Introduction
Cytochrome p450 (CYP) epoxygenases, CYP2C and CYP2J subfamilies enzymes, play important role in fatty acid metabolism [1]. In the heart, CYP2J is abundant and predominantly involved in the biosynthesis of epocyeicosatrienoic acids (5,6-, 8,9-, 11,12-, and 14,15-EETs) from arachidonic acid [2]. EETs play important role in cellular signaling and possess numerous biological activities in the cardiovascular system including vasodilatory, anti-inflammatory, anti-fibrotic, natriuretic and anti-apoptotic effects [3, 4]. Modulation of EET-levels can occur via reincorporation into phospholipid membranes or by β-oxidation to smaller reactive epoxides [4–6]; however, the predominant pathway occurs through metabolism to inactive vicinal diols by epoxide hydrolases [4, 6, 7]. Two major epoxide hydrolases are found in mammalian tissues, microsomal epoxide hydrolase (mEH) and soluble epoxide hydrolase (sEH or Ephx2) [8].
Accumulating evidence indicates that EETs have important functional roles in ischemia reperfusion (I/R) injury [9–13]. Studies from our group and others have demonstrated cardioprotective effects of EETs [1, 13–15]. Studies reported that mice with the targeted deletion of the Ephx2 gene (sEH knockout, sEH null) had enhanced postischemic recovery of left ventricular function, which was mediated by activation of the PI3K pathway and K+ channels [10, 14]. Inhibition of sEH, using pharmacological inhibitors (sEHis) also protects the heart against I/R injury [12, 13]. Cardiomyocyte specific over-expression of CYP2J2 in CYP2J2 Tr mice leads to improved functional recovery and reduced infarct size after ischemia [9, 16]. Moreover, treatment with exogenous EETs has also been demonstrated to be protective against I/R injury [1, 17]. According to current knowledge, the cardioprotective mechanism(s) of EETs suggest involvement of signaling pathways including phosphoinositide 3-kinase (PI3K) – Akt, increased secretion of cardiac hormones, and activation of cardiac ion channels such as ATP-sensitive K+ channels and BKCa channels [1, 9, 10, 14, 17].
The growing elderly population has significantly increased interest in age-related diseases, particularly related to the heart. Importantly, this population has a higher risk of cardiovascular disease, which is reflected by death rates of approximately 1000 times higher in individuals who are 85–89 years old compared to those of 25–29 years of age [18]. The increased death rate can be explained by an increased susceptibility of aged hearts to stress compared to younger counterparts [19, 20]. Indeed, aging causes a significant reduction in the heart’s ability to tolerate damage stemming from I/R injury [19, 20]. Consequences of aging not only lower the heart’s ability to resist I/R injury but also decrease the effectiveness of cardioprotective strategies [21]. Therefore, it is important to evaluate the effectiveness of cardioprotective strategies in aged animal models.
While the cardioprotective effects of EETs are well studied in young animal models, there is a lack of information about EET-induced cardioprotection in aged animals. Therefore, in the present study, we examined the effect of aging on EET-induced cardioprotection using young and aged; CYP2J2 Tr and sEH null mice. We demonstrate that aged sEH null mice are protected against I/R injury while aged CYP2J2 Tr mice are not. Moreover, our data suggest the loss of protective effects in aged CYP2J2 Tr mice can be prevented by sEHi. Taken together, these data suggest that sEHi and therefore EETs can protect the aged mouse hearts against I/R injury.
Material and Methods
Animals
Mouse colonies with targeted disruption of the Ephx2 gene (sEH null) and cardiac myocyte-specific over expression of human CYP2J2 (CYP2J2 Tr) backcrossed onto a C57BL6 genetic background for more than 10 generations were maintained at the University of Alberta, sEH null and CYP2J2 Tr mice have been previously described [1, 9, 10, 22]. C57BL6 mice were purchased from Charles River Laboratories (Pointe Claire, PQ). All experiments used male and female mice aged 2–3 months (young) or 11–13 months (aged) and were treated in accordance with the guidelines of Health Science Laboratory Animal Services (HSLAS), University of Alberta. The experiments conformed with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Isolated heart perfusions
Hearts were perfused in the Langendorff mode as previously published [9, 10]. Briefly, hearts were perfused with Krebs-Henseleit buffer for 40 min of baseline and subjected to 30 min of global no flow ischemia followed by 40 min of reperfusion. For some experiments, hearts were perfused with trans-4-[4-(3-adamantan-1-y1-ureido)-cyclohexyloxy]-benzoic acid (t-AUCB; sEH inhibitor, 100 nM), MS-PPOH (CYP epoxygenase inhibitoe, 50 µM) (Cayman Chemicals, Ann Arbor, MI, USA). The percentage of left ventricular developed pressure (%LVDP) at 40 min of reperfusion (R40), as compared to baseline LVDP, was taken as a marker for recovery of contractile function. After 40 min of reperfusion, hearts were immediately frozen and stored below −80°C.
Tissue homogenization and sub-cellular fractionation
Cytosolic and microsomal fractions were prepared from frozen mouse hearts as described [23]. For preparation of subcellular fractions, tissues were minced and homogenized in homogenization buffer containing (sucrose 250 mM, TrisHCL 10 mM, EDTA 1 mM, sodium orthovanadate 1 mM, sodium flouride 1 mM, aproptinin 10 µL/L, leupeptin 2 µL/L, pepstatin 100 µL/L). The homogenate was centrifuged at 700 × g for 10 min to remove the debris and the supernatant was again centrifuged to 10,000 × g for 20 min at 4 °C. The resulting supernatant (S-9) fraction and pellet were separated. The supernatant obtained from further centrifugation of the S-9 fraction at 100,000 × g for 1 h at 4 °C was used as the cytosolic fraction and pellet was used as microsomal fraction. The protein concentration of these fractions was determined colorimetrically using a Bio-Rad protein assay kit (Mississauga, ON, Canada) using bovine serum albumin as a standard.
Epoxide hydrolase activity analysis
Heart cytosol or microsomes (1 mg of protein/mL) were incubated in the incubation buffer (5 mM magnesium chloride hexahydrate dissolved in 0.5 M potassium phosphate buffer, pH = 7.4) at 37°C in a shaking water bath (50 rpm). A pre-equilibration period of 5 min was performed. 14,15-EET was added to a final concentration of 50 µM and incubated for 30 min. The reaction was terminated by the addition of 600 µL of ice-cold acetonitrile followed by the internal standard, 4-hydroxybenzophenone. 14,15-EET and 14,15-DHET were extracted by 1 mL of ethyl acetate twice and dried using a speed vacuum (Thermo Fisher Scientific).
Extracted 14,15-EET and 14,15-DHET were analyzed using the LC-ESI-MS (Micromass ZQ 4000 spectrometer; Waters, Milford, MA) method as described previously [24]. Briefly, the mass spectrometer was operated in negative ionization mode with single ion recorder acquisition. The nebulizer gas was acquired from an in-house high-purity nitrogen source. The source temperature was set at 150°C, and the voltages of the capillary and the cone were 3.51 kV and 25 V, respectively. The samples (10 µL) were separated on a reverse-phase C18 column (Kromasil, 250 × 3.2 mm) using a linear gradient mobile phase system with a mobile phase of water-acetonitrile with 0.005% acetic acid at a flow rate of 0.2 mL/min. The mobile phase system started at 60% acetonitrile, linearly increased to 80% acetonitrile in 30 min, increased to 100% acetonitrile in 5 min, and held for 5 min.
Protein phosphatase 2a activity assay
PP2A activity was determined in heart samples with the ‘PP2A Immunoprecipitation Phosphatase Assay Kit’ by Millipore (Billerica, MA, USA) according to manufacturer’s guidelines. Briefly, cytosolic fractions were incubated with 4 µg of anti-PP2Ac antibodies and protein A/G plus agarose at 4°C with continuous shaking 1200 RPM for 2 hr. Immuno-complexes were washed three times with TBS and one time with PP2A buffer (250 mM imidazole, 1 mM EGTA, 0.1% β-mercaptoethanol and 0.5 mg/mL BSA). Phosphatase activity was assayed by suspending the final pellet in 80 µL PP2A buffer containing 750 mM phophopeptide substrate RRA(pT)VA for 10 minutes at 37°C. 25 µL of supernatant was incubated with 100 µL of the molybdate dye solution. The reaction was carried out at room temperature for 10 min and absorbance was read at 600 nm.
