Summary
SMC condensin complexes play a central role in organizing and compacting chromosomes in all domains of life [1, 2]. In the bacterium Bacillus subtilis, cells lacking SMC are viable only during slow growth and display decondensed chromosomes, suggesting that SMC complexes function throughout the genome [3, 4]. Here, we show that rapid inactivation of SMC or its partner protein ScpB during fast growth leads to a failure to resolve newly replicated origins and a complete block to chromosome segregation. Importantly, the loss of origin segregation is not due to an inability to unlink pre-catenated sister chromosomes by Topoisomerase IV. In support of the idea that ParB-mediated recruitment of SMC complexes to the origin is important for their segregation, cells with reduced levels of SMC that lack ParB are severely impaired in origin resolution. Finally, we demonstrate that origin segregation is a task shared by the condensin complex and the parABS partitioning system. We propose that origin-localized SMC constrains adjacent DNA segments along their lengths, drawing replicated origins in on themselves and away from each other. This SMC-mediated lengthwise condensation, bolstered by the parABS system, drives origin segregation.
Keywords: Chromosome segregation, SMC, condensin, Topoisomerase IV, ParA
Results and Discussion
The SMC condensin complex plays a central role in chromosome condensation and segregation in all eukaryotes and virtually all bacteria [1, 2]. In vitro, these highly conserved complexes constrain DNA [5–7] but how they function to organize and segregate chromosomes in vivo remains poorly understood. In the bacterium Bacillus subtilis, the role of the SMC complex has been inferred by the phenotypes associated with loss of function mutants [3, 4, 8, 9]. Cells lacking SMC or its partner proteins ScpA and ScpB are inviable during fast growth. However, under slow growth conditions, the null mutants have decondensed chromosomes and an increased frequency of anucleate cells. These findings have lead to the idea that SMC complexes function throughout the B. subtilis chromosome. Interestingly, SMC complexes are enriched adjacent to the origin of replication in B. subtilis in a manner that depends, in part, on the partitioning protein ParB (called Spo0J) bound to its centromere-like sequences called parS [10, 11]. The functional relevance of this enrichment has remained unclear. Here, we demonstrate a critical role for origin-localized SMC in resolving and segregating origins during fast growth. We further show that during slow growth, the parABS partitioning system provides enough origin segregation activity to support chromosome segregation in the absence of SMC. Thus, ParB bound to parS serves as a central hub in origin segregation. This nucleoprotein complex recruits condensin complexes to the origin and is acted upon by ParA resulting in efficient origin resolution and segregation.
Origin segregation is lost upon rapid inactivation of condensin subunits
To investigate how SMC mutants impact chromosome organization and segregation in B. subtilis, we visualized chromosomes (called nucleoids) and replication origins in cells lacking SMC under permissive growth conditions, in which growth rates were reduced in minimal medium and/or at reduced temperature. As reported previously, a proportion of the cells lacked DNA [3, 4, 12] (Figure S1A). However, we also observed a striking heterogeneity in nucleoid morphology and cell size under all conditions tested (Figure 1A, S1A) precluding quantitative analysis of chromosome organization. To circumvent this problem, we isolated temperature sensitive mutants (Figure 1B, S1B and see Supplemental Experimental Procedures) and constructed degradable alleles [13] of smc and scpB to assess chromosome organization and segregation upon rapid inactivation of the condensin complex.
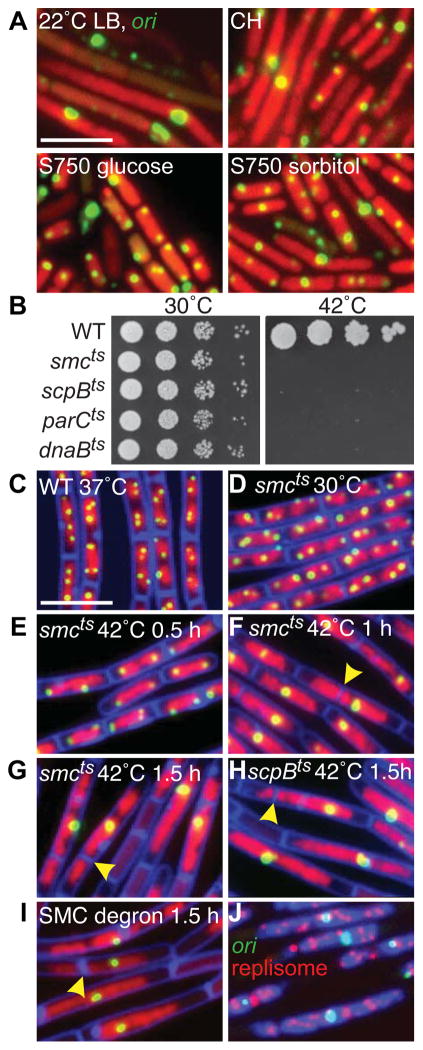
Figure 1. SMC complexes are required for origin segregation.
