Abstract
Endometriosis pain is a very common and extremely disabling condition whose mechanism is still poorly understood. While increased levels of leptin have been reported in patients with endometriosis, their contribution to endometriosis pain has not been explored. Using a rodent model of endometriosis we provide evidence for an estrogen-dependent contribution of leptin in endometriosis-induced pain. Rats implanted with autologous uterine tissue onto the gastrocnemius muscle developed endometriosis-like lesions and local chronic pain. Compared to eutopic uterine tissue, leptin mRNA and protein were up-regulated in the endometriosis-like lesions. Intramuscular injection of recombinant leptin in naive rats produced dose-dependent local mechanical hyperalgesia and nociceptor sensitization to mechanical stimulation. Ovariectomy attenuated the mechanical hyperalgesia induced by recombinant leptin, in rats treated with vehicle compared to those treated with 17β-estradiol replacement, at 1 and 24 h after leptin injection. Finally, intralesional injections of a pegylated leptin receptor (Ob-R) antagonist or of an inhibitor of Janus kinase2, which transduces the Ob-R signal of leptin receptor, markedly attenuated pain in the endometriosis model. Taken together these data support the hypothesis that leptin, generated in ectopic endometrial lesions produces mechanical hyperalgesia by acting on nociceptors innervating the lesion. This sensitivity to leptin is dependent on estrogen levels. Thus, interventions targeting leptin signaling, especially in combination with interventions that lower estrogen levels, might be useful for the treatment of endometriosis pain.
Keywords: endometriosis, leptin, chronic pelvic pain, ob gene, nociceptor, mechanical hyperalgesia
Introduction
Endometriosis is a chronic disease characterized by the presence of endometrial stromal and glandular tissue outside of the uterus, whose major clinical manifestations are infertility and chronic pelvic pain (Giudice, 2010). The pathophysiology of endometriosis is complex, involving many cytokines and pro-inflammatory mediators (Giudice, 2010). Among those mediators, growing evidence suggests a role for leptin in the establishment of lesions and the expression of symptoms related to endometriosis (Bedaiwy et al., 2006, Wu et al., 2007, Barcz et al., 2008, Styer et al., 2008). Leptin, a 16 kD adipokine, is the product of the ob (obese) gene (Zhang et al., 1994) and initially was mainly associated to the regulation food intake and energy expenditure (Friedman and Halaas, 1998). More recently, it has been recognized as an important mediator of immune regulation, angiogenesis and inflammation (Wu et al., 2007, Styer et al., 2008, Babaei et al., 2011). The pro-inflammatory and neo-angiogenic properties of leptin have been shown to be important for the formation and persistence of endometriotic lesions. For instance, aberrant expression of leptin mRNA and protein in endometrial stromal cells is involved in the genesis and progression of endometriosis (Wu et al., 2007). Indeed, it has been shown that leptin stimulates the growth of human endometriotic epithelial cells (Oh et al., 2013). Furthermore, experimental antagonism of leptin receptor in a murine model of endometriosis inhibits lesion proliferation, early vascular recruitment and neo-angiogenesis (Styer et al., 2008).
Many attempts have been made to assess the role of leptin in clinical symptoms of endometriosis: while the relationship of leptin levels to fertility is equivocal (Wertel et al., 2005, Bedaiwy et al., 2006, Barcz et al., 2008), its relationship to pain has been more consistently demonstrated (Mahutte et al., 2003, Bedaiwy et al., 2006). The leptin receptor (Ob-R) is expressed in afferent pathways involved in pain processing, namely dorsal root ganglion (DRG) neurons (Chen et al., 2006, Murphy et al., 2013) and superficial dorsal horn neurons and glia (Lim et al., 2009). Acting on peripheral macrophages (Maeda et al., 2009) or dorsal horn neurons (Lim et al., 2009, Tian et al., 2011) leptin induces mechanical allodynia, contributing to models of neuropathic pain. Conversely, Ob-R antagonists inhibit nerve injury-induced mechanical allodynia (Lim et al., 2009), and Ob-R (Wright et al., 2007) or leptin (Maeda et al., 2009) null mutant animals fail to develop neuropathic pain-like behaviors. While these data suggest that leptin might play a role in chronic pain, its contribution to endometriosis pain has yet to be studied. Since estrogen, a well-established player in endometriosis (Giudice, 2010), up-regulates the expression of Ob-R in DRG neurons (Chen et al., 2006), we explored the hypothesis that leptin released from endometriosis lesions acts on nociceptors to induce an estrogen-dependent persistent mechanical hyperalgesia.
Experimental Procedures
Animals
Adult female Sprague Dawley rats (10-12 weeks old, 220–240 g at arrival; Charles River, Hollister, CA) were used in these experiments. They were housed in the Animal Care Facility at the University of California San Francisco, under environmentally controlled conditions (lights on 07:00–19:00 h; room temperature 21–23°C) with food and water available ad libitum. Upon completion of experiments, rats were killed by pentobarbital overdose followed by cervical dislocation. Animal care and use conformed to NIH guidelines (NIH Guide for the Care and Use of Laboratory Animals).
Ethical approval
The University of California San Francisco Committee on Animal Research approved all experimental protocols. Concerted effort was made to minimize number and suffering of experimental animals, in accordance with the principle of the minimal sample size stated in the Ethical Guidelines for investigations of Experimental Pain in Conscious Animals (Zimmermann, 1983).
Drugs
Unless otherwise stated, all chemicals used in these experiments were obtained from Sigma-Aldrich (St. Louis, MO). Stock solution of recombinant rat leptin (rrleptin, R&D systems, Minneapolis, MN) was made in 0.9% NaCl containing 0.5% BSA, stored at −20°C and diluted in 0.9% NaCl-0.5% BSA immediately before injection. A pegylated leptin antagonist (rrleptin triple mutant, ProSpec-Tany Technogene, Rehovot, Israel), was diluted in D-PBS containing 0.1% BSA immediately before injection. 17β-estradiol (Calbiochem, La Jolla, CA) was solubilized in sesame oil and kept at room temperature. Stock solution of the JAK2 inhibitor tyrphostin (AG490) was stored at −20°C in 100% DMSO, and diluted in 0.9% NaCl (10% DMSO final concentration) immediately before injection.
