Abstract
Astrocytes have not been a major therapeutic target for the treatment of stroke, with most research emphasis on the neuron. Given the essential role that astrocytes play in maintaining physiological function of the central nervous system and the very rapid and sensitive reaction astrocytes have in response to cerebral injury or ischemic insult, we propose to replace the neurocentric view for treatment with a more nuanced astrocytic centered approach. In addition, after decades of effort in attempting to develop neuroprotective therapies, which target reduction of the ischemic lesion, there are no effective clinical treatments for stroke, aside from thrombolysis with tissue plasminogen activator, which is used in a small minority of patients. A more promising therapeutic approach, which may affect nearly all stroke patients, may be in promoting endogenous restorative mechanisms, which enhance neurological recovery. A focus of efforts in stimulating recovery post stroke is the use of exogenously administered cells. The present review focuses on the role of the astrocyte in mediating the brain network, brain plasticity, and neurological recovery post stroke. As a model to describe the interaction of a restorative cell-based therapy with astrocytes, which drives recovery from stroke, we specifically highlight the subacute treatment of stroke with multipotent mesenchymal stromal cell therapy.
Keywords: stroke, marrow stromal cells, microRNA, exosomes, Shh, tPA, restoration, plasticity
Introduction
Stroke is a devastating neurological disease with limited functional recovery and is one of the leading causes of death and disability worldwide. Currently, the only approved stroke therapy is thrombolysis induced by intravenous administration of recombinant tissue plasminogen activator (tPA; Alberts and Naidech, 2013; Marler, 1995). However, because of a short therapeutic time window (<4.5 h), only a small fraction of patients benefit from this treatment (Fang et al., 2010). In the past two decades, many therapeutic targets have been pursued and improved neurological sequelae in experimental animal models of stroke; whereas, clinical trials have failed to demonstrate a corresponding benefit (Balami et al., 2013; Sutherland et al., 2012). Reasons for this failure and inconsistency between laboratory studies and human clinical trials are many, and include, inappropriate clinical translation of laboratory studies, particularly with regard to therapeutic window and dosing, and the historical primary focus on neuroprotection. In the acute phase of stroke, neuroprotective treatments aim to reduce rapidly progressing cell damage and to reduce the volume of cerebral infarction and secondary cell death, whether by necrosis or apoptosis. In addition, most clinical trials were often performed using a single drug with single purported mechanism of action specifically targeting, the neuron. To treat stroke, we have to reconceptualize and redefine our therapeutic targets. Acute neuroprotective treatments for stroke fight a temporal battle of salvaging cerebral tissue before the onset of death, as well as a physiological impediment of delivery of therapy to tissue which has inadequate blood flow. Thus, a more promising therapeutic approach would be to promote remodeling of the central nervous system (CNS) via neurovascular plasticity, and thereby to foster neurological recovery. To accomplish this and to broaden treatment targets, we must consider therapeutic approaches that benefit multiple cell types, and in our view, particularly, astrocytes (Bang et al., 2005; Bhasin et al., 2011; Chopp and Li, 2002; Clarke and Barres, 2013; Dharmasaroja, 2009; Hermann and Chopp, 2012; Lee et al., 2010; Li and Chopp, 2009; Suarez-Monteagudo et al., 2009; Zhou, 2011). Stroke affects all cellular elements of the brain, that is, vascular cells, neurons, astrocytes, oligodendrocytes, microglia, and ependymocytes. Astrocytes are likely to be essential targets for manipulation, because they are the most abundant cells in the adult CNS and greatly outnumber neurons (Bignami, 1991), and are in contact with and interact and affect all parenchymal cells. Nevertheless, among all brain cells, astrocytes are probably the least understood in terms of cell biology and function, and their role in neurological recovery.
In the delayed subacute and chronic phases of stroke, restorative treatments designed to enhance neuroplasticity and to remodel the intact CNS through selective cellular or molecular modifications, which stimulate intrinsic restorative pathways and thereby promote neurological recovery should be the primary focus of therapeutic efforts. These new restorative therapies, which will impact intact parenchymal cells, and primarily, astrocytes, can then be applied days, weeks and even later after stroke; thus, all stroke patients, will be treated, without tight time constraints. Among potential restorative therapies, exogenous cell-based therapies have been proposed to ameliorate post-stroke deficits. Multipotent mesenchymal stromal cells (MSCs) have emerged as a strong candidate (Bang et al., 2005; Bhasin et al., 2011; Chen et al., 2003a, b, 2001b; Chopp and Li, 2002; Dharmasaroja, 2009; Hermann and Chopp, 2012; Lee et al., 2010; Li and Chopp, 2009; Li et al., 2008b, 2002b, 2001b, 2005b, 2006; Shen et al., 2007a,b; Suarez-Monteagudo et al., 2009; Zhou, 2011). We hypothesize that MSCs stimulate the neural repair process, especially via activating the major endogenous repair mediators, astrocytes, in the CNS. This approach contrasts with the predominant neurocentric view of stroke therapy. In this manuscript, we focus on the astrocyte as a mediator of neurological recovery post stroke and describe the means by which astrocytes impact neural remodeling. We describe how the astrocyte, outside the core lesion, reacts to an ischemic insult, how the astrocyte interacts with parenchymal cells as well as some important aspects of astrocyte physiology which may impact neurological recovery after stroke. In addition, as a means to describe how a restorative therapy affects astrocytes and astrocytes thereby contribute to brain plasticity and neurite remodeling, we discuss in detail how MSCs promote neurological recovery post-stroke via their interaction with astrocytes.
Astrocytes Post-Stroke
Astrocytes have diverse and important functions in many aspects of ischemic brain damage (Rossi et al., 2007). Astrocytes are coupled to one another into a cellular network (homocellular and heterocellular junctions) via gap junction intercellular communication (GJIC; Rouach et al., 2000), through which they can pass various metabolites. The role of astrocyte junctions in stroke remains controversial, with evidence that both beneficial and harmful substances may pass through them and influence stroke in opposite ways (Nakase et al., 2004). Astrocytic end-feet cover almost the entire surface of capillaries of the adult brain (Kerr, 2000) and are integral to the formation and integrity of the blood brain barrier (BBB). Astrocytic finely branching processes envelop all cellular components throughout the CNS, and contact all parts of neurons, for example, soma, dendrites, axons, and synaptic terminals. The close association between astrocytes and pre-synaptic and postsynaptic terminals as well as their ability to integrate synaptic activity and release neuromodulators has been termed the “tripartite synapse” (Araque et al., 1999). Synaptic modulation by astrocytes takes place because of this three-part association (Halassa et al., 2007a,b; Parpura et al., 2012; Stevens, 2008; Verkhratsky and Parpura, 2010). Neurons rely on astrocytes to instruct the formation of their synapses (Clarke and Barres, 2013). Astrocytes, thus, function as a syncytium of interconnected cells in the CNS (Li et al., 1998; Nagy and Rash, 2000; Naus et al., 2001; Pekny and Nilsson, 2005; Scemes et al., 2000; Siushansian et al., 2001) and discrete microdomains within the astrocytic syncytium may interact autonomously with one another and with neurons (Giaume and Liu, 2012; Verkhratsky, 2010). Perisynaptic and perivascular processes are the major characteristics of astrocytes, in which astrocytes wire the brain (Simon and Nicolelis, 2012). Theoretically, vascular cells belong to the circulatory system, instead of the nervous system (Kerr, 2000); therefore, in this mini-review, we focus on the astrocyte-neuron network. A key concept that we highlight here is that astrocytes are morphologically highly elaborated cells, establishing associations through their fine processes with practically tripartite synapses in the CNS. Individual astrocytes in the hippocampus of the grey matter of rodents touch up to 100,000 synapses (Bushong et al., 2002; Halassa et al., 2007b) and they extend to considerable volumes of up to 80,000 μm3, which correspond to a 40-fold volume of their soma (Bushong et al., 2002; Halassa et al., 2007b; Pfrieger and Slezak, 2012). Human protoplasmic astrocytes are even larger and more complex. This astrocytic complexity has permitted the increased functional competence of the adult human brain (Oberheim et al., 2009). Thus, close morphological associations, together with the fact that astrocytes express a multitude of ion channels, transporters, and membrane receptors, endow these cells with the unique capability to sense and influence diverse CNS functions (Berry et al., 2002; Giaume and McCarthy, 1996; Jourdain et al., 2007; Lynn et al., 2001; Mazzanti et al., 2001; Pekny and Nilsson, 2005; Pellerin and Magistretti, 2004; Rouach et al., 2004; Trendelenburg and Dirnagl, 2005; Ullian et al., 2001). Several forms of astrocytes exist in the CNS, classically including fibrous (in white matter), protoplasmic (in gray matter), and radial (Marin-Padilla, 1995; Shannon et al., 2007). We, in this review, have only used an umbrella term, astrocyte, neglecting the complexity, variety and distribution of assorted astrocytes, likely with different responses to restorative cell-base therapies. Further investigation is, therefore, called-for to more deeply determine the response of the wonderful diversity of astrocytes to restorative therapy. This may provide a richer opportunity to develop more effective therapies for stroke and other neurological diseases.
Complete cerebral blood flow cessation after stroke causes irreversible injury to all cell types in the ischemic core, because the supply of glucose and oxygen ceases. In contrast, in the ischemic boundary zone (IBZ), where oxygen and glucose delivery is partly maintained, astrocytes may survive for a prolonged period compared with neurons (Swanson et al., 2004; Zhao and Rempe, 2010). Compared with neurons, cultured astrocytes are more resistant to oxygen and glucose deprivation (OGD; Panickar and Norenberg, 2005). In addition, stroke can alter astrocyte function. Studies have shown that, in the acute phase after ischemic stroke, astrocytes become “reactive.” In the reactive process, the astrocytes exhibit hypertrophied, interdigitated processes and inhibit axonal regeneration by participating in the formation of the glial scar (Sofroniew, 2005), in which contains many different inhibitory molecules including chondroitin sulfate proteoglycans (CSPGs), a major barrier against axonal regeneration (Morgenstern et al., 2002). Astrocytes can also produce a variety of proinflammatory cytokines. Astrocytic gap junctions may remain open following ischemia (Cotrina et al., 1998), allowing substances such as proapoptotic factors to spread through the syncytium, thereby expanding the size of the infarct (Siushansian et al., 2001), and decreased astrogliosis often correlates with decreased infarct size (Chen et al., 2008; Fang et al., 2006; Zhao et al., 2011).
However, studies also report that the glial scar, the rapidly expanding astrocytic processes create both physical and functional walls surrounding the ischemic core, which extend the time available for marshalling endogenous repair mechanisms, for example, redirection of blood flow to still salvageable parts of the brain and redirection of neurite sprouting and synapse formation to build a new circuitry. In addition to their role in glial scar formation, astrocytes also respond to ischemia with functions important for neuroprotection and neurorestoration. These include protecting spared tissue from further damage, rebuilding the BBB, taking up excess glutamate (Mazzanti et al., 2001), and producing neurotrophic factors (Anderson et al., 2003; Swanson et al., 2004). For example, after ischemic stroke, neurons have less endogenous antioxidants and neurons are far more susceptible to ischemic damage than neighboring astrocytes (Garcia et al., 1993). Astrocytes are important in neuronal antioxidant defense and secrete growth factors and astrocytes upregulate glucose transporters in order to provide energy to stressed/dying neurons. A growing body of data demonstrate that astrocytes also play an important role to promote neurorestoration in the chronic phase after injury (Zhao and Rempe, 2010). Astrocytes effect long-term recovery after brain injury, through neurite outgrowth, synaptic plasticity, or neuron regeneration (Larsson et al., 2004; Privat, 2003), which are influenced by astrocyte surface molecule expression and trophic factor release (Chen and Swanson, 2003). Astrocytes promote brain plasticity and recovery from stroke (Gao et al., 2005a,b; Li et al., 2008a; Trendelenburg and Dirnagl, 2005; Xin et al., 2006; Zhang et al., 2006). Profound synaptic plasticity occurs in the IBZ, which improves functional outcome after stroke (Carmichael, 2003; Chen et al., 2003c; Nudo, 2007; Shen et al., 2006). Astrocytes have prominent roles in modifying synaptic plasticity and formation of new synapses (Barker and Ullian, 2010). Astrocytes make extensive contacts with synaptic sites where they release soluble factors that can increase synapse number, provide synaptic insulation restricting the spread of neuro-transmitter to neighboring synapses, and release neuroactive compounds, gliotransmitters, that can directly influence synaptic transmission (Halassa et al., 2007a). During periods of synaptogenesis, astrocyte processes are highly mobile and may contribute to the stabilization of new synapses. As our understanding of the extent of their influence at the synapse unfolds, it is clear that astrocytes are important in neural repair (Sofroniew, 2005). Emphasis should therefore be shifted from the neuron to the astrocyte as the mediator of neurovascular plasticity and neurological recovery. An overview of the most current findings on the diverse roles played by astrocytes in the CNS function and dysfunction, the connections that the astrocyte makes with other cells of the brain that are essential for a variety of important neural functions, is warranted.
Gene-Modified Astrocytes Post Stroke
Understanding astrocyte-neuron interactions in vivo requires dedicated experimental approaches to independently manipulate the astrocyte. Recently, genetic approaches to study glial cells in the rodent brain have been reviewed by Pfrieger and Slezak (2012), they include: (1) astrocyte-specific gene overexpression of a wide range of cellular components; (2) visualization of astrocytes, where fluorescent protein expression is driven by fragments of murine and human promoters to underline the importance of regional patterns of promoter activities; (3) elimination of astrocytes, which eliminates glial fibrillary acidic protein (GFAP)-positive progenitor cells; and 4) astrocyte-specific gene ablation using the Cre/loxP system. The majority of transgenic lines generated so far use fragments of the Gfap promoter to target constitutively active Cre to astrocytes (Pfrieger and Slezak, 2012).
