Abstract
We investigated the relationship between basal ganglia volume and treatment response to the atypical antipsychotic medication risperidone in unmedicated patients with schizophrenia. Basal ganglia volumes included the bilateral caudate, putamen, and pallidum and were measured using the Freesurfer automated segmentation pipeline in 23 subjects. Also, baseline symptom severity, duration of illness, age, gender, time off medication, and exposure to previous antipsychotic were measured. Treatment response was significantly correlated with all three regions of the bilateral basal ganglia (caudate, putamen, and pallidum), baseline symptom severity, duration of illness, and age but not gender, time off antipsychotic medication, or exposure to previous antipsychotic medication. The caudate volume was the basal ganglia region that demonstrated the strongest correlation with treatment response and was significantly negatively correlated with patient age. Caudate volume was not significantly correlated with any other measure. We demonstrated a novel finding that the caudate volume explains a significant amount of the variance in treatment response over the course of six-weeks of risperidone pharmacotherapy even when controlling for baseline symptom severity and duration of illness.
Keywords: Schizophrenia, Treatment response, Basal ganglia, Caudate, Risperidone, Magnetic resonance imaging (MRI)
1. Introduction
The schizophrenia patient population has a variable response to antipsychotic drugs (APDs). Between 20% and 30% of patients are categorized as treatment-resistant, showing little to no improvement following treatment with APDs (Conley and Buchanan, 1997). Considering that schizophrenia is a severe mental illness with a world-wide prevalence of approximately 1% (Jablensky et al., 1992), this variability in treatment response is a major problem in the field of psychiatry. Currently, it takes clinicians several weeks to decide if a treatment is ineffective and initiate an alternate treatment therapy (van den Oord et al., 2009). During these extended trial periods, patients can remain symptomatic and thus at increased risk of hospitalization (van den Oord et al., 2009). The discovery of neural biomarkers using non-invasive magnetic resonance imaging (MRI) to predict treatment response could improve both the speed and quality of treatment in psychiatric diseases such as schizophrenia. The first step in creating usable biomarkers for the clinic is to discover variables that are associated with variance in factors such as treatment response. Later these factors can be tested at the individual level in larger groups of patients using predictive techniques that employ bootstrapping techniques to yield metrics such as sensitivity and specificity. The scope of this article is not to conduct predictive modeling at the individual subject level but to further investigate if basal ganglia volume warrants such larger studies.
The goal of this study was to investigate whether the volumes of regions of the basal ganglia are associated with treatment response to an atypical APD in patients with schizophrenia. Volumes were measured from scans obtained before treatment, when patients were unmedicated, and treatment response was evaluated following 6 weeks of treatment with risperidone. Previous work has shown that the volumes of the subcortical regions included in the basal ganglia are associated with treatment response to APDs (Buchsbaum et al., 2003; Okugawa et al., 2007; Molina et al., 2010; Li et al., 2011; Molina et al., 2011). However, none of the previous articles have controlled for other factors that can also be used to explain variance in treatment response.
Treatment response to APD therapy has also been shown to be associated with baseline symptom severity (Bartkó et al., 1990; McEvoy et al., 1991; Cuesta et al., 1994; Crespo-Facorro et al., 2007), duration of psychotic illness (McEvoy et al., 1991; Keck et al., 1995), age (Keck et al., 1995), and gender (Ghafari et al., 2013; Seeman, 1997). Interestingly, lower severity of general psychopathology before exposure to medication is associated with poorer treatment response (Crespo-Facorro et al., 2007), a finding that is likely related to a floor effect. Studies have also indicated that shorter illness duration and younger age are associated with better treatment response (McEvoy et al., 1991; Keck et al., 1995). For these reasons, we investigated whether measurements of basal ganglia volume significantly improve the fit of a hierarchical multiple linear regression analysis that controls for these demographic variables.
We also took in consideration factors that could affect basal ganglia volume, such as time spent off APDs and exposure to previous APDs (Navari and Dazzan, 2009; Leung et al., 2011; Haijma et al., 2012). Since treatment with APDs can cause increases in basal ganglia volume (Navari and Dazzan, 2009; Leung et al., 2011; Haijma et al., 2012), we recorded exposure to previous APDs. We investigated patients’ time off APDs as well because it has been shown that volume changes associated with APD treatment are reversible after cessation of treatment (Meshul and Casey, 1989).