Immunoblot analysis
Protein was resolved on SDS-polyacrylamide gels, transferred to nitrocellulose membranes and immunoblotted as previously described [9]. Immunoblots were prepared using cytosolic (50 µg protein) or microsomal (25 µg protein) fractions and probed with antibodies to sEH (Catalogue# sc-22344, Santacruz biotechnology, CA, USA), cAMP activated protein kinase (AMPK) (Catalogue# 2532, Cell Signaling Technology, Inc., MA, USA), phospho- AMPK (pThr172-AMPK) (Catalogue# 2535, Cell Signaling Technology, Inc., MA, USA), CYP2J2 and GAPDH (Catalogue# 2118, Cell Signaling Technology, Inc., MA, USA). Anti-CYP2J2 was provided by Dr. Darryl Zeldin and has been previously described [9]. GAPDH and prohibitin were used as loading controls for cytosolic and mitochondrial fraction, respectively. Relative band intensities were assessed by densitometry using Image J (NIH, USA). Protein expression in vehicle treated controls were taken as 100% and compared with treated group.
Eicosanoid analysis
Eicosanoid analysis was performed using liquid chromatography, tandem mass spectroscopy (LC/MS/MS) as described previously [16]. Briefly, each minute 1 mL of perfusate was collected from last 20 min of baseline, spiked with 30 ng PGE2-d4, 10,11-DiHN, and 10,11-EpHep (Cayman Chemical Company, Ann Arbor, MI, USA) as internal standards, mixed with 0.1 vol of 1% acetic acid in 50% methanol, and extracted by serial passage through Oasis HLB C18 3mL columns (Waters, Milford, MA, USA). Liquid chromatography of the sample was performed with an Agilent 1200 Series capillary HPLC (Agilent Technologies, Santa Clara, CA, USA). Negative ion electrospray ionization tandem mass spectrometry was used for detection.
Protein carbonyl assay
Protein carbonyl ELISA was performed to measure protein oxidation or oxidative stress. Carbonyl groups (aldehydes and ketones) are produced upon oxidation of protein side chains in response to oxidative stress. These protein carbonyl moieties are chemically stable and they are suitable markers for oxidative stress [25]. ELISA was performed using OxiSlect™ protein carbonyl ELISA kit (Cell Biolabs, Inc., San Diego, CA, USA). The assay was performed according to manufacturer’s instructions. The frozen (−80° C) heart samples were powdered in liquid nitrogen using mortar and pastel. Proteins from the powdered samples were extracted using the homogenization buffer and centrifuged at 10000 × g for 20 min at 4 °C. Total protein in the supernatant was measured by Bradford assay. All the samples were diluted to 10 µg/mL using PBS. Nucleic acids were removed by incubating the sample with streptomycin sulfate (1%) for 30 min at room temp. Nuclei acid precipitates were removed by centrifuging the samples at 6000 × g for 10 min at 4 °C. 100 µL of the diluted samples were analyzed for protein carbonylation according to manufacturer’s protocol.
Statistical analysis
Values expressed as mean ± standard error of mean (SEM). Statistical significance was determined by the unpaired Student’s t-test and one-way ANOVA. To determine whether significant difference exists between the groups, Newman-Keuls post-hoc test was performed. Values were considered significant if p < 0.05.
Results
Post-ischemic cardiac function in young and aged sEH null mice
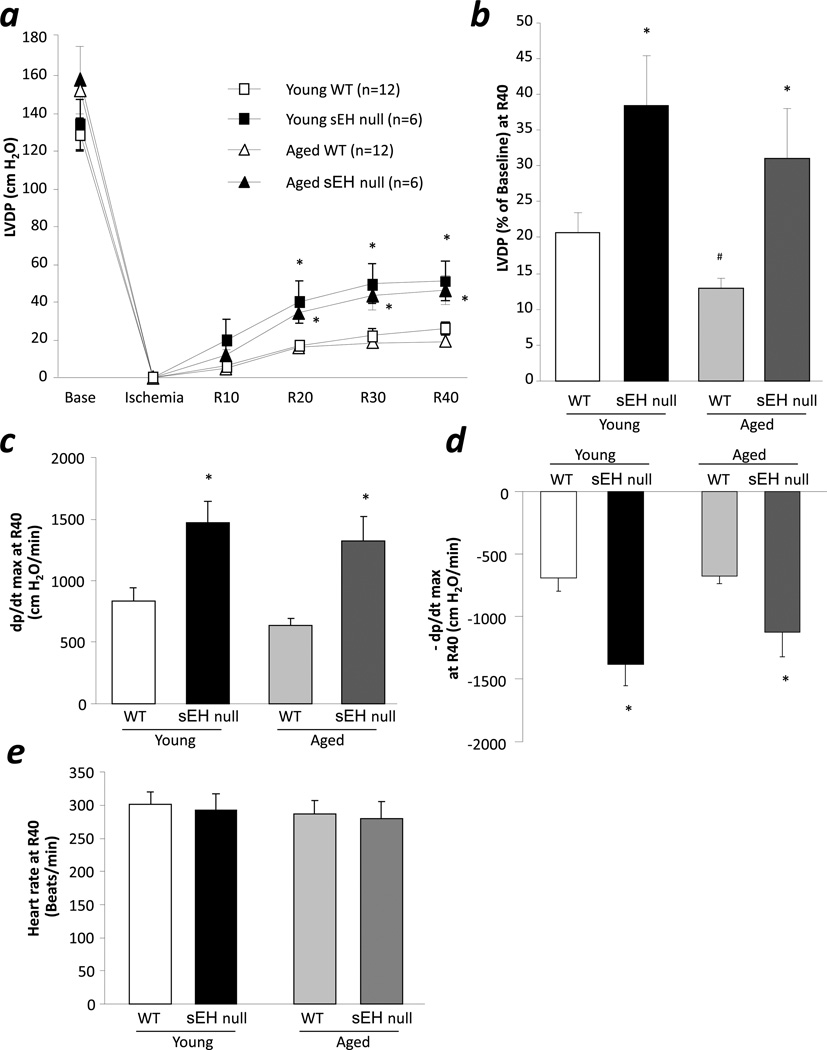
Consistent with our previous observations [10], sEH null and WT mice had normal baseline contractile function (Fig 1a). Furthermore, young sEH null hearts had improved postischemic LVDP recovery following 30min of global no-flow ischemia and 40min reperfusion compared to WT hearts (38.5% vs. 20.7%, respectively, p<0.05) (Fig 1b). Similarly, improved postischemic left ventricular function was observed in aged sEH null mice (31.1% vs. 12.9%, respectively, p<0.05) (Fig 1a, 1b). Consistent with previous reports of decreased tolerance of aged hearts against ischemic injury [26, 27], we observed significant reduction in postischemic functional recovery of aged WT as compared to young WT (Fig 1b). Both, young and aged, sEH null mouse hearts demonstrated significantly higher contraction and relaxation rate compared to age matched WT (Fig 1c, 1d). No difference was observed in heart rate between any groups (Fig 1e).
Figure 1.
Effect of aging on postischemic contractile function in sEH null mice. a, LVDP at baseline, at the end of ischemia, and at reperfusion (R10, R20, R30, and R40) in young and aged, WT and sEH null hearts. b, LVDP recovery at 40 min of reperfusion in young and aged, WT and sEH null hearts. c. Rate of contraction in young and aged, WT and sEH null hearts. d. Rate of relaxation in young and aged, WT and sEH null hearts. e. Heart rate in young and aged, WT and sEH null hearts. Values represent mean ± SEM; n=6–12 per group; *, p<0.05 vs. WT; #, p<0.05 vs. young animal of same genotype.