The use of GFAP antibodies and promoters are valuable in studying astrocytes after stroke. In response to stroke, major features of reactive astrocytes include upregulation of GFAP and vimentin and re-expression of nestin (Fuchs and Cleveland, 1998; Li and Chopp, 1999; Li et al., 2005). GFAP, vimentin and nestin are constituents of intermediate filaments (IFs), which are part of the cytoskeleton. Investigators generated knock-out (KO) mice for GFAP, by which astrocytic structure and function are abnormal in adult Gfap−/−mice and Gfap−/−mice are highly susceptible to cerebral ischemia (Nawashiro et al., 2000). Since the astrocyte processes contact synapses and modulate synaptic function, Gfap−/−mice show alteration in long-term potentiation in neurons after transient ischemia, suggesting that GFAP has an important role in astrocyte-neural interactions (Tanaka et al., 2002). Thus, GFAP is necessary for the integrity of CNS architecture and its long-term maintenance. To eliminate IFs in their specific “reactive” state, studies have shown that astrocytes in mice deficient for double GFAP and vimentin (Gfap−/−vim−/−) cannot form IFs. Inactivation of the Gfap gene, but not that of vimentin, improves neuronal survival and neurite growth (Menet et al., 2001). The double KO astrocytes present many features of immaturity and greatly improve survival and neurite growth by increasing cell-cell contact and secrete diffusible factors. Glial scar formation appeared normal after brain lesions in Gfap−/− or vim−/−mice, but was impaired in Gfap−/−vim−/−mice that developed less dense scars frequently accompanied by bleeding (Pekny et al., 2007). These results indicate that GFAP and vimentin are required for proper glial scar formation in the injured CNS and that some degree of functional overlap exists between these IF proteins (Pekny et al., 1999). The role of reactive astrocytes has been studied in brain ischemia by using the available Gfap−/−vim−/−mice (Li et al., 2008a). Seven days after middle cerebral artery occlusion (MCAo), infarct volume was 210–350% higher in Gfap−/−vim−/− than in wild-type (WT-) mice; Gfap−/−, vim−/−, and WT-mice had the same infarct volume. Gfap−/−vim−/−mice have larger infarct volume than WT-controls, which suggests that reactive astrocytes are protective in brain ischemia and limit the extent of the infarct. A recent in vitro study also showed that Gfap−/− vim−/− astrocytes exposed to OGD and reperfusion exhibited increased cell death and conferred lower degree of protection to cocultured neurons than WT astrocytes (de Pablo et al., 2013). This observation stands in sharp contrast to the reported adverse roles played by the glial scar and associated astrocytic activation in promoting ischemic cell damage (Askalan et al., 2006; Bush et al., 1999). The absence of IFs affects vesicle trafficking in astrocytes. Gfap−/−vim−/−astrocytes have a decreased number of vesicles displaying directional mobility and fewer vesicles that travel for a long distance compared with WT-astrocytes. This suggests that IFs may act as a structure supporting highly mobile vesicles in astrocytes. Gfap−/−vim−/−mice have impaired astrocyte activation, and decreased glutamate uptake abilities after ischemia (Li et al., 2008a). Studies of Gfap−/−vim−/−mice provide evidence suggesting that reactive astrocytes are beneficial on all accounts after stroke.
Cell-Therapy Post Stroke
Worldwide, tissue engineering and cell-based therapies are at the forefront of the regenerative medicine agenda, and researchers are addressing key diseases, including stroke with these therapies. Cell therapy can be categorized by their embryonic, fetal or adult origin, and the later two can be further identified by their tissue of origin. Cell transplantation has shown promise in reducing neurological deficits associated with stroke. Embryonic neural progenitor cells transplanted in a model of MCAo in rats demonstrated potent therapeutic effects examined behaviorally, along with neuroradiological assessment using magnetic resonance imaging (MRI; Takahashi et al., 2008). Fetal cortical cells survive after stroke in adult rats, and the adult hosts have a regenerative capacity sufficient to innervate the grafted tissue (Grabowski et al., 1992). Because of ethical dilemmas and practical concerns, increased attention has been directed to adult derived cells for therapeutic application to neural injury. Endogenous neural progenitor cells (NPCs) are activated in response to ischemia, both in rodents (Arvidsson et al., 2002; Zhang et al., 2002a) and humans (Jin et al., 2006). MRI analysis of ferromagnetic labeled adult subventricular zone (SVZ) cells intracisternally into stroked rats 48 h after MCAo (Zhang et al., 2003) showed that SVZ cells targeted the IBZ, and these cells selectively migrated within the cerebrospinal fluid (CSF) into parenchyma. Neurological function evaluation showed that the deficit ameliorating effect of SVZ cell treatment was apparent at 28 days after stroke. Transplanted neural stem cells also migrated to the lesion, and differentiated into neurons with axons that projected to appropriate targets and expressed appropriate neurotransmitters and receptors (Magavi and Macklis, 2002; Patkar et al., 2012). The most appropriate type of cell to be used in brain ischemic therapies, as well as their sources, remain a matter of intense research (Bang et al., 2005; Chen et al., 2001b; Chopp and Li, 2002). A good candidate cell should, in principle, display high plasticity to generate diverse benefits, and low risk to cause undesired outcomes. One of the more exciting emerging therapies for improving functional recovery after stroke is the use of bone marrow derived stromal cells (MSCs) (Chopp and Li, 2002; Joyce et al., 2010; Li and Chopp, 2009). MSCs are a mixed cell population, including stem and progenitor cells (Bang et al., 2005; Chopp and Li, 2002). MSCs are currently a strong candidate therapy in stroke, since they are easily isolated and can be expanded in culture from humans without ethical and technical problems (Chen et al., 2001b; Chopp and Li, 2002; Joyce et al., 2010). The feasibility and safety of MSCs have been extensively tested and demonstrated in preclinical studies and in clinical trials of many diseases (Bang et al., 2005; Horwitz et al., 1999, 2001; Koc et al., 1999, 2000; Le Blanc et al., 2004; Wakitani et al., 2004; Wollert et al., 2004). Using the MCAo model in rodents, MSCs transplanted into rodent brain intracerebrally (Li et al., 2000; Zhao et al., 2006), intraarterially (Li et al., 2001; Zhang et al., 2012), intracisternally (Zhang et al., 2002b, 2012), intravenously (Chen et al., 2001c; Gutierrez-Fernandez et al., 2013; Horita et al., 2006; Pavlichenko et al., 2008; Ukai et al., 2007), or lumber intrathecally (Zhang et al., 2012) after stroke, significantly improve neurological outcome. The MSCs escape immune system surveillance and survive in the rodent ischemic brain, and specifically migrate into the IBZ after transplantation (Chen et al., 2001a; Irons et al., 2004; Lee et al., 2003; Li et al., 2002). Although the mechanisms underlying this targeted movement are not fully understood, studies suggest that chemotactic factors are responsible (Wang et al., 2002a,b). MSCs are capable of homing to an area of ischemia by sensing stromal cell derived factor-1 (SDF-1), which is highly expressed in ischemic tissue, including astrocytes (Wang et al., 2012), with their chemokine (C-X-C motif) receptor 4 (CXCR4) receptors (Cui et al., 2007; Shen et al., 2007b; Tsai et al. 2011). Most of the administered MSCs present in the brain localize to the IBZ, which is potentially salvageable, and multiple processes of cell repair are initiated.
Although some MSCs express proteins phenotypic of neural cells (Chen et al., 2001b; Kopen et al., 1999), it is highly unlikely that benefit is derived by replacement of infarct tissue with transdifferentiated MSCs (2002 Lancet). The MSCs that are employed in this therapy are not necessarily stem cells, but progenitor and differentiated cells that escape immune system surveillance and survive in the CNS, even for transplantation of allogeneic (Caplan, 2007; Li et al., 2006; Yang et al., 2010) or xenogeneic MSCs (Li et al., 2002; Yang et al., 2010). After treatment of stroke with MSCs, brain derived neurotrophic factor (BDNF) and nerve growth factor (NGF) among other trophic factors significantly increased and apoptotic cells significantly decreased in the IBZ (Li et al., 2002). MSCs secrete other bioactive factors that amplify endogenous repair mechanisms. MSCs as “small molecular factories” dynamically facilitate CNS repair, which include decreasing apoptotic cell death (Chen et al., 2003a; Joyce et al., 2010), and promotion of neurological recovery via increasing angiogenesis, development of new blood vessels, (Joyce et al., 2010; Zacharek et al., 2007), neurogenesis, development of new CNS cells, (Aizman et al., 2013; Gutierrez-Fernandez et al., 2013; Shen et al., 2010), synaptogenesis, formation of new synapses between neurons, (Gutierrez-Fernandez et al., 2013; Shen et al., 2007a), and promoting glial (Li et al., 2005; Yang et al., 2010), neuronal (Dezawa et al., 2005; Li et al., 2005), and blood vascular (Chen et al., 2003b; Parr et al., 2007) remodeling. It is likely that the functional improvements as a result of MSC treatment are due to combined action via multiple cellular and molecular mechanisms to affect the intact CNS responds to stroke (Hermann and Chopp, 2012). We and others have demonstrated that in rodents following stroke and treatments, axonal remodeling highly correlates with behavioral outcome (Chen et al., 2002b; Lee et al., 2004; Liu et al., 2007, 2010; Papadopoulos et al., 2002). MSCs markedly enhanced inter-hemispheric and intracortical connections (Liu et al., 2010) and increased axonal sprouting and rewiring into the denervated spinal cord (Liu et al., 2007, 2011), suggesting MSCs facilitate functional recovery after stroke. Interestingly, we compared the effect of treatment of stroke with MSCs from stroke rats and normal rats on functional outcome (Zacharek et al., 2010); the former is superior to the latter for the neurorestorative treatment of stroke, indicating a major effect of stroke on the entire body. Therefore, multiple events act in concert to induce neurite remodeling and reestablish new functional synaptic networks that may be causally related to changes in functional outcome. Persistent focus on cell-based therapies post stroke as a means to replace or augment the generation of parenchymal cells undermines their therapeutic potential.
As described above, there are multitudes of affects at both cellular and molecular levels evoked by the treatment of stroke with a cell-based therapy that promote functional recovery. Although it has traditionally been appealing to selectively identify those effects that primarily contribute to the enhanced functional recovery, it is highly likely that the physiological manifestations of plasticity, for example, angiogenesis, neurogenesis, and neurite outgrowth are coupled (Carmichael, 2010; Zhang and Chopp, 2009), and therefore attempts to isolate the particular molecular and physiological indices specifically inducing recovery may not be possible. Likewise, the molecular pathways driving aspects of plasticity are interdependent, and it is unlikely that such an interactive environment would permit a simplified mono-mechanistic or hierarchical approach to discriminate selective events and molecular mediators of recovery. However, a close astrocytic and vascular coupling may underlie the neurovascular remodeling that leads to improved neurological outcome after MSC treatment of stroke. Vascular stimulation and angiogenesis are driven by astrocytic expression of angiogenic proteins, for example, vascular endothelial growth factor (VEGF) and Angiopoietin 1 (Ang1), that may orchestrate recovery (Zhang and Chopp, 2002). Activated and angiogenic blood vessels generate factors, for example, BDNF, glial-derived neurotrophic factor (GDNF), and VEGF that stimulate neurogenesis within the SVZ and promote the vasculature-mediated migration of neuronal precursors toward the ischemic areas (Grade et al., 2013). The newly generated cells within the SVZ then migrate to the vasculature. This migration is fostered by vascular expression of SDF-1, which signals to its receptor CXCR4 on the migrating neuroblasts (Liu et al., 2008). Reactive astrocytes, which are widespread throughout the damaged area, ensheath blood vessels, and express TrkB, a high-affinity receptor for BDNF, suggesting that these glial cells trap extracellular neurotrophic factors. Importantly, this pattern of expression is reminiscent of the adult rostral migratory stream, where TrkB-expressing astrocytes bind and sequester vasculature-derived BDNF, leading to the entry of migrating cells into the stationary phase (Grade et al., 2013). Consequently, the migrating neuroblasts localize to activated vasculature and are driven to differentiation by VEGF-B expressed in the vasculature (Madri, 2009; Teng et al., 2008). The rewiring, that is, neurite outgrowth may also be mediated by astrocytic driven vascular stimulation, Ang 1, expressed in astrocytes (Koyama et al., 2012) and activated endothelial cells (Cui et al., 2013), although an angiogenic agent, also promotes neurite outgrowth (Yan et al., 2012). In addition, astrocytic and vascular expression of tPA from astrocytes (Xin et al., 2010) and endothelial cells (Correa et al., 2011) contributes to neurite outgrowth/remodeling and fosters neurological recovery post stroke (Shen et al., 2011). Although there is a complex web of interaction of the CNS structural and molecular mediators of recovery, our goal here, however, is to illuminate an important, if not primary role of the astrocytes in promoting neurological recovery after cell-based treatment of stroke. Many restorative events coalesce around the astrocytes as promoting plasticity and recovery. However, as a caveat, the temporal sequence of events that constitute the multifaceted restorative dynamic with the astrocyte at its core is not known. The described sequential means of interaction, although elegant and scientifically appealing has not been confirmed in vivo, and whether the described trajectory of events are specific for restoration, or whether competing or complementary cellular interactions also contribute the neurovascular remodeling post stroke therapy is not known. A way to obtain insight into the possibility of concurrent multipathway restorative trajectories that promote recovery is my introducing microRNAs (Section “MicroRNAs (miRNAs) as a molecule-mediator shuttled by microvesicles/exosomes after MSC-therapy”). It is our hypothesis that the varied molecular signals driving restorative events may derive from the communication of microRNAs via microvesicles between the exogenously administered cells and parenchymal cells, primarily the astrocytes. It is reasonable to focus on the astrocyte because the astrocyte is the most abundant and possibly the most interactive of the parenchymal cells, bridging neurons, and endothelial cells.