To our knowledge, no previous study has investigated whether subcortical volumes of the basal ganglia, as measured with a fully automated algorithm such as Freesurfer, are associated with response to a commonly prescribed atypical APD, risperidone. The Freesurfer technique has the advantage of reducing operator bias and also being a technique that could easily be transitioned into a clinical setting.
In this study, we investigated whether basal ganglia volume was able to significantly explain variance in treatment response after controlling for factors such as baseline symptom severity and duration of illness, and whether age, time off APDs, or exposure to previous APDs was related to basal ganglia volume or treatment response. Based on previous findings (Buchsbaum et al., 2003; Li et al., 2011), we hypothesized that (1) larger volume of basal ganglia regions (caudate, putamen, and pallidum) in unmedicated participants would be associated with greater treatment response over 6 weeks of APD treatment and (2) regions within the basal ganglia would account for a significant amount of variance in treatment response even after controlling for other non-neural variables.
2. Methods
2.1. Subjects
Thirty participants with a DSM-IV-TR defined diagnosis of schizophrenia or schizoaffective disorder, not currently taking antipsychotic medication (within 10 days), and seeking treatment at the University of Alabama at Birmingham were recruited for this study (Table 1). We did not ask patients to discontinue antipsychotic drugs to enroll in our study. Diagnoses were established using participants’ medical records and the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger et al., 1994). The diagnosis was made as a consensus reached by a board-certified psychiatrist and a trained Master’s level program manager. The program manager also administered the Brief Psychiatric Rating Scale (BPRS) (Overall and Gorham, 1962), which was used to characterize symptom severity and response to antipsychotic treatment. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) (Randolph, 2010) was used to characterize general cognitive function.
Table 1.
Demographic and clinical data of patients with schizophrenia.
| Mean (SD) n = 23 |
|
|---|---|
| Age (years) | 32.48 (11.24) |
| Age of onset (years) | 20.57 (5.43) |
| Gender (male/female) | 18/5 |
| Duration of illness (years) | 11.91 (9.53) |
| Parental SESa | 7.17 (5.32) |
| RBANS total indexb | 67.96 (11.51) |
| BPRSc Total | 46.52 (11.37) |
| BPRS Positived | 12.52 (4.21) |
| BPRS Negativee | 7.30 (2.65) |
| Antipsychotic medication naïve | 8/23 (34.8%) |
| Time since previous antipsychotic (months)f | 19.39 (38.62) |
| Previous atypical treatmentsg, h | 1.13 (1.39) |
| Previous typical treatmentsg,i | 0.48 (0.79) |
Parental socioeconomic status ranks determined from Diagnostic Interview for Genetic Studies, DIGS (1–18 scale); higher rank (lower numerical value) corresponds to higher socioeconomic status.
Repeatable Battery for the Assessment of Neuropsychological Status
Brief Psychiatric Rating Scale scores recorded at the baseline
Positive scale consisted of conceptual disorganization, hallucinations, unusual thought content, and suspiciousness.
Negative scale consisted of emotional withdrawal, motor retardation, and blunted affect.
Total amount of time since patient last took an antipsychotic medication, which was determined from the patients medical history.
Total number of times that the patient was previously treated with either typical or atypical antipsychotic medication.
Range of previous atypical treatments was 0 – 5 previous treatments.
Range of previous typical treatments was 0 – 3 previous treatments.
The exclusion criteria were other major medical conditions, substance abuse within the past 6 months, previous serious head injury, neurological disorders, loss of consciousness, and pregnancy. The University of Alabama at Birmingham Institutional Review Board approved the study, and all participants gave written informed consent. See Fig. 1 for a flowchart of the subject-exclusion process. Six subjects were excluded because they did not complete the 6-week treatment period, and one subject was excluded because the magnetic resonance imaging (MRI) data contained too much noise because of motion and could not be analyzed with the Freesurfer pipeline. This left a final group of 23 subjects with schizophrenia.
Fig. 1.
Flowchart showing criteria used to exclude subjects from study and reach final sample size.