Post-ischemic cardiac function in young and aged CYP2J2 Tr mice
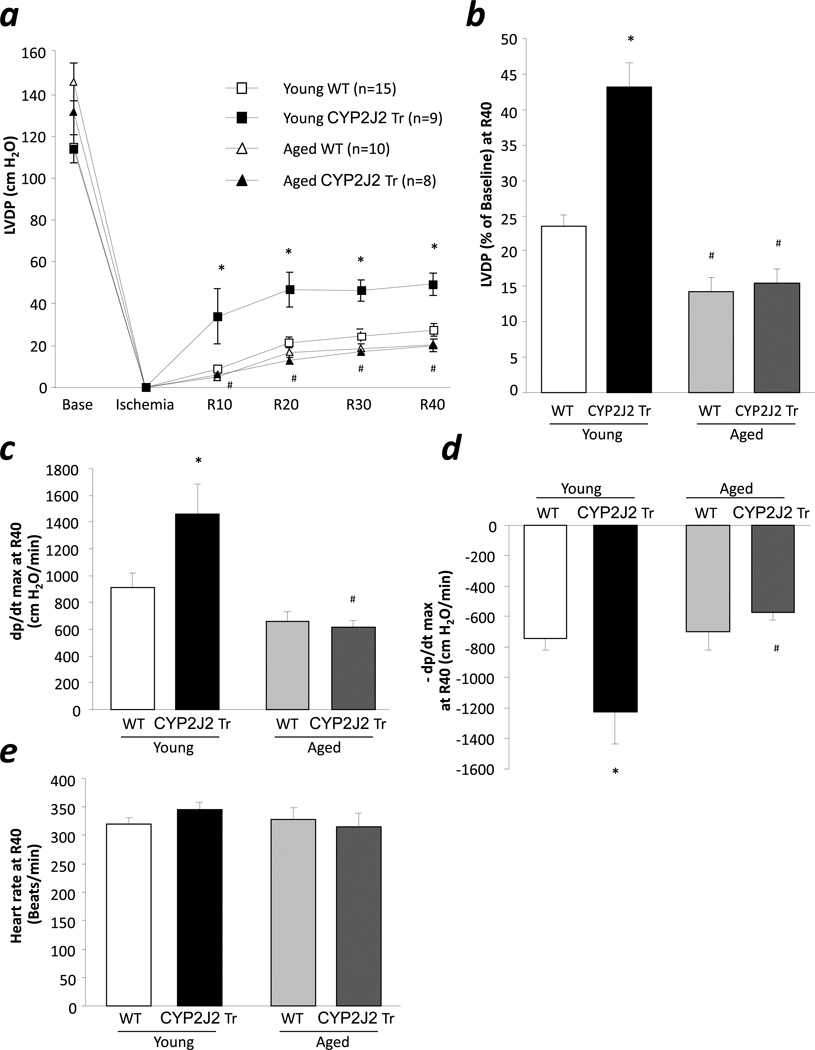
Young and aged CYP2J2 Tr and WT mice had normal baseline contractile function as measured by LVDP (Fig 2a). Following ischemia, significant improvement in contractile function was observed in young CYP2J2 Tr mice compared to age matched WT (43.1% vs. 23.3%, respectively, p<0.05) (Fig 2a, 2b). Consistent with increased postischemic LVDP, we observed significantly improved postischemic contraction and relaxation rate of young CYP2J2 Tr mouse hearts compared to WT hearts. Surprisingly, opposite to the improved postischemic functional recovery in aged sEH null mice, hearts from aged CYP2J2 Tr mice did not show improved postischemic functional recovery compared to WT mice (15.4% vs. 14.8%, respectively). In addition, a significant decrease in postischemic contraction and relaxation rate was observed in aged CYP2J2 Tr hearts compared to young CYP2J2 Tr mouse hearts (Fig 2c, 2d). No difference was observed in heart rate between any groups (Fig 2e). Taken together, these data suggest that targeted deletion of sEH but not CYP2J2 overexpression protects the aged mouse hearts against I/R injury. Further, we investigated the reason for loss of cardioprotection in aged CYP2J2 Tr mice.
Figure 2.
Effect of aging on postischemic contractile function in CYP2J2 Tr mice. a, LVDP at baseline, at the end of ischemia, and at reperfusion (R10, R20, R30, and R40) in young and aged, WT and CYP2J2 Tr hearts. b, LVDP recovery at 40 min of reperfusion in young and aged, WT and CYP2J2 Tr hearts. c. Rate of contraction, d. Rate of relaxation and, e. Heart rate in young and aged, WT and CYP2J2 Tr hearts. Values represent mean ± SEM; n=8–15 per group; *, p<0.05 vs. WT; #, p<0.05 vs. young animal of same genotype.
sEHi and postischemic functional recovery in aged CYP2J2 Tr mice
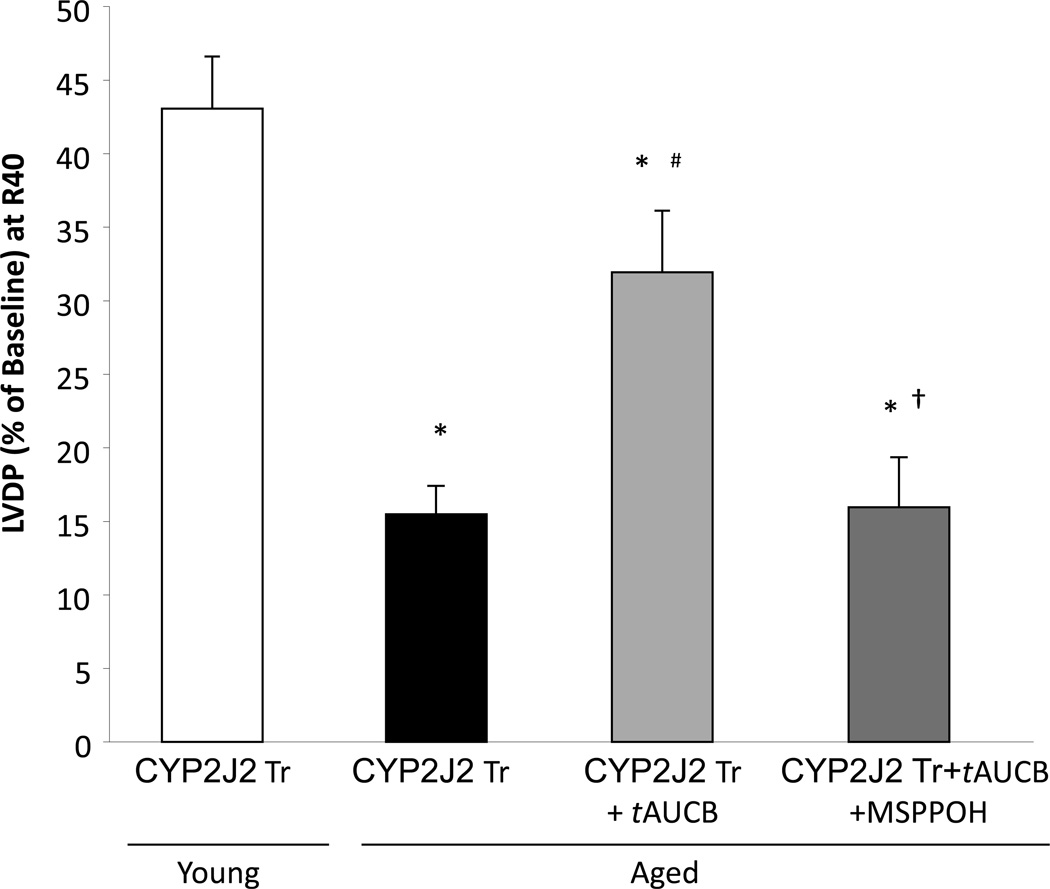
Previous reports suggest that aging is associated with increased epoxide hydrolase expression in soluble fraction of the heart [28]. To study the role of sEH, we perfused the aged CYP2J2 Tr mouse hearts with soluble epoxide hydrolase inhibitor t-AUCB (100 nM). At this concentration t-AUCB has been demonstrated to have protective effects against I/R injury [12]. Perfusion with t-AUCB resulted in significant improvement of postischemic functional recovery in aged CYP2J2 Tr hearts compared to controls (33.9% vs. 15.4%, respectively, p<0.05) (Fig 3). This improvement in postischemic functional recovery was inhibited by co-perfusion of aged CYP2J2 Tr hearts with t-AUCB and MS-PPOH (CYP epoxygenase inhibitor) (15.9%) (Fig 3). These data confirms that t-AUCB induced cardioprotection in aged CYP2J2 Tr mice is due to inhibition of eicosanoid metabolism. Moreover, sEH inhibition and thereby EETs can protect the aged mouse hearts against I/R injury.
Figure 3.