Astrocytes as Mediators of MSC-Therapy
Astrocytes in the developing brain direct neurites through their synthesis of cell surface and extracellular matrix (ECM) molecules (Powell et al., 1997). An account of neuronal development is beyond the scope of this review; nevertheless, neuronal plasticity in the adult animal may use mechanisms that are active during development (Levitan and Kaczmarek, 2002). In the adult animal after stroke, axons may also acquire their potential for outgrowth from neighboring astrocytes and establish contacts with existing circuits in the CNS (Matsaas and Tsacopoulos, 1999). For example, GDNF, one member of the transforming growth factor-beta (TGFβ) family, signals via the cognate receptors, for example, GDNF-receptor alpha-1 (Hase et al., 1999; Sarabi et al., 2003). The latter receptors are expressed on a variety of neurons. Astrocytic endogenous GDNF production is enhanced in the IBZ by MSC transplantation after stroke in adult rats (Shen et al., 2010). Some axons were reoriented parallel to GFAP-positive processes of reactive astrocytes (Li et al., 2006; Shen et al., 2007b). MSCs can stimulate neurotrophins and growth factors including VEGF (Chen et al., 2003b; Gutierrez-Fernandez et al., 2013), basic fibroblast growth factor (Chen et al., 2003a; Wakabayashi et al., 2010), and BDNF (Alder et al., 2012; Kurozumi et al., 2005), within astrocytes in response to the ischemic brain environment (Chen et al., 2002d; Qu et al., 2007). Moreover, MSCs also diminish glial scar formation after stroke (Li et al., 2005; Pavlichenko et al., 2008). MSCs upregulate expression of GJIC protein connexin-43 and bone morphogenetic proteins 2 and 4 (BMP2/4) after stroke in rats (Zhang et al., 2006). Bone morphogenetic proteins affect cell proliferation and differentiation. Our understanding of the molecular triggers and mechanisms underlying the induction of CNS plasticity mediated by reactive astrocytes has been substantially expanded by strong complementary in vitro data (Chen et al., 2002c; Gao et al., 2005a,b; Qu et al., 2007; Xin et al., 2006). In vitro studies demonstrate that MSCs significantly reduce astrocyte apoptosis, and increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase (PI3K/Akt) and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult (Gao et al., 2005b), which suggest that MSCs exert protective roles on astrocytes that have otherwise been irreparably compromised. MSCs enhance connexin 43 GJIC communication in cultured astrocytes (Gao et al., 2005a). MSCs induce BMP2/4 production in OGD astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells (Xin et al., 2006). In vitro data support the hypothesis that reactive astrocytes promote brain plasticity and recovery from stroke, and that the beneficial effects of reactive astrocytes are enhanced in the ischemic brain after MSC transplantation (Aizman et al., 2009; Barzilay et al., 2013; Croft and Przyborski, 2009; Gao et al., 2005b).
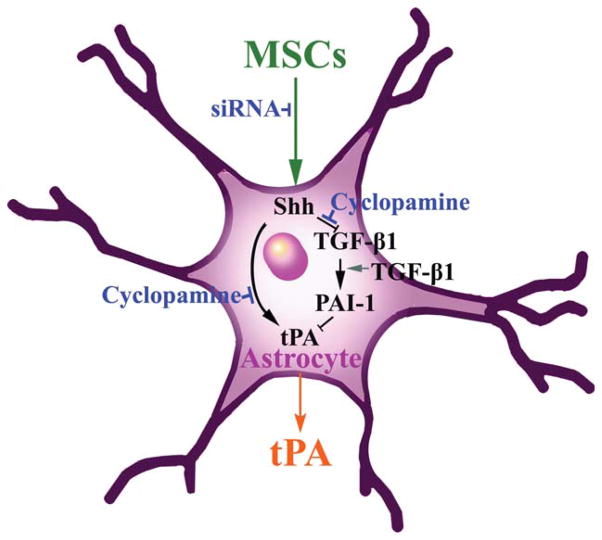
PAs are extracellular serine proteases that catalyze the conversion of the proteolytically inactive zymogen, plasminogen, into the serine protease, plasmin (Collen, 1980). In the CNS, tPA is the major PA (Davies et al., 1998; Sappino et al., 1993; Teesalu et al., 2003) and inhibition of the tPA activity occurs through PA inhibitor -1 (PAI-1). Pekny and his colleagues demonstrate that PAI-1 was significantly down-regulated in the Gfap−/−Vim−/−double knockout mice compared with WT-mice (Li et al., 2008a). In addition to trophic-growth factors induced by MSCs, white matter changes in response to MSC treatment are mediated by astrocytes via increased tPA activity (Shen et al., 2011; Xin et al., 2010, 2011). Exogenous MSCs may therefore provide therapeutic benefit via astrocytes to affect the tPA level (Fig. 1), and thereby, promote neurite remodeling (Fig. 2) in the CNS, which fosters improvement in neurological function. Using genetically modified tPA−/−mice compared with WT-mice, MSCs were found to diminish PAI-1 in astrocytes, which increases tPA activity in the IBZ after MCAo (Shen et al., 2011; Xin et al., 2010). In vitro data suggest that the MSC mediated increased activation of tPA in astrocytes promotes neurite outgrowth after ischemia (Xin et al., 2010); and MSCs significantly increase tPA expression and concomitantly decrease PAI-1 expression in astrocytes (Xin et al., 2011). Another and complementary approach that may elucidate the ability of the brain to activate many restorative and associated processes, is the role of transcription factors stimulated in astrocytes by cell therapy. We and others have also shown that MSCs and other stem/progenitor cells affect transcription factors (Chang et al., 2011; Guo et al., 2012; Xin et al., 2011). Thus, our observation that MSCs upregulate the sonic hedgehog (Shh) pathway, a major morphogen playing a vital role in brain development (Kasai et al., 2004), within astrocytes similarly provides a means to affect multiple processes and molecular pathways (Ding et al., 2013; Xin et al., 2011). Increasing Shh, permits the expression of Gli transcription factors, which leads to the activation of tPA and likely the generation of other proteins (Cayuso and Marti, 2005; Machold et al., 2003; Stecca and Ruiz i Altaba, 2005; Xin et al., 2011). tPA, as noted, promotes brain plasticity, via its proteolytic and nonproteolytic pathways (Lee et al., 2007; Wind et al., 2002). The proteolytic plasminogen/plasmin function of tPA cleaves the precursor forms of neurotrophins, for example, pro-BDNF and pro-NGF, respectively, to the active forms of BDNF and NGF. These trophic factors promote neurite remodeling (Bernd, 2008; Crutcher, 1986; Edgar, 1985; Fahnestock et al., 2004; Wozniak, 1993). tPA also activates the nonproteolytic pathways, for example, N-methyl-D-Aspartate receptor (NMDAR). The NMDAR can subsequently enhance neurite remodeling (Aoki et al., 1998). Thus, our approach is not to tease out which molecules are vital to stimulating recovery and turning on multiple interactive restorative events but rather to enhance appreciation of the role of the astrocyte in mediating these events. Astrocytes likely play a key role possibly also via tPA, in mediating neurite remodeling and functional recovery after treatment of stroke with MSCs. It would be interesting to determine whether activation of astrocytes is a necessary condition for mediating MSC stimulation of tPA and the Shh-Gli transcription factors.
FIGURE 1.
Schematic of proposed mechanisms underlying MSC-enhanced tPA. Our previous studies show that administration of MSCs significantly increased tPA in astrocytes in the ipsilateral IBZ and coculture of MCS with neural parenchymal cells upregulated mRNA and protein levels of Shh and tPA, which were abolished by the small interfering RNA (siRNA) that reduces the expression of the Shh gene (siRNA-Shh), and cyclopamine that specifically blocks Shh signaling (Chen et al., 2002a; Cooper et al., 1998). These data suggest that MSC stimulation of the Shh pathway mediates this increase of tPA. Our in vivo data support the observation that administration of MSCs not only increases tPA but also significantly reduces PAI-1 expression in astrocytes. The TGF-β pathway regulates PAI-1 expression (Docagne et al., 2002; Milei et al., 2004). Our in vitro data show that MSCs repress the TGF-β pathway in astrocytes, which may lead to reduced PAI-1 expression. Blockage of the TGF-β pathway in astrocytes by a TGF-β neutralizing antibody abolished PAI-1 induced by coculture of MSCs and astrocytes. These data suggest that MSCs interact with astrocytes to enhance tPA and concurrently reduce PAI-1 via the TGF-β pathway. The spatiotemporal pattern in the adult IBZ of how the Shh pathway upregulates tPA expression in parenchymal cells and the TGF-β pathway regulates PAI-1 expression in activated astrocytes, have not been fully investigated.
FIGURE 2.
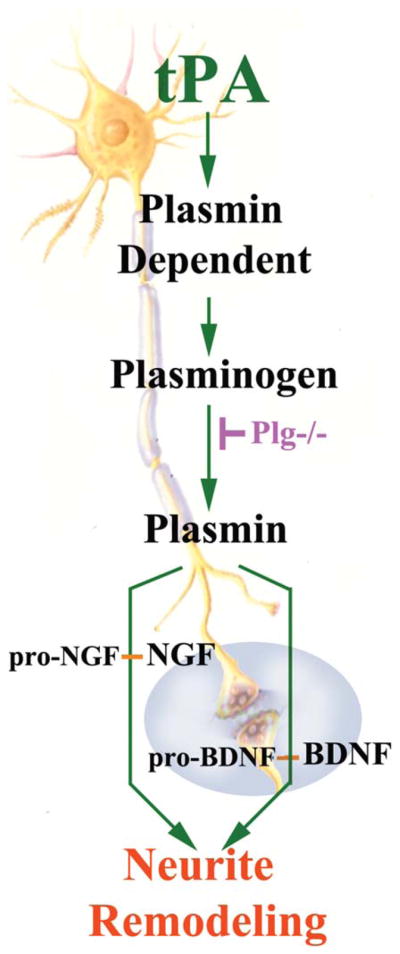
Schematic of proposed mechanisms underlying tPA-enhanced neurite outgrowth. tPA secreted by astrocytes after stroke with MSC treatment activates neurite outgrowth, possibly, via both proteolytic and non proteolytic pathways. Plg−/− mice may be employed (unpublished data) to investigate the non proteolytic effects of tPA on neurite outgrowth after stroke and MSC treatment.
Neurocan is one of the main components of CSPGs expressed in the glial scar (McKeon et al., 1999). As CSPGs inhibit axonal outgrowth (Cafferty et al., 2007), implying that reactive astrocytes impact local axonal outgrowth and repair processes in the damaged CNS (Morgenstern et al., 2002; Snow et al., 2001). Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of MSCs in the ischemic rat brain (Shen et al., 2008). Although cell-based therapy impacting astrocytes may indirectly facilitate neurite outgrowth by reducing CSPGs, and thereby create a permissive environment for neurite outgrowth (Shen et al., 2008), the signals that astrocytes receive from the exogenous cells that downregulate astrocytic CSPGs are not known. In addition, there are a paucity of studies on how the exogenous cell therapy and the activated astrocyte interact with the ECM, and thereby, directly or indirectly, facilitate neurite remodeling. We, however, provide some insight into the potential interactions of the MSC, astrocyte, and ECM triad mediating neurite remodeling, by investigating the effects of the MSC stimulation of tPA within the astrocyte (Xin et al., 2010, 2011). Among ways tPA may interact with the ECM is to cleave inactive pro-trophic factors, for example, pro-BDNF, pro-NGF, residing within the ECM into active trophic factors. Thus, the ECM may play a direct and active role in stimulation neurite remodeling via tPA (Tsirka, 2002; Yoshida et al., 1992).
MicroRNAs (miRNAs) as a Molecule-Mediator Shuttled by Microvesicles/Exosomes after MSC-Therapy
We propose that the varied molecular signals driving restorative events may derive from the communication of miRNAs via microvesicles between the exogenously administered cells and parenchymal cells, primarily the astrocytes (Fig. 3). MiRNAs are short ribonucleic acid (usually 18 to 25 nucleotides) molecules found in eukaryotic cells. They are non-protein coding transcripts that post-transcriptionally control gene expression via binding to complementary sequences on target messenger RNA (mRNA) transcripts and result in mRNA degradation or translational repression and gene silencing (Bartel 2004, 2009). By affecting gene regulation, miRNAs are likely involved in most biological processes (Brennecke et al., 2003; Chen et al., 2004; Cuellar and McManus, 2005; Harfe et al., 2005; Kim et al., 2005). MiR-NAs act as master switches regulating the translation of many genes and they play significant roles in many regulatory mechanisms (Agnati et al., 2010; Cai et al., 2009). The human genome may encode over 1,000 miRNAs (Bartel, 2004), which target about 60% of mammalian genes (Friedman et al., 2009; Lewis et al., 2005) and are abundant in many human cell types (Lim et al., 2003).
FIGURE 3.