(A) Heterogeneous nucleoid morphologies and cell sizes in the SMC null mutant grown under permissive conditions. Representative images of Δsmc (strain BWX2208) grown at 22°C in LB, casein hydrolysates (CH), minimal medium (S750) supplemented with glucose or sorbitol. The nucleoids (red) and the origins (green) were visualized with HbsU-GFP and TetR-mCherry bound to a tetO array inserted adjacent to the origin. (B) Spot-dilutions of indicated temperature-sensitive mutants grown on LB-agar plates at permissive (30°C) and restrictive (42°C) temperatures. Representative images of DAPI-stained nucleoids (red) and origin foci (green) in wild-type cells (BWX811) grown in CH medium at 37°C (C); smcts (BWX2090) grown in CH medium at 30°C (D) and after shift to 42°C (E–G); scpBts (BWX2092) grown in CH medium for 1.5 h at 42°C (H); smc-ssrA (BWX1497) 1.5 h after induction of SspB grown in CH medium at 37°C (I). 85% (n=1273) of the smcts cells; 81% (n=1336) of the scpBts cells; and 85% (n=1392) of smc-ssrA cells had a single origin focus or cluster of foci at hour 1.5. Membranes (false-colored blue) were stained with FM4-64. Yellow carets highlight septum formation on top of the nucleoid. (J) Origin (green), replisome foci (red, DnaX-YFP), and DAPI-stained nucleoids (blue) in the smc-ssrA strain (BWX1771) 1.5 h after induction of SspB in CH medium at 37°C. Scale bars are 4 μm. Strains harboring wild-type copies of smc, scpB, or parC with a linked antibiotic resistance gene displayed normal chromosome organization and segregation when grown at 42°C (Figure S1F). See also Figure S1.
We first monitored the replication origins (using TetR-CFP bound to a tetO operator array [14]) upon rapid inactivation of a temperature-sensitive SMC mutant. Under permissive growth conditions (rich medium at 30°C, doubling time (τ) =56±2 min), the morphology of the nucleoid and the subcellular localization of the origins were similar to wild-type (Figure 1CD). At the restrictive temperature (42°C), the cells continued to grow (τ=29± 1 min) and divide for the duration of the experiment but contained larger and more extended nucleoids (Figure 1E–G). Strikingly, in most cells, a single bright origin focus or a cluster of foci was present at or near the center of these extended nucleoids. In some cases, a division septum guillotined the DNA mass (Figure 1FG). As a result of nucleoid bisection and DNA degradation [3], anucleate cells accumulated in the population. Similar phenotypes were observed using the ScpB(ts) mutant and in strains in which SMC or ScpB contained an SsrA tag and the E. coli adaptor protein SspB that targets SsrA-tagged proteins to the ClpXP proteosome was expressed [13] (Figure 1HI, S1C–E). Consistent with the idea that the elongated nucleoid was composed of multiple chromosomes and the bright origin focus was made up of several unsegregated origins, the cells contained multiple replisome foci (Figure 1J) and the origin foci at hour 1.5 had, on average, a 4–8-fold increase in fluorescence intensity compared to the cells grown at the permissive temperature. These results indicate that during rapid growth the condensin complex plays an essential role in origin individualization and segregation. Re-examination of origin foci in the SMC null mutant (Figure 1A) clearly shows that origin segregation is also impaired under permissive growth conditions.