2.2. Treatment response
Treatment response over the course of the 6-week period was measured with the BRPS total score. The total scale, which was administered by a trained rater, comprises 20 items scored on a 1–7 Likert scale. Treatment response was defined as the absolute change in BPRS total score from baseline (off medication) to week 6 of treatment. To correct for the patients’ differences in initial symptom severity, baseline BPRS total scores were entered in the first step of the hierarchical regression analysis.
2.3. Image analysis
Volumetric segmentation of structural MRI data was performed using the Freesurfer image analysis suite, which is documented and freely available online (version 4.5.0, http://surfer.nmr.mgh.harvard.edu). The main Freesurfer pipeline involves removal of non-brain tissue, registration to Talairach space, segmentation of subcortical white and gray matter, intensity normalization, identification of gray matter and white matter boundaries, topology correction, and registration to a spherical atlas.
Each of the resulting cortical maps was visually inspected to detect errors. Errors in the segmentation protocol were fixed using an automated algorithm, gcut, which adjusted the threshold boundary between brain and non-brain tissue such as dura mater and scalp. One subject who still had substantial mistakes after this step was excluded. The Freesurfer pipeline generated a total of 50 regional subcortical volumes. Only the three volumes of the basal ganglia (caudate, putamen, and pallidum) were analyzed for this study. Subcortical volumetric measures from the left and right hemisphere were averaged to reduce the number of comparisons. In total, three MRI volumes (caudate, putamen, and pallidum) from the Freesurfer output were used for further analysis. All subcortical volumes were normalized by each subject’s intracranial volume (Westman et al., 2012), which is based on an affine transform in Freesurfer. This segmentation approach has been used previously for biomarker discovery (Thambisetty et al., 2010).
2.4. Demographic variables analyzed
To test our second hypothesis that the volume of regions within the basal ganglia would explain variance in treatment response even after controlling additional variables, we measured baseline symptom severity and illness duration. Baseline symptom severity was measured as the BPRS total score at the baseline time period while patients were unmedicated. Illness duration was obtained based on the patient’s self-reported start of psychotic illness and by verifying this information against the patient’s medical records. The illness duration was determined by a trained program manager and a board-certified psychiatrist. Additional variables included age, gender, time off APD, and number of different atypical or typical APDs received. The number of antipsychotics previously received was determined by reviewing the patient’s medical records and by tallying up the number of medication trials (typical or atypical) the patient received since the beginning of the illness. Number of previous antipsychotic trials was used as an estimate for previous antipsychotic exposure because medical records did not allow for the calculation of chlorpromazine equivalents or a corresponding dose-years measurement (Andreasen et al., 2010). Correlations between each of these variables and both treatment response and basal ganglia volume were evaluated.
2.5. Statistical analysis
All data analyzed for this article were analyzed using the Statistical Package for the Social Sciences (SPSS) (version 20). All of the basal ganglia volumes and BPRS total scores were analyzed for outliers ≥ 3.3 standardized z-scores. The assumptions of multiple regression were tested, including normality, linearity, multicollinearity, and homoscedasticity. Pearson product-moment correlation coefficients were used to test the relationships between treatment response and basal ganglia volumes (bilateral caudate, left caudate, right caudate, bilateral putamen, and bilateral pallidum) as well as among the basal ganglia volumes. Also, correlations were used to test the relationship between treatment response and other factors (baseline symptom severity, duration of illness, caudate volume, age, gender, time off medication, and previous antipsychotic exposure) to determine which other non-volumetric factors should be controlled for in the following hierarchical multiple linear regression. Correlation analyses of the caudate volume with other factors were conducted to evaluate if any other variables measured were associated with baseline caudate volume.
The relationship between treatment response and gender was tested using an independent t-test. To assess laterality of the caudate, a paired sample t-test was used to compare the left and right caudate volumes. An independent-samples t-test was conducted to compare the caudate volumes for the medication-naïve and previously medicated subjects. The basal ganglia volume that had the most significant correlation with treatment response (caudate volume) was entered into the hierarchical multiple linear regression analysis. Hierarchical multiple linear regression analyses were used to test the association between the caudate volume and treatment response while controlling for baseline symptom severity and duration of illness.
3. Results
3.1. Subject demographics
The schizophrenia group analyzed in this study consisted mainly of previously medicated subjects (n = 15) but also contained antipsychotic-naïve subjects (n = 8) (Table 1). Also, all patients recruited for this study were off medication at study entry. Our study consisted mostly of male subjects (18/23). The mean time off medication was 19.4 months. None of the subjects showed BPRS total scores that were determined to be outlier values.