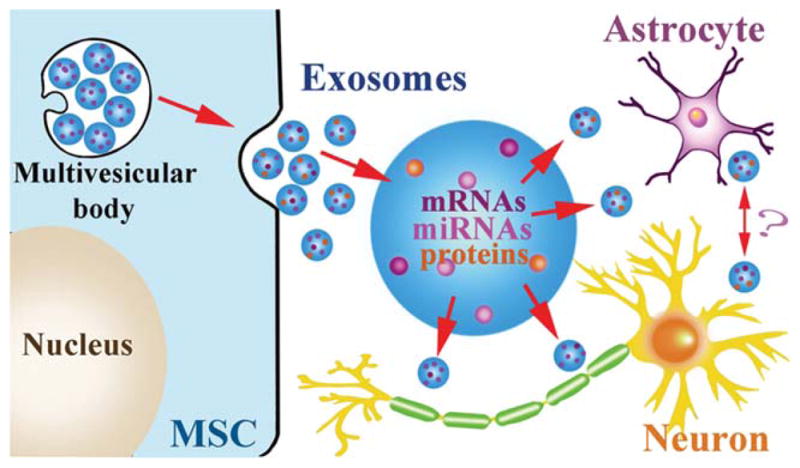
Exosomes shuttle miRNAs: The fusion of multivesicular bodies with the plasma membrane of MSCs and the release of their intraluminal vesicles as exosomes. Exosomes shuttle miR-NAs, mRNAs, and proteins from MSCs to astrocytes and neurons, modulating gene translation in these cellular targets. Whether the exosome targeted astrocytes and neurons subsequently release exosomes in response to the MSC generated absorbed exosome, is not known.
Microvesicles/Exosomes may provide the vehicles by which MSCs communicate with neural cells and modify miRNAs? Microvesicles are fragments of plasma membrane ranging from 50 to 1000 nm shed from almost all cell types (Breakefield et al., 2011). Microvesicles play a role in intercellular communication and can transport mRNA, miRNA, and proteins between cells. Exosomes are formed by inward budding of late endosomes, producing multivesicular bodies, and are released into the environment by fusion of the multivesicular bodies with the plasma membrane. Cells can communicate intercellularly by the secretion of exosomes from multivesicular bodies into the extracellular space. Increasing evidence indicates that exosomes transporting miRNAs play an important role in cell-to-cell communication (Lotvall and Valadi, 2007; Mathivanan et al., 2010; Record et al., 2011; Smalheiser, 2007; Valadi et al., 2007). Exosomes are membrane vesicles sized 40–100 nm in diameter and are secreted by a wide range of cell types (Leung and Brown, 2010; Mathivanan et al., 2010; Simpson et al., 2009; Stoorvogel et al., 2002; van Niel et al., 2006). They contain RNA molecules including mRNAs and miRNAs, which can be transferred between cells and thus affect the protein production of recipient cells (Katakowski et al., 2010; Pegtel et al., 2010; Zomer et al., 2010). Our data demonstrate that miR-133b levels were increased in MSCs and in their released exosomes after MSCs were exposed to brain extracts from rats subjected to MCAo, and the miR-133b was transferred to primary cultured astrocytes and neurons via exosomes (Xin et al., 2012). Exosomal miR-133b from MSCs significantly increased the neurite branch number and total neurite length (Xin et al., 2012). Compared with administration of normal MSCs, administration of MSCs with increased or decreased miR-133b (MSCs modified using lentivirus with miR-133b knocked in or knocked down) resulted in promotion or inhibition of neurite outgrowth, respectively (Xin et al., 2012). MiR-133b is active in several regulatory processes (Dreyer, 2010; Kim et al., 2007; Sanchez-Simon et al., 2010). Connective tissue growth factor (CTGF), a major inhibitor of axonal growth at injury sites in the CNS in mammals, is regulated by miR-133 (Duisters et al., 2009; White and Jakeman, 2008). The transfer of miR-133b from MSCs to astrocytes via exosomes may downregulate CTGF expression, thin the glial scar, and benefit neurite outgrowth. MiR-133 also downregulates ras homolog gene family member A (RhoA) protein expression (Care et al., 2007; Chiba et al., 2009), and since the inhibition of RhoA enhances regrowth of the corticospinal tract after spinal cord injury (Dergham et al., 2002; Ellezam et al., 2002), miR-133b appears essential for neurite outgrowth and functional recovery after spinal cord injury in adult zebrafish (Yu et al., 2011). Increasing miR-133b transfer from MSC exosomes may regulate target genes like RhoA in neurons that stimulate neurite outgrowth and thereby improve functional recovery after stroke. Further in vivo studies on how miR133-b affects neurite outgrowth after stroke are needed. Based on these data, MSC treatment promotes neurite outgrowth and benefits functional recovery at least partly by transferring miRNAs (here represented with miR-133b) to parenchymal cells via exosomes. Thus, a way to gain insight into the simultaneous appearance post cell-based treatment of multiple molecular cascades stimulating interactive restorative events may be by the transfer of miRNAs between exogenously administered cells and astrocytes. MiRNAs are master molecular switches, concurrently affecting translation of, possibly, hundreds of mRNAs (Agnati et al., 2010; Cai et al., 2009). In initial studies, we have demonstrated that MSCs transfer miR-133b to parenchymal cells (Xin et al., 2012). We emphatically note, however, that this transfer of miR-133b is not to the exclusion of the affect of MSCs or other cell-based therapies on other miRNAs and clusters of miRNAs. However, this observation provides the conceptual basis of simultaneously altering many processes.
Studies of how MSCs communicate with the parenchyma via microvesicles/exosomes are in their infancy. There are many possible ways the exogenously administered cell can transfer information to the parenchymal cell. The “packaging” of these bits of regulatory molecules and information, such as miRNAs, may not necessarily be mediated by microvesicle-exosome; miRNAs may be transferred between cells and are found in body fluids simply bound to high-density lipoprotein (HDL) and Argonaute (Chen et al., 2012). Thus, the work on miRNA and exosomes, while provocative, should primarily stimulate the concept that exogenously administered cells and parenchymal cells communicate by transferring information and regulatory genes, via multiple, possibly, overlapping pathways. Thus, how the astrocyte alters the environment in response to a restorative therapy, like a cell-based therapy, how it passes information to parenchymal cells and impacts tissue function by means of microvessicles/exosome/HDL packets of genetic information, remain largely unexplored.
Summary and Conclusions
In the setting of ischemia, astrocytes perform multiple functions, making them excellent candidates as therapeutic targets to improve outcome following stroke and in other CNS injuries. The older neurocentric view of the CNS has changed radically with the growing understanding of the many essential functions of astrocytes. Continued elucidation of the complex interactions involved in modulating the astrocytic responses may enable novel therapeutic approaches that translate successfully into clinical efficacy. Although few studies have specifically targeted astrocytes for repair after stroke, there is some evidence that this can be a successful strategy. For example, recent results indicate that enhancing astrocyte resistance to ischemic stress by overexpressing protective proteins or antioxidant enzyme results improved survival of hippocampal CA1 neurons following forebrain ischemia (Xu et al., 2010). Thus, specific attention should be paid to how these treatments may alter astrocyte response or viability. A detailed understanding of the astrocytic response, as well as the timing and location of the changes, is necessary to develop effective drug treatment strategies for stroke patients.
Cultured autologous MSCs are safe when injected intravenously in stroke patients (Bang et al., 2005; Bhasin et al., 2011; Dharmasaroja, 2009; Lee et al. 2010; Li et al., 2008b; Suarez-Monteagudo et al., 2009; Zhou, 2011), and the grafted groups showed higher frequencies of functional recovery. Clinical studies are currently ongoing or planned (http://www.clinicaltrials.gov), making it increasingly important to use preclinical animal studies to understand the biological mechanisms underlying cell-mediated tissue restoration. From the perspective of cell-based therapies, the interaction of MSCs, as a model of a general cell therapy, with parenchymal cells, is important, because benefit appears to derive from the stimulation of parenchymal cells by the exogenous cells, with the MSCs acting almost as catalysts for parenchymal cell response. Our data demonstrate that MSCs secrete and induce within parenchymal cells biomaterial factors that create a favorable environment to reduce apoptosis, and promote angiogenesis, synaptogenesis, and neurogenesis. MSC transplantation facilitates and amplifies endogenous neuroprotective and neurorestorative mechanisms that act in concert to form a CNS repair/remodeling compendium. Astrocytes likely play active roles in mediating CNS plasticity and neurological recovery post stroke. MSCs and neural cells communicate, and that intercellular communication between MSCs and neurons and astrocytes may occur via MSC-exosomes mediated miRNAs, e.g., miR-133b. MSC transfer of miR-133b to astrocytes via exosomes may downregulate the CTGF expression and thin the glial scar. MSC mediated down regulation of RhoA level in neurons may directly benefit neurite outgrowth and thereby improve functional recovery after stroke. We also provide the molecular bases for concurrently activating mediators of the multifaceted aspects of plasticity, whether via miRs packaged within lipid nanoparticles like exosomes, or stimulating morphogens, like Shh, which release multiple Gli transcription factors thereby altering important proteins, such as the neurite inducing tPA.
The CNS response to stroke is a multicellular process that changes continually over time and is regulated by a multitude of extracellular and intracellular molecular signaling events. There is controversy on the roles of reactive astrocytes after stroke, promoting or inhibiting axonal regeneration. Restorative cell-based treatment for stroke, such as MSC-treatment, appears effective and its efficacy is likely mediated by stimulation of and interaction with astrocytes. This MSC-via-astrocyte approach, if successful, because of the absence of an acute time-window requirement, will permit us to treat all patients with stroke. Further elucidation of the vital roles of astrocytes in facilitating neurological recovery will not only promote the clinical application of MSCs for the treatment of stroke, but also lead us to a new therapeutic target for CNS diseases.
This discussion and review of the literature on the role of the astrocyte in mediating neurological recovery post stroke have neglected some vital clinically relevant information. Stroke is a disease of aging, with most patients having comorbidities, such as diabetes, hypertension, and underlying vascular disease and cancer. How the astrocyte responds to stroke under these conditions of aging or comorbidities has not been investigated. Preclinically, we have found that early (i.e., 1 day) treatment of stroke with MSCs in diabetic rats provides no therapeutic benefit, and there is evidence of adverse effects, such as the induction of atherosclerotic-like lesions (Chen et al., 2011). How the astrocyte responds to a restorative cell therapy under conditions of diabetes, represents just a single clinically compelling issue. We tend to idealize the laboratory studies and investigate genetically identical young, male animals to obtain proof-of-principle information on important biological responses to stroke. Using this oversimplified idealized approach, we have demonstrated that the CNS is capable of restorative responses which can be amplified by a variety of cell-based and pharmacological therapies, largely mediated by the powerful astrocyte. But, it is imperative to investigate therapeutic responses under clinically relevant conditions of aging and comorbidities, before we can truly assert an understanding of the role of the astrocyte in mediating neurological recovery post stroke.
Acknowledgments
Grant sponsor: National Institutes of Health (NIH); Grant number: R01AG037506; R01NS066041.