Global chromosome segregation is blocked upon rapid inactivation of condensin
Next, we investigated the organization and segregation of loci on the left and right arms of the chromosome upon inactivation of SMC. We began by monitoring the replication origin and a locus at −87°. After shifting the cells to the non-permissive temperature, the origins localized in a single bright focus close to the middle of the nucleoid while the −87° marker formed clusters of foci (Figure 2A, S2AB). In a strain in which we visualized the left and right arms using operator arrays inserted at ±87°, clusters of −87° foci and separately +87° foci were observed in opposite cell halves (Figure 2A, S2AB). Finally, a strain containing two arrays on the same chromosome arm (at −87° and −120°) had clusters of foci in the same cell half (Figure 2A, S2AB). Similar results were obtained using the ScpB(ts) mutant (Figure S2C). These data indicate that upon condensin inactivation, DNA synthesis persists but replicated chromosomes fail to segregate. Furthermore, the presence of clusters of foci at origin-distal positions compared to the single unresolved focus at the origin (Figure 1G–I) suggests that an unidentified factor helps maintain origin cohesion [12]. Finally, these data indicate that the replicated chromosomes in cells lacking SMC lie on top of each other (or are intermingled) in a left-ori-right configuration, similar to what has been observed in E. coli during slow growth [15, 16].
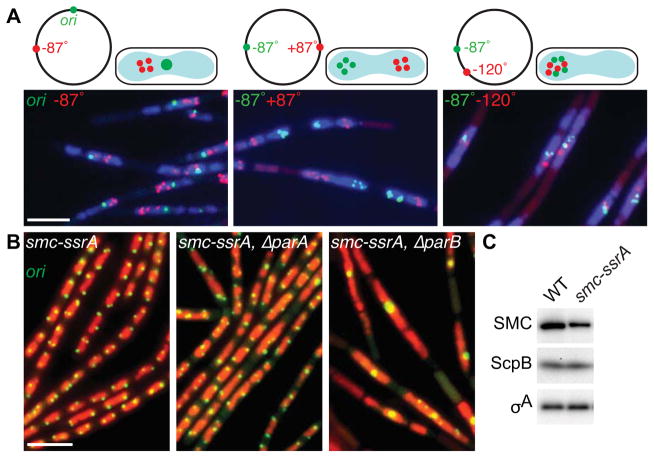
Figure 2. ParB-mediated recruitment of SMC promotes efficient chromosome segregation.
(A) Bulk chromosome segregation is blocked upon SMC inactivation. Representative images of DAPI-stained nucleoids (blue) and indicated chromosomal loci (green and red) in the smcts mutant (BWX2378, BWX2116, BWX2110) grown in CH medium for 1.5 h after shifting to 42°C. Schematic representations of the loci analyzed and their subcellular locations are shown above the micrographs. 72–76% (n>1320 per strain) had nucleoids and foci similar to those shown in (A). (B) Origin segregation is impaired in ParB mutants when SMC levels are reduced. Representative micrographs of DAPI-stained nucleoids (false-colored red) and origins (green) in cells (BWX1497, left panel) harboring an smc-ssrA fusion that results in a 2.5-fold reduction in SMC levels. The cells in the middle panel (BWX2551) harbor an in-frame deletion of parA and those in the right panel (BWX1569) lack parB. 2% (n=1165) of smc-ssrA cells; 8% (n=1038) of ΔparA, smc-ssrA cells; and 34% (n=1214) of ΔparB, smc-ssrA cells had a single bright origin focus. 5%, 13%, and 80%, respectively had an unsegregated nucleoid. Representative images of ParA and ParB mutants that have wild-type levels of SMC and quantitative analysis of nucleoid size in all strains can be found in Figure S2D. Scale bars are 4 μm. (C) Immunoblot analysis of SMC, ScpB, and a loading control (σA) in wild-type and the smc-ssrA mutant under conditions in which the adaptor protein is not induced. See also Figure S2.
ParB-mediated enrichment of condensin at replicated origins promotes their segregation
The failure to segregate replicated origins upon inactivation of SMC suggests that condensin acts at the origin. SMC is recruited to this site by the partitioning protein ParB [10, 11]. In the absence of ParB, origin-localized SMC complexes are significantly reduced and loci in the origin region are less well organized [10, 11], however, origin segregation is not significantly impaired [12] (Figure S2D). We reasoned that if ParB-mediated recruitment of SMC were important for origin individualization, then cells in which SMC levels were reduced would be more sensitive to the loss of ParB. To test this, we used the SMC-SsrA fusion under conditions in which the adaptor protein SspB was not induced resulting in ~2.5-times lower levels of SMC-SsrA compared to wild-type (Figure 2C). Importantly, origin resolution and segregation were similar to wild-type in this background (Figure 2B, S2D). Strikingly, deletion of parB resulted in a significant proportion of cells with large nucleoids in which the replicated origins failed to resolve (Figure 2B, S2D). Importantly, these phenotypes were principally due to the absence of ParB-mediated recruitment of SMC rather than the loss of the parABS partitioning system, because cells lacking ParA that are not impaired in SMC recruitment [11] displayed mild origin segregation defects in the SMC-SsrA background (Figure 2B, S2D). Thus, ParB-mediated recruitment of condensin complexes to the origin promotes origin individualization and efficient chromosome segregation.
