10.1056/NEJMoa0904971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jacobsohn DA, Duerst R, Tse W, Kletzel M. Reduced intensity haemopoietic stem-cell transplantation for treatment of non-malignant diseases in children. The Lancet. 2004;364:156–162. doi: 10.1016/S0140-6736(04)16628-2. [DOI] [PubMed] [Google Scholar]
- 17.Krishnamurti L, Kharbanda S, Biernacki MA, et al. Stable Long-Term Donor Engraftment following Reduced-Intensity Hematopoietic Cell Transplantation for Sickle Cell Disease. Biology of Blood and Marrow Transplantation. 2008;14:1270–1278. doi: 10.1016/j.bbmt.2008.08.016. [DOI] [PubMed] [Google Scholar]
- 18.Shenoy S, Grossman WJ, DiPersio J, et al. A novel reduced-intensity stem cell transplant regimen for nonmalignant disorders. Bone Marrow Transplant. 2004;35:345–352. doi: 10.1038/sj.bmt.1704795. [DOI] [PubMed] [Google Scholar]
- 19.Kamani NR, Walters MC, Carter S, et al. Unrelated Donor Cord Blood Transplantation for Children with Severe Sickle Cell Disease: Results of One Cohort from the Phase II Study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Biology of Blood and Marrow Transplantation. 2012;18:1265–1272. doi: 10.1016/j.bbmt.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruggeri A, Eapen M, Scaravadou A, et al. Umbilical Cord Blood Transplantation for Children with Thalassemia and Sickle Cell Disease. Biology of Blood and Marrow Transplantation. 2011;17:1375–1382. doi: 10.1016/j.bbmt.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Radhakrishnan K, Bhatia M, Geyer MB, et al. Busulfan, Fludarabine, and Alemtuzumab Conditioning and Unrelated Cord Blood Transplantation in Children with Sickle Cell Disease. Biology of Blood and Marrow Transplantation. 2013;19:676–677. doi: 10.1016/j.bbmt.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 22.Satwani P, Jin Z, Duffy D, et al. Transplantation-Related Mortality, Graft Failure, and Survival after Reduced-Toxicity Conditioning and Allogeneic Hematopoietic Stem Cell Transplantation in 100 Consecutive Pediatric Recipients. Biology of Blood and Marrow Transplantation. 2013;19:552–561. doi: 10.1016/j.bbmt.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 23.Rubinstein P, Dobrila L, Rosenfield RE, et al. Processing and Cryopreservation of Placental/Umbilical Cord Blood for Unrelated Bone Marrow Reconstitution. Proc Natl Acad Sci U S A. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad VK, Mendizabal A, Parikh SH, et al. Unrelated donor umbilical cord blood transplantation for inherited metabolic disorders in 159 pediatric patients from a single center: influence of cellular composition of the graft on transplantation outcomes. Blood. 2008;112:2979–2989. doi: 10.1182/blood-2008-03-140830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leen AM, Bollard CM, Mendizabal AM, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121:5113–5123. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Szabolcs P, Park K-D, Reese M, Marti L, Broadwater G, Kurtzberg J. Absolute Values of Dendritic Cell Subsets in Bone Marrow, Cord Blood, and Peripheral Blood Enumerated by a Novel Method. Stem Cells. 2003;21:296–303. doi: 10.1634/stemcells.21-3-296. [DOI] [PubMed] [Google Scholar]
- 27.Szabolcs P, Park K-D, Reese M, Marti L, Broadwater G, Kurtzberg J. Coexistent naïve phenotype and higher cycling rate of cord blood T cells as compared to adult peripheral blood. Experimental Hematology. 2003;31:708–714. doi: 10.1016/s0301-472x(03)00160-7. [DOI] [PubMed] [Google Scholar]
- 28.Kanda J, Chiou L-W, Szabolcs P, et al. Immune Recovery in Adult Patients after Myeloablative Dual Umbilical Cord Blood, Matched Sibling, and Matched Unrelated Donor Hematopoietic Cell Transplantation. Biology of Blood and Marrow Transplantation. 2012;18:1664–1676. e1661. doi: 10.1016/j.bbmt.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Szabolcs P, Park K-D, Marti L, et al. Superior depletion of alloreactive T cells from peripheral blood stem cell and umbilical cord blood grafts by the combined use of trimetrexate and interleukin-2 immunotoxin. Biology of Blood and Marrow Transplantation. 2004;10:772–783. doi: 10.1016/j.bbmt.