Surgical model of endometriosis
The model of surgically-induced muscle endometriosis used here has been previously described (Alvarez et al., 2012), regardless of the estrous cycle status of the rats. Briefly, female rats were pre-medicated with a mixture of ketamine hydrochloride and xylazine (80 and 6 mg/kg, s.c., respectively) and anesthetized with isoflurane. One cm of the right uterine horn was excised and placed in a Petri dish containing 0.9% NaCl. A full thickness 3 × 3 mm square of this uterine was implanted onto the gastrocnemius muscle, applying single stitches using 5-0 nylon. Muscle and skin incisions were sutured, separately, with 5-0 nylon single stitches. The sham surgical procedure was similar but the uterus was left intact and not implanted on the surface of the gastrocnemius.
Ovariectomy
Rats pre-medicated with a ketamine hydrochloride-xylazine mixture and carprofen (Rimadyl, Pfizer Animal Health, NY, 5 mg/kg, s.c.) and anesthetized with isoflurane. Under aseptic conditions the ovaries were exposed, isolated, ligated and thereafter excised. After checking for hemostasis, the oviduct and uterine horn were replaced into the peritoneal cavity and incisions were closed in two planes with 5-0 nylon sutures. Animals received a daily injection of carprofen the next 3 days; sutures were removed 7 days after surgery. Experiments were performed 15 days after ovariectomy.
Measurement of mechanical hyperalgesia
Mechanical nociceptive threshold in the ectopic endometrial lesion or gastrocnemius muscle was quantified using a manually-driven digital force transducer (Chatillon DFI2; Amtek Inc., Largo, FL), as previously described (Alvarez et al., 2010) Alvarez et al., 2012; Dina et al., 2008). Data are reported as a mean of 3 readings and are expressed in milliNewtons (mN). Behavioral data collection was performed unblind to treatment condition.
Intramuscular and intralesional injections
Intramuscular/intralesional injections were performed as previously reported (Alvarez et al., 2012; Dina et al., 2008). Briefly, rats were anesthetized with isoflurane to facilitate the injection of drugs (volume 20 μl). The injection site was previously shaved and scrubbed with alcohol. Intramuscular injections were performed into the belly of the gastrocnemius muscle, whereas the lesions at the implant site were localized by palpation and the tip of the needle directed to the base of the implant. The injection site was marked with a fine-tip indelible ink pen.
In vivo single fiber electrophysiology
Recordings were performed as previously described (Alvarez et al., 2012). Briefly, fine fascicles of axons were dissected from the gastrocnemius-soleus nerve, and placed on a recording electrode. Single units, first detected by mechanical stimulation of the gastrocnemius muscle with a small blunt-tipped glass bar, were subsequently confirmed by electrical stimulation of the mechanical receptive field. Bipolar stimulating electrodes were then placed and held on the center of the receptive field of the muscle afferent by a micromanipulator (Narishige model MM-3, Tokyo, Japan). All recorded muscle afferents had conduction velocities in the range of type III (conduction velocity 2.5–30 m/s) or type IV (conduction velocity <2.5 m/s) fibers (Diehl et al., 1993). Mechanical threshold, determined with calibrated von Frey hairs (VFH Ainsworth, London, UK), was defined as the lowest force that elicited at least 2 spikes within 1 second, in at least 50% of trials. Sustained (60 s) suprathreshold (10 g) mechanical stimulation was accomplished by use of a mechanical stimulator that consisted of a force-measuring transducer (Entran, Fairfield, NJ, USA) with a blunt plastic tip that was applied by a micromanipulator (BC-3 and BE-8, Narishige) on the center of the afferent's receptive field for 60 s. Rat recombinant leptin (500 ng/5 μl) or its vehicle (0.9% NaCl-0.5% BSA, 5 μl) were injected into the receptive field. Mechanical threshold was measured 30 min after injection, and the response to 10 g stimulus recorded 30 and 60 min after the injection.
Quantitative one-step multiplex RT-PCR
Total RNA from the endometrial lesion and uterine tissue from sham-operated control rats was extracted using Trizol reagent (Invitrogen, Carlsbad, CA) with the PureLinkTM RNA mini kit (Ambion) according to the manufacturer's instruction. The amount of RNA was quantified with a spectrophotometer (UV-160, Shimadzu) and cDNA preparation was carried out with 1 μg of total RNA/sample and the superscript III platinum one-step quantitative RT-PCR system (Invitrogen). The PCR-primers used for the amplification of rat leptin according to NCBI database-entry NM_013076 were: F1: 5’-TGAGTTTGTCCAAGATGGACC-3’ and B1: 5’-CATTCAGGGCTAAGGTCCAA-3’ (Invitrogen). To compensate for variations in the quality or quantity of the samples a one-step multiplex RT-PCR with S18 rRNA as an endogenous standard was performed and the amplification product of the leptin gene (292 bp) normalized to the PCR product of the S18 rRNA (489 bp). Pilot experiments were performed to optimize for: 1) Annealing temperature (56.9°C); and, 2) Number of PCR-cycles (33-42); and 3) Ratio of S18 rRNA primer to competimersTM (Ambion; 1:9). The PCR-products were separated on 2% agarose gels and visualized by ethidium bromide. Images of the gels were acquired with the ChemiImager system and analyzed with AlphaEaseFC Software (Alpha Innotech Corporation, San Leandro, CA).