SMC-mediated origin individualization is not dependent on Topoisomerase IV
The failure to resolve the origins upon inactivation of SMC in B. subtilis was reminiscent of the segregation defects in temperature-sensitive mutations in parE and parC (encoding the subunits of Topoisomerase IV (Topo IV)) in E. coli [17]. Intriguingly, the E. coli SMC analog (called MukB) has been shown to stimulate some of Topo IV’s activities [18–20]. Accordingly, we wondered whether the failure to individualize the origins upon SMC inactivation was due to impaired Topo IV activity. To explore this possibility, we generated a temperature-sensitive mutant of ParC (Figure 1B) and monitored segregation of chromosomal loci at the non-permissive temperature. As expected, upon inactivation of Topo IV the nucleoid mass increased in size (Figure 3). Consistent with the idea that the replicated arms remain intertwined in the absence of the decatenase, chromosomal loci at ±87° formed clusters of foci in opposite cell halves and chromosomal loci at −87° and −120° formed adjacent clusters (Figure 3A). However and surprisingly, the origins were able to individualize and segregate in this mutant background (Figure 3B) indicating that condensin does not act through Topo IV to mediate origin resolution.
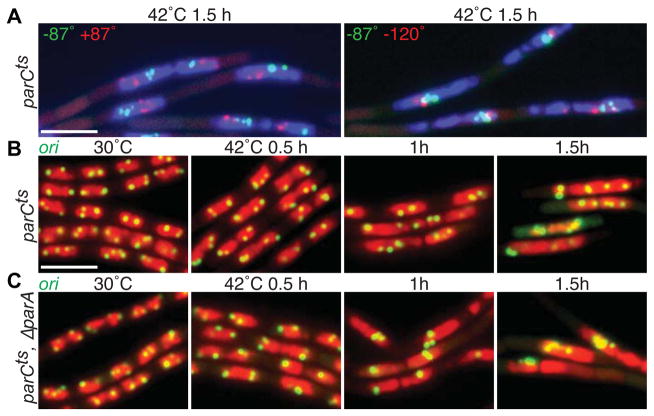
Figure 3. Topoisomerase IV is essential for bulk chromosome segregation but is not necessary to resolve replicated origins.
(A) Representative images of DAPI-stained nucleoids (blue) and indicated chromosomal loci (green and red) in a parCts mutant (BWX2112 and BWX2106) grown in CH medium for 1.5 h after shifting to 42°C. Clusters of green and red foci reflect a failure to segregate the replicated chromosomes. 70–75% (n>1190 per strain) had nucleoids and foci similar to those shown in (A). (B) Representative images of nucleoids (red) and origin loci (green) in a parCts mutant (BWX2082) grown at 30°C and after shift to 42°C. (C) The same time course as in (B) with a parCts mutant that also lacks ParA (BWX2574). Origin foci are more often clustered in the absence of ParA although this is not as pronounced as upon SMC inactivation (Figure 1). 70% (n=1143) of parCts and 19% (n=1238) of ΔparA, parCts had nucleoids with 3 or more well-resolved foci or clusters of foci at hour 1.5. Scale bars are 4 μm.
Inhibition of replication suppresses the chromosome segregation defect in SMC depleted cells
During slow growth, SMC null mutants segregate their chromosomes, albeit inefficiently [3, 4] (Figure 1A, S1A). We therefore wondered whether the unsegregated chromosomes that accumulate upon SMC inactivation could be segregated if new rounds of DNA synthesis were halted. To address this, we analyzed chromosome segregation in a strain harboring a degradable allele of SMC and a temperature sensitive replication initiation mutant (dnaBts) [21]. Cells were grown at 30°C to allow normal replication and growth. Degradation of SMC was then induced for 1 h to generate unsegregated nucleoids. Finally, replication initiation was blocked by shifting the cells to 42°C. Strikingly, over the next 1.5 hours the DNA mass resolved into segregated chromosomes containing a single replication origin (Figure 4A). Similar results were obtained using the ScpB degradable allele (Figure S3A). Moreover, replicated and unsegregated loci on the left and right arms also resolved into a single focus after replication was inhibited (Figure S3B). These results support the idea that condensin complexes become increasingly important for chromosome segregation as the rate DNA synthesis increases.
