Abstract
We previously demonstrated that the severe cytokine storm and pathology associated with RSV infection following intramuscular vaccination of cotton rats with FI-RSV Lot 100 could be completely abolished by formulating the vaccine with the mild TLR4 agonist and adjuvant, monophosphoryl lipid A (MPL). Despite this significant improvement, the vaccine failed to blunt viral replication in the lungs. Since MPL is a weak TLR4 agonist, we hypothesized that its adjuvant activity was mediated by modulating the innate immune response of respiratory tract resident macrophages. Therefore, we developed a new vaccine preparation with purified, baculovirus expressed, partially purified, anchorless RSV F protein formulated with synthetic MPL that was administered to cotton rats intranasally, followed by an intradermal boost. This novel formulation and heterologous “prime/boost” route of administration resulted in decreased viral titers compared to that seen in animals vaccinated with F protein alone. Furthermore, animals vaccinated by this route showed no evidence of enhanced lung pathology upon RSV infection. This indicates that MPL acts as an immune modulator that protects the host from vaccine-enhanced pathology, and reduces RSV replication in the lower respiratory tract when administered by a heterologous prime/boost immunization regimen.
INTRODUCTION
RSV, the most significant cause of serious lower respiratory tract infection in infants and young children [1], results in 75,000–125,000 hospitalizations [2] and ~500 deaths yearly in the USA [3]. RSV has more recently been identified as an increasing cause of morbidity and mortality in the elderly [4, 5], transplant patients, and immunodeficiency disease patients [6, 7]. RSV is relatively stable antigenically, yet most adults are re-infected every few years, suggesting that natural immunity is not long-lasting [8]. Since children, the elderly, and the immunosuppressed are at a much higher risk for severe disease, an immune pathological mechanism has long been suspected. In children, severe RSV disease is often associated with prematurity, bronchopulmonary dysplasia (BPD), or congenital heart disease (CHD) [9]. In addition, RSV infection in early infancy has been correlated with development of allergic and asthmatic symptoms later in life [10]. In the elderly, infections can be severe and are mostly associated with a debilitated immune system [4, 5]. Unquestionably, the two passively administered antibodies licensed for use, RespiGam® and Synagis®, provide significant prophylactic protection to high-risk infants [11, 12]. However, due to the high cost of antibody prophylaxis, the U. S. A. is the only country that routinely administers this drug to high-risk infants. Therefore, in the absence of a safe, effective vaccine, sanitary education on avoiding RSV is the only option for reducing infection in healthy infants, children, the elderly, and immunosuppressed populations with the majority of RSV-related hospitalizations [13].
The idea that the immune response plays an adverse role in RSV-induced disease is based largely on the observation that, in early clinical trials, vaccination of infants with formalin-inactivated RSV (FI-RSV) resulted in enhanced susceptibility to develop severe lower respiratory tract involvement upon subsequent RSV infection [14–17]. Subsequent studies have shown that purified RSV Fusion (F) protein administered by the intramuscular (i.m.) route also leads to vaccine-enhanced disease [18, 19].
Monophosphoryl lipid A (MPL) is a synthetic lipid A analog that is a weak Toll-like receptor 4 (TLR4) agonist [20, 21]. MPL is licensed for two vaccines [22], and has demonstrated a safe profile when co-administered with different RSV vaccine preparations [23, 24]. In fact, we previously reported that immunization of cotton rats with the original Lot 100 FI-RSV, formulated with MPL, blunted both the cytokine storm and the enhanced lung pathology elicited by subsequent RSV infection, but did not inhibit viral replication in the lungs [25]. However, these preparations were administered intramuscularly (i.m.). To improve the efficacy of these formulations, we engineered a recombinant, anchorless RSV F protein that we partially purified and formulated it with MPL. This new formulation was tested by combining different routes of administration (e.g., intranasal, i.n.; and intradermal, i.d). Our results indicate that intranasal vaccination followed by an intradermal boost enhances protection against RSV challenge, maintaining a safe profile of lung histology and reduced lung virus titers in the cotton rat model.
MATERIALS AND METHODS
Animals
Inbred Sigmodon hispidus cotton rats were obtained from a colony maintained at Sigmovir Biosystems, Inc. (Rockville, MD). Four-eight week-old animals were used for all experiments. Animals were housed in large polycarbonate cages and were fed a standard diet of rodent chow and water ad libitum. The colony was monitored for antibodies to paramyxoviruses and rodent viruses, and no such antibodies were found. All studies were conducted under applicable laws and guidelines and after approval from the Sigmovir Biosystems, Inc. Institutional Animal Care and Use Committee.