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Davis CC, Marti LC, Sempowski GD, Jeyaraj DA, Szabolcs P. Interleukin-7 Permits Th1/Tc1 Maturation and Promotes Ex vivo Expansion of Cord Blood T Cells: A Critical Step toward Adoptive Immunotherapy after Cord Blood Transplantation. Cancer Research. 2010;70:5249–5258. doi: 10.1158/0008-5472.CAN-09-2860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood. 2007;110:4543–4551. doi: 10.1182/blood-2007-05-092130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gooley T, Leisenring W, Crowley J, Storer B. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 33.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 34.Shulman H, Sullivan K, Weiden P, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–217. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 36.Parkman R, Cohen G, Carter SL, et al. Successful Immune Reconstitution Decreases Leukemic Relapse and Improves Survival in Recipients of Unrelated Cord Blood Transplantation. Biology of Blood and Marrow Transplantation. 2006;12:919–927. doi: 10.1016/j.bbmt.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Moretta A, Maccario R, Fagioli F, et al. Analysis of immune reconstitution in children undergoing cord blood transplantation. Experimental Hematology. 2001;29:371–379. doi: 10.1016/s0301-472x(00)00667-6. [DOI] [PubMed] [Google Scholar]
- 38.Réard C, Barlogis V, Mialou V, et al. Lymphocyte subset reconstitution after unrelated cord blood or bone marrow transplantation in children. British Journal of Haematology. 2011;152:322–330. doi: 10.1111/j.1365-2141.2010.08409.x. [DOI] [PubMed] [Google Scholar]
- 39.Thomson BG, Robertson KA, Gowan D, et al. Analysis of engraftment, graft-versus-host disease, and immune recovery following unrelated donor cord blood transplantation. Blood. 2000;96:2703–2711. [PubMed] [Google Scholar]
- 40.Comans-Bitter WM, de Groot R, van den Beemd R, et al. Immunophenotyping of blood lymphocytes in childhoodReference values for lymphocyte subpopulations. The Journal of Pediatrics. 1997;130:388–393. doi: 10.1016/s0022-3476(97)70200-2. [DOI] [PubMed] [Google Scholar]
- 41.Bizzetto R, Bonfim C, Rocha V, et al. Outcomes after related and unrelated umbilical cord blood transplantation for hereditary bone marrow failure syndromes other than Fanconi anemia. Haematologica. 2011;96:134–141. doi: 10.3324/haematol.2010.027839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frangoul H, Wang L, Harrell FE, Jr, Manes B, Calder C, Domm J. Unrelated umbilical cord blood transplantation in children with immune deficiency: results of a multicenter study. Bone Marrow Transplant. 2009;45:283–288. doi: 10.1038/bmt.2009.137. [DOI] [PubMed] [Google Scholar]
- 43.Gennery A, Cant AJ. Cord blood stem cell transplantation in primary immune deficiencies. Curr Opin Allergy Clin Immunol. 2007;7:528–534. doi: 10.1097/ACI.0b013e3282f1d6b6. [DOI] [PubMed] [Google Scholar]
- 44.Bhattacharya A, Slatter MA, Chapman CE, et al. Single centre experience of umbilical cord stem cell transplantation for primary immunodeficiency. Bone Marrow Transplant. 2005;36:295–299. doi: 10.1038/sj.bmt.1705054. [DOI] [PubMed] [Google Scholar]
- 45.Fernandes JF, Rocha V, Labopin M, et al. Transplantation in patients with SCID: mismatched related stem cells or unrelated cord blood? Blood. 2012;119:2949–2955. doi: 10.1182/blood-2011-06-363572. [DOI] [PubMed] [Google Scholar]
- 46.Adamkiewicz T, Mehta P, Boyer M, et al. Transplantation of unrelated placental blood cells in children with high-risk sickle cell disease. Bone Marrow Transplant. 2004;34:405–411. doi: 10.1038/sj.bmt.1704606. [DOI] [PubMed] [Google Scholar]
- 47.Adamkiewicz TV, Szabolcs P, Haight A, et al. Unrelated cord blood transplantation in children with sickle cell disease: Review of four-center experience. Pediatric Transplantation. 2007;11:641–644. doi: 10.1111/j.1399-3046.2007.00725.x. [DOI] [PubMed] [Google Scholar]
- 48.Chow RY, Jaing TH, Chan LL, et al. Unrelated Cord Blood Transplantation (CBT) of 101 Hemoglobinopathy (HGB) Patients. Biology of Blood and Marrow Transplantation. 2012;18:S268. [Google Scholar]