References
- Agnati LF, Guidolin D, Guescini M, Genedani S, Fuxe K. Understanding wiring and volume transmission. Brain Res Rev. 2010;64:137–159. doi: 10.1016/j.brainresrev.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Aizman I, McGrogan M, Case CC. Quantitative microplate assay for studying mesenchymal stromal cell-induced neuropoiesis. Stem Cells Transl Med. 2013;2:223–232. doi: 10.5966/sctm.2012-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizman I, Tate CC, McGrogan M, Case CC. Extracellular matrix produced by bone marrow stromal cells and by their derivative, SB623 cells, supports neural cell growth. J Neurosci Res. 2009;87:3198–3206. doi: 10.1002/jnr.22146. [DOI] [PubMed] [Google Scholar]
- Alberts MJ, Naidech AM. tPA and warfarin: Time to move forward. Neurology. 2013;80:514–515. doi: 10.1212/WNL.0b013e31827b1b7c. [DOI] [PubMed] [Google Scholar]
- Alder J, Kramer BC, Hoskin C, Thakker-Varia S. Brain-derived neurotrophic factor produced by human umbilical tissue-derived cells is required for its effect on hippocampal dendritic differentiation. Dev Neurobiol. 2012;72:755–765. doi: 10.1002/dneu.20980. [DOI] [PubMed] [Google Scholar]
- Anderson MF, Blomstrand F, Blomstrand C, Eriksson PS, Nilsson M. Astrocytes and stroke: Networking for survival? Neurochem Res. 2003;28:293–305. doi: 10.1023/a:1022385402197. [DOI] [PubMed] [Google Scholar]
- Aoki C, Bredt DS, Fenstemaker S, Lubin M. The subcellular distribution of nitric oxide synthase relative to the NR1 subunit of NMDA receptors in the cerebral cortex. Prog Brain Res. 1998;118:83–97. doi: 10.1016/s0079-6123(08)63202-1. [DOI] [PubMed] [Google Scholar]
- Araque A, Sanzgiri RP, Parpura V, Haydon PG. Astrocyte-induced modulation of synaptic transmission. Can J Physiol Pharmacol. 1999;77:699–706. [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Askalan R, Deveber G, Ho M, Ma J, Hawkins C. Astrocytic-inducible nitric oxide synthase in the ischemic developing human brain. Pediatr Res. 2006;60:687–692. doi: 10.1203/01.pdr.0000246226.89215.a6. [DOI] [PubMed] [Google Scholar]
- Balami JS, Chen R, Sutherland BA, Buchan AM. Thrombolytic agents for acute ischaemic stroke treatment: The past, present and future. CNS Neurol Disord Drug Targets. 2013;12:145–154. doi: 10.2174/18715273113129990057. [DOI] [PubMed] [Google Scholar]
- Bang OY, Lee JS, Lee PH, Lee G. Autologous mesenchymal stem cell transplantation in stroke patients. Ann Neurol. 2005;57:874–882. doi: 10.1002/ana.20501. [DOI] [PubMed] [Google Scholar]
- Barker AJ, Ullian EM. Astrocytes and synaptic plasticity. Neuroscientist. 2010;16:40–50. doi: 10.1177/1073858409339215. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: Target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzilay R, Ganz J, Sadan O, Ben-Zur T, Bren Z, Hinden N, Taler M, Lev N, Gil-Ad I, Weizman A, Offen D. Mesenchymal stem cells protect from sub-chronic phencyclidine insult in vivo and counteract changes in astrocyte gene expression in vitro. Eur Neuropsychopharmacol. 2013;23:1115–1123. doi: 10.1016/j.euroneuro.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Bernd P. The role of neurotrophins during early development. Gene Expr. 2008;14:241–250. doi: 10.3727/105221608786883799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M, Butt AM, Wilkin G, Perry H. Structure and function of glia in the central nervous system. New York: Georgina Bentliff; 2002. pp. 77–79. [Google Scholar]
- Bhasin A, Srivastava MV, Kumaran SS, Mohanty S, Bhatia R, Bose S, Gaikwad S, Garg A, Airan B. Autologous mesenchymal stem cells in chronic stroke. Cerebrovasc Dis Extra. 2011;1:93–104. doi: 10.1159/000333381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A. Glial cells in the central nervous system. Discuss Neurosci. 1991;8:1–45. [Google Scholar]
- Breakefield XO, Frederickson RM, Simpson RJ. Gesicles: Microvesicle cookies for transient information transfer between cells. Mol Ther. 2011;19:1574–1576. doi: 10.1038/mt.2011.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J, Hipfner DR, Stark A, Russell RB, Cohen SM. Bantam encodes a developmentally regulated microRNA that controls cell proliferation and regulates the proapoptotic gene hid in Drosophila. Cell. 2003;113:25–36. doi: 10.1016/s0092-8674(03)00231-9. [DOI] [PubMed] [Google Scholar]
- Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T, Svendsen CN, Mucke L, Johnson MH, Sofroniew MV. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 1999;23:297–308. doi: 10.1016/s0896-6273(00)80781-3. [DOI] [PubMed] [Google Scholar]
- Bushong EA, Martone ME, Jones YZ, Ellisman MH. Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci. 2002;22:183–192. doi: 10.1523/JNEUROSCI.22-01-00183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cafferty WB, Yang SH, Duffy PJ, Li S, Strittmatter SM. Functional axonal regeneration through astrocytic scar genetically modified to digest chondroitin sulfate proteoglycans. J Neurosci. 2007;27:2176–2185. doi: 10.1523/JNEUROSCI.5176-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Yu X, Hu S, Yu J. A brief review on the mechanisms of miRNA regulation. Genomics Proteomics Bioinformatics. 2009;7:147–154. doi: 10.1016/S1672-0229(08)60044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- Care A, Catalucci D, Felicetti F, Bonci D, Addario A, Gallo P, Bang ML, Segnalini P, Gu Y, Dalton ND, Elia L, Latronico MV, Høydal M, Autore C, Russo MA, Dorn GW, 2nd, Ellingsen O, Ruiz-Lozano P, Peterson KL, Croce CM, Peschle C, Condorelli G. MicroRNA-133 controls cardiac hypertrophy. Nat Med. 2007;13:613–618. doi: 10.1038/nm1582. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003;9:64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- Carmichael ST. Targets for neural repair therapies after stroke. Stroke. 2010;41:S124–S126. doi: 10.1161/STROKEAHA.110.597146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayuso J, Marti E. Morphogens in motion: growth control of the neural tube. J Neurobiol. 2005;64:376–387. doi: 10.1002/neu.20169. [DOI] [PubMed] [Google Scholar]
- Chang SJ, Weng SL, Hsieh JY, Wang TY, Chang MD, Wang HW. MicroRNA-34a modulates genes involved in cellular motility and oxidative phosphorylation in neural precursors derived from human umbilical cord mesenchymal stem cells. BMC Med Genomics. 2011;4:65. doi: 10.1186/1755-8794-4-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CZ, Li L, Lodish HF, Bartel DP. MicroRNAs modulate hematopoietic lineage differentiation. Science. 2004;303:83–86. doi: 10.1126/science.1091903. [DOI] [PubMed] [Google Scholar]
- Chen G, Frokiaer J, Pedersen M, Nielsen S, Si Z, Pang Q, Stodkilde-Jorgensen H. Reduction of ischemic stroke in rat brain by alpha melanocyte stimulating hormone. Neuropeptides. 2008;42:331–338. doi: 10.1016/j.npep.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Katakowski M, Chen X, Wang L, Lu D, Lu M, Gautam SC, Chopp M. Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res. 2003a;73:778–786. doi: 10.1002/jnr.10691. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001a;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 2001b;32:1005–1011. doi: 10.1161/01.str.32.4.1005. [DOI] [PubMed] [Google Scholar]
- Chen J, Sanberg PR, Li Y, Wang L, Lu M, Willing AE, Sanchez-Ramos J, Chopp M. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001c;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- Chen J, Ye X, Yan T, Zhang C, Yang XP, Cui X, Cui Y, Zacharek A, Roberts C, Liu X, Dai X, Lu M, Chopp M. Adverse effects of bone marrow stromal cell treatment of stroke in diabetic rats. Stroke. 2011;42:3551–3558. doi: 10.1161/STROKEAHA.111.627174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, Lu M, Zhu Z, Chopp M. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003b;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang Y, Wang L, Jiang H, Zhang C, Lu M, Katakowski M, Feldkamp CS, Chopp M. Statins induce angiogenesis, neurogenesis, and synaptogenesis after stroke. Ann Neurol. 2003c;53:743–751. doi: 10.1002/ana.10555. [DOI] [PubMed] [Google Scholar]
- Chen JK, Taipale J, Cooper MK, Beachy PA. Inhibition of Hedgehog signaling by direct binding of cyclopamine to Smoothened. Genes Dev. 2002a;16:2743–2748. doi: 10.1101/gad.1025302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci USA. 2002b;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Katakowski M, Li Y, Lu D, Wang L, Zhang L, Chen J, Xu Y, Gautam S, Mahmood A, Chopp M. Human bone marrow stromal cell cultures conditioned by traumatic brain tissue extracts: Growth factor production. J Neurosci Res. 2002c;69:687–691. doi: 10.1002/jnr.10334. [DOI] [PubMed] [Google Scholar]
- Chen X, Li Y, Wang L, Katakowski M, Zhang L, Chen J, Xu Y, Gautam SC, Chopp M. Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology. 2002d;22:275–279. doi: 10.1046/j.1440-1789.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- Chen X, Liang H, Zhang J, Zen K, Zhang CY. Horizontal transfer of microRNAs: Molecular mechanisms and clinical applications. Protein Cell. 2012;3:28–37. doi: 10.1007/s13238-012-2003-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Chiba Y, Tanabe M, Goto K, Sakai H, Misawa M. Down-regulation of miR-133a contributes to up-regulation of Rhoa in bronchial smooth muscle cells. Am J Respir Crit Care Med. 2009;180:713–719. doi: 10.1164/rccm.200903-0325OC. [DOI] [PubMed] [Google Scholar]
- Chopp M, Li Y. Treatment of neural injury with marrow stromal cells. Lancet Neurol. 2002;1:92–100. doi: 10.1016/s1474-4422(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collen D. On the regulation and control of fibrinolysis. Edward Kowalski Memorial Lecture. Thromb Haemost. 1980;43:77–89. [PubMed] [Google Scholar]
- Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- Correa F, Gauberti M, Parcq J, Macrez R, Hommet Y, Obiang P, Hernangomez M, Montagne A, Liot G, Guaza C, Maubert E, Ali C, Vivien D, Docagne F. Tissue plasminogen activator prevents white matter damage following stroke. J Exp Med. 2011;208:1229–1242. doi: 10.1084/jem.20101880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotrina ML, Kang J, Lin JH, Bueno E, Hansen TW, He L, Liu Y, Nedergaard M. Astrocytic gap junctions remain open during ischemic conditions. J Neurosci. 1998;18:2520–2537. doi: 10.1523/JNEUROSCI.18-07-02520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft AP, Przyborski SA. Mesenchymal stem cells expressing neural antigens instruct a neurogenic cell fate on neural stem cells. Exp Neurol. 2009;216:329–341. doi: 10.1016/j.expneurol.2008.12.010. [DOI] [PubMed] [Google Scholar]
- Crutcher KA. The role of growth factors in neuronal development and plasticity. CRC Crit Rev Clin Neurobiol. 1986;2:297–333. [PubMed] [Google Scholar]
- Cuellar TL, McManus MT. MicroRNAs and endocrine biology. J Endocrinol. 2005;187:327–332. doi: 10.1677/joe.1.06426. [DOI] [PubMed] [Google Scholar]
- Cui X, Chen J, Zacharek A, Li Y, Roberts C, Kapke A, Savant-Bhonsale S, Chopp M. Nitric oxide donor upregulation of stromal cell-derived factor-1/chemokine (CXC motif) receptor 4 enhances bone marrow stromal cell migration into ischemic brain after stroke. Stem Cells. 2007;25:2777–2785. doi: 10.1634/stemcells.2007-0169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X, Chopp M, Zacharek A, Cui Y, Roberts C, Chen J. The neurorestorative benefit of GW3965 treatment of stroke in mice. Stroke. 2013;44:153–161. doi: 10.1161/STROKEAHA.112.677682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies BJ, Pickard BS, Steel M, Morris RG, Lathe R. Serine proteases in rodent hippocampus. J Biol Chem. 1998;273:23004–23011. doi: 10.1074/jbc.273.36.23004. [DOI] [PubMed] [Google Scholar]
- de Pablo Y, Nilsson M, Pekna M, Pekny M. Intermediate filaments are important for astrocyte response to oxidative stress induced by oxygen-glucose deprivation and reperfusion. Histochem Cell Biol. 2013;140:81–91. doi: 10.1007/s00418-013-1110-0. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezawa M, Hoshino M, Nabeshima Y, Ide C. Marrow stromal cells: implications in health and disease in the nervous system. Curr Mol Med. 2005;5:723–732. doi: 10.2174/156652405774641070. [DOI] [PubMed] [Google Scholar]
- Dharmasaroja P. Bone marrow-derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci. 2009;16:12–20. doi: 10.1016/j.jocn.2008.05.006. [DOI] [PubMed] [Google Scholar]
- Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, Chopp M. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J Cereb Blood Flow Metab. 2013;33:1015–1024. doi: 10.1038/jcbfm.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Docagne F, Nicole O, Gabriel C, Fernandez-Monreal M, Lesne S, Ali C, Plawinski L, Carmeliet P, MacKenzie ET, Buisson A, Vivien D. Smad3-dependent induction of plasminogen activator inhibitor-1 in astrocytes mediates neuroprotective activity of transforming growth factor-beta 1 against NMDA-induced necrosis. Mol Cell Neurosci. 2002;21:634–644. doi: 10.1006/mcne.2002.1206. [DOI] [PubMed] [Google Scholar]