EET-induced cardioprotection in aged mice. LVDP recovery at 40 min of reperfusion in aged CYP2J2 Tr mice treated with tAUCB (100 nM) or tAUCB (100nM) and MS-PPOH (50 µM). Values represent mean ± SEM; n=5–9 per group; *, p<0.05 vs. young animal of same genotype; #, p<0.05 vs vehicle control; †,p<0.05 vs tAUCB treated.
sEH expression and activity analysis
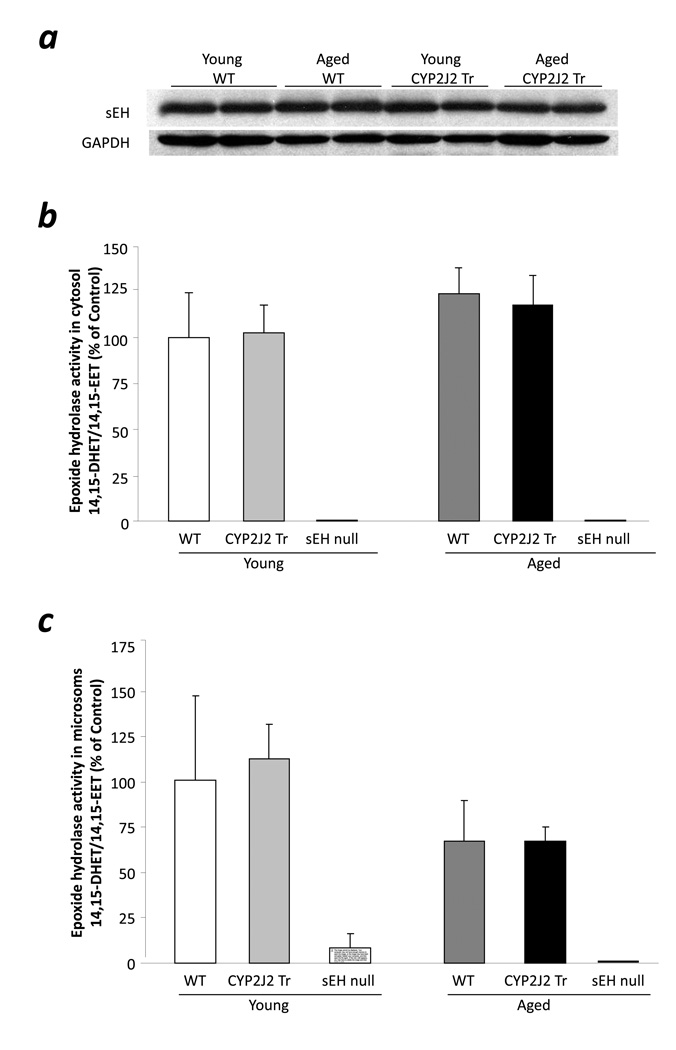
To further confirm the role of sEH, we analyzed cytosolic and microsomal fractions of young and aged mouse hearts for epoxide hydrolase activity and sEH expression. Interestingly, no difference was observed in sEH expression in cytosol of young or aged CYP2J2 Tr or WT hearts (Fig 4a). In addition, no sEH expression was observed in young or aged sEH null mice hearts. Consistent with the protein expression, no difference was observed in the epoxide hydrolase activity in cytosol or microsomal fractions of any groups (Fig 4b, 4c). No epoxide hydrolase activity was observed in cytosol or microsomal fractions of young or aged sEH null heart samples (Fig 4b, 4c). These data confirm that the loss of protective effects in aged CYP2J2 Tr mouse hearts is not due to higher sEH activity in aged hearts. However, the improved postischemic function in aged sEH null and t-AUCB treated aged CYP2J2 Tr mouse hearts suggests that inhibition of EET-metabolism can protect the aged hearts against I/R injury.
Figure 4.
sEH activity and expression in ischemia reperfused mouse hearts. a, Representative immunoblot of sEH in cytosol fractions of young WT, aged WT, young CYP2J2 Tr and aged CYP2J2 Tr hearts. b. Epoxide hydrolase activity in cytosol fractions of young WT, aged WT, young CYP2J2 Tr and aged CYP2J2 Tr hearts. Values represent mean±SEM; n=5 per group. c, Epoxide hydrolase activity in microsomal fractions of young WT, aged WT, young CYP2J2 Tr and aged CYP2J2 Tr hearts. Values represent mean±SEM; n=5 per group.
Eicosanoid analysis
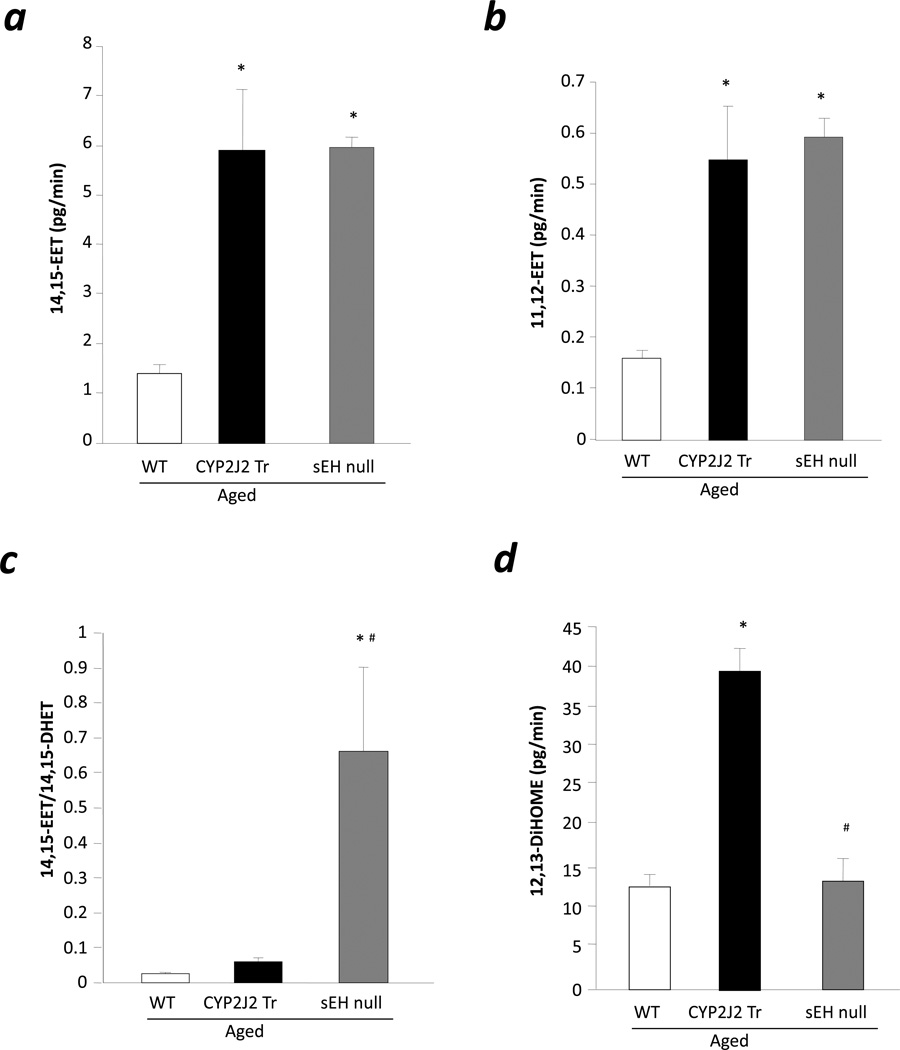
To investigate whether the levels of EETs were altered in aged CYP2J2 Tr mouse hearts, we analyzed eicosanoid levels in heart perfusates during baseline. Levels of 14,15-EET and 11,12-EET were higher in aged CTP2J2 Tr (4.26 fold and 3.45 fold) and sEH null (4.3 fold and 3.73 fold) hearts compared to aged WT hearts (Fig 5a, 5b). Moreover, the 14,15-EET:14,15-DHET ratio was significantly higher in aged sEH null mouse hearts compared to aged CYP2J2 Tr and WT hearts suggesting lower epoxide hydrolase activity in sEH null hearts compared to CYP2J2 Tr and WT hearts (Fig 5c). There was no difference between aged CYP2J2 Tr and WT hearts for 14,15-EET:14,15-DHET ratio (Fig 5c). These data suggests that aged CYP2J2 Tr and sEH null mice produce similar levels of EETs although aged sEH null mice are protected against I/R injury and aged CYP2J2 Tr are not.
Figure 5.
Eicosanoid levels in heart perfusate. a, 14,15-EET levels, b. 11,12-EET levels, c. 14,15-EET:14,15-DHET ratio and d. 12,13-DiHOME levels in the heart perfusate collected duting last 20 min of baseline. Values represent mean±SEM; n=3–6 per group; *, p<0.05 vs. WT; #, p<0.05 vs. aged CYP2J2 Tr.