Vaccine formulations
FI-RSV vaccine Lot 100 preparation was produced in the mid-1960s by Pfizer, Inc. for the National Institutes of Health under contract PH43-63-582 and was previously described. Mock vaccination was performed intramuscularly with PBS. Monophosphoryl lipid A (synthetic, MPL) was obtained from Avanti Polar Lipids Inc (Cat.# 699800P, lot# SMPLA-12). A 2 mg/ml stock was made in saline/0.2% triethylamine. The anchorless F protein gene sequence (F protein of RSV A/Long GenBank Accession #FJ614815) lacks the 25 amino acids at the N terminus encompassing the F protein signal sequence, and the last 49 amino acids at the C-terminus that include the transmembrane and the intracellular domain. In the N-terminal end, a bombyxin signal sequence, and in C-terminal end, an enterokinase cleavage site, and HISx6 sequence were included to facilitate processing and purification of the protein. The anchorless F protein was produced by Chesapeake PERL (Savage, MD) using a recombinant baculovirus system and infection in insect larvae. Protein was obtained using a one-step purification on a Ni-sepharose column. The purity of RSV F protein in the preparation was estimated to be ≥50% based on Coomassie staining and Western blot analyses (data not shown). Recombinant F protein was used at a concentration 10 μg/ml, alone or formulated with different concentrations of MPL. MPL was admixed with F protein before vaccination.
Viruses and viral assays
The prototype Long strain of RSV was obtained from American Type Culture Collection (ATCC VR-26, Manassas, VA). Virus was propagated in HEp-2 cells and serially plaque-purified to reduce defective-interfering particles. The single pool of virus containing 107.6 pfu/ml was used for all experiments. Viral titers in the lungs of RSV-infected animals were determined as described elsewhere [26].
Histopathology
Lungs were prepared for histologic analysis as previously described [27]. Each lung section was analyzed and scored blindly for one of the four parameters of pulmonary inflammatory changes: peribronchiolitis (inflammatory cells surrounding a bronchiole), perivasculitis (inflammatory cells surrounding a small blood vessel), alveolitis (inflammatory cells within alveolar spaces), and interstitial pneumonitis (increased thickness of alveolar walls associated with inflammatory cells).
Experimental design
The first set of experiments was designed to determine whether vaccination with MPL given i.n. was able to enhance the protection generated by vaccination with recombinant F protein against RSV challenge. A control group of cotton rats was immunized i.n. with 100 μl of PBS, pH 7.4, on days 0 and 21. The experimental groups of animals were vaccinated on the same days i.n. with either 100 μl of anchorless RSV F protein (1.25 μg/animal; F i.n/i.n) or RSV F protein admixed with MPL (F=1.25 μg/animal, MPL= 5 μg/animal; F+MPL i.n/i.n). Immunizations and challenge were performed under light isoflurane anesthesia. Animals were sacrificed by CO2 inhalation at 5 days post-infection (5 animals per group). Challenge of all animals was performed using a dose of RSV/A Long of 105.5 pfu/animal. The lungs were removed from the thorax. The right lung was inflated with 10% formalin for histopathology. The nose and the upper lobe of the left lung were dissected for determination of viral titers.
The second set of experiments was designed to evaluate the effect of the route of immunization during F+MPL immunizations. Two experimental groups corresponding to animals that were immunized (day 0) and boosted (day 21) with F+MPL, i.n. (group F+MPL i.n./i.n.), and animals that were immunized i.n. and boosted with the same preparation i.d. (F+MPL i.n./i.d.) were compared. Two control groups were included: the first group remained unvaccinated until challenge (primary RSV infection), whereas the second group was infected on day 0 with RSV i.n. (secondary infection control). On day 49, all groups were challenged with 1×106 pfu RSV A Long per animal i.n. Animals were sacrificed at day 5 after challenge (5 animals per group). Lungs and noses were removed and processed as described above.
In a third set of experiments, vaccination with F+MPL using the i.n/i.d. routes of administration was compared to vaccination with the original FI-RSV Lot 100 vaccine diluted 1:125 and given i.m [25]. MPL at two concentrations (15 or 50 μg/animal) was co-administered with recombinant F protein. Two additional control groups were included for comparison: one corresponding to naïve animals and another corresponding to animals that were previously infected and immune. After RSV challenge of all animals (day 48), analysis was performed at day 1 (peak expression of several cytokines), day 4 (peak viral replication), and day 6 (peak of lung histopathology).