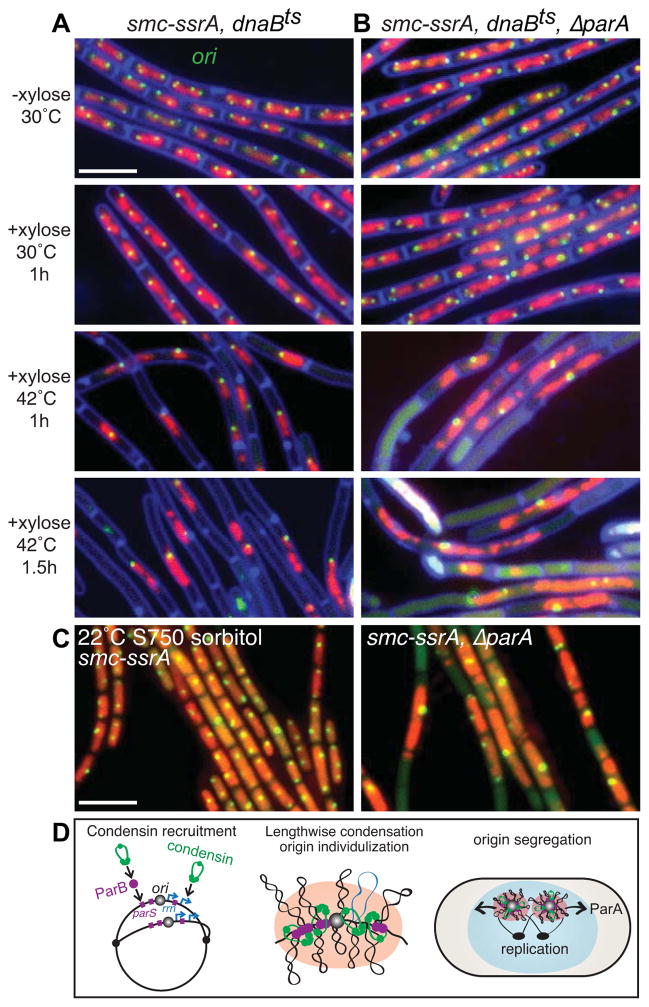
Figure 4. The parABS partitioning system and the SMC complex contribute to origin segregation.
(A) Chromosome resolution and segregation occur in the absence of SMC if new rounds of replication are blocked. Representative micrographs of DAPI-stained nucleoids (false-colored red) and origin foci (green) in a strain (BWX1527) harboring the smc-ssrA degradable allele and a temperature-sensitive replication initiation mutant (dnaBts). After induction of SMC-SsrA degradation for 1 h most cells had unsegregated nucleoids with unresolved origin foci. Inhibition of replication leads to resolution and segregation of the chromosomes if the parABS system is intact (61%; n=1284) (A) but not if ParA is absent (strain BWX2558) (29%; n=1262) (B). (C) Cells lacking SMC and ParA grown under permissive conditions have severe defects in origin segregation. Cells with intact parABS (BWX1497) and lacking parA (BWX2551) were induced to degraded SMC-SsrA under permissive growth conditions (minimal S750 medium supplemented with sorbitol at 22°C). Representative images of nucleoids (false-colored red) and origin foci (green) are shown. 11% (n=1595) of smc-ssrA cells and 51% (n=1287) of ΔparA, smc-ssrA cells had unsegregated nucleoids. 2% and 30%, respectively had a single bright origin focus. Scale bars are 4 μm. (D) Schematic model depicting origin segregation in B. subtilis. The SMC condensin complex (green) is recruited to the origin by ParB (purple) bound to origin-proximal parS sites and is enriched at highly transcribed genes including ribosomal RNA genes (rrn) (blue) that reside adjacent to the origin [10, 11]. Compaction of contiguous DNA segments leads to individualization of the sister origins that are resolved and segregated, in part, through the action of ParA acting on ParB/parS complexes. See also Figure S3.