CYP epoxygenases can metabolize linoleic acid to 9,10-epoxy-12-octadecenoate (9,10-EpOME) and 12,13-epoxy-9-octadecenoate (12,13-EpOME) which is further metabolized to dihydro-metabolites (9,10-, and 12,13-DiHOME, respectively) by sEH [29]. The detrimental effect of DiHOME on postischemic functional recovery has been reported [16]. Interestingly, aged CYP2J2 Tr mice hearts produce significantly higher levels of 12,13-DiHOME compared to age matched WT (Fig 5d), while aged sEH mice has significantly lower levels of 12,13-DiHOME (Fig 5d). It is important to note that such differences were not observed in 9,10-DiHOME, indicating a regioisomer specific effect. Together, the data suggest that increased 12,13-DiHOME levels in aged CYP2J2 Tr mice might be responsible for the loss of cardioprotective effects in these mice.
Analysis of oxidative stress markers
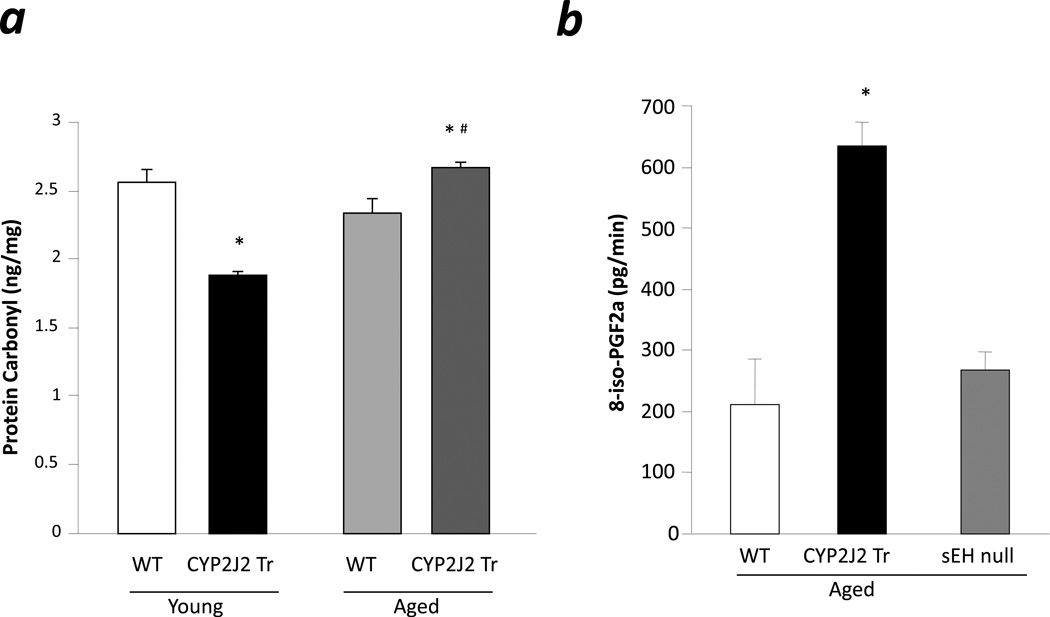
Recently, Edin et al. demonstrated that endothelial expression of human cytochrome P450 epoxygenase CYP2C8 (Tie2-CYP2C8) increases susceptibility to I/R injury in isolated mouse heart, potentially resulting from increased DiHOME and ROS production [16]. To investigate ROS production in aged mice, we analyzed levels of protein carbonylation in postischemic heart samples, as a marker of oxidative stress [25]. Young CYP2J2 Tr mice had significantly lower levels of protein carbonyl as compared to age matched WT (Fig 6a). No difference was observed in protein carbonyl levels between young and aged WT. Interestingly, aged CYP2J2 Tr mice had significantly higher protein carbonyl levels compared to age matched controls (Fig 6a). Furthermore, the loss of cardioprotective effects in aged CYP2J2 Tr hearts was accompanied by a significant increase in protein carbonyl levels compared to hearts from young CYP2J2 Tr mice (Fig 6a). Further evidence for increased stress was observed in perfusate from preischemic hearts of aged mice. A significant increase in 8-iso-PGF2α levels, a marker of ROS, was observed in aged CYP2J2 Tr hearts compared to age matched WT (Fig 6b). Conversely, perfusate from aged sEH null hearts had lower levels of 8-iso-PGF2α compared to aged CYP2J2 Tr mice (Fig 6b). Thus suggesting a higher level of oxidative stress occurs in aged CYP2J2 Tr hearts.
Figure 6.
Oxidative stress marker. a. Protein carbonyl levels were measured in the cytosolic fraction of the ischemia reperfused hearts of young WT, aged WT, young CYP2J2 Tr and aged CYP2J2 Tr mice. Values represent mean ± SEM; n=3 per group; *, p<0.05 vs. WT; #, p<0.05 vs. young animal of same genotype. b. 8-iso-PGF2α levels in the heart perfusate collected duting last 20 min of baseline. Values represent mean±SEM; n=3–6 per group; *, p<0.05 vs. WT.
Protein phosphatase 2A (PP2A) activity analysis
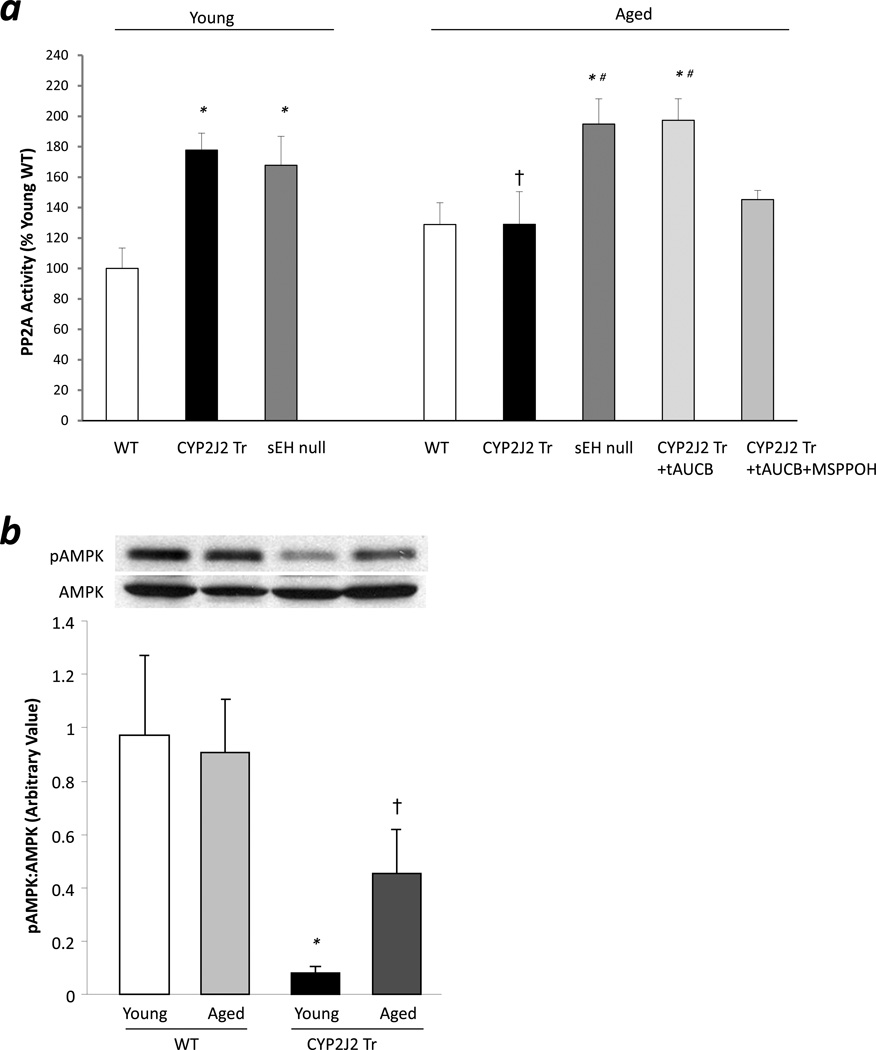
Previous studies have demonstrated that EETs increases PP2A activity in muscle cells [30, 31]. Hence, we analyzed PP2A activity in young and aged CYP2J2 Tr, sEH null or WT heart samples. Significant increase in PP2A activity was observed in young CYP2J2 Tr and young sEH null mouse hearts compared to age matched WT hearts (Fig 7a). Aged CYP2J2 Tr mouse hearts failed to demonstrate increase in PP2A activity compared to age matched WT (Fig 7a). However, significant increases in PP2A activities were observed in hearts from aged sEH null mice and aged CYP2J2 Tr mice perfused with t-AUCB compared to aged matched controls. In addition, co-perfusion of t-AUCB with MS-PPOH in aged CYP2J2 Tr hearts attenuated the increased PP2A activity (Fig 7a). AMPK is a target for PP2A, as such, to confirm changes in PP2A activity, we analyzed pThr172-AMPK expression in these heart samples. Consistent with increased PP2A activity in young CYP2J2 Tr mouse hearts, significant decrease in pThr172-AMPK expression was observed while higher pThr172-AMPK expression was observed in aged CYP2J2 Tr mouse hearts (Fig 7b).