RNA isolation and RT-PCR analysis
Lung tissue (lingular lobe) was flash-frozen in liquid nitrogen and homogenized in 0.5 ml RLT-buffer (Qiagen) with β-mercaptoethanol using a TissueLyzer LT (two 5 mm beads per sample; 50 Hz; 2 min, Qiagen). Lung RNA was isolated using a RNeasy kit (Qiagen, cat#74106). Reverse transcription was performed using a SuperScript II Reverse Transcriptase (Invitrogen SSIIRT, cat #18064-071). cDNA was diluted in water to give ratio 1 μg RNA of the original RNA per 100 μl final volume. Three μl of cDNA per reaction were used for real-time PCR using iQ SybrGreen Supermix (BioRad cat#170-8882). All reactions were done in duplicate. Primers and conditions for MX-2 gene expression were described previously [28]. mRNA expression was normalized to β-actin as a housekeeping gene. Primers for β-actin in this study were: 5'-CCCATTGAACACGGCATTGTC-3' (forward) and 5'-TGTCACGCACGATTTCCCTCTC-3' (reverse).
Statistical Analysis
Viral titers were calculated as geometric means ± standard errors for all animals in a group at a given time post-infection. Pulmonary scores were expressed as the arithmetic mean ± standard error for all animals in a group. Student t-test was applied to determine statistically significant differences between two groups, using an unpaired, two-tailed test.
RESULTS
Baculovirus-derived, anchorless F protein (F) was formulated without or with the TLR4 agonist adjuvant, MPL. Cotton rats were vaccinated i.n. with F protein alone or F plus MPL on days 0 and 14. Fourteen days later (28 days after the first vaccination), animals were challenged with RSV i.n. (1×105.5 pfu) and sacrificed 5 days after infection to quantify viral replication in the nose and in the lung and to determine the extent of lung histopathology. Animals vaccinated with recombinant F protein alone via the intranasal route exhibited some lung protection against subsequent RSV challenge, compared with mock-vaccinated and RSV-challenged animals. However, animals vaccinated with F protein formulated with MPL showed enhanced protection as evidenced by reduced virus titers in the lung, indicating that MPL can enhance immunity to RSV F when administered i.n. (Figure 1). While the same trend was observed for the nose, neither of these preparations resulted in a statistically significant reduction of virus titers in the nose.
Figure 1.
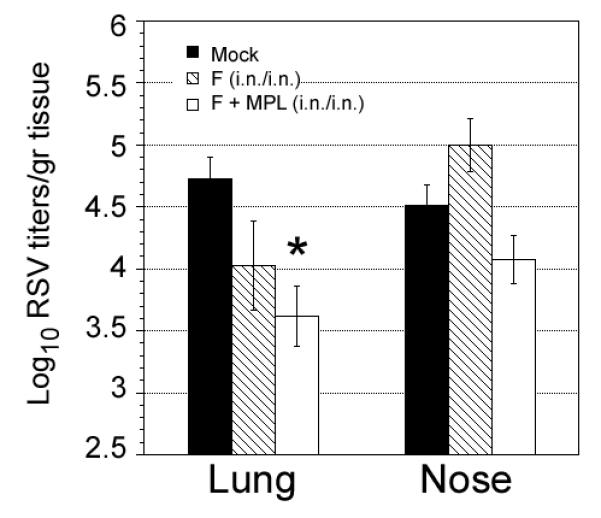
Vaccination of cotton rats intranasally with anchorless F protein formulated with monophosphoryl lipid A (MPL) boosts protection against RSV infection. Cotton rats (n=5/group) were PBS-vaccinated (Mock) or vaccinated with 1.25 mg of baculovirus-expressed anchorless RSV F protein formulated with or without MPL (5 μg/rat) on day 0 and day 14. All animals were challenged on day 28 with RSV/A Long (105.5 pfu, i.n) and sacrificed on day 5 p.i. Log10 of RSV lung and nose titers are presented. Group marked with *represents p<0.01 when compared with the mock-vaccinated group.
To study further the effect of the route of immunization with the anchorless F protein formulated with MPL, cotton rats were initially vaccinated by the i.n. route using F protein adjuvanted with MPL. Three weeks later, animals were boosted with the same preparation of F+MPL, but this time, one group received a second vaccination i.n., whereas the second group received vaccination intradermally (i.d.). Animals vaccinated i.n. and boosted i.d. showed enhanced protection and reduced lung histopathology when compared with animals vaccinated twice by the i.n. route (Figure 2). A control group vaccinated with the F protein without MPL i.n., followed by an i.d. boost was included to determine the value of adding MPL to the vaccine preparation. When compared to unvaccinated controls, the reduction in viral titers in animals vaccinated in this fashion was negligible for the nose and 0.2 Log10 for the lungs.