Immunoblotting analysis
To determine whether the changes in the nociceptive responses of rats with a surgical implanted endometrial lesion are due to local increase in leptin level, a Western blot analysis was performed. Tissue biopsies from eutopic and ectopic (cystic lesions) uterine tissue of rats implanted with autologous uterine tissue were harvested and transferred into cold homogenization buffer (150 mM NaCl, 10mM EDTA, 2% SDS, 50 mM Tris-HCl pH 7.4) supplemented with a 2x protease inhibitor cocktail (Roche Diagnostics, Indianapolis, IN). The tissue biopsies were homogenized on ice with a hand-held Teflon-glass homogenizer and extracted by a 15 min centrifugation at 20.000g in a tabletop centrifuge. Protein determination was performed using the micro BCA Protein Assay Kit (Pierce, Rockford, IL) with BSA as the standard. 40 μg of proteins/sample were denatured by boiling in sample buffer (3% SDS, 10% (v/v) Glycerol, 5% (v/v) β-mercaptoethanol, 0.025% bromphenol blue, 62.5 mM Tris-HCl, pH 6.8) for 10 min and electrophoresed on 4 to 15% pre-cast polyacrylamide gels (Biorad, Hercules, CA) in 25 mM Tris containing 192 mM glycine and 0.1% SDS. Proteins were electrophoretically transferred to a nitrocellulose membrane by using the semi-dry method (transfer time 1h at 10V, with 47.6 mM Tris, 38.9 mM glycine, 0.038% SDS and 20% (v/v) Methanol). The nitrocellulose membranes were saturated by shaking in blocking buffer [5% BSA in Tris-buffered saline containing 0.1% Tween20 (TBST)] for 1 h at room temperature (RT) and probed with rabbit anti-leptin (sc-842, 1:500, Santa Cruz Biotechnology, Santa Cruz, CA), or rabbit anti-β-actin (ab 8227, 1:500; Abcam, Cambridge, MA) antibodies in blocking buffer at 4°C overnight. After washing with TBST (3 times at RT, 15 min each) the leptin blot was probed with a biotinylated goat anti-rabbit antibody (1:1000 in blocking buffer, Jackson Immunoresearch, West Grove, PA) for 2 h at RT while the β-actin blot was probed with a horseradish peroxidase (HRP) conjugated anti-rabbit antibody (NA934V, 1:5000 in blocking buffer, GE Healthcare, Piscataway, NJ) for 2 h at RT. The blots were washed with TBST (3 times at RT, 15 min each) and the leptin blot was finally probed with Steptavidin conjugated HRP (S2438, 1:2500 in blocking buffer) for 1 h under shaking at RT. After washing the leptin Western blot with TBST (3 times at RT, 15 min each), immunoreactivity was visualized using the enhanced chemiluminescence detection system (Pierce, Rockford, IL). Results were analyzed by computer-assisted densitometry and levels of leptin immunoreactivity were normalized with respect to the β-actin control levels in each sample.
Statistical analysis
Group data are expressed as mean ± SEM of n independent observations. Statistical comparisons were made using GraphPad Prism 5.0 statistical software (GraphPad Software, Inc., La Jolla, CA, USA). The Student's t-test was used to compare 1 or 2 independent samples, whereas analysis of variance (ANOVA) followed by Tukey's or Dunnett's multiple comparison tests were used for comparing multiple treatments. The mechanical hyperalgesia induced by the implant of ectopic uterine tissue was analyzed by means of a two-way repeated measures analysis of variance (ANOVA), with one within subjects-factor (time) and one between-subjects factor (surgical procedure with two levels, uterine tissue implant or sham procedure), followed by Bonferroni's multiple comparisons test. Data were tested for normality using the D'Agostino and Pearson omnibus normality test; if data did not pass the normality test for Gaussian distribution, Welch correction for the Student's t-test was used. Mann-Whitney's test was used for comparison of mechanical threshold data obtained from in vivo single fiberelectrophysiology. A P-value < 0.05 was considered statistically significant.
Results
Induction of endometriosis
All rats uneventfully recovered from surgery. Inspection of the operated hind limb on day 10 after surgery revealed the presence of a subcutaneous lesion at the implant site in all uterine tissue-implanted, but not in sham operated, control rats, regardless of their preoperative estrous cycle status. As we previously reported, surgical exposure of the muscle on day 14 after surgery revealed cystic lesions in all uterine tissue implanted rats, but not in sham operated rats, with a reddish-brown to amber fluid in the cysts’ cavities (Alvarez et al., 2012).
Development of mechanical hyperalgesia
Ten days after surgery, rats implanted with autologous uterus (endometriosis model), but not sham surgery (control), exhibited a significant decrease in mechanical nociceptive threshold (mechanical hyperalgesia) at the site of the implant (−41.1 ± 1.1%, n = 6, and −0.8 ± 1%, n = 6, respectively; two-way ANOVA followed by Bonferroni's post-hoc test, P < 0.001, Fig. 1A). Fourteen days after surgery, rats submitted to the endometriosis pain model (−50.4 ± 2%, n = 6), but not control rats (0.4 ± 0.6%, n = 6), exhibited a further increase in mechanical hyperalgesia over the implanted gastrocnemius muscle, compared to pre-surgical baseline (two-way ANOVA followed by Bonferroni's post-hoc test, P < 0.001, Fig. 1A). Consistent with our previous observations, this hyperalgesia remained unattenuated at postoperative day 21 (−57.1 ± 1.8%, n = 6, two-way ANOVA followed by Bonferroni's post-hoc test, P < 0.001, Fig. 1A). At this time point, mechanical nociceptive threshold in sham operated rats was still unchanged compared to pre-surgery baseline (1.4 ± 0.8%, n=6, two-way ANOVA followed by Bonferroni's post-hoc test, P > 0.05).
Figure 1.
Surgical implant of ectopic uterine tissue on the gastrocnemius muscle produces persistent mechanical hyperalgesia and increased local levels of leptin transcripts. (A) Compared to baseline (Base) values, mechanical hyperalgesia was already present at day 10 post-implantation after unilateral transplantation of endometrium and remained unattenuated at day 21 after surgery. In contrast, rats submitted to a sham surgical procedure in the gastrocnemius muscle did not exhibit significant hyperalgesia at any time point tested; ***P < 0.001. (B) Quantitative one-step multiplex RT-PCR for leptin mRNA. Total RNA from the endometriosis-like lesion or uterine tissue from sham-operated control rats was compared 14 d post-surgery. After RT-PCR amplification, the basal concentration of mRNA encoding leptin (292 bp) was almost undetectable in eutopic endometrium (lane 1), whereas leptin mRNA was expressed in high levels in cystic lesions from implanted rats (lane 2). S18 rRNA was used as an endogenous standard (489 bp). (C) Western blot analysis. Protein extracts from ectopic (lane 1) and eutopic (lane 2) uterine tissue were submitted to Western blot analysis, using β-actin as an internal loading control.
Leptin mRNA and protein in endometriosis lesions
The expression of leptin transcripts was evaluated in cystic lesions and eutopic uterus obtained from implanted rats at day 14 after surgery. Compared to eutopic endometrium (lane 1, n = 1) there was a 92% increase in the leptin mRNA expression in ectopic endometrium (lane 2, n = 1) over the full linear range from 33 to 42 cycles (Fig.1B). In good agreement with this, we also observed an increased leptin-like immunoreactivity in protein extracts from ectopic endometrial tissue (96.2 ± 58.5%, n = 5, in duplicate), compared with eutopic endometrial tissue (n = 5, P = 0.03) in Western blot analysis (Fig. 1C).