Figure 7.
PP2A activity analysis. a. PP2A activity was measured in the cytosolic fractions of the ischemia reperfused hearts. Values represent mean ± SEM; n=3–7 per group; *, p<0.05 vs. age matched WT; #, p<0.05 vs aged CYP2J2 Tr; †,p<0.05 vs young animal of same genotype. b. Representative immunoblot and densitometry demonstrating expression of pThr172-AMPK in cytosolic fraction of ischemia reperfused mice hearts. Values represent mean ± SEM; n=6 per group; *, p<0.05 vs. age matched WT; †,p<0.05 vs young animal of same genotype.
Discussion
Numerous reports have demonstrated the cardioprotective effects of EETs in young animal models; however, it remains unknown whether EETs can trigger cardioprotection in aged animals [9, 10, 14]. Evidence suggests that many cardioprotective strategies, such as ischemic and anesthetic preconditioning, are lost in aged animals [21, 32, 33]. In the current study, we demonstrate that targeted deletion of sEH resulted in cardioprotection towards I/R injury in aged animals. The decreased postischemic functional recovery observed in aged mice with cardiomyocyte specific over-expression of CYP2J2 was attributed to increased DiHOME levels, oxidative stress and activation of PP2A. Importantly, our data demonstrated that the pharmacological inhibition of sEH, and thereby EETs, can improve postischemic cardiac functional recovery in aged mice. Taken together, these data suggest inhibition of sEH can be an effective strategy for protecting aged hearts against I/R injury.
There is a growing body of evidence suggesting the effectiveness of cardioprotective strategies, including preconditioning, postconditioning and pharmacological conditioning, decreases with increasing age [21, 32, 33]. Schulmann et al reported the effect of aging on various cardioprotective strategies toward I/R injury [32]. In the study, the authors utilized young, mid-aged and aged rats to assess the infarct size reduction properties of ischemic preconditioning and pharmacological conditioning mimetics such as selective adenosin A1 agonist (CCPA), PKC analogue (DOG) and mitochondrial KATP channel opener (diazoxide). Ischemic preconditioning effectively reduced the infarct size following I/R in young animals; however, it was less effective in middle-aged animals and ineffective in aged animals. Similarly, pharmacological conditioning mimetics were ineffective in aged animals [32]. Other animal models have demonstrated blunted effects of preconditioning, postconditioning and pharmacological conditioning against I/R injury upon aging [21, 33]. Similarly, we observed loss of cardioprotection in aged CYP2J2 Tr mice. Interestingly, aged sEH null mice and t-AUCB treated age CYP2J2 Tr mice demonstrated improved postischemic left ventricular function. Together, our results suggest that EETs are cardioprotective in aged animals and inhibition of sEH can be an effective strategy to induce cardioprotection in aged myocardium.
Several factors may be involved in loss of cardioprotection in aged CYP2J2 Tr mice including increased epoxide hydrolase activity, increased production of detrimental molecules and impaired response to cardioprotective stimuli. Studies demonstrated increased epoxide hydrolase expression in the soluble fraction of the left ventricle of middle aged and aged rats [28]. Therefore, we hypothesized that increased metabolism of EETs due to increased epoxide hydrolase activity is responsible for the loss of cardioprotection in aged CYP2J2 Tr mice. Improved postischemic function in aged CYP2J2 Tr mice following perfusion with t-AUCB further supported the hypothesis. However, no difference in sEH expression or activity in the present study suggested the involvement of factors other than increased activity of sEH in the loss of cardioprotection.
CYP2J2 can effectively metabolize linoleic acid to EpOMEs, which have been shown to produce adverse cardiac effects in various animal models [34–36]. In anaesthetized dog model, infusion of EpOMEs causes significant reduction in aortic flow, aortic pressure, contractility and heart rate in dose dependent manner [35]. However, in absence of sEH activity, EpOME do not exert their cytotoxic effects [37]. Reports suggest that the toxic effects of EpOMEs are due to their metabolites, DiHOME, and therefore EpOMEs are considered as protoxins [36–38]. DiHOMEs but not EpOMEs inhibits electrical activity in isolated rat ventricular myocytes [36]. In addition, sEH null mice have been demonstrated to have significantly higher EpOME:DiHOME ratios and are protected against I/R injury suggesting that DiHOMEs can be detrimental to the heart [10]. In experimental models of myocardial ischemia, DiHOME levels rise from 1–3 µg/g of tissue to 4–22 µg/g of tissue [39]. DiHOMEs but not EpOME depress Na+ channels, K+ channels and trigger mitochondrial dysfunction [36, 40, 41]. DiHOMEs are responsible for a massive burst of ROS in vascular endothelial cells [42]. Furthermore, perfusion of isolated mice hearts with DiHOME results in decreased postischemic functional recovery suggesting DiHOME may contribute to the detrimental effects of myocardial ischemia [16]. In the present study, higher levels of DiHOME in aged CYP2J2 Tr mice correlate with loss of cardioprotection, while aged sEH null mice had lower levels of DiHOME and better postischemic cardiac function. This data suggests that inhibition of sEH can prevent the formation of DiHOMEs and protect the heart from the detrimental effects of DiHOMEs. Improved postischemic functional recovery in t-AUCB treated aged CYP2J2 Tr mice, further suggests that DiHOMEs are involved in the loss of cardioprotection in aged CYP2J2 mice. Therefore, inhibiting sEH can increase EET-levels and potentially prevent the formation of DiHOME, which both can contribute to cardioprotection in aged mice.
Interestingly, we observed higher levels of 12,13-DiHOME and not 9,10-DiHOME, indicating a regioisomer specific increase in metabolism. Data from previous studies have demonstrated that CYP2J2 produces more 12,13-EpOME as compared to 9,10-EpOME [9, 43]. Using recombinant enzymes, Moran et al. demonstrated that CYP2J2 metabolizes linoleic acid in regioselective manner and produces 12,13-EpOME: 9,10-EpOME in 3:2 ratio [43]. Similarly, higher levels of 12,13-DiHOME, but not 9,10-DiHOME, were observed in the media of cardiomyocytes isolated from the CYP2J2 Tr mice as compared to WT [9]. This data further suggest that the cardiomyocyte specific overexpression of CYP2J2 increases production of the specific regioisomer, 12,13-EpOME. Therefore, in the present study, the detriments effects of the DiHOME are attributed to specific 12,13-DiHOME regioisomer.
Adverse effects of CYP epoxygenase activity have been demonstrated in I/R heart studies suggesting an increased production of ROS results from uncoupling of CYP reactions. For example, inhibition of CYP2C by sulphaphenazole reduces infarct size in isolated rat hearts. The protective effects are due to suppression of CYP2C-induced ROS production during reperfusion and reduce tissue damage [44]. Recently, Edin et al. reported increased generation of 8-iso-PGF2α in the cardiac perfusate of mice with endothelial cell specific expression of human CYP2C8 (Tie2-CYP2C8) [16]. Moreover, 8-iso-PGF2α was associated with decreased postischemic functional recovery in Tie2-CYP2C8 mice, which was improved after the treatment with the ROS scavenger (N-acetyl-cysteine; NAC) or the antioxidant enzyme (superoxide dismutase) [16]. Conversely, previous studies demonstrate a low tendency of CYP2J2 for generating ROS and inhibitory effects of EETs on ROS production [45, 46]. Consistent with these reports, young CYP2J2 Tr mice had significantly lower levels of protein carbonyl following I/R. However, aged CYP2J2 Tr mice had significantly higher protein carbonyl levels than age matched WT and young CYP2J2 Tr. Similarly, we observed that isolated heart perfusate from aged CYP2J2 Tr mice had increased levels in 8-iso-PGF2α suggesting higher oxidative stress in these mice. These data suggest that the aging process correlates with increased markers of oxidative stress in mice overexpressing cardiac CYP2J2.