Figure 2.
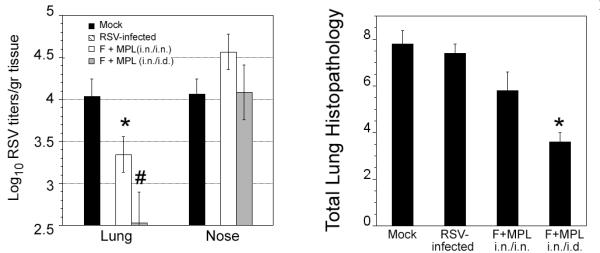
Vaccination with RSV F protein formulated with MPL enhanced protection when the booster vaccination is administered by the i.d. route. Cotton rats (n=5/group) were vaccinated i.n with mock vaccine (100 μl of PBS); infected with RSV/A long; or vaccinated with RSV F+MPL i.n. on day 0. A second immunization (boost) was performed with the same preparations on day 21, but in the case of F+MPL vaccine, was given i.n. or i.d. On day 49, all animals were challenged with RSV/A Long i.n. and sacrificed on day 5 to determine lung and nose viral titers (A). * represent p<0.05 and represents p<0.01 when compared with the mock-vaccinated group. Total histopathology score was compared in animals vaccinated with F-MPL i.n. or i.d. (B) Total histopathology scores are combined scores for peribronchiolitis, perivasculitis, interstitial pneumonia, and alveolitis. * represents p<0.001 when compared with the mock-vaccinated group.
To confirm and extend the finding of improved performance of the F protein vaccine formulated with MPL and given first by the i.n. route, followed by an i.d. boost, we performed a vaccination study with 2 different doses MPL (15 and 50 μg/animal). The generation of neutralizing antibodies and lung cytokine expression, as well as the kinetics of viral replication and lung histopathology, were analyzed. For this experiment, three control groups were included: one group was left uninfected (naïve animals) to determine basal levels of cytokine expression and lung histopathology. Another control group was infected i.n. with RSV at the time of the first immunization. These immune animals have high neutralizing antibody titers and are protected against subsequent RSV challenge. Animals vaccinated with FI-RSV i.m. were also included as control for vaccine-enhanced lung histopathology and cytokine storm. Vaccination with F+ MPL resulted in the generation of detectable anti-RSV neutralizing antibody (Figure 3A). Animals from each experimental group were sacrificed on day 1, day 4, and day 6 post-infection. Animals vaccinated with F+MPL showed significant reduction of viral titers on day 4 post-infection (Figure 3B). The extent of reduction was 1 Log10 when measured at the peak of viral titers and all the groups showed no significant viral replication in the lung on day 6 post-infection (p.i.)(data not shown). When RSV gene expression was monitored by qPCR, viral gene expression was significantly reduced in vaccinated animals compared to mock-treated control group (Figure 3C). Most importantly, all groups vaccinated i.n. and boosted i.d. with the anchorless recombinant RSV F protein formulated with MPL showed a statistically significant decrease in viral-induced interstitial pneumonitis and alveolitis that was reduced to levels below those found in infected, naïve animals and comparable to the levels found in immune animals (Figure 3D).
Figure 3.