3.2. Correlations between treatment response and basal ganglia volumes
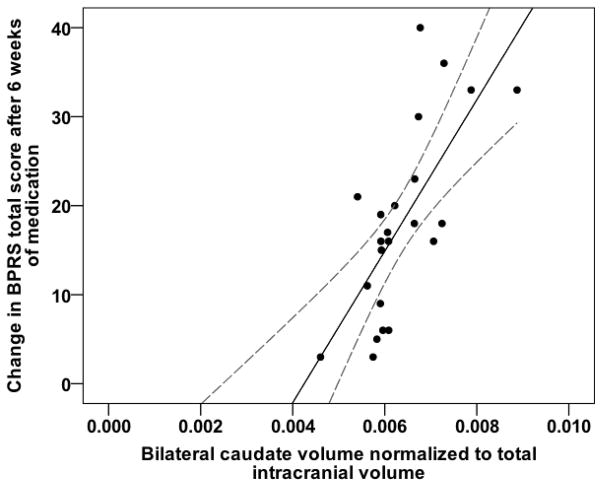
None of the basal ganglia volumes were determined to be outliers. Analyses revealed that there were no violations of the assumptions of normality, linearity, multicollinearity or homoscedasticity. The relationship between subcortical volumes and treatment response showed significant positive correlations for all volumes (bilateral caudate nucleus: r= 0.71, P < 0.005; left caudate: r = 0.66, P < 0005; right caudate: r = 0.75, P < 0.005; putamen: r = 0.61, P < 0.05; pallidum: r = 0.48, P < 0.05). Greater volume of the bilateral caudate before the start of antipsychotic treatment was associated with greater improvements in BPRS total scores (Fig. 2).
Fig. 2.
Scatter plot showing the positive correlation between the volume of the bilateral caudate and treatment response to antipsychotic medication. Solid line is a best-fit linear regression line and dashed lines are 95% confidence intervals around the mean.
The subcortical volumes also showed strong correlations among themselves: bilateral caudate-putamen (r = 0.81, P < 0.05), bilateral caudate-pallidum (r = 0.81, P < 0.05), left caudate-right caudate (r = 0.96, P < 0.001), and putamen-pallidum (r = 0.86, P < 0.05). To avoid collinearity, only the bilateral caudate, which had the one of the strongest correlations with treatment response, was entered into the following hierarchical multiple regression.
3.3. Relationship between treatment response, caudate volume, and other demographic factors
The main correlations of focus were the ones involving treatment response and other factors, which was the first row in Table 2. We found that treatment response was significantly positively correlated with baseline symptom severity and significantly negatively correlated with illness duration and age. Since illness duration and age were significantly correlated, but illness duration was more highly correlated with treatment response, we controlled for baseline symptom severity and illness duration in the hierarchical regression. Treatment response was not significantly correlated with time off APD or previous APD exposure. There was no significant difference in the treatment response scores for males (M = 16.22, SD = 9.72) and females (M = 24.40, SD = 12.58; t (21) = −1.57, P = 0.30, two-tailed). The magnitude of the differences in the means (mean difference = −8.18, 95% confidence interval = −19.03 to 2.68) was relatively large (eta squared = 0.11).
Table 2.
Pearson product-moment correlations between treatment response, caudate volume, and other factors.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. Treatment responsea | -- | 0.71* | −0.53* | −0.44* | 0.77* | 0.02 | 0.11 | −0.26 | −0.15 |
| 2. Caudate volumeb | -- | −0.42* | −0.41 | 0.63* | 0.00 | −0.05 | −0.22 | −0.21 | |
| 3. Duration of illness (years) | -- | 0.88* | −0.48* | 0.05 | 0.33 | 0.11 | 0.21 | ||
| 4. Age (years) | -- | −0.43* | 0.03 | 0.42* | 0.19 | 0.31 | |||
| 5. Baseline symptom severityc | -- | −0.04 | 0.01 | −0.37 | −0.31 | ||||
| 6. Time off medication (months)d | -- | −0.13 | −0.42 | −0.30 | |||||
| 7. Previous FGAse | -- | 0.40 | 0.72* | ||||||
| 8. Previous SGAsf | -- | 0.91* | |||||||
| 9. Total previous antipsychoticsg | -- |
BPRS Total score change from the baseline to week 6
Bilateral caudate volume normalized to total intracranial volume
BPRS Total score at the baseline before antipsychotic medication
Time since patients’ last antipsychotic medication treatment
Number of FGAs previously received
Number of SGAs previously received
Number of FGAs and/or SGAs previously received
P < 0.05
Abbreviations: FGA, first generation antipsychotics; SGA, second generation antipsychotics
The second row in Table 2 shows factors associated with variance in caudate volumes. The caudate volume was significantly negatively correlated with duration of illness and positively with baseline symptom severity. The caudate volume did not significantly correlate with any other factors including age, time off APD, or previous APD exposure.