EET-mediated signaling is known to cause activation of several protein kinases such as PI3K, Akt, PKCε and PKA, where kinase inhibition will significantly attenuate EET-mediated effects [1, 9, 10, 14]. In contrast, the current study demonstrated that EETs can activate PP2A, which can result in dephosphorylation and inhibition of protein kinases such as Akt, AMPK and Erk [47]. Limited evidence for EET activation of PP2A has been demonstrated in vascular smooth muscle cells [30, 31]. We observed loss of PP2A activation in aged CYP2J2 Tr mice that is consistent with the loss of EET-induced cardioprotection. Moreover, PP2A activation was evident in young and aged sEH null mice and aged CYP2J2 Tr mice treated with t-AUCB, demonstrating correlation between increased PP2A activity and improved postischemic functional recovery. Low PP2A activity in aged CYP2J2 Tr mice co-perfused with t-AUCB and MS-PPOH further confirms that decreased postischemic functional recovery due to inhibition of EET-synthesis involves decreased PP2A activity. Together, our results indicate that decreased PP2A activity in addition to DiHOME and ROS may be responsible for loss of cardioprotection in aged CYP2J2 Tr mice.
Conclusion
We report both young and aged sEH null mice are protected against I/R injury. While an abrogated cardioprotective response of EETs in aged CYP2J2 Tr mice is prevented by inhibition of sEH. Moreover, loss of cardioprotection in aged CYP2J2 Tr mice is due to increased DiHOME levels, oxidative stress and decreased PP2A activation all of which can be prevented by inhibition of sEH. Therefore, we conclude that while preconditioning and other pharmacological agents become ineffective in aged animal models, EETs and sEHi can protect the heart against I/R injury in aged mice.
Acknowledgments
Source of Funding
KRC is the recipient of the Doctoral Research Award from the Heart and Stroke Foundation of Canada and the Studentship award from the Alberta Innovates- Health Solutions. JMS is the recipient of a New Investigator Award from the Heart and Stroke Foundation of Canada and a Health Scholar Award from the Alberta Innovates- Health Solutions. This work was supported by grants from Heart and Stroke Foundation of Canada (JMS) and Canadian Institutes of Health Research (JMS).
Footnotes
Conflict of Interest
None declared.
References
- 1.Chaudhary KR, Batchu SN, Das D, Suresh MR, Falck JR, Graves JP, et al. Role of B-type natriuretic peptide in epoxyeicosatrienoic acid-mediated improved post-ischaemic recovery of heart contractile function. Cardiovasc Res. 2009;83:362–370. doi: 10.1093/cvr/cvp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu S, Moomaw CR, Tomer KB, Falck JR, Zeldin DC. Molecular cloning and expression of CYP2J2, a human cytochrome P450 arachidonic acid epoxygenase highly expressed in heart. J Biol Chem. 1996;271:3460–3468. doi: 10.1074/jbc.271.7.3460. [DOI] [PubMed] [Google Scholar]
- 3.Seubert JM, Zeldin DC, Nithipatikom K, Gross GJ. Role of epoxyeicosatrienoic acids in protecting the myocardium following ischemia/reperfusion injury. Prostaglandins Other Lipid Mediat. 2007;82:50–59. doi: 10.1016/j.prostaglandins.2006.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spector AA, Norris AW. Action of epoxyeicosatrienoic acids on cellular function. Am J Physiol Cell Physiol. 2007;292:C996–C1012. doi: 10.1152/ajpcell.00402.2006. [DOI] [PubMed] [Google Scholar]
- 5.Fang X, Weintraub NL, Oltman CL, Stoll LL, Kaduce TL, Harmon S, et al. Human coronary endothelial cells convert 14,15-EET to a biologically active chain-shortened epoxide. Am J Physiol Heart Circ Physiol. 2002;283:H2306–H2314. doi: 10.1152/ajpheart.00448.2002. [DOI] [PubMed] [Google Scholar]
- 6.Fang X, Weintraub NL, McCaw RB, Hu S, Harmon SD, Rice JB, et al. Effect of soluble epoxide hydrolase inhibition on epoxyeicosatrienoic acid metabolism in human blood vessels. Am J Physiol Heart Circ Physiol. 2004;287:H2412–H2420. doi: 10.1152/ajpheart.00527.2004. [DOI] [PubMed] [Google Scholar]
- 7.Schmelzer KR, Kubala L, Newman JW, Kim IH, Eiserich JP, Hammock BD. Soluble epoxide hydrolase is a therapeutic target for acute inflammation. Proc Natl Acad Sci U S A. 2005;102:9772–9777. doi: 10.1073/pnas.0503279102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decker M, Arand M, Cronin A. Mammalian epoxide hydrolases in xenobiotic metabolism and signalling. Arch Toxicol. 2009;83:297–318. doi: 10.1007/s00204-009-0416-0. [DOI] [PubMed] [Google Scholar]
- 9.Seubert J, Yang B, Bradbury JA, Graves J, Degraff LM, Gabel S, et al. Enhanced postischemic functional recovery in CYP2J2 transgenic hearts involves mitochondrial ATP-sensitive K+ channels and p42/p44 MAPK pathway. Circ Res. 2004;95:506–514. doi: 10.1161/01.RES.0000139436.89654.c8. [DOI] [PubMed] [Google Scholar]
- 10.Seubert JM, Sinal CJ, Graves J, DeGraff LM, Bradbury JA, Lee CR, et al. Role of soluble epoxide hydrolase in postischemic recovery of heart contractile function. Circ Res. 2006;99:442–450. doi: 10.1161/01.RES.0000237390.92932.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gross GJ, Baker JE, Hsu A, Wu HE, Falck JR, Nithipatikom K. Evidence for a role of opioids in epoxyeicosatrienoic acid-induced cardioprotection in rat hearts. Am J Physiol Heart Circ Physiol. 2010;298:H2201–H2207. doi: 10.1152/ajpheart.00815.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chaudhary KR, Abukhashim M, Hwang SH, Hammock BD, Seubert JM. Inhibition of soluble epoxide hydrolase by trans-4-[4-(3-adamantan-1-yl-ureido)-cyclohexyloxy]-benzoic acid is protective against ischemia-reperfusion injury. J Cardiovasc Pharmacol. 2010;55:67–73. doi: 10.1097/FJC.0b013e3181c37d69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motoki A, Merkel MJ, Packwood WH, Cao Z, Liu L, Iliff J, et al. Soluble epoxide hydrolase inhibition and gene deletion are protective against myocardial ischemia-reperfusion injury in vivo. Am J Physiol Heart Circ Physiol. 2008;295:H2128–H2134. doi: 10.1152/ajpheart.00428.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhanasekaran A, Gruenloh SK, Buonaccorsi JN, Zhang R, Gross GJ, Falck JR, et al. Multiple antiapoptotic targets of the PI3K/Akt survival pathway are activated by epoxyeicosatrienoic acids to protect cardiomyocytes from hypoxia/anoxia. Am J Physiol Heart Circ Physiol. 2008;294:H724–H735. doi: 10.1152/ajpheart.00979.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang YX, Zeng XJ, Lu LQ, Ma LQ, Jiang DQ, Mu J, et al. Effects of 11, 12-epoxyeicosatrienoic acid preconditioning and postconditioning on Ca(2+)- handling proteins in myocardial ischemia/reperfusion injury in rats. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2007;29:787–791. [PubMed] [Google Scholar]
- 16.Edin ML, Wang Z, Bradbury JA, Graves JP, Lih FB, DeGraff LM, et al. Endothelial expression of human cytochrome P450 epoxygenase CYP2C8 increases susceptibility to ischemia-reperfusion injury in isolated mouse heart. Faseb J. 2011;25:3436–3447. doi: 10.1096/fj.11-188300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bodiga S, Zhang R, Jacobs DE, Larsen BT, Tampo A, Manthati VL, et al. Protective actions of epoxyeicosatrienoic acid: dual targeting of cardiovascular PI3K and KATP channels. J Mol Cell Cardiol. 2009;46:978–988. doi: 10.1016/j.yjmcc.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaudhary KR, El-Sikhry HE, Seubert JM. Mitochondria and the aging heart. J Geriatr Cardiol. 2011;8:159–167. doi: 10.3724/SP.J.1263.2011.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu M, Zhang P, Chen M, Zhang W, Yu L, Yang XC, et al. Aging might increase myocardial ischemia / reperfusion-induced apoptosis in humans and rats. Age (Dordr) 2011 doi: 10.1007/s11357-011-9259-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mariani J, Ou R, Bailey M, Rowland M, Nagley P, Rosenfeldt F, et al. Tolerance to ischemia and hypoxia is reduced in aged human myocardium. J Thorac Cardiovasc Surg. 2000;120:660–667. doi: 10.1067/mtc.2000.106528. [DOI] [PubMed] [Google Scholar]