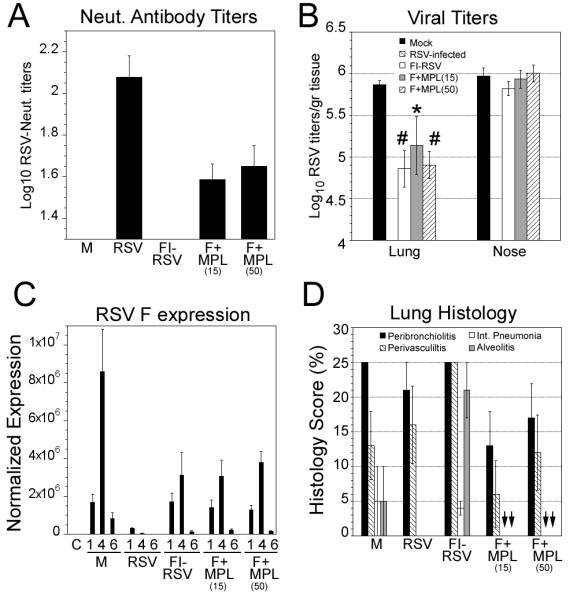
F protein vaccine formulated with MPL increased neutralizing antibody titers against RSV, and reduced lung viral replication and histopathology. Cotton rats were vaccinated with F+MPL (at 15 and 50 μg/rat) i.n., followed by i.d. boosting 21 days later. Control animals were PBS-vaccinated (M, naïve animals), or infected with RSV i.n. (RSV). An additional control was included that corresponded to animals vaccinated twice i.m. with the FI-RSV vaccine Lot 100 in a dilution of 1:125. All animals were challenged with RSV/A Long 48 days after the first vaccination. (A) RSV neutralization antibody titers from sera obtained before challenge (day 48). (B) Viral titers of lung and nose obtained 4 days post-challenge (peak viral replication). (C) RSV F protein gene expression in lungs of animals challenged on day 48 with RSV/A Long. Significant differences in the expression are detected on day 4 and day 6 post challenge. (D) Lung histopathology scores on day 6 post-RSV challenge (peak lung pathology). Scores of interstitial pneumonia and alveolitis are blunted in animals vaccinated with F+MPL (arrows).
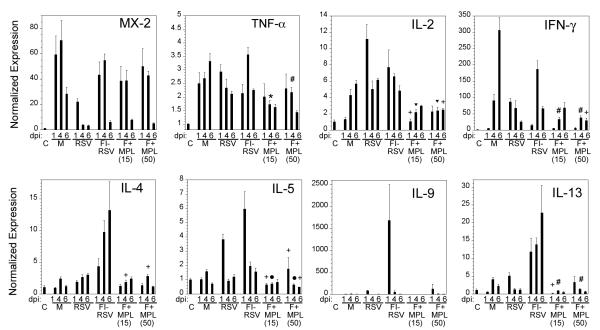
Lung cytokine expression was previously used to determine the anti-inflammatory effect of MPL in FI-RSV vaccine preparations following RSV infection [25, 29] and was used in this work to evaluate the effect of MPL addition to the F protein-based vaccine. Strong type I interferon-activated Mx-2 expression was seen in animals infected after mock or vaccine immunizations, but not in re-challenged cotton rats (Figure 4). This indicates that MPL does not restrict expression of important antiviral genes involved in the type I interferon response and previously shown to be activated by RSV infection [28]. However, as previously shown [25], inclusion of MPL into the vaccine strongly repressed expression of Th2 cytokines. Expression of IL-4, IL-5, IL-9, and IL-13 was strongest in animals vaccinated with FI-RSV Lot 100. Importantly, animals vaccinated with F protein and MPL showed very low expression of these cytokines (i.e., IL-4, IL-5, and IL-13 showed statistically significant reduction, whereas, in the case of IL-9, it was strongly reduced in 4 of 5 animals tested), correlating with the observed strong reduction in lung pathology. The only exception was the expression of IL-10, which was increased in naïve, RSV-challenged animals on day 6 post-infection compared to F+MPL-vaccinated, RSV-challenged animals (data not shown). Levels of pro-inflammatory gene expression (IL-2, IFN-γ, and TNF-α) were also significantly reduced in F+MPL-vaccinated animals.
Figure 4.
Cytokine gene expression induced by RSV infection of animals vaccinated with FI-RSV is blunted in animals immunized with F+MPL i.n/i.d. Cytokine expression in the lungs of infected, re-infected, and vaccinated groups at days 1, 4, and 6 post-RSV challenged. Significant reduction of Th1 (TNF-α, IL-2, IFN-γ) and Th2 (IL-4, IL-5, and IL-13) cytokine expression is achieved by MPL formulation of the RSV F protein vaccine. Student t-test comparisons were performed between F+MPL and FI-RSV groups. Significance is indicated as:  p<0.0001, *, p<0.001; #, p<0.005; •, p,0.01; +, p<0.05.
p<0.0001, *, p<0.001; #, p<0.005; •, p,0.01; +, p<0.05.
Overall, our data demonstrate that the anchorless F protein of RSV, formulated with MPL and given i.n./i.d., strongly enhanced immunity against RSV without increasing histopathology or inducing cytokine storm upon RSV challenge.