- Dreyer JL. New insights into the roles of microRNAs in drug addiction and neuroplasticity. Genome Med. 2010;2:92. doi: 10.1186/gm213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duisters RF, Tijsen AJ, Schroen B, Leenders JJ, Lentink V, van der Made I, Herias V, van Leeuwen RE, Schellings MW, Barenbrug P, Maessen JG, Heymans S, Pinto YM, Creemers EE. miR-133 and miR-30 regulate connective tissue growth factor: Implications for a role of microRNAs in myocardial matrix remodeling. Circ Res. 2009;104:170–178. doi: 10.1161/CIRCRESAHA.108.182535. 6p following 178. [DOI] [PubMed] [Google Scholar]
- Edgar D. Nerve growth factors and molecules of the extracellular matrix in neuronal development. J Cell Sci Suppl. 1985;3:107–113. doi: 10.1242/jcs.1985.supplement_3.11. [DOI] [PubMed] [Google Scholar]
- Ellezam B, Dubreuil C, Winton M, Loy L, Dergham P, Selles-Navarro I, McKerracher L. Inactivation of intracellular Rho to stimulate axon growth and regeneration. Prog Brain Res. 2002;137:371–380. doi: 10.1016/s0079-6123(02)37028-6. [DOI] [PubMed] [Google Scholar]
- Fahnestock M, Yu G, Coughlin MD. ProNGF: a neurotrophic or an apoptotic molecule? Prog Brain Res. 2004;146:101–110. doi: 10.1016/S0079-6123(03)46007-X. [DOI] [PubMed] [Google Scholar]
- Fang MC, Cutler DM, Rosen AB. Trends in thrombolytic use for ischemic stroke in the United States. J Hosp Med. 2010;5:406–409. doi: 10.1002/jhm.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang SH, Wei EQ, Zhou Y, Wang ML, Zhang WP, Yu GL, Chu LS, Chen Z. Increased expression of cysteinyl leukotriene receptor-1 in the brain mediates neuronal damage and astrogliosis after focal cerebral ischemia in rats. Neuroscience. 2006;140:969–979. doi: 10.1016/j.neuroscience.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Cleveland DW. A structural scaffolding of intermediate filaments in health and disease. Science. 1998;279:514–519. doi: 10.1126/science.279.5350.514. [DOI] [PubMed] [Google Scholar]
- Gao Q, Katakowski M, Chen X, Li Y, Chopp M. Human marrow stromal cells enhance connexin43 gap junction intercellular communication in cultured astrocytes. Cell Transplant. 2005a;14:109–117. doi: 10.3727/000000005783983205. [DOI] [PubMed] [Google Scholar]
- Gao Q, Li Y, Chopp M. Bone marrow stromal cells increase astrocyte survival via upregulation of phosphoinositide 3-kinase/threonine protein kinase and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathways and stimulate astrocyte trophic factor gene expression after anaerobic insult. Neuroscience. 2005b;136:123–134. doi: 10.1016/j.neuroscience.2005.06.091. [DOI] [PubMed] [Google Scholar]
- Garcia JH, Yoshida Y, Chen H, Li Y, Zhang ZG, Lian J, Chen S, Chopp M. Progression from ischemic injury to infarct following middle cerebral artery occlusion in the rat. Am J Pathol. 1993;142:623–635. [PMC free article] [PubMed] [Google Scholar]
- Giaume C, Liu X. From a glial syncytium to a more restricted and specific glial networking. J Physiol Paris. 2012;106:34–39. doi: 10.1016/j.jphysparis.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Giaume C, McCarthy KD. Control of gap-junctional communication in astrocytic networks. Trends Neurosci. 1996;19:319–325. doi: 10.1016/0166-2236(96)10046-1. [DOI] [PubMed] [Google Scholar]
- Grabowski M, Brundin P, Johansson BB. Fetal neocortical grafts implanted in adult hypertensive rats with cortical infarcts following a middle cerebral artery occlusion: ingrowth of afferent fibers from the host brain. Exp Neurol. 1992;116:105–121. doi: 10.1016/0014-4886(92)90159-n. [DOI] [PubMed] [Google Scholar]
- Grade S, Weng YC, Snapyan M, Kriz J, Malva JO, Saghatelyan A. Brain-derived neurotrophic factor promotes vasculature-associated migration of neuronal precursors toward the ischemic striatum. PLoS One. 2013;8:e55039. doi: 10.1371/journal.pone.0055039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo F, Lv S, Lou Y, Tu W, Liao W, Wang Y, Deng Z. Bone marrow stromal cells enhance the angiogenesis in ischaemic cortex after stroke: Involvement of notch signalling. Cell Biol Int. 2012;36:997–1004. doi: 10.1042/CBI20110596. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Fernandez M, Rodriguez-Frutos B, Ramos-Cejudo J, Teresa Vallejo-Cremades M, Fuentes B, Cerdan S, Diez-Tejedor E. Effects of intravenous administration of allogenic bone marrow- and adipose tissue-derived mesenchymal stem cells on functional recovery and brain repair markers in experimental ischemic stroke. Stem Cell Res Ther. 2013;4:11. doi: 10.1186/scrt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Haydon PG. The tripartite synapse: Roles for gliotransmission in health and disease. Trends Mol Med. 2007a;13:54–63. doi: 10.1016/j.molmed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG. Synaptic islands defined by the territory of a single astrocyte. J Neurosci. 2007b;27:6473–6477. doi: 10.1523/JNEUROSCI.1419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harfe BD, McManus MT, Mansfield JH, Hornstein E, Tabin CJ. The RNaseIII enzyme Dicer is required for morphogenesis but not patterning of the vertebrate limb. Proc Natl Acad Sci USA. 2005;102:10898–10903. doi: 10.1073/pnas.0504834102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hase A, Suzuki H, Arahata K, Akazawa C. Expression of human GFR alpha-1 (GDNF receptor) at the neuromuscular junction and myelinated nerves. Neurosci Lett. 1999;269:55–57. doi: 10.1016/s0304-3940(99)00419-x. [DOI] [PubMed] [Google Scholar]
- Hermann DM, Chopp M. Promoting brain remodelling and plasticity for stroke recovery: Therapeutic promise and potential pitfalls of clinical translation. Lancet Neurol. 2012;11:369–380. doi: 10.1016/S1474-4422(12)70039-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita Y, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Intravenous administration of glial cell line-derived neurotrophic factor gene-modified human mesenchymal stem cells protects against injury in a cerebral ischemia model in the adult rat. J Neurosci Res. 2006;84:1495–1504. doi: 10.1002/jnr.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- Horwitz EM, Prockop DJ, Gordon PL, Koo WW, Fitzpatrick LA, Neel MD, McCarville ME, Orchard PJ, Pyeritz RE, Brenner MK. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001;97:1227–31. doi: 10.1182/blood.v97.5.1227. [DOI] [PubMed] [Google Scholar]
- Irons H, Lind JG, Wakade CG, Yu G, Hadman M, Carroll J, Hess DC, Borlongan CV. Intracerebral xenotransplantation of GFP mouse bone marrow stromal cells in intact and stroke rat brain: Graft survival and immunologic response. Cell Transplant. 2004;13:283–294. doi: 10.3727/000000004783983990. [DOI] [PubMed] [Google Scholar]
- Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, Greenberg DA. Evidence for stroke-induced neurogenesis in the human brain. Proc Natl Acad Sci USA. 2006;103:13198–13202. doi: 10.1073/pnas.0603512103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M, Domercq M, Matute C, Tonello F, Gundersen V, Volterra A. Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci. 2007;10:331–339. doi: 10.1038/nn1849. [DOI] [PubMed] [Google Scholar]
- Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, Nolta JA. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–946. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai K, Takahashi M, Osumi N, Sinnarajah S, Takeo T, Ikeda H, Kehrl JH, Itoh G, Arnheiter H. The G12 family of heterotrimeric G proteins and Rho GTPase mediate Sonic hedgehog signalling. Genes Cells. 2004;9:49–58. doi: 10.1111/j.1356-9597.2004.00701.x. [DOI] [PubMed] [Google Scholar]
- Katakowski M, Buller B, Wang X, Rogers T, Chopp M. Functional microRNA is transferred between glioma cells. Cancer Res. 2010;70:8259–8263. doi: 10.1158/0008-5472.CAN-10-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr JB. Atlas of functional histology. London: Mosby; 2000. [Google Scholar]
- Kim J, Inoue K, Ishii J, Vanti WB, Voronov SV, Murchison E, Hannon G, Abeliovich A. A MicroRNA feedback circuit in midbrain dopamine neurons. Science. 2007;317:1220–1224. doi: 10.1126/science.1140481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JB, Yu YM, Kim SW, Lee JK. Anti-inflammatory mechanism is involved in ethyl pyruvate-mediated efficacious neuroprotection in the post-ischemic brain. Brain Res. 2005;1060:188–192. doi: 10.1016/j.brainres.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Koc ON, Gerson SL, Cooper BW, Dyhouse SM, Haynesworth SE, Caplan AI, Lazarus HM. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J Clin Oncol. 2000;18:307–316. doi: 10.1200/JCO.2000.18.2.307. [DOI] [PubMed] [Google Scholar]
- Koc ON, Peters C, Aubourg P, Raghavan S, Dyhouse S, DeGasperi R, Kolodny EH, Yoseph YB, Gerson SL, Lazarus HM, Caplan AI, Watkins PA, Krivit W. Bone marrow-derived mesenchymal stem cells remain host-derived despite successful hematopoietic engraftment after allogeneic transplantation in patients with lysosomal and peroxisomal storage diseases. Exp Hematol. 1999;27:1675–1681. doi: 10.1016/s0301-472x(99)00101-0. [DOI] [PubMed] [Google Scholar]
- Kopen GC, Prockop DJ, Phinney DG. Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA. 1999;96:10711–10716. doi: 10.1073/pnas.96.19.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama Y, Maebara Y, Hayashi M, Nagae R, Tokuyama S, Michinaga S. Endothelins reciprocally regulate VEGF-A and angiopoietin-1 production in cultured rat astrocytes: implications on astrocytic proliferation. Glia. 2012;60:1954–1963. doi: 10.1002/glia.22411. [DOI] [PubMed] [Google Scholar]
- Kurozumi K, Nakamura K, Tamiya T, Kawano Y, Ishii K, Kobune M, Hirai S, Uchida H, Sasaki K, Ito Y, Kato K, Honmou O, Houkin K, Date I, Hamada H. Mesenchymal stem cells that produce neurotrophic factors reduce ischemic damage in the rat middle cerebral artery occlusion model. Mol Ther. 2005;11:96–104. doi: 10.1016/j.ymthe.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Larsson A, Wilhelmsson U, Pekna M, Pekny M. Increased cell proliferation and neurogenesis in the hippocampal dentate gyrus of old GFAP(−/−)Vim(−/−) mice. Neurochem Res. 2004;29:2069–2073. doi: 10.1007/s11064-004-6880-2. [DOI] [PubMed] [Google Scholar]
- Le Blanc K, Rasmusson I, Sundberg B, Gotherstrom C, Hassan M, Uzunel M, Ringden O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- Lee HY, Hwang IY, Im H, Koh JY, Kim YH. Non-proteolytic neurotrophic effects of tissue plasminogen activator on cultured mouse cerebrocortical neurons. J Neurochem. 2007;101:1236–1247. doi: 10.1111/j.1471-4159.2007.04417.x. [DOI] [PubMed] [Google Scholar]
- Lee J, Kuroda S, Shichinohe H, Ikeda J, Seki T, Hida K, Tada M, Sawada K, Iwasaki Y. Migration and differentiation of nuclear fluorescence-labeled bone marrow stromal cells after transplantation into cerebral infarct and spinal cord injury in mice. Neuropathology. 2003;23:169–180. doi: 10.1046/j.1440-1789.2003.00496.x. [DOI] [PubMed] [Google Scholar]
- Lee JK, Kim JE, Sivula M, Strittmatter SM. Nogo receptor antagonism promotes stroke recovery by enhancing axonal plasticity. J Neurosci. 2004;24:6209–6217. doi: 10.1523/JNEUROSCI.1643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Hong JM, Moon GJ, Lee PH, Ahn YH, Bang OY. A long-term follow-up study of intravenous autologous mesenchymal stem cell transplantation in patients with ischemic stroke. Stem Cells. 2010;28:1099–1106. doi: 10.1002/stem.430. [DOI] [PubMed] [Google Scholar]
- Leung E, Brown JD. Biogenesis of the signal recognition particle. Biochem Soc Trans. 2010;38:1093–1098. doi: 10.1042/BST0381093. [DOI] [PubMed] [Google Scholar]
- Levitan IB, Kaczmarek LK. The Neuron: Cell and Molecular Biology. New York: Oxford University Press; 2002. [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li L, Lundkvist A, Andersson D, Wilhelmsson U, Nagai N, Pardo AC, Nodin C, Stahlberg A, Aprico K, Larsson K, Yabe T, Moons L, Fotheringham A, Davies I, Carmeliet P, Schwartz JP, Pekna M, Kubista M, Blomstrand F, Maragakis N, Nilsson M, Pekny M. Protective role of reactive astrocytes in brain ischemia. J Cereb Blood Flow Metab. 2008a;28:468–481. doi: 10.1038/sj.jcbfm.9600546. [DOI] [PubMed] [Google Scholar]
- Li WE, Ochalski PA, Hertzberg EL, Nagy JI. Immunorecognition, ultra-structure and phosphorylation status of astrocytic gap junctions and connexin43 in rat brain after cerebral focal ischaemia. Eur J Neurosci. 1998;10:2444–2463. doi: 10.1046/j.1460-9568.1998.00253.x. [DOI] [PubMed] [Google Scholar]
- Li WY, Choi YJ, Lee PH, Huh K, Kang YM, Kim HS, Ahn YH, Lee G, Bang OY. Mesenchymal stem cells for ischemic stroke: Changes in effects after ex vivo culturing. Cell Transplant. 2008b;17:1045–1059. doi: 10.3727/096368908786991551. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chen XG, Wang L, Gautam SC, Xu YX, Katakowski M, Zhang LJ, Lu M, Janakiraman N, Chopp M. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Zhang CL, Wang L, Lu D, Katakowski M, Gao Q, Shen LH, Zhang J, Lu M, Chopp M. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M. Temporal profile of nestin expression after focal cerebral ischemia in adult rat. Brain Res. 1999;838:1–10. doi: 10.1016/s0006-8993(99)01502-4. [DOI] [PubMed] [Google Scholar]