- 21.Boengler K, Schulz R, Heusch G. Loss of cardioprotection with ageing. Cardiovasc Res. 2009;83:247–261. doi: 10.1093/cvr/cvp033. [DOI] [PubMed] [Google Scholar]
- 22.Batchu SN, Law E, Brocks DR, Falck JR, Seubert JM. Epoxyeicosatrienoic acid prevents postischemic electrocardiogram abnormalities in an isolated heart model. J Mol Cell Cardiol. 2009;46:67–74. doi: 10.1016/j.yjmcc.2008.09.711. [DOI] [PubMed] [Google Scholar]
- 23.Baines CP, Zhang J, Wang GW, Zheng YT, Xiu JX, Cardwell EM, et al. Mitochondrial PKCepsilon and MAPK form signaling modules in the murine heart: enhanced mitochondrial PKCepsilon-MAPK interactions and differential MAPK activation in PKCepsilon-induced cardioprotection. Circ Res. 2002;90:390–397. doi: 10.1161/01.res.0000012702.90501.8d. [DOI] [PubMed] [Google Scholar]
- 24.Aboutabl ME, Zordoky BN, El-Kadi AO. 3-methylcholanthrene and benzo(a)pyrene modulate cardiac cytochrome P450 gene expression and arachidonic acid metabolism in male Sprague Dawley rats. Br J Pharmacol. 2009;158:1808–1819. doi: 10.1111/j.1476-5381.2009.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta. 2003;329:23–38. doi: 10.1016/s0009-8981(03)00003-2. [DOI] [PubMed] [Google Scholar]
- 26.Peart JN, See Hoe L, Pepe S, Johnson P, Headrick JP. Opposing effects of age and calorie restriction on molecular determinants of myocardial ischemic tolerance. Rejuvenation research. 2012;15:59–70. doi: 10.1089/rej.2011.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abete P, Ferrara N, Cioppa A, Ferrara P, Bianco S, Calabrese C, et al. Preconditioning does not prevent postischemic dysfunction in aging heart. Journal of the American College of Cardiology. 1996;27:1777–1786. doi: 10.1016/0735-1097(96)00070-8. [DOI] [PubMed] [Google Scholar]
- 28.Dai Q, Escobar GP, Hakala KW, Lambert JM, Weintraub ST, Lindsey ML. The left ventricle proteome differentiates middle-aged and old left ventricles in mice. J Proteome Res. 2008;7:756–765. doi: 10.1021/pr700685e. [DOI] [PubMed] [Google Scholar]
- 29.Thompson DA, Hammock BD. Dihydroxyoctadecamonoenoate esters inhibit the neutrophil respiratory burst. J Biosci. 2007;32:279–291. doi: 10.1007/s12038-007-0028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dimitropoulou C, West L, Field MB, White RE, Reddy LM, Falck JR, et al. Protein phosphatase 2A and Ca2+-activated K+ channels contribute to 11,12-epoxyeicosatrienoic acid analog mediated mesenteric arterial relaxation. Prostaglandins Other Lipid Mediat. 2007;83:50–61. doi: 10.1016/j.prostaglandins.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Imig JD, Dimitropoulou C, Reddy DS, White RE, Falck JR. Afferent arteriolar dilation to 11, 12-EET analogs involves PP2A activity and Ca2+-activated K+ Channels. Microcirculation. 2008;15:137–150. doi: 10.1080/10739680701456960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulman D, Latchman DS, Yellon DM. Effect of aging on the ability of preconditioning to protect rat hearts from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2001;281:H1630–H1636. doi: 10.1152/ajpheart.2001.281.4.H1630. [DOI] [PubMed] [Google Scholar]
- 33.Boengler K, Konietzka I, Buechert A, Heinen Y, Garcia-Dorado D, Heusch G, et al. Loss of ischemic preconditioning's cardioprotection in aged mouse hearts is associated with reduced gap junctional and mitochondrial levels of connexin 43. Am J Physiol Heart Circ Physiol. 2007;292:H1764–H1769. doi: 10.1152/ajpheart.01071.2006. [DOI] [PubMed] [Google Scholar]
- 34.Sisemore MF, Zheng J, Yang JC, Thompson DA, Plopper CG, Cortopassi GA, et al. Cellular characterization of leukotoxin diol-induced mitochondrial dysfunction. Arch Biochem Biophys. 2001;392:32–37. doi: 10.1006/abbi.2001.2434. [DOI] [PubMed] [Google Scholar]
- 35.Fukushima A, Hayakawa M, Sugiyama S, Ajioka M, Ito T, Satake T, et al. Cardiovascular effects of leukotoxin (9, 10-epoxy-12-octadecenoate) and free fatty acids in dogs. Cardiovasc Res. 1988;22:213–218. doi: 10.1093/cvr/22.3.213. [DOI] [PubMed] [Google Scholar]
- 36.Stimers JR, Dobretsov M, Hastings SL, Jude AR, Grant DF. Effects of linoleic acid metabolites on electrical activity in adult rat ventricular myocytes. Biochim Biophys Acta. 1999;1438:359–368. doi: 10.1016/s1388-1981(99)00064-5. [DOI] [PubMed] [Google Scholar]
- 37.Moghaddam MF, Grant DF, Cheek JM, Greene JF, Williamson KC, Hammock BD. Bioactivation of leukotoxins to their toxic diols by epoxide hydrolase. Nat Med. 1997;3:562–566. doi: 10.1038/nm0597-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zheng J, Plopper CG, Lakritz J, Storms DH, Hammock BD. Leukotoxin-diol: a putative toxic mediator involved in acute respiratory distress syndrome. Am J Respir Cell Mol Biol. 2001;25:434–438. doi: 10.1165/ajrcmb.25.4.4104. [DOI] [PubMed] [Google Scholar]
- 39.Dudda A, Spiteller G, Kobelt F. Lipid oxidation products in ischemic porcine heart tissue. Chem Phys Lipids. 1996;82:39–51. doi: 10.1016/0009-3084(96)02557-1. [DOI] [PubMed] [Google Scholar]
- 40.Harrell MD, Stimers JR. Differential effects of linoleic Acid metabolites on cardiac sodium current. J Pharmacol Exp Ther. 2002;303:347–355. doi: 10.1124/jpet.102.038166. [DOI] [PubMed] [Google Scholar]
- 41.Xiao YF, Kang JX, Morgan JP, Leaf A. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A. 1995;92:11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Viswanathan S, Hammock BD, Newman JW, Meerarani P, Toborek M, Hennig B. Involvement of CYP 2C9 in mediating the proinflammatory effects of linoleic acid in vascular endothelial cells. J Am Coll Nutr. 2003;22:502–510. doi: 10.1080/07315724.2003.10719328. [DOI] [PubMed] [Google Scholar]
- 43.Moran JH, Mitchell LA, Bradbury JA, Qu W, Zeldin DC, Schnellmann RG, et al. Analysis of the cytotoxic properties of linoleic acid metabolites produced by renal and hepatic P450s. Toxicology and applied pharmacology. 2000;168:268–279. doi: 10.1006/taap.2000.9053. [DOI] [PubMed] [Google Scholar]
- 44.Granville DJ, Tashakkor B, Takeuchi C, Gustafsson AB, Huang C, Sayen MR, et al. Reduction of ischemia and reperfusion-induced myocardial damage by cytochrome P450 inhibitors. Proc Natl Acad Sci U S A. 2004;101:1321–1326. doi: 10.1073/pnas.0308185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu L, Chen C, Gong W, Li Y, Edin ML, Zeldin DC, et al. Epoxyeicosatrienoic acids attenuate reactive oxygen species level, mitochondrial dysfunction, caspase activation, and apoptosis in carcinoma cells treated with arsenic trioxide. J Pharmacol Exp Ther. 2011;339:451–463. doi: 10.1124/jpet.111.180505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang B, Graham L, Dikalov S, Mason RP, Falck JR, Liao JK, et al. Overexpression of cytochrome P450 CYP2J2 protects against hypoxia-reoxygenation injury in cultured bovine aortic endothelial cells. Mol Pharmacol. 2001;60:310–320. doi: 10.1124/mol.60.2.310. [DOI] [PubMed] [Google Scholar]
- 47.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]