DISCUSSION
The results presented show that a baculovirus-expressed, anchorless F protein works as a strong antigen when formulated with monophosphoryl lipid A and administered in a heterologous prime-boost strategy, i.e., intranasally, followed by an i.d. boost. In addition, we have demonstrated that this combination of antigen and adjuvant is safe by comparing this preparation to the response of cotton rats immunized with the original FI-RSV Lot 100 vaccine that was previously tested in a human clinical trial and generated highly undesirable pathologic responses.
Previously, we reported that MPL is a strong immunomodulator that reversed the pathology of RSV-challenged, FI-RSV-vaccinated animals from one of strong pathology to either mild or non-existent pathology [25, 29]. Herein, the potential of MPL is now exploited to generate a new vaccine based on recombinant purified, anchorless F protein. Similar to what has been previously shown using the FI-RSV Lot 100 vaccine, MPL achieves a strong immunomodulatory effect by reducing Th1 and Th2 cytokine expression in the lung of challenged animals. However, in this work we show that MPL, when formulated with anchorless, recombinant F protein, enhances the protective response as evidenced by reduced lung virus titers as well. While there has been controversy about the role of contaminants in the FI-RSV in the induction of vaccine-enhanced disease [30], the levels of pathology induced by FI-RSV Lot 100 in the absence of MPL are consistently much greater than when formulated with MPL as an adjuvant [25]. Therefore, we have used FI-RSV immunization alone as the “standard” for vaccine-enhanced disease in this model system.
Adjuvants that signal through other TLRs have been used in combination with the F protein of RSV and administered intranasally. Mucosal immunization of cotton rats with native RSV F protein co-adjuvanted with CpG ODN (a TLR9 agonist) only resulted in modest protection from viral challenge, but did not prevent the development of enhanced pulmonary pathology [31].
Detection of virus in the lungs of infected animals by plaque assay and qRT-PCR revealed a significant reduction in virus load in RSV-challenged cotton rats vaccinated with F+MPL compared with mock-vaccinated animals, suggesting more effective clearance of the virus induced by vaccination. The observation that vaccination with F+MPL did not eliminate viral replication in the lungs completely may be the result of the low dose F used in the vaccine formulations. Protection following immunization with F subunit vaccines is typically observed with antigen doses greater than 1 μg [32–34]. Therefore, higher doses of the recombinant F may be required for complete protection when co-formulated with MPL, but also will require strict scrutiny of the effect of increased dosing on lung pathology upon challenge. These studies are currently underway.
Recently, MPL was used in the formulation of another RSV vaccine based on nucleocapsid-depleted RSV membranes (virosomes) [35]. This vaccine was tested in cotton rats and shown to be safe and protective However, the complexity of such a vaccine will likely result in obstacles related to production for use in people. For this reason, the recombinant anchorless F protein used in these studies represents a considerable advance over mixed subunit vaccines.
Previously, a recombinant, modified, RSV F protein vaccine was generated in baculovirus and tested in cotton rats [36]. Although this vaccine showed complete protection from viral replication after challenge, the i.m route of immunization will most likely find obstacles to circumvent the immune suppressive effect of anti-RSV antibodies present in infants (maternal antibodies) or in adults (natural anti-RSV antibodies). However, this study and those of others have demonstrated the role of prefusion F protein to enhanced antigenicity [37–38] and this new information could be pivotal for improving our vaccine.
Taken together, these results suggest that the co-formulation of recombinant, anchorless F protein with MPL for i.n. delivery followed by an i.d. boost, may provide the next step in the development of a safe and effective vaccine for the prevention of RSV infection, and may have significant implications for the development of a novel human RSV vaccines. One example is the enhancement of maternal immunity that can support extended protection in babies. Previous F protein-based vaccines tested during pregnancy show that only 10% of pregnant women developed a ≥4-fold rise in neutralizing titers [39], indicating that new delivery and adjuvants systems for F protein will be needed to tackle protection of babies with this strategy. The success of our vaccine in protecting cotton rats in the absence of vaccine-enhanced disease indicates that this approach may be applicable during pregnancy.
Highlights
Developed a recombinant RSV F-protein based subunit vaccine formulated with monophosphoryl lipid A (MPL).
Vaccine reduced viral replication in the lungs of cotton rats when given in a prime-boost regimen through the intranasal/intradermal route, respectively.
Animals vaccinated showed no evidence of exacerbation of lung pathology or induction of cytokine storm upon RSV challenge.