The parABS partitioning system and the SMC complex contribute to origin segregation
Although our data indicate that the condensin complex plays a central role in promoting origin segregation, previous work suggests that the parABS partitioning system also functions in this capacity [11, 12]. In support of this idea, we found that origin segregation in the absence of Topo IV was reduced in a ParA mutant (Figure 3C). To further explore the role of the parABS module in origin segregation, we investigated whether it was required for the successful resolution of the unsegregated chromosomes in the cells in which SMC was degraded and DNA synthesis was blocked (Figure 4A). We generated a strain lacking ParA that harbors a degradable allele of SMC and the dnaBts mutant. When we induced SMC degradation and then blocked new rounds of replication by shifting to 42°C, most of the origins and the unsegregated chromosomes failed to resolve as they had in the ParA+ strain (Figure 4B). Thus, a critical role for the parABS partitioning system is specifically revealed under conditions in which SMC is absent and replication is blocked.
Grossman and co-workers previously reported synthetic interactions between mutations in parA (or parB) and the genes encoding components of the condensin complex [3, 12]. The double mutants were found to have a significant increase in the production of anucleate cells. These data were interpreted in the context of the SMC null phenotype and its proposed role in condensation of the chromosome as a whole. Our findings raise the possibility that these synthetic phenotypes are a direct consequence of a failure to segregate replicated origins. To test this, we constructed a strain lacking ParA and SMC. However, The double mutant was so infirmed that it could not stably maintain fluorescent reporters and/or operator arrays. Accordingly, we used a ParA mutant that harbors the smc-ssrA degradable allele. SMC was degraded from ParA+ and ΔparA strains grown under permissive growth conditions (minimal medium at 22°C) and the origins were analyzed by fluorescence microscopy. ParA+ cells resembled the smc null mutant with aberrant and heterogeneous nucleoids and some bright origin foci (Figure 4C). By contrast, most cells lacking ParA had elongated nucleoids with a single bright origin focus (Figure 4C). Furthermore, as reported previously, many cells also had septa that bisected unsegregated nucleoids resulting in anucleated cells [3]. These results indicate that the parABS system becomes critical for origin segregation in the absence of SMC and highlights the complementary roles played by the condensin complex and the ParAB/parS module in segregating replicated origins.
Conclusions
Our results support a model in which origin-localized SMC complexes constrain contiguous DNA segments drawing the origin region in on itself and away from its sister origin (Figure 4D) [22]. This so-called lengthwise condensation [23, 24] supplemented by the parABS partitioning system could overcome factors that maintain origin cohesion [12] and promote efficient origin individualization and segregation. Once the origins have been segregated, further lengthwise compaction mediated by SMC and/or other factors like small nucleoid-associated proteins or negative supercoiling likely drives segregation of the bulk DNA [22]. Finally, we have shown that in the absence of SMC, the parABS system, which normally plays a supporting role in segregating origins, becomes critical. Thus, ParB bound to parS serves as a central hub in origin segregation. This nucleoprotein complex recruits the SMC condensin complex to the origin and is acted upon by the ParA ATPase [25, 26]. Together, the activities of these two molecular machines ensure efficient resolution and segregation of replicated origins.
Experimental Procedures
General Methods
B. subtilis strains were derived from the prototrophic strain PY79 [27]. E. coli SspB was induced with 0.5% xylose or 0.5mM IPTG as specified for degradation of SsrA-tagged proteins [13]. At least two biological replicates were performed for all experiments and >500 cells per strain were scored for each experiment. The generation and identification of temperature-sensitive mutants are described in Supplemental Experimental Procedures. Strains, plasmids and oligonucleotides used in this study are listed in Supplemental Tables S1–3.
Fluorescence microscopy
Fluorescence microscopy was performed with a Nikon Ti microscope equipped with Plan Apo 100x/1.4NA phase contrast oil objective and a CoolSnapHQ2 CCD camera (Photometrics). Cells were immobilized on 2% agarose pads containing growth media. Membranes were stained with TMA-DPH (0.01 mM) or FM4-64 (3 μg/ml). DNA was visualized with DAPI (2 μg/ml) (Molecular Probes) or an Hbsu-GFP fusion (a merodiploid) that had no impact on growth or nucleoid morphology. Images were cropped and adjusted using MetaMorph software (Molecular Devices). Final figure preparation was performed in Adobe Illustrator.