3.4. Hierarchical multiple linear regression of treatment response
In the hierarchical multiple regression analysis, baseline symptom severity and duration of illness, which were entered at step 1, explained 62.9% of the variance in treatment response. After entry of the bilateral normalized caudate volume at Step 2, the total variance explained by the model as a whole was 69.9%, F(3, 19)= 14.68, P < 0.001. The caudate volume explained an additional 7.0% of the variance in the BPRS total change scores; R squared change = 0.07, F change (1, 19) = 4.39, P = 0.05.
3.5. Comparison of caudate volumes in previously medicated vs. medication-naïve subjects
There was a trend level difference in the bilateral caudate volumes for previously medicated (M = 0.0031, SD = 0.0004) versus medication-naïve subjects (M = 0.0034, SD = 0.0005; t (21) = −1.87, P = 0.08, two-tailed). The magnitude of the differences in the means (mean difference = −0.00035, 95% confidence interval = −0.0007 to 0.00004) was fairly large (eta squared = 0.14).
4. Discussion
In this study, we confirmed two hypotheses as follows: (1) volumes from three regions of the basal ganglia (caudate, putamen, and pallidum) showed significant associations with treatment response to an APD, and (2) volume of the caudate significantly contributed to the variance in treatment response after controlling for baseline symptom severity and duration of illness. Neither treatment response nor caudate volumes were related to time off APD or previous exposure to APD.
Our finding that basal ganglia volumes are associated with treatment response is consistent with previous work (Buchsbaum et al., 2003; Molina et al., 2003; Okugawa et al., 2007; Molina et al., 2010) (Table 3). Three of the previous studies (Buchsbaum et al., 2003; Okugawa et al., 2007; Molina et al., 2010) that have investigated the relationship between basal ganglia volumes and treatment response have found a similar positive correlation between larger basal ganglia volumes and treatment response. However, the direction of our result was not consistent with a more recent study (Molina et al., 2011), which showed that reduced volume of the putamen was associated with greater treatment response. This study’s use of a different volume-analysis technique (i.e., voxel-based morphometry (VBM) versus our use of Freesurfer) or treatment regimen (i.e., risperidone and olanzapine versus our use of risperidone only) could have contributed to the discrepancy. VBM is different from FreeSurfer in that the former classifies voxels as either gray matter, white matter, or cerebrospinal fluid, while the latter is based on surface-based representation and inter-subject registration (Winkler et al., 2010).
Table 3.
Summary of studies investigating relationship between the basal ganglia and treatment response to antipsychotics in schizophrenia
| Author (year)a | Sample sizea | Patient medication status | Segmentation method | Finding |
|---|---|---|---|---|
| Buchsbaum et al. (2003) | 37 | NT | Manual tracing | Patients with a better outcome to medication were characterized by larger relative mean putamen volumes. Caudate size was not related to treatment outcome. |
| Molina et al. (2003) | 19 | NT | Semi-automated segmentation routine | None of the brain measures predicted response to treatment |
| Okugawa et al. (2007) | 10 | NTb | Semi-automated segmentation routine | Patients with schizophrenia showed significant increases in caudate volume after treatment with olanzapine that was accompanied by significant reductions in symptom severity as measured by PANSS |
| Molina et al. (2010) | 44 | NT | Voxel-based morphometry (VBM) | Patients that responded poorly to antipsychotic medication had decreased grey matter in subcortical structures such as the bilateral striatum and in the thalamus. |
| Molina et al. (2011) | 30 | 25 NT and 5 NN | VBM | Inverse association between striatal size and the degree of clinical improvement. This finding was specifically localized to the bilateral putamen. |
| Geerts et al. (2012) | NAc | NT | NA | A detailed computer-based mechanistic disease model of schizophrenia that included striatal circuits including medium spiny neurons as components of the cortico-striatal-thalamo-cortical loop correctly predicted the lower performance of a highly selective low-affinity D2 experimental APD therapy. |
Articles are listed in the references section
Patients had not received medication for 1 year
Study was a mathematical simulation study.