- Li Y, Chopp M. Marrow stromal cell transplantation in stroke and traumatic brain injury. Neurosci Lett. 2009;456:120–123. doi: 10.1016/j.neulet.2008.03.096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Chopp M, Chen J, Wang L, Gautam SC, Xu YX, Zhang Z. Intra-striatal transplantation of bone marrow nonhematopoietic cells improves functional recovery after stroke in adult mice. J Cereb Blood Flow Metab. 2000;20:1311–1319. doi: 10.1097/00004647-200009000-00006. [DOI] [PubMed] [Google Scholar]
- Li Y, McIntosh K, Chen J, Zhang C, Gao Q, Borneman J, Raginski K, Mitchell J, Shen L, Zhang J, Lu D, Chopp M. Allogeneic bone marrow stromal cells promote glial-axonal remodeling without immunologic sensitization after stroke in rats. Exp Neurol. 2006;198:313–325. doi: 10.1016/j.expneurol.2005.11.029. [DOI] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Weinstein EG, Abdelhakim A, Yekta S, Rhoades MW, Burge CB, Bartel DP. The microRNAs of Caenorhabditis elegans. Genes Dev. 2003;17:991–1008. doi: 10.1101/gad.1074403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Santra M, Hozeska-Solgot A, Zhang RL, Wang L, Teng H, Lu M, Zhang ZG. Functional response to SDF1 alpha through over-expression of CXCR4 on adult subventricular zone progenitor cells. Brain Res. 2008;1226:18–26. doi: 10.1016/j.brainres.2008.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Qu R, Shen L, Gao Q, Zhang X, Lu M, Savant-Bhonsale S, Borneman J, Chopp M. Axonal sprouting into the denervated spinal cord and synaptic and postsynaptic protein expression in the spinal cord after transplantation of bone marrow stromal cell in stroke rats. Brain Res. 2007;1149:172–180. doi: 10.1016/j.brainres.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang RL, Cui Y, Chopp M. Bone marrow stromal cells promote skilled motor recovery and enhance contralesional axonal connections after ischemic stroke in adult mice. Stroke. 2011;42:740–744. doi: 10.1161/STROKEAHA.110.607226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Li Y, Zhang ZG, Cui X, Cui Y, Lu M, Savant-Bhonsale S, Chopp M. Bone marrow stromal cells enhance inter- and intracortical axonal connections after ischemic stroke in adult rats. J Cereb Blood Flow Metab. 2010;30:1288–1295. doi: 10.1038/jcbfm.2010.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn BD, Rempel JL, Nagy JI. Enrichment of neuronal and glial connexins in the postsynaptic density subcellular fraction of rat brain. Brain Res. 2001;898:1–8. doi: 10.1016/s0006-8993(01)02062-5. [DOI] [PubMed] [Google Scholar]
- Machold R, Hayashi S, Rutlin M, Muzumdar MD, Nery S, Corbin JG, Gritli-Linde A, Dellovade T, Porter JA, Rubin LL, Dudek H, McMahon AP, Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telence-phalic stem cell niches. Neuron. 2003;39:937–950. doi: 10.1016/s0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- Madri JA. Modeling the neurovascular niche: implications for recovery from CNS injury. J Physiol Pharmacol. 2009;60(Suppl 4):95–104. [PubMed] [Google Scholar]
- Magavi SS, Macklis JD. Induction of neuronal type-specific neurogenesis in the cerebral cortex of adult mice: Manipulation of neural precursors in situ. Brain Res Dev Brain Res. 2002;134:57–76. doi: 10.1016/s0165-3806(01)00316-9. [DOI] [PubMed] [Google Scholar]
- Marin-Padilla M. Prenatal development of fibrous (white matter), protoplasmic (gray matter), and layer I astrocytes in the human cerebral cortex: a Golgi study. J Comp Neurol. 1995;357:554–572. doi: 10.1002/cne.903570407. [DOI] [PubMed] [Google Scholar]
- Marler JR. Tissue plasminogen activator for acute ischemic stroke. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Mathivanan S, Ji H, Simpson RJ. Exosomes: Extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–1920. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Matsaas R, Tsacopoulos M. The functional roles of glial cells in health and disease: Dialogue between glia and neurons. New York: Kluwer Academic; 1999. [Google Scholar]
- Mazzanti M, Sul JY, Haydon PG. Glutamate on demand: astrocytes as a ready source. Neuroscientist. 2001;7:396–405. doi: 10.1177/107385840100700509. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J Neurosci. 1999;19:10778–10788. doi: 10.1523/JNEUROSCI.19-24-10778.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menet V, Gimenez Y, Ribotta M, Chauvet N, Drian MJ, Lannoy J, Colucci-Guyon E, Privat A. Inactivation of the glial fibrillary acidic protein gene, but not that of vimentin, improves neuronal survival and neurite growth by modifying adhesion molecule expression. J Neurosci. 2001;21:6147–6158. doi: 10.1523/JNEUROSCI.21-16-06147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milei J, Cao G, Grana DR, Toblli JE. Plasminogen activator inhibitor-1 and transforming growth factor-beta 1 in carotid glomus and autonomic ganglia from spontaneously hypertensive rats. J Hypertens. 2004;22:1351–1359. doi: 10.1097/01.hjh.0000125434.28861.9b. [DOI] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res. 2002;137:313–332. doi: 10.1016/s0079-6123(02)37024-9. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Rash JE. Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res Brain Res Rev. 2000;32:29–44. doi: 10.1016/s0165-0173(99)00066-1. [DOI] [PubMed] [Google Scholar]
- Nakase T, Sohl G, Theis M, Willecke K, Naus CC. Increased apoptosis and inflammation after focal brain ischemia in mice lacking connexin43 in astrocytes. Am J Pathol. 2004;164:2067–2075. doi: 10.1016/S0002-9440(10)63765-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naus CC, Ozog MA, Bechberger JF, Nakase T. A neuroprotective role for gap junctions. Cell Commun Adhes. 2001;8:325–328. doi: 10.3109/15419060109080747. [DOI] [PubMed] [Google Scholar]
- Nawashiro H, Brenner M, Fukui S, Shima K, Hallenbeck JM. High susceptibility to cerebral ischemia in GFAP-null mice. J Cereb Blood Flow Metab. 2000;20:1040–1044. doi: 10.1097/00004647-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Nudo RJ. Postinfarct cortical plasticity and behavioral recovery. Stroke. 2007;38:840–845. doi: 10.1161/01.STR.0000247943.12887.d2. [DOI] [PubMed] [Google Scholar]
- Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panickar KS, Norenberg MD. Astrocytes in cerebral ischemic injury: Morphological and general considerations. Glia. 2005;50:287–298. doi: 10.1002/glia.20181. [DOI] [PubMed] [Google Scholar]
- Papadopoulos CM, Tsai SY, Alsbiei T, O’Brien TE, Schwab ME, Kartje GL. Functional recovery and neuroanatomical plasticity following middle cerebral artery occlusion and IN-1 antibody treatment in the adult rat. Ann Neurol. 2002;51:433–441. doi: 10.1002/ana.10144. [DOI] [PubMed] [Google Scholar]
- Parpura V, Heneka MT, Montana V, Oliet SH, Schousboe A, Haydon PG, Stout RF, Jr, Spray DC, Reichenbach A, Pannicke T, Pekny M, Pekna M, Zorec R, Verkhratsky A. Glial cells in (patho)physiology. J Neurochem. 2012;121:4–27. doi: 10.1111/j.1471-4159.2012.07664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr AM, Tator CH, Keating A. Bone marrow-derived mesenchymal stromal cells for the repair of central nervous system injury. Bone Marrow Transplant. 2007;40:609–619. doi: 10.1038/sj.bmt.1705757. [DOI] [PubMed] [Google Scholar]
- Patkar S, Tate R, Modo M, Plevin R, Carswell HV. Conditionally immortalised neural stem cells promote functional recovery and brain plasticity after transient focal cerebral ischaemia in mice. Stem Cell Res. 2012;8:14–25. doi: 10.1016/j.scr.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Pavlichenko N, Sokolova I, Vijde S, Shvedova E, Alexandrov G, Krouglyakov P, Fedotova O, Gilerovich EG, Polyntsev DG, Otellin VA. Mesenchymal stem cells transplantation could be beneficial for treatment of experimental ischemic stroke in rats. Brain Res. 2008;1233:203–213. doi: 10.1016/j.brainres.2008.06.123. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, de Gruijl TD, Wurdinger T, Middeldorp JM. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci USA. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Johansson CB, Eliasson C, Stakeberg J, Wallen A, Perlmann T, Lendahl U, Betsholtz C, Berthold CH, Frisen J. Abnormal reaction to central nervous system injury in mice lacking glial fibrillary acidic protein and vimentin. J Cell Biol. 1999;145:503–514. doi: 10.1083/jcb.145.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- Pekny M, Wilhelmsson U, Bogestal YR, Pekna M. The role of astrocytes and complement system in neural plasticity. Int Rev Neurobiol. 2007;82:95–111. doi: 10.1016/S0074-7742(07)82005-8. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Neuroenergetics: Calling upon astrocytes to satisfy hungry neurons. Neuroscientist. 2004;10:53–62. doi: 10.1177/1073858403260159. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Slezak M. Genetic approaches to study glial cells in the rodent brain. Glia. 2012;60:681–701. doi: 10.1002/glia.22283. [DOI] [PubMed] [Google Scholar]
- Powell EM, Fawcett JW, Geller HM. Proteoglycans provide neurite guidance at an astrocyte boundary. Mol Cell Neurosci. 1997;10:27–42. doi: 10.1006/mcne.1997.0629. [DOI] [PubMed] [Google Scholar]
- Privat A. Astrocytes as support for axonal regeneration in the central nervous system of mammals. Glia. 2003;43:91–93. doi: 10.1002/glia.10249. [DOI] [PubMed] [Google Scholar]
- Qu R, Li Y, Gao Q, Shen L, Zhang J, Liu Z, Chen X, Chopp M. Neurotrophic and growth factor gene expression profiling of mouse bone marrow stromal cells induced by ischemic brain extracts. Neuropathology. 2007;27:355–363. doi: 10.1111/j.1440-1789.2007.00792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M, Subra C, Silvente-Poirot S, Poirot M. Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol. 2011;81:1171–1182. doi: 10.1016/j.bcp.2011.02.011. [DOI] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Glowinski J, Giaume C. Activity-dependent neuronal control of gap-junctional communication in astrocytes. J Cell Biol. 2000;149:1513–1526. doi: 10.1083/jcb.149.7.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Koulakoff A, Giaume C. Neurons set the tone of gap junctional communication in astrocytic networks. Neurochem Int. 2004;45:265–272. doi: 10.1016/j.neuint.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Sanchez-Simon FM, Zhang XX, Loh HH, Law PY, Rodriguez RE. Morphine regulates dopaminergic neuron differentiation via miR-133b. Mol Pharmacol. 2010;78:935–942. doi: 10.1124/mol.110.066837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino AP, Madani R, Huarte J, Belin D, Kiss JZ, Wohlwend A, Vassalli JD. Extracellular proteolysis in the adult murine brain. J Clin Invest. 1993;92:679–685. doi: 10.1172/JCI116637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarabi A, Chang CF, Wang Y, Tomac AC, Hoffer BJ, Morales M. Differential expression of the cell line-derived neurotrophic factor (GDNF) receptor GFRalpha1 in heterozygous Gfralpha1 null-mutant mice after stroke. Neurosci Lett. 2003;341:241–245. doi: 10.1016/s0304-3940(03)00195-2. [DOI] [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: Fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci. 2000;20:1435–1445. doi: 10.1523/JNEUROSCI.20-04-01435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C, Salter M, Fern R. GFP imaging of live astrocytes: regional differences in the effects of ischaemia upon astrocytes. J Anat. 2007;210:684–692. doi: 10.1111/j.1469-7580.2007.00731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke. 2007a;38:2150–2156. doi: 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Cui Y, Zhang C, Kapke A, Lu M, Savant-Bhonsale S, Chopp M. Therapeutic benefit of bone marrow stromal cells administered 1 month after stroke. J Cereb Blood Flow Metab. 2007b;27:6–13. doi: 10.1038/sj.jcbfm.9600311. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chen J, Zhang J, Vanguri P, Borneman J, Chopp M. Intracarotid transplantation of bone marrow stromal cells increases axon-myelin remodeling after stroke. Neuroscience. 2006;137:393–399. doi: 10.1016/j.neuroscience.2005.08.092. [DOI] [PubMed] [Google Scholar]
- Shen LH, Li Y, Chopp M. Astrocytic endogenous glial cell derived neurotrophic factor production is enhanced by bone marrow stromal cell transplantation in the ischemic boundary zone after stroke in adult rats. Glia. 2010;58:1074–1081. doi: 10.1002/glia.20988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Li Y, Gao Q, Savant-Bhonsale S, Chopp M. Down-regulation of neurocan expression in reactive astrocytes promotes axonal regeneration and facilitates the neurorestorative effects of bone marrow stromal cells in the ischemic rat brain. Glia. 2008;56:1747–1754. doi: 10.1002/glia.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen LH, Xin H, Li Y, Zhang RL, Cui Y, Zhang L, Lu M, Zhang ZG, Chopp M. Endogenous tissue plasminogen activator mediates bone marrow stromal cell-induced neurite remodeling after stroke in mice. Stroke. 2011;42:459–464. doi: 10.1161/STROKEAHA.110.593863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon SA, Nicolelis MAL. Astrocytes: Wiring the Brain. Tayor & Francis Group: CRC Press; 2012. [Google Scholar]
- Simpson RJ, Lim JW, Moritz RL, Mathivanan S. Exosomes: Proteomic insights and diagnostic potential. Expert Rev Proteomics. 2009;6:267–283. doi: 10.1586/epr.09.17. [DOI] [PubMed] [Google Scholar]