MPL acts as an adjuvant and immunomodulatory in the F-protein based vaccine
ACKNOWLEDGEMENTS
We would like to thanks to Charles Smith, and Freddy and Ana Rivera for animal care. This work has been supported by grants AI057570 and AI18797 for JCGB and SNV, respectively.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Welliver RC. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J Pediatr. 2003;143:S112. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- [2].Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-associated hospitalizations among US children, 1980–1996. Jama. 1999;282:1440. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- [3].Shay DK, Holman RC, Roosevelt GE, Clarke MJ, Anderson LJ. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J Infect Dis. 2001;183:16. doi: 10.1086/317655. [DOI] [PubMed] [Google Scholar]
- [4].Han LL, Alexander JP, Anderson LJ. Respiratory syncytial virus pneumonia among the elderly: an assessment of disease burden. J Infect Dis. 1999;179:25. doi: 10.1086/314567. [DOI] [PubMed] [Google Scholar]
- [5].Falsey AR, Walsh EE. Respiratory syncytial virus infection in elderly adults. Drugs & aging. 2005;22:577. doi: 10.2165/00002512-200522070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Harrington RD, Hooton TM, Hackman RC, Storch GA, Osborne B, Gleaves CA, et al. An outbreak of respiratory syncytial virus in a bone marrow transplant center. J Infect Dis. 1992;165:987. doi: 10.1093/infdis/165.6.987. [DOI] [PubMed] [Google Scholar]
- [7].Bowden RA. Respiratory virus infections after marrow transplant: the Fred Hutchinson Cancer Research Center experience. Am J Med. 1997;102:27. doi: 10.1016/s0002-9343(97)00007-7. [DOI] [PubMed] [Google Scholar]
- [8].Henderson FW, Collier AM, Clyde WA, Jr., Denny FW. Respiratory-syncytial-virus infections, reinfections and immunity. A prospective, longitudinal study in young children. N Engl J Med. 1979;300:530. doi: 10.1056/NEJM197903083001004. [DOI] [PubMed] [Google Scholar]
- [9].Collins PL, Crowe JE. Respiratory syncytial virus and metapneumovirus. 5th ed Lippincott-Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- [10].Sigurs N, Bjarnason R, Sigurbergsson F, Kjellman B, Bjorksten B. Asthma and immunoglobulin E antibodies after respiratory syncytial virus bronchiolitis: a prospective cohort study with matched controls. Pediatrics. 1995;95:500. [PubMed] [Google Scholar]
- [11].The Prevent-RSV Study Group Reduction of respiratory syncytial virus hospitalization among premature infants and infants with bronchopulmonary dysplasia using respiratory syncytial virus immune globulin prophylaxis. Pediatrics. 1997;99:93. doi: 10.1542/peds.99.1.93. [DOI] [PubMed] [Google Scholar]
- [12].The IMpact-RSV Study Group Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531. [PubMed] [Google Scholar]
- [13].AAP Summary of Recommendations of the AAP Committee on Infectious Diseases. AAP News. 2002;21:232. [Google Scholar]
- [14].Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- [15].Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am J Epidemiol. 1969;89:449. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- [16].Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am J Epidemiol. 1969;89:435. doi: 10.1093/oxfordjournals.aje.a120956. [DOI] [PubMed] [Google Scholar]
- [17].Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am J Epidemiol. 1969;89:405. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- [18].Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine. 1990;8:497. doi: 10.1016/0264-410x(90)90253-i. [DOI] [PubMed] [Google Scholar]
- [19].Delgado MF, Coviello S, Monsalvo AC, Melendi GA, Hernandez JZ, Batalle JP, et al. Lack of antibody affinity maturation due to poor Toll-like receptor stimulation leads to enhanced respiratory syncytial virus disease. Nat Med. 2009;15:34. doi: 10.1038/nm.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Qureshi N, Takayama K, Ribi E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J Biol Chem. 1982;257:11808. [PubMed] [Google Scholar]
- [21].Baldridge JR, Crane RT. Monophosphoryl lipid A (MPL) formulations for the next generation of vaccines. Methods. 1999;19:103. doi: 10.1006/meth.1999.0834. [DOI] [PubMed] [Google Scholar]