Local leptin induces mechanical hyperalgesia
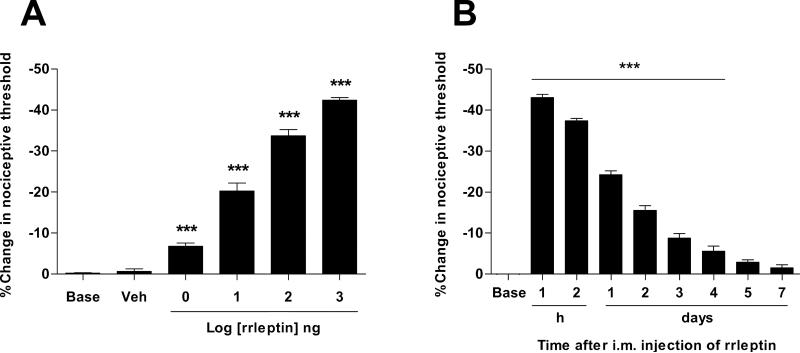
To determine the effect of leptin on mechanical nociceptive threshold we injected recombinant rat leptin (rrleptin) into the gastrocnemius muscle of naïve rats. The intramuscular (i.m.) injection of rrleptin produced dose-dependent mechanical hyperalgesia (1 ng - 1 μg), after administration in a cumulative dosing protocol with sequentially higher doses administered at 30 min intervals (Fig. 2A). To study the time course of rrleptin-induced mechanical hyperalgesia, a single injection of 1 μg dose of rrleptin was performed (Fig. 2B). This dose produced a decrease in mechanical nociceptive threshold that reached its maximum by 60 min (−43 ± 0.8, P < 0.001, n = 6, Fig. 2B) and while diminished was still significant for 4 days (−5.5 ± 1.3, P < 0.001, n = 6, Fig. 2B).
Figure 2.
Local injection of leptin produces persistent mechanical hyperalgesia. (A) Cumulative dose–response curve for the effect of i.m. rrleptin on mechanical nociceptive threshold following administration into the belly of the gastrocnemius muscle in the rat. Incremental doses from 1 ng to 1 μg were administered at 30 min intervals. (B) The time-effect curve for hyperalgesia induced by 1 μg of rrleptin was investigated from 60 min to 7 days (n = 6). Hyperalgesia reached a maximum 1 h. post-injection and lasted for 4 days. ***P < 0.001.
Local leptin sensitizes nociceptors
Single-fiber recordings were made from nociceptors innervating the gastrocnemius muscle of female rats. Compared to baseline, the injection of rrleptin (500 ng/5 μl) into the peripheral receptive field caused an increase in action potential firing in response to a supra-threshold mechanical stimulus (10 g) applied for 60 s in recordings taken 30 min and 1 h after injection (Fig. 3A-D). The number of spikes measured over 60 s of stimulation increased from 66.5 ± 10.3 pre-injection (n = 20), to 107.2 ± 18.3 after 30 min. (P < 0.05, n = 20, paired t-test) or to 289.1 ± 74.3 after 1 h (P < 0.01, n = 20, paired t-test) after rrleptin injection (Fig. 3A). The analysis of the time course of the response to sustained mechanical stimulation shows that, compared to pre-treatment control (64.2 ± 10.2 action potentials/10 s stimulus, n = 20, Fig. 3A,B), a significant increase in the number of spikes elicited in the first 10 s during the application of the stimulus as recorded 30 min (91.5 ± 12.7 action potentials/10 s stimulus, n = 20, P < 0.01, Student's t-test with Welch's correction, Fig. 3A,C) or 1 h (166.7 ± 29.3 action potentials/10 s stimulus, n = 20, P < 0.01, Student's t-test with Welch's correction, Fig. 3A,D) after rrleptin injection. The number of spikes recorded during the last 50 s of the 1 min. stimulus was not significantly changed 30 min. after rrleptin injection (15.7 ± 11.4 action potentials/50 to 60-s stimulus, n = 20, P > 0.05, Student's t-test with Welch's correction, Fig. 3A,C) compared to pre-injection control (2.3 ± 1 action potentials/50 to 60 s stimulus, n = 20, Fig. 3A,B). However, 1 h after rrleptin injection, this parameter was significantly increased (122.4 ± 52.2 action potentials/50 to 60 s stimulus, n = 20, P < 0.05, Student's t-test with Welch's correction, Fig. 3A,D). In contrast, the injection of the rrleptin vehicle (0.9% NaCl-0.5% BSA, 5 μl) did not produce significant changes on total action potential firing in response to a supra-threshold mechanical stimulus compared to pre-injection control (46 ± 21.2, n = 5), at 30 (64.8 ± 19.7, P > 0.05, n = 5, paired t-test) or 60 min (67.6 ± 36.6, P > 0.05, n = 5, paired t-test) after injection. The analysis of the time course of the response to sustained mechanical stimulation didn't show significant differences during the first 10 s, when pre-treatment control (44.4 ± 19.7 action potentials/10 s stimulus), is compared to responses obtained 30 (63.2 ± 18.5 action potentials/10 s stimulus, P > 0.05, n = 5) or 60 min (61 ± 30.1 action potentials/50 to 60 s stimulus, P > 0.05, n = 5) after vehicle injection. Similarly, no changes in the last 50 s of the response to sustained stimulation were observed during when comparing pre-injection control (1.6 ± 1.6 action potentials/50 to 60 s stimulus, n = 5) to responses obtained 30 (1.6 ± 1.6 action potentials/50 to 60 s stimulus, P > 0.05, n = 5) or 60 min (6.6 ± 6.6 action potentials/50 to 60 s stimulus, P > 0.05, n = 5) after vehicle injection. Finally, the injection of rrleptin or its vehicle had no effect on the threshold to elicit nociceptor activity or its conduction velocity (data not shown).
Figure 3.
Leptin produces nociceptor sensitization. (A) Thirty min. after injection, rrleptin significantly increased the number of spikes in recordings obtained during the early part of the stimulation period (first 10 s, n = 20, P < 0.01), as well as total response (60 s, n = 20, P < 0.05), compared to pre-rrleptin injection values. One hour after injection, rrleptin not only increased the number of spikes in recordings obtained during the early part of the stimulation period (first 10 s, n = 20, P < 0.01), but also in its late component (last 50 s, n = 20, P < 0.05) and total response (60 s, n = 20, P < 0.05) compared to pre-injection values. This increased response at 1 h. after rrleptin injection was also evident in histograms depicting the time-course of the nociceptor response, representing recordings obtained pre and post rrleptin injection (B-D); *P < 0.05; **P < 0.01.