Supplementary Material
Highlights.
The SMC condensin complex plays a central role in origin resolution and chromosome segregation in Bacillus subtilis.
Cells in which Topoisomerase IV has been rapidly inactivated are able to segregate their origins, indicating that SMC-dependent origin resolution does not require the unlinking of pre-catenated sister chromosomes.
ParB-mediated enrichment of condensin complexes adjacent to the origin of replication is important for origin resolution and segregation.
Origin segregation is a task shared by SMC condensin complexes and the parABS partitioning system.
Acknowledgments
We thank members of the Rudner and Bernhardt labs, and Viknesh Sivanathan for stimulating discussions and support. We thank Stephan Gruber for communicating results prior to publication. We thank Alan Grossman, Kevin Griffith, Dirk Landgraf, David Sherratt and Rodrigo Reyes-Lamothe for reagents. This work was supported by the National Institute of Health Grant GM086466 (D.Z.R.), X.W. was a long-term fellow of the Human Frontier Science Program, O.W.T. was supported by the Harvard College PRISE and Herchel Smith Research Programs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hirano T. At the heart of the chromosome: SMC proteins in action. Nat Rev Mol Cell Biol. 2006;7:311–322. doi: 10.1038/nrm1909. [DOI] [PubMed] [Google Scholar]
- 2.Nasmyth K, Haering CH. The structure and function of SMC and kleisin complexes. Annu Rev Biochem. 2005;74:595–648. doi: 10.1146/annurev.biochem.74.082803.133219. [DOI] [PubMed] [Google Scholar]
- 3.Britton RA, Lin DC, Grossman AD. Characterization of a prokaryotic SMC protein involved in chromosome partitioning. Genes Dev. 1998;12:1254–1259. doi: 10.1101/gad.12.9.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moriya S, Tsujikawa E, Hassan AK, Asai K, Kodama T, Ogasawara N. A Bacillus subtilis gene-encoding protein homologous to eukaryotic SMC motor protein is necessary for chromosome partition. Mol Microbiol. 1998;29:179–187. doi: 10.1046/j.1365-2958.1998.00919.x. [DOI] [PubMed] [Google Scholar]
- 5.Cui Y, Petrushenko ZM, Rybenkov VV. MukB acts as a macromolecular clamp in DNA condensation. Nat Struct Mol Biol. 2008;15:411–418. doi: 10.1038/nsmb.1410. [DOI] [PubMed] [Google Scholar]
- 6.Hirano M, Hirano T. Opening closed arms: long-distance activation of SMC ATPase by hinge-DNA interactions. Mol Cell. 2006;21:175–186. doi: 10.1016/j.molcel.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 7.Kimura K, Rybenkov VV, Crisona NJ, Hirano T, Cozzarelli NR. 13S condensin actively reconfigures DNA by introducing global positive writhe: implications for chromosome condensation. Cell. 1999;98:239–248. doi: 10.1016/s0092-8674(00)81018-1. [DOI] [PubMed] [Google Scholar]
- 8.Mascarenhas J, Soppa J, Strunnikov AV, Graumann PL. Cell cycle-dependent localization of two novel prokaryotic chromosome segregation and condensation proteins in Bacillus subtilis that interact with SMC protein. EMBO J. 2002;21:3108–3118. doi: 10.1093/emboj/cdf314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soppa J, Kobayashi K, Noirot-Gros MF, Oesterhelt D, Ehrlich SD, Dervyn E, Ogasawara N, Moriya S. Discovery of two novel families of proteins that are proposed to interact with prokaryotic SMC proteins, and characterization of the Bacillus subtilis family members ScpA and ScpB. Mol Microbiol. 2002;45:59–71. doi: 10.1046/j.1365-2958.2002.03012.x. [DOI] [PubMed] [Google Scholar]
- 10.Gruber S, Errington J. Recruitment of condensin to replication origin regions by ParB/SpoOJ promotes chromosome segregation in B. subtilis. Cell. 2009;137:685–696. doi: 10.1016/j.cell.2009.02.035. [DOI] [PubMed] [Google Scholar]
- 11.Sullivan NL, Marquis KA, Rudner DZ. Recruitment of SMC by ParB-parS organizes the origin region and promotes efficient chromosome segregation. Cell. 2009;137:697–707. doi: 10.1016/j.cell.2009.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee PS, Grossman AD. The chromosome partitioning proteins Soj (ParA) and Spo0J (ParB) contribute to accurate chromosome partitioning, separation of replicated sister origins, and regulation of replication initiation in Bacillus subtilis. Mol Microbiol. 2006;60:853–869. doi: 10.1111/j.1365-2958.2006.05140.x. [DOI] [PubMed] [Google Scholar]