Abbreviations: APD, antipsychotic drug; D2, D-2 dopamine receptor; NN, neuroleptic naïve; NT, neuroleptic treated; PANSS, Positive and Negative Syndrome Scale, symptom severity
While many early studies showed that antipsychotic treatment is associated with volumetric increases in the caudate (Breier et al., 1992; Chakos et al., 1994; Keshavan et al., 1994; Chakos et al., 1995), more recent studies have also identified volume reductions. A study of the second generation antipsychotic (SGA) quetiapine in antipsychotic-naïve patients found volume decreases in the striatum (caudate and putamen) after 6 months of treatment (Ebdrup et al., 2011). In addition, medication-naïve patients with schizophrenia appear to have smaller caudate volumes compared with healthy controls (Ebdrup et al., 2010). A recent review of 13 longitudinal MRI studies found that volumetric changes in the basal ganglia can vary considerably depending on the drug’s receptor-binding profile and the dosing regimen used (Ebdrup et al., 2013). Future work should focus on studying specific antipsychotic drugs rather than on the comparison between first and second generation APDs.
We did not find a correlation between patients’ basal ganglia volumes and the number of different APDs to which patients had been exposed. This metric may not have been a sensitive enough measure of patients’ cumulative exposure to APDs. A more precise metric is dose-years (Andreasen et al., 2010). In order to calculate this metric, one must calculate chlorpromazine equivalents for each medication, multiply each chlorpromazine equivalent by the dose period in years, and then add up all the resulting figures to yield a cumulative measure of dose-years exposure. Our data did not allow us to do that; we will plan to carry out this measure in future work.
To further explore the variance in basal ganglia volumes, we compared caudate volumes in previously medicated versus medication-naïve subjects, but we did not find significant differences. This finding, however, could have been the result of a lack of power. Indeed, large-scale studies trying to investigate the relationship between recent onset schizophrenia and brain structure are often conducted in medication-naïve subjects to avoid the confounding effects of previous antipsychotic exposure (Fusar-Poli et al., 2012).
The variance in basal ganglia volumes among the schizophrenia patients off-medication should be considered in light of the recent meta-analyses evaluating the effects of illness progression or medication exposure on brain volumes, including those of the basal ganglia in schizophrenia (Table 4). The studies focusing on illness progression suggest that, relative to findings in controls, caudate volumes are reduced in first episode patients with schizophrenia, while no reduction is observed in chronic patients (Ellison-Wright et al., 2008). An interpretation of this finding is that chronic patients, who have taken multiple courses of APD therapy, show increased basal ganglia volume. The meta-analyses which have focused on medication exposure indicate that striatal volumes are reduced before APD exposure and then are subsequently increased following therapy (Navari and Dazzan, 2009; Leung et al., 2011; Haijma et al., 2012). Longitudinal studies have shown that while first episode patients have decreased caudate volume, the volume may return to a normal level with antipsychotic treatment (Chua et al., 2009; Leung et al., 2011) or may even exceed that of healthy controls (Glahn et al., 2008).
Table 4.
Summary of brain volume meta-analyses in schizophrenia involving progression of illness or medication exposure
| Study goal | Author (year)a | Sample sizeb | Patient status | Contrast | Finding |
|---|---|---|---|---|---|
| Illness progression | Woods (2005) | 982 | FES and Chronic | HC vs. SZ | WB volume reduced |
| Steen (2006) | 1424 | FES | HC vs. FES | WB volume reduced and ventricular volume is increased | |
| Ellison-Wright (2008) | 1556 | FES and Chronic | HC vs. SZ | Bilateral reductions in caudate GM present in FES but not chronic SZ | |
| Glahn (2008) | 1195 | FES and Chronic