Leptin-induced mechanical hyperalgesia is estrogen-dependent
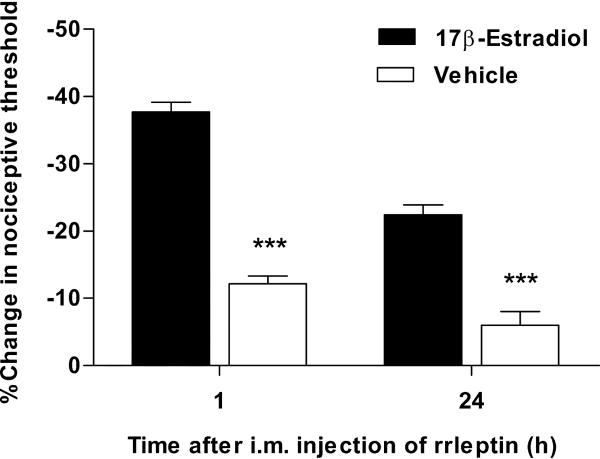
Since the expression of Ob-R in DRG neurons depends on estrogen levels (Chen et al., 2006), we asked whether the mechanical hyperalgesia induced by leptin is also estrogen dependent. Fifteen days after ovariectomy nociceptive mechanical thresholds were assessed and rats received either 17 β-estradiol (10 μg/0.1 ml, n = 6) or vehicle (sesame oil 0.1 ml, n = 6) s.c. in the base of neck for 3 consecutive days. Twenty four hours after the last injection of 17 β-estradiol or vehicle, rats received a single dose of rrleptin (100 ng/20 μl) into the gastrocnemius muscle. Rats treated with 17 β-estradiol replacement, exhibited a marked mechanical hyperalgesia in readings obtained at 1 (−37.7 ± 1.4%, n = 6, Fig. 4) or 24 h (−22.4 ± 1.5%, n = 6, Fig. 4) after rrleptin injection. However, compared to estradiol-treated rats, vehicle-injected rats exhibited only a modest hyperalgesia as measured at 1 h (−12.2 ± 1.2%, n = 6, P < 0.001, Student's t-test, Fig. 4) or 24 h (−6 ± 2%, n = 6, P < 0.001, Student's t-test, Fig. 4) after rrleptin injection, indicating that estrogen is necessary for full expression of acute and persistent leptin-induced mechanical hyperalgesia.
Figure 4.
Leptin-induced hyperalgesia is estrogen-dependent. Ovariectomized rats were treated with either 17β-estradiol or vehicle for 3 consecutive days. One day after, they received an i.m. injection of rrleptin (1 μg/20 μl) and muscle mechanical nociceptive threshold was measured 1 or 24 h after rrleptin injection. Rats without estrogen replacement exhibited a marked attenuation of the nociceptive response induced by rrleptin; ** P < 0.001.
Effects of intralesional injection of an Ob-R antagonist
Next, we asked whether the intralesional injection of an Ob-R antagonist could inhibit the hyperalgesia observed in the endometriosis model. Compared to baseline threshold observed in the endometriosis pain model (−46 ± 4.1%, n = 5), single injection of pegylated rat Ob-R antagonist (3 μg/20 μl) produced a significant inhibition of mechanical hyperalgesia appearing 1 h after injection (−29.2 ± 3.4, n = 5, P < 0.05, Fig. 5A) and lasting for at least 24 h (−23.2 ± 2.6, n = 5, P < 0.0001, Fig. 5A). Two additional daily intralesional injections of Ob-R antagonist were administered in order to explore whether this could produce an increase in the amplitude or duration of the antihyperalgesic effect observed after a single injection. The mechanical hyperalgesia was significantly inhibited for at least 72 h after last injection (−34.7 ± 2.5, n = 5, P < 0.05, Fig. 5B). To evaluate whether an eventual contribution of off-site actions on the antihyperalgesic effect observed, we injected the Ob-R antagonist or its vehicle into the contralateral gastrocnemius muscle of an additional group of implanted rats. Compared to pre-injection values −58.9 ± 1, n = 4, Fig. 5C) no significant changes in the mechanical nociceptive threshold were observed after contralateral injection of vehicle (−56.6 ± 1, n = 5, P < 0.05, Fig. 5C). Equally, no significant changes in mechanical hyperalgesia were observed at 30 min (−57.7 ± 2, n = 4, P < 0.05, Fig. 5C), 1 h (−56.6 ± 1.1, n = 4, P < 0.05, Fig. 5C) or 24 h (−58.7 ± 1, n = 4, P < 0.05, Fig. 5C) after injection of the pegylated Ob-R antagonist.
Figure 5.
Intralesional leptin receptor antagonist inhibits mechanical hyperalgesia in rats submitted to a model of endometriosis pain. Two weeks after surgical implant of autologous uterine tissue, rats displayed a marked mechanical hyperalgesia (EMS, endometriosis pain model). (A) Single intralesional injection of leptin receptor pegylated antagonist (3 μg/20 μl), but not vehicle (Veh), produced an attenuation of mechanical hyperalgesia at 1 h after injection which reached a maximal effect 24 h. after injection; (B) Repeated intralesional injections of leptin receptor pegylated antagonist (3 μg/20 μl*3) produced an inhibition of mechanical hyperalgesia lasting at least 3 days after last injection. (C) Administration of leptin receptor pegylated antagonist (3 μg/20 μl) into the contralateral gastrocnemius muscle was devoid of effect. *P < 0.05; ***P < 0.001.
No significant changes in body weight were noticed during the treatment with the pegylated Ob-R antagonist.
Effect of a JAK2 inhibitor
Given that local blocking of Ob-R attenuated mechanical hyperalgesia in the endometriosis pain model, we asked whether local disruption of an Ob-R intracellular signalling pathway could also inhibit hyperalgesia in this model. We explored the involvement of the main Ob-R-associated intracellular signalling pathway, involving the Janus kinase2/Signal Transducer and Activator of Transcription 3 (JAK2/STAT3) (Myers et al., 2008). Compared to baseline threshold observed in the endometriosis pain model (−57.1 ± 1.8%, n = 6), local injection of the JAK2 inhibitor tyrphostin AG490 (5 μg/20 μl) significantly attenuated the mechanical hyperalgesia at 1 h (−38.8 ± 3.5%, n = 6, P < 0.001, one-way ANOVA followed by Dunnett's post-hoc test) and 24 h (−40.7 ± 2.9%, n = 6, P < 0.001, one-way ANOVA followed by Dunnett's post-hoc test) after AG490 local injection (Fig. 6).