- 13.Griffith KL, Grossman AD. Inducible protein degradation in Bacillus subtilis using heterologous peptide tags and adaptor proteins to target substrates to the protease ClpXP. Mol Microbiol. 2008;70:1012–1025. doi: 10.1111/j.1365-2958.2008.06467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lau IF, Filipe SR, Soballe B, Okstad OA, Barre FX, Sherratt DJ. Spatial and temporal organization of replicating Escherichia coli chromosomes. Mol Microbiol. 2003;49:731–743. doi: 10.1046/j.1365-2958.2003.03640.x. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen HJ, Ottesen JR, Youngren B, Austin SJ, Hansen FG. The Escherichia coli chromosome is organized with the left and right chromosome arms in separate cell halves. Mol Microbiol. 2006;62:331–338. doi: 10.1111/j.1365-2958.2006.05346.x. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Liu X, Possoz C, Sherratt DJ. The two Escherichia coli chromosome arms locate to separate cell halves. Genes Dev. 2006;20:1727–1731. doi: 10.1101/gad.388406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Reyes-Lamothe R, Sherratt DJ. Modulation of Escherichia coli sister chromosome cohesion by topoisomerase IV. Genes Dev. 2008;22:2426–2433. doi: 10.1101/gad.487508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Stewart NK, Berger AJ, Vos S, Schoeffler AJ, Berger JM, Chait BT, Oakley MG. Escherichia coli condensin MukB stimulates topoisomerase IV activity by a direct physical interaction. Proc Natl Acad Sci U S A. 2010;107:18832–18837. doi: 10.1073/pnas.1008678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hayama R, Marians KJ. Physical and functional interaction between the condensin MukB and the decatenase topoisomerase IV in Escherichia coli. Proc Natl Acad Sci U S A. 2010;107:18826–18831. doi: 10.1073/pnas.1008140107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayama R, Bahng S, Karasu ME, Marians KJ. The MukB-ParC interaction affects the intramolecular, not intermolecular, activities of topoisomerase IV. J Biol Chem. 2013;288:7653–7661. doi: 10.1074/jbc.M112.418087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rokop ME, Auchtung JM, Grossman AD. Control of DNA replication initiation by recruitment of an essential initiation protein to the membrane of Bacillus subtilis. Mol Microbiol. 2004;52:1757–1767. doi: 10.1111/j.1365-2958.2004.04091.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Montero Llopis P, Rudner DZ. Organization and segregation of bacterial chromosomes. Nat Rev Genet. 2013;14:191–203. doi: 10.1038/nrg3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alipour E, Marko JF. Self-organization of domain structures by DNA-loop-extruding enzymes. Nucleic Acids Res. 2012;40:11202–11212. doi: 10.1093/nar/gks925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marko JF. Linking topology of tethered polymer rings with applications to chromosome segregation and estimation of the knotting length. Phys Rev E Stat Nonlin Soft Matter Phys. 2009;79:051905. doi: 10.1103/PhysRevE.79.051905. [DOI] [PubMed] [Google Scholar]
- 25.Ptacin JL, Lee SF, Garner EC, Toro E, Eckart M, Comolli LR, Moerner WE, Shapiro L. A spindle-like apparatus guides bacterial chromosome segregation. Nat Cell Biol. 2010;12:791–798. doi: 10.1038/ncb2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang LC, Vecchiarelli AG, Han YW, Mizuuchi M, Harada Y, Funnell BE, Mizuuchi K. ParA-mediated plasmid partition driven by protein pattern self-organization. EMBO J. 2013;32:1238–1249. doi: 10.1038/emboj.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youngman PJ, Perkins JB, Losick R. Genetic transposition and insertional mutagenesis in Bacillus subtilis with Streptococcus faecalis transposon Tn917. Proc Natl Acad Sci U S A. 1983;80:2305–2309. doi: 10.1073/pnas.80.8.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.