- [22].Rappuoli R, Mandl CW, Black S, De Gregorio E. Vaccines for the twenty-first century society. Nat Rev Immunol. 2011;11:865. doi: 10.1038/nri3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Garcon N, Segal L, Tavares F, Van Mechelen M. The safety evaluation of adjuvants during vaccine development: the AS04 experience. Vaccine. 2011;29:4453. doi: 10.1016/j.vaccine.2011.04.046. [DOI] [PubMed] [Google Scholar]
- [24].Garcon N, Van Mechelen M. Recent clinical experience with vaccines using MPL- and QS-21-containing adjuvant systems. Expert Rev Vaccines. 2011;10:471. doi: 10.1586/erv.11.29. [DOI] [PubMed] [Google Scholar]
- [25].Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JC. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine. 2006;24:5027. doi: 10.1016/j.vaccine.2006.03.064. [DOI] [PubMed] [Google Scholar]
- [26].Prince GA, Jenson AB, Horswood RL, Camargo E, Chanock RM. The pathogenesis of respiratory syncytial virus infection in cotton rats. Am J Pathol. 1978;93:771. [PMC free article] [PubMed] [Google Scholar]
- [27].Prince GA, Prieels JP, Slaoui M, Porter DD. Pulmonary lesions in primary respiratory syncytial virus infection, reinfection, and vaccine-enhanced disease in the cotton rat (Sigmodon hispidus) Lab Invest. 1999;79:1385. [PubMed] [Google Scholar]
- [28].Pletneva LM, Haller O, Porter DD, Prince GA, Blanco JC. Induction of type I interferons and interferon-inducible Mx genes during respiratory syncytial virus infection and reinfection in cotton rats. J Gen Virol. 2008;89:261. doi: 10.1099/vir.0.83294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Prince GA, Denamur F, Deschamps M, Garcon N, Prieels JP, Slaoui M, et al. Monophosphoryl lipid A adjuvant reverses a principal histologic parameter of formalin-inactivated respiratory syncytial virus vaccine-induced disease. Vaccine. 2001;19:2048. doi: 10.1016/s0264-410x(00)00417-5. [DOI] [PubMed] [Google Scholar]
- [30].Prince GA, Curtis SJ, Yim KC, Porter DD. Vaccine-enhanced respiratory syncytial virus diseasse in cotton rats following immunization with Lot 100 or a newly prepared reference vaccine. J Gen Virol. 2001;82:2881. doi: 10.1099/0022-1317-82-12-2881. [DOI] [PubMed] [Google Scholar]
- [31].Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. J Virol. 2003;77:13156. doi: 10.1128/JVI.77.24.13156-13160.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hancock GE, Hahn DJ, Speelman DJ, Hildreth SW, Pillai S, McQueen K. The pulmonary immune response of Balb/c mice vaccinated with the fusion protein of respiratory syncytial virus. Vaccine. 1994;12:267. doi: 10.1016/0264-410x(94)90204-6. [DOI] [PubMed] [Google Scholar]
- [33].Hancock GE, Speelman DJ, Frenchick PJ, Mineo-Kuhn MM, Baggs RB, Hahn DJ. Formulation of the purified fusion protein of respiratory syncytial virus with the saponin QS-21 induces protective immune responses in Balb/c mice that are similar to those generated by experimental infection. Vaccine. 1995;13:391. doi: 10.1016/0264-410x(95)98263-a. [DOI] [PubMed] [Google Scholar]
- [34].Walsh EE. Humoral, mucosal, and cellular immune response to topical immunization with a subunit respiratory syncytial virus vaccine. J Infect Dis. 1994;170:345. doi: 10.1093/infdis/170.2.345. [DOI] [PubMed] [Google Scholar]
- [35].Kamphuis T, Shafique M, Meijerhof T, Stegmann T, Wilschut J, de Haan A. Efficacy and safety of an intranasal virosomal respiratory syncytial virus vaccine adjuvanted with monophosphoryl lipid A in mice and cotton rats. Vaccine. 2013;31:2169. doi: 10.1016/j.vaccine.2013.02.043. [DOI] [PubMed] [Google Scholar]
- [36].Smith G, Raghunandan R, Wu Y, Liu Y, Massare M, Nathan M, et al. Respiratory syncytial virus fusion glycoprotein expressed in insect cells form protein nanoparticles that induce protective immunity in cotton rats. PLoS ONE. 2012;7:e50852. doi: 10.1371/journal.pone.0050852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].McLellan JS, Chen M, Leung S, Graepel KW, Du X, Yang Y, et al. Structure of RSV fusion glycoprotein trimer bound to a prefusion-specific neutralizing antibody. Science. 2013;340:1113. doi: 10.1126/science.1234914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Magro M, Mas V, Chappell K, Vazquez M, Cano O, Luque D, Terron MC, Melero JA, Palomo C. Neutralizing antibodies against the preactive form of respiratory syncytial virus fusion protein offer unique possibilities for clinical intervention. PNAS. 2012;109(8):3089. doi: 10.1073/pnas.1115941109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21:3465. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]