Figure 6.
Inhibition of leptin receptor signalling attenuates mechanical hyperalgesia in rats submitted to a model of endometriosis pain. Two weeks after surgical implant of autologous uterine tissue, rats displayed a marked mechanical hyperalgesia (EMS, endometriosis pain model). In these rats, a single local injection of the selective inhibitor of JAK2 (AG490), but not vehicle (Veh), produced an attenuation of mechanical hyperalgesia between 1 to 24 h. after injection; ***P < 0.001.
Discussion
Leptin is a well-recognized pro-inflammatory and mitogenic agent (Lago et al., 2008, Styer et al., 2008), which plays a pivotal role in the establishment and viability of endometriosis lesions (Styer et al., 2008). Its role in endometriosis pain, however, has not been explored.
Raised leptin levels in endometriosis lesions
Consistent with our previous report, rats implanted with autologous uterus on the gastrocnemius muscle developed endometriotic-like cystic lesions and long-lasting primary mechanical hyperalgesia (Alvarez et al., 2012). We now also report that, compared to eutopic uterine tissue, endometriotic-like cystic lesions exhibit increased levels of leptin mRNA and protein. While leptin mRNA is barely detectable and leptin protein is absent in eutopic endometrium (Kitawaki et al., 2000, Wu et al., 2002), leptin mRNA and protein levels are markedly increased in endometriosis lesions (Wu et al., 2002). Furthermore, leptin protein levels are increased in peritoneal fluid of endometriosis patients (Matarese et al., 2000, De Placido et al., 2001, Mahutte et al., 2003, Bedaiwy et al., 2006, Gungor et al., 2009). Indeed, leptin mRNA and protein levels are extremely low in eutopic endometrium of both healthy controls and endometriosis patients (Lima-Couy et al., 2004). On the other hand, mice lacking functional Ob-R fail to develop endometriosis-like lesions, and preventive treatment with an Ob-R antagonist inhibits lesion establishment and development (Styer et al., 2008). Our results are consistent with these observations and indicate that implant of uterine tissue in gastrocnemius muscle induces local synthesis and release of leptin from endometriosis-like lesions. Of note, the increased levels of leptin in peritoneal fluid of endometriosis patients are not necessarily associated with an increase in plasma levels of leptin (Vigano et al., 2002, Bedaiwy et al., 2006, Gungor et al., 2009), suggesting that the main contribution of leptin to endometriosis pain is due to a local effect.
Leptin produces persistent hyperalgesia and nociceptor sensitization
Since Ob-R expression has been observed in sensory neurons from the DRG (Chen et al., 2006) and nodose ganglia (Buyse et al., 2001, Burdyga et al., 2002), we evaluated the effects of a single local leptin injection on mechanical nociceptive threshold and in vivo electrophysiology experiments. Intramuscular leptin produced persistent mechanical hyperalgesia, consistent with previous studies showing that local release of leptin after sciatic nerve injury is able to induce mechanical allodynia (Maeda et al., 2009). Importantly, the mechanical hyperalgesia observed in the present experiments was inhibited by ovariectomy and re-established by estrogen administration, indicating estrogen dependency in leptin-induced hyperalgesia. This leptin-estrogen interaction might be explained by the estrogen regulation of Ob-R expression in DRG neurons, mediated by the estrogen receptor α (Chen et al., 2006). Such co-expression of estrogen and leptin receptors is also observed in the hypothalamus (Del Bianco-Borges et al., 2010), where estrogen also modulates Ob-R expression (Meli et al., 2004) and sensitivity to leptin (Ainslie et al., 2001).
Local leptin injection also produced persistent nociceptor sensitization to mechanical stimulation, suggesting that leptin can sensitize nociceptors. This is consistent with studies reporting leptin-induced sensitization to mechanical stimuli in sensory neurons from the nodose ganglia innervating the gastrointestinal tract (Li et al., 2011) and increased firing rate in nerve fibres of sensory neurons innervating white adipose tissue (Niijima, 1998, Murphy et al., 2013).
Besides its direct effects on nociceptors, indirect effects of leptin might also contribute to hyperalgesia displayed by rats with the endometriosis pain model. For instance, stromal endometrial cells treated with leptin increase their production of monocyte chemoattractant protein 1 and interleukin-6 (Fukuda et al., 2003), two well-established pro-nociceptive mediators (Dina et al., 2008, Bogen et al., 2009). Leptin also produces the expression of inducible nitric oxide synthase and matrix metalloproteinase-9 in cultured macrophages (Maeda et al., 2009), which are also important pro-nociceptive agents in persistent pain. Thus, the local effect of leptin is probably due to a combination of actions over neuronal and non-neuronal elements present in the endometriosis lesion.
Leptin receptor antagonism or inhibition of its signalling pathway blocks hyperalgesia
Intralesional administration of a pegylated leptin receptor antagonist produced a marked and persistent inhibition of mechanical hyperalgesia. Since it was produced by local administration –contralateral injection was devoid of any effect on mechanical hyperalgesia– and the maximal effect of this antagonist was observed 1 day after injection, its effect is probably due to an action on nociceptors in the lesion and/or to inhibition of the action of leptin on non-neuronal cells present in ectopic endometrial lesions. Pegylated leptin peptide receptor antagonists have also been used to inhibit leptin-dependent control of appetite/energy expenditure (Elinav et al., 2009) and to inhibit the establishment and viability of endometriosis-like lesions (Styer et al., 2008). However, we did not observe changes in body weight after repeated injections of leptin antagonist, further supporting the suggestion that the doses used in the present experiments the effects observed are related to a local action in the endometrial lesion. The antinociceptive effects observed here were fully reversed 5 days after last antagonist injection, suggesting that viability of endometriosis lesions was not compromised by such intralesional treatment.
The inhibition of JAK2/STAT3, a leptin receptor intracellular signalling pathway, also produced a persistent inhibition of mechanical hyperalgesia in rats submitted to the endometriosis model. Of note, previous studies using AG 490 have shown attenuation of nociceptive responses induced by exogenous leptin administration (Lim et al., 2009, Tian et al., 2011) or by nerve injury-induced local release of leptin (Maeda et al., 2009), indicating that the JAK2/STAT3 signalling pathway might play a role in the induction of nociceptive responses by leptin. While leptin effects have been shown to involve other signalling pathways (Myers et al., 2008, Tian et al., 2011, Oh et al., 2013), whether they play a role in endometriosis-induced pain remains to be determined.