- Siushansian R, Bechberger JF, Cechetto DF, Hachinski VC, Naus CC. Connexin43 null mutation increases infarct size after stroke. J Comp Neurol. 2001;440:387–394. doi: 10.1002/cne.1392. [DOI] [PubMed] [Google Scholar]
- Smalheiser NR. Exosomal transfer of proteins and RNAs at synapses in the nervous system. Biol Direct. 2007;2:35. doi: 10.1186/1745-6150-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow DM, Mullins N, Hynds DL. Nervous system-derived chondroitin sulfate proteoglycans regulate growth cone morphology and inhibit neurite outgrowth: A light, epifluorescence, and electron microscopy study. Microsc Res Tech. 2001;54:273–286. doi: 10.1002/jemt.1140. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- Stecca B, Ruiz i Altaba A. Brain as a paradigm of organ growth: Hedgehog-Gli signaling in neural stem cells and brain tumors. J Neurobiol. 2005;64:476–490. doi: 10.1002/neu.20160. [DOI] [PubMed] [Google Scholar]
- Stevens B. Neuron-astrocyte signaling in the development and plasticity of neural circuits. Neurosignals. 2008;16:278–288. doi: 10.1159/000123038. [DOI] [PubMed] [Google Scholar]
- Stoorvogel W, Kleijmeer MJ, Geuze HJ, Raposo G. The biogenesis and functions of exosomes. Traffic. 2002;3:321–330. doi: 10.1034/j.1600-0854.2002.30502.x. [DOI] [PubMed] [Google Scholar]
- Suarez-Monteagudo C, Hernandez-Ramirez P, Alvarez-Gonzalez L, Garcia-Maeso I, de la Cuetara-Bernal K, Castillo-Diaz L, Bringas-Vega ML, Martinez-Aching G, Morales-Chacon LM, Baez-Martin MM, Sánchez-Catasús C, Carballo-Barreda M, Rodríguez-Rojas R, Gömez-Fernńdez L, Alberti-Amador E, Macías-Abraham C, Balea ED, Rosales LC, Del Valle Pérez L, Ferrer BB, Gonzłez RM, Bergado JA. Autologous bone marrow stem cell neuro-transplantation in stroke patients. An open study. Restor Neurol Neurosci. 2009;27:151–161. doi: 10.3233/RNN-2009-0483. [DOI] [PubMed] [Google Scholar]
- Sutherland BA, Minnerup J, Balami JS, Arba F, Buchan AM, Kleinschnitz C. Neuroprotection for ischaemic stroke: translation from the bench to the bedside. Int J Stroke. 2012;7:407–418. doi: 10.1111/j.1747-4949.2012.00770.x. [DOI] [PubMed] [Google Scholar]
- Swanson RA, Ying W, Kauppinen TM. Astrocyte influences on ischemic neuronal death. Curr Mol Med. 2004;4:193–205. doi: 10.2174/1566524043479185. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yasuhara T, Shingo T, Muraoka K, Kameda M, Takeuchi A, Yano A, Kurozumi K, Agari T, Miyoshi Y, Kinugasa K, Date I. Embryonic neural stem cells transplanted in middle cerebral artery occlusion model of rats demonstrated potent therapeutic effects, compared to adult neural stem cells. Brain Res. 2008;1234:172–182. doi: 10.1016/j.brainres.2008.07.086. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Katoh A, Oguro K, Shimazaki K, Gomi H, Itohara S, Masuzawa T, Kawai N. Disturbance of hippocampal long-term potentiation after transient ischemia in GFAP deficient mice. J Neurosci Res. 2002;67:11–20. doi: 10.1002/jnr.10004. [DOI] [PubMed] [Google Scholar]
- Teesalu T, Kulla A, Asser T, Simisker A, Vaheri A. Plasminogen activators in CNS physilogy and disease. In: Waisman DM, editor. Plasminogen, activation and regulation. New York: Kuwer Academic/Plenum Publishers; 2003. [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trendelenburg G, Dirnagl U. Neuroprotective role of astrocytes in cerebral ischemia: Focus on ischemic preconditioning. Glia. 2005;50:307–320. doi: 10.1002/glia.20204. [DOI] [PubMed] [Google Scholar]
- Tsai LK, Wang Z, Munasinghe J, Leng Y, Leeds P, Chuang DM. Mesenchymal stem cells primed with valproate and lithium robustly migrate to infarcted regions and facilitate recovery in a stroke model. Stroke. 2011;42:2932–2939. doi: 10.1161/STROKEAHA.110.612788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirka SE. Tissue plasminogen activator as a modulator of neuronal survival and function. Biochem Soc Trans. 2002;30:222–225. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007;24:508–520. doi: 10.1089/neu.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- van Niel G, Porto-Carreiro I, Simoes S, Raposo G. Exosomes: A common pathway for a specialized function. J Biochem. 2006;140:13–21. doi: 10.1093/jb/mvj128. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology of neuronal-glial networking. Neurochem Int. 2010;57:332–343. doi: 10.1016/j.neuint.2010.02.002. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Parpura V. Recent advances in (patho)physiology of astroglia. Acta Pharmacol Sin. 2010;31:1044–1054. doi: 10.1038/aps.2010.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Nagai A, Sheikh AM, Shiota Y, Narantuya D, Watanabe T, Masuda J, Kobayashi S, Kim SU, Yamaguchi S. Transplantation of human mesenchymal stem cells promotes functional improvement and increased expression of neurotrophic factors in a rat focal cerebral ischemia model. J Neurosci Res. 2010;88:1017–1025. doi: 10.1002/jnr.22279. [DOI] [PubMed] [Google Scholar]
- Wakitani S, Mitsuoka T, Nakamura N, Toritsuka Y, Nakamura Y, Horibe S. Autologous bone marrow stromal cell transplantation for repair of full-thickness articular cartilage defects in human patellae: Two case reports. Cell Transplant. 2004;13:595–600. doi: 10.3727/000000004783983747. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Y, Chen J, Gautam SC, Zhang Z, Lu M, Chopp M. Ischemic cerebral tissue and MCP-1 enhance rat bone marrow stromal cell migration in interface culture. Exp Hematol. 2002a;30:831–836. doi: 10.1016/s0301-472x(02)00829-9. [DOI] [PubMed] [Google Scholar]
- Wang L, Li Y, Chen X, Chen J, Gautam SC, Xu Y, Chopp M. MCP-1, MIP-1, IL-8 and ischemic cerebral tissue enhance human bone marrow stromal cell migration in interface culture. Hematology. 2002b;7:113–117. doi: 10.1080/10245330290028588. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang J, Li Y, Yang GY. Roles of chemokine CXCL12 and its receptors in ischemic stroke. Curr Drug Targets. 2012;13:166–172. doi: 10.2174/138945012799201603. [DOI] [PubMed] [Google Scholar]
- White RE, Jakeman LB. Don’t fence me in: Harnessing the beneficial roles of astrocytes for spinal cord repair. Restor Neurol Neurosci. 2008;26:197–214. [PMC free article] [PubMed] [Google Scholar]
- Wind T, Hansen M, Jensen JK, Andreasen PA. The molecular basis for anti-proteolytic and non-proteolytic functions of plasminogen activator inhibitor type-1: Roles of the reactive centre loop, the shutter region, the flexible joint region and the small serpin fragment. Biol Chem. 2002;383:21–36. doi: 10.1515/BC.2002.003. [DOI] [PubMed] [Google Scholar]
- Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: The BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- Wozniak W. Brain-derived neurotrophic factor (BDNF): role in neuronal development and survival. Folia Morphol (Warsz) 1993;52:173–181. [PubMed] [Google Scholar]
- Xin H, Li Y, Buller B, Katakowski M, Zhang Y, Wang X, Shang X, Zhang ZG, Chopp M. Exosome-mediated transfer of miR-133b from multipotent mesenchymal stromal cells to neural cells contributes to neurite outgrowth. Stem Cells. 2012;30:1556–1564. doi: 10.1002/stem.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Chen X, Chopp M. Bone marrow stromal cells induce BMP2/4 production in oxygen-glucose-deprived astrocytes, which promotes an astrocytic phenotype in adult subventricular progenitor cells. J Neurosci Res. 2006;83:1485–1493. doi: 10.1002/jnr.20834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Shen LH, Liu X, Hozeska-Solgot A, Zhang RL, Zhang ZG, Chopp M. Multipotent mesenchymal stromal cells increase tPA expression and concomitantly decrease PAI-1 expression in astrocytes through the sonic hedgehog signaling pathway after stroke (in vitro study) J Cereb Blood Flow Metab. 2011;31:2181–2188. doi: 10.1038/jcbfm.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin H, Li Y, Shen LH, Liu X, Wang X, Zhang J, Pourabdollah-Nejad DS, Zhang C, Zhang L, Jiang H, Zhang ZG, Chopp M. Increasing tPA activity in astrocytes induced by multipotent mesenchymal stromal cells facilitate neurite outgrowth after stroke in the mouse. PLoS One. 2010;5:e9027. doi: 10.1371/journal.pone.0009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Emery JF, Ouyang YB, Voloboueva LA, Giffard RG. Astrocyte targeted overexpression of Hsp72 or SOD2 reduces neuronal vulnerability to forebrain ischemia. Glia. 2010;58:1042–1049. doi: 10.1002/glia.20985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan T, Chopp M, Ye X, Liu Z, Zacharek A, Cui Y, Roberts C, Buller B, Chen J. Niaspan increases axonal remodeling after stroke in type 1 diabetes rats. Neurobiol Dis. 2012;46:157–164. doi: 10.1016/j.nbd.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Wei X, Li J, Heine LA, Rosenwasser R, Iacovitti L. Changes in host blood factors and brain glia accompanying the functional recovery after systemic administration of bone marrow stem cells in ischemic stroke rats. Cell Transplant. 2010;19:1073–1084. doi: 10.3727/096368910X503415. [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Dereski MO, Garcia JH, Hetzel FW, Chopp M. Photoactivated Photofrin II: Astrocytic swelling precedes endothelial injury in rat brain. J Neuropathol Exp Neurol. 1992;51:91–100. doi: 10.1097/00005072-199201000-00011. [DOI] [PubMed] [Google Scholar]
- Yu YM, Gibbs KM, Davila J, Campbell N, Sung S, Todorova TI, Otsuka S, Sabaawy HE, Hart RP, Schachner M. MicroRNA miR-133b is essential for functional recovery after spinal cord injury in adult zebrafish. Eur J Neurosci. 2011;33:1587–1597. doi: 10.1111/j.1460-9568.2011.07643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, Chen J, Cui X, Li A, Li Y, Roberts C, Feng Y, Gao Q, Chopp M. Angiopoietin1/Tie2 and VEGF/Flk1 induced by MSC treatment amplifies angiogenesis and vascular stabilization after stroke. J Cereb Blood Flow Metab. 2007;27:1684–1691. doi: 10.1038/sj.jcbfm.9600475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, Shehadah A, Chen J, Cui X, Roberts C, Lu M, Chopp M. Comparison of bone marrow stromal cells derived from stroke and normal rats for stroke treatment. Stroke. 2010;41:524–530. doi: 10.1161/STROKEAHA.109.568881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, Chopp M. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience. 2006;141:687–695. doi: 10.1016/j.neuroscience.2006.04.054. [DOI] [PubMed] [Google Scholar]
- Zhang L, Li Y, Romanko M, Kramer BC, Gosiewska A, Chopp M, Hong K. Different routes of administration of human umbilical tissue-derived cells improve functional recovery in the rat after focal cerebral ischemia. Brain Res. 2012;1489:104–112. doi: 10.1016/j.brainres.2012.10.017. [DOI] [PubMed] [Google Scholar]
- Zhang R, Wang Y, Zhang L, Zhang Z, Tsang W, Lu M, Chopp M. Sildenafil (Viagra) induces neurogenesis and promotes functional recovery after stroke in rats. Stroke. 2002a;33:2675–2680. doi: 10.1161/01.str.0000034399.95249.59. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Chopp M. Vascular endothelial growth factor and angiopoietins in focal cerebral ischemia. Trends Cardiovasc Med. 2002;12:62–66. doi: 10.1016/s1050-1738(01)00149-9. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Chopp M. Neurorestorative therapies for stroke: underlying mechanisms and translation to the clinic. Lancet Neurol. 2009;8:491–500. doi: 10.1016/S1474-4422(09)70061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZG, Jiang Q, Zhang R, Zhang L, Wang L, Arniego P, Ho KL, Chopp M. Magnetic resonance imaging and neurosphere therapy of stroke in rat. Ann Neurol. 2003;53:259–263. doi: 10.1002/ana.10467. [DOI] [PubMed] [Google Scholar]
- Zhang ZG, Zhang L, Jiang Q, Chopp M. Bone marrow-derived endothelial progenitor cells participate in cerebral neovascularization after focal cerebral ischemia in the adult mouse. Circ Res. 2002b;90:284–288. doi: 10.1161/hh0302.104460. [DOI] [PubMed] [Google Scholar]
- Zhao CZ, Zhao B, Zhang XY, Huang XQ, Shi WZ, Liu HL, Fang SH, Lu YB, Zhang WP, Tang FD, Wei EQ. Cysteinyl leukotriene receptor 2 is spatiotemporally involved in neuron injury, astrocytosis and microgliosis after focal cerebral ischemia in rats. Neuroscience. 2011;189:1–11. doi: 10.1016/j.neuroscience.2011.05.066. [DOI] [PubMed] [Google Scholar]
- Zhao MZ, Nonoguchi N, Ikeda N, Watanabe T, Furutama D, Miyazawa D, Funakoshi H, Kajimoto Y, Nakamura T, Dezawa M, Shibata MA, Otsuki Y, Coffin RS, Liu WD, Kuroiwa T, Miyatake S. Novel therapeutic strategy for stroke in rats by bone marrow stromal cells and ex vivo HGF gene transfer with HSV-1 vector. J Cereb Blood Flow Metab. 2006;26:1176–1188. doi: 10.1038/sj.jcbfm.9600273. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Rempe DA. Targeting astrocytes for stroke therapy. Neurotherapeutics. 2010;7:439–451. doi: 10.1016/j.nurt.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S. From bone to brain: Human skeletal stem cell therapy for stroke. Cent Nerv Syst Agents Med Chem. 2011;11:157–163. doi: 10.2174/187152411796011376. [DOI] [PubMed] [Google Scholar]
- Zomer A, Vendrig T, Hopmans ES, van Eijndhoven M, Middeldorp JM, Pegtel DM. Exosomes: Fit to deliver small RNA. Commun Integr Biol. 2010;3:447–450. doi: 10.4161/cib.3.5.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]