- 49.Lang PJ, Mueller I, Teltschik H, et al. Low Transplant Related Toxicity and Mortality after Transplantation of CD3/CD19 Depleted Haploidentical Stem Cells with Reduced Intensity Conditioning in Children. Blood. 2007;110:3076. [Google Scholar]
- 50.Hara J, Osugi Y, Ohta H, et al. Double-conditioning regimens consisting of thiotepa, melphalan and busulfan with stem cell rescue for the treatment of pediatric solid tumors. Bone Marrow Transplant. 1998;22:7–12. doi: 10.1038/sj.bmt.1701283. [DOI] [PubMed] [Google Scholar]
- 51.Ciurea SO, Qureshi S, Rondon G, et al. Sustained Engraftment Using Fludarabine, Melphalan and Thiotepa Conditioning for Haploidentical Stem Cell Transplantation. Blood. 2007;110:5081. [Google Scholar]
- 52.Terenzi A, Lubin I, Lapidot T, et al. Enhancement of T cell-depleted bone marrow allografts in mice by thiotepa. Transplantation. 1990;50:717–720. [PubMed] [Google Scholar]
- 53.Down JD, Westerhof GR, Boudewijn A, Setroikromo R, Ploemacher RE. Thiotepa improves allogeneic bone marrow engraftment without enhancing stem cell depletion in irradiated mice. Bone Marrow Transplant. 1998;21:327–330. doi: 10.1038/sj.bmt.1701103. [DOI] [PubMed] [Google Scholar]
- 54.Rosales F, Peylan-Ramu N, Cividalli G, et al. The role of thiotepa in allogeneic bone marrow transplantation for genetic diseases. Bone Marrow Transplant. 1999;23:861–865. doi: 10.1038/sj.bmt.1701758. [DOI] [PubMed] [Google Scholar]
- 55.Locatelli F, Rocha V, Reed W, et al. Related umbilical cord blood transplantation in patients with thalassemia and sickle cell disease. Blood. 2003;101:2137–2143. doi: 10.1182/blood-2002-07-2090. [DOI] [PubMed] [Google Scholar]
- 56.Mazur M, Kurtzberg J, Halperin EC, Ciocci G, Szabolcs P. Transplantation of a Child With Sickle Cell Anemia With an Unrelated Cord Blood Unit After Reduced Intensity Conditioning. J Pediatr Hematol Oncol. 2006;28:840–844. doi: 10.1097/MPH.0b013e31802d3e53. [DOI] [PubMed] [Google Scholar]
- 57.Uchida N, Friera AM, He D, Reitsma MJ, Tsukamoto AS, Weissman IL. Hydroxyurea Can Be Used to Increase Mouse c-kit+Thy-1. 1loLin-/loSca-1+ Hematopoietic Cell Number and Frequency in Cell Cycle In Vivo. Blood. 1997;90:4354–4362. [PubMed] [Google Scholar]
- 58.Page KM, Zhang L, Mendizabal A, et al. Total Colony-Forming Units Are a Strong, Independent Predictor of Neutrophil and Platelet Engraftment after Unrelated Umbilical Cord Blood Transplantation: A Single-Center Analysis of 435 Cord Blood Transplants. Biology of Blood and Marrow Transplantation. 2011;17:1362–1374. doi: 10.1016/j.bbmt.2011.01.011. [DOI] [PubMed] [Google Scholar]
- 59.Eapen M, Klein JP, Ruggeri A, et al. Is allele-level HLA-matching relevant for single umbilical cord blood transplants? Bone Marrow Transplant. 2013;48:2. [Google Scholar]
- 60.Bacigalupo A, Ballen K, Rizzo D, et al. Defining the Intensity of Conditioning Regimens: Working Definitions. Biology of Blood and Marrow Transplantation. 2009;15:1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Baker KS, Filipovich AH, Gross TG, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2008;42:175–180. doi: 10.1038/bmt.2008.133. [DOI] [PubMed] [Google Scholar]
- 62.Cooper N, Rao K, Gilmour K, et al. Stem cell transplantation with reduced-intensity conditioning for hemophagocytic lymphohistiocytosis. Blood. 2006;107:1233–1236. doi: 10.1182/blood-2005-05-1819. [DOI] [PubMed] [Google Scholar]
- 63.Sawada A, Ohga S, Ishii E, et al. Feasibility of reduced-intensity conditioning followed by unrelated cord blood transplantation for primary hemophagocytic lymphohistiocytosis: a nationwide retrospective analysis in Japan. International Journal of Hematology. 2013;98:223–230. doi: 10.1007/s12185-013-1391-z. [DOI] [PubMed] [Google Scholar]
- 64.Hwang EI, Camitta MGW, Vinesett R, et al. Pericardial effusion following unrelated umbilical cord blood transplantation: Analysis in a cohort of 423 pediatric patients transplanted at a single center. Biol Blood Marrow Transplant. 2009;15:73. [Google Scholar]
- 65.Lee F, Madrigal A, Shaw B, Saudemont A. Study of the effects of Campath on cord blood and peripheral blood cells. Bone Marrow Transplant. 2013;48:241. [Google Scholar]