In summary, the present results support the hypothesis that leptin released from endometriosis lesions acts locally to produce mechanical hyperalgesia. The pro-algesic effect of leptin is dependent on estrogen levels. This suggests that therapeutic interventions targeting leptin actions and its dependence on estrogen might be useful for the treatment of endometriosis pain.
Highlights.
Ectopic uterine tissue implants induce endometriosis-like lesions and pain in rats.
Leptin released from endometriosis lesions produces primary mechanical hyperalgesia.
Intralesional blocking of leptin receptor, or its signaling, inhibits endometriosis pain.
The pro-algesic effect of leptin is estrogen-dependent.
Targeting leptin actions may be useful for the treatment of endometriosis pain.
Acknowledgements
Authors thank D. Mendoza for excellent technical assistance. This work was supported by N.I.H. (grants AR063312, AR048821).
Abbreviations
- BSA
bovine serum albumin
- D-PBS
Dulbecco's phosphate buffered saline
- JAK2
Janus kinase2
- Ob-R
leptin receptor
- RT-PCR
reverse transcriptase polymerase chain reaction
- STAT3
signal transducer and activator of transcription 3
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: P.A., O.B. and J.D.L. designed experiments; P.A., O.B. and X.C. performed experiments; P.A., O.B. and X.C. performed statistical analyses; P.A., O.B., L.C.G. and J.D.L. wrote the paper.
None of the authors have conflict of interest.
References
- Ainslie DA, Morris MJ, Wittert G, Turnbull H, Proietto J, Thorburn AW. Estrogen deficiency causes central leptin insensitivity and increased hypothalamic neuropeptide Y. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25:1680–1688. doi: 10.1038/sj.ijo.0801806. [DOI] [PubMed] [Google Scholar]
- Alvarez P, Chen X, Hendrich J, Irwin JC, Green PG, Giudice LC, Levine JD. Ectopic uterine tissue as a chronic pain generator. Neuroscience. 2012;225:269–282. doi: 10.1016/j.neuroscience.2012.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Levine JD, Green PG. Eccentric exercise induces chronic alterations in musculoskeletal nociception in the rat. The European journal of neuroscience. 2010;32:819–825. doi: 10.1111/j.1460-9568.2010.07359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babaei A, Zarkesh-Esfahani SH, Bahrami E, Ross RJ. Restricted leptin antagonism as a therapeutic approach to treatment of autoimmune diseases. Hormones (Athens) 2011;10:16–26. doi: 10.14310/horm.2002.1289. [DOI] [PubMed] [Google Scholar]
- Barcz E, Milewski L, Radomski D, Dziunycz P, Kaminski P, Roszkowski PI, Malejczyk J. A relationship between increased peritoneal leptin levels and infertility in endometriosis. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2008;24:526–530. doi: 10.1080/09513590802288200. [DOI] [PubMed] [Google Scholar]
- Bedaiwy MA, Falcone T, Goldberg JM, Sharma RK, Nelson DR, Agarwal A. Peritoneal fluid leptin is associated with chronic pelvic pain but not infertility in endometriosis patients. Hum Reprod. 2006;21:788–791. doi: 10.1093/humrep/dei376. [DOI] [PubMed] [Google Scholar]
- Bogen O, Dina OA, Gear RW, Levine JD. Dependence of monocyte chemoattractant protein 1 induced hyperalgesia on the isolectin B4-binding protein versican. Neuroscience. 2009;159:780–786. doi: 10.1016/j.neuroscience.2008.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdyga G, Spiller D, Morris R, Lal S, Thompson DG, Saeed S, Dimaline R, Varro A, Dockray GJ. Expression of the leptin receptor in rat and human nodose ganglion neurones. Neuroscience. 2002;109:339–347. doi: 10.1016/s0306-4522(01)00474-2. [DOI] [PubMed] [Google Scholar]
- Buyse M, Ovesjo ML, Goiot H, Guilmeau S, Peranzi G, Moizo L, Walker F, Lewin MJ, Meister B, Bado A. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. The European journal of neuroscience. 2001;14:64–72. doi: 10.1046/j.0953-816x.2001.01628.x. [DOI] [PubMed] [Google Scholar]
- Chen HP, Fan J, Cui S. Detection and estrogen regulation of leptin receptor expression in rat dorsal root ganglion. Histochemistry and cell biology. 2006;126:363–369. doi: 10.1007/s00418-006-0170-9. [DOI] [PubMed] [Google Scholar]
- De Placido G, Alviggi C, Carravetta C, Pisaturo ML, Sanna V, Wilding M, Lord GM, Matarese G. The peritoneal fluid concentration of leptin is increased in women with peritoneal but not ovarian endometriosis. Hum Reprod. 2001;16:1251–1254. doi: 10.1093/humrep/16.6.1251. [DOI] [PubMed] [Google Scholar]
- Del Bianco-Borges B, Cabral FJ, Franci CR. Co-expression of leptin and oestrogen receptors in the preoptic-hypothalamic area. Journal of neuroendocrinology. 2010;22:996–1003. doi: 10.1111/j.1365-2826.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- Diehl B, Hoheisel U, Mense S. The influence of mechanical stimuli and of acetylsalicylic acid on the discharges of slowly conducting afferent units from normal and inflamed muscle in the rat. Experimental brain research Experimentelle Hirnforschung Experimentation cerebrale. 1993;92:431–440. doi: 10.1007/BF00229031. [DOI] [PubMed] [Google Scholar]
- Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinav E, Niv-Spector L, Katz M, Price TO, Ali M, Yacobovitz M, Solomon G, Reicher S, Lynch JL, Halpern Z, Banks WA, Gertler A. Pegylated leptin antagonist is a potent orexigenic agent: preparation and mechanism of activity. Endocrinology. 2009;150:3083–3091. doi: 10.1210/en.2008-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Fukuda J, Nasu K, Sun B, Shang S, Kawano Y, Miyakawa I. Effects of leptin on the production of cytokines by cultured human endometrial stromal and epithelial cells. Fertility and sterility. 2003;80(Suppl 2):783–787. doi: 10.1016/s0015-0282(03)00776-3. [DOI] [PubMed] [Google Scholar]
- Giudice LC. Clinical practice. Endometriosis. The New England journal of medicine. 2010;362:2389–2398. doi: 10.1056/NEJMcp1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gungor T, Kanat-Pektas M, Karayalcin R, Mollamahmutoglu L. Peritoneal fluid and serum leptin concentrations in women with primary infertility. Archives of gynecology and obstetrics. 2009;279:361–364. doi: 10.1007/s00404-008-0744-y. [DOI] [PubMed] [Google Scholar]
- Kitawaki J, Koshiba H, Ishihara H, Kusuki I, Tsukamoto K, Honjo H. Expression of leptin receptor in human endometrium and fluctuation during the menstrual cycle. The Journal of clinical endocrinology and metabolism. 2000;85:1946–1950. doi: 10.1210/jcem.85.5.6567. [DOI] [PubMed] [Google Scholar]
- Lago R, Gomez R, Lago F, Gomez-Reino J, Gualillo O. Leptin beyond body weight regulation--current concepts concerning its role in immune function and inflammation. Cellular immunology. 2008;252:139–145. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu X, Zhou S, Owyang C. Low-affinity CCK-A receptors are coexpressed with leptin receptors in rat nodose ganglia: implications for leptin as a regulator of short-term satiety. American journal of physiology Gastrointestinal and liver physiology. 2011;300:G217–227. doi: 10.1152/ajpgi.00356.2010. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lim G, Wang S, Zhang Y, Tian Y, Mao J. Spinal leptin contributes to the pathogenesis of neuropathic pain in rodents. The Journal of clinical investigation. 2009;119:295–304. doi: 10.1172/JCI36785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima-Couy I, Cervero A, Bonilla-Musoles F, Pellicer A, Simon C. Endometrial leptin and leptin receptor expression in women with severe/moderate endometriosis. Molecular human reproduction. 2004;10:777–782. doi: 10.1093/molehr/gah115. [DOI] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Kobayashi Y, Ikuta T, Ozaki M, Kishioka S. Leptin derived from adipocytes in injured peripheral nerves facilitates development of neuropathic pain via macrophage stimulation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13076–13081. doi: 10.1073/pnas.0903524106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahutte NG, Matalliotakis IM, Goumenou AG, Vassiliadis S, Koumantakis GE, Arici A. Inverse correlation between peritoneal fluid leptin concentrations and the extent of endometriosis. Hum Reprod. 2003;18:1205–1209. doi: 10.1093/humrep/deg233. [DOI] [PubMed] [Google Scholar]
- Matarese G, Alviggi C, Sanna V, Howard JK, Lord GM, Carravetta C, Fontana S, Lechler RI, Bloom SR, De Placido G. Increased leptin levels in serum and peritoneal fluid of patients with pelvic endometriosis. The Journal of clinical endocrinology and metabolism. 2000;85:2483–2487. doi: 10.1210/jcem.85.7.6703. [DOI] [PubMed] [Google Scholar]
- Meli R, Pacilio M, Raso GM, Esposito E, Coppola A, Nasti A, Di Carlo C, Nappi C, Di Carlo R. Estrogen and raloxifene modulate leptin and its receptor in hypothalamus and adipose tissue from ovariectomized rats. Endocrinology. 2004;145:3115–3121. doi: 10.1210/en.2004-0129. [DOI] [PubMed] [Google Scholar]
- Murphy KT, Schwartz GJ, Nguyen NL, Mendez JM, Ryu V, Bartness TJ. Leptin Sensitive Sensory Nerves Innervate White Fat. American journal of physiology Endocrinology and metabolism. 2013 doi: 10.1152/ajpendo.00021.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annual review of physiology. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Niijima A. Afferent signals from leptin sensors in the white adipose tissue of the epididymis, and their reflex effect in the rat. Journal of the autonomic nervous system. 1998;73:19–25. doi: 10.1016/s0165-1838(98)00109-x. [DOI] [PubMed] [Google Scholar]
- Oh HK, Choi YS, Yang YI, Kim JH, Leung PC, Choi JH. Leptin receptor is induced in endometriosis and leptin stimulates the growth of endometriotic epithelial cells through the JAK2/STAT3 and ERK pathways. Molecular human reproduction. 2013;19:160–168. doi: 10.1093/molehr/gas055. [DOI] [PubMed] [Google Scholar]
- Styer AK, Sullivan BT, Puder M, Arsenault D, Petrozza JC, Serikawa T, Chang S, Hasan T, Gonzalez RR, Rueda BR. Ablation of leptin signaling disrupts the establishment, development, and maintenance of endometriosis-like lesions in a murine model. Endocrinology. 2008;149:506–514. doi: 10.1210/en.2007-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Wang S, Ma Y, Lim G, Kim H, Mao J. Leptin enhances NMDA-induced spinal excitation in rats: A functional link between adipocytokine and neuropathic pain. Pain. 2011;152:1263–1271. doi: 10.1016/j.pain.2011.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano P, Somigliana E, Matrone R, Dubini A, Barron C, Vignali M, di Blasio AM. Serum leptin concentrations in endometriosis. The Journal of clinical endocrinology and metabolism. 2002;87:1085–1087. doi: 10.1210/jcem.87.3.8286. [DOI] [PubMed] [Google Scholar]
- Wertel I, Gogacz M, Polak G, Jakowicki J, Kotarski J. Leptin is not involved in the pathophysiology of endometriosis-related infertility. European journal of obstetrics, gynecology, and reproductive biology. 2005;119:206–209. doi: 10.1016/j.ejogrb.2004.08.004. [DOI] [PubMed] [Google Scholar]
- Wright DE, Johnson MS, Arnett MG, Smittkamp SE, Ryals JM. Selective changes in nocifensive behavior despite normal cutaneous axon innervation in leptin receptor-null mutant (db/db) mice. Journal of the peripheral nervous system : JPNS. 2007;12:250–261. doi: 10.1111/j.1529-8027.2007.00144.x. [DOI] [PubMed] [Google Scholar]
- Wu MH, Chen KF, Lin SC, Lgu CW, Tsai SJ. Aberrant expression of leptin in human endometriotic stromal cells is induced by elevated levels of hypoxia inducible factor-1alpha. The American journal of pathology. 2007;170:590–598. doi: 10.2353/ajpath.2007.060477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MH, Chuang PC, Chen HM, Lin CC, Tsai SJ. Increased leptin expression in endometriosis cells is associated with endometrial stromal cell proliferation and leptin gene up-regulation. Molecular human reproduction. 2002;8:456–464. doi: 10.1093/molehr/8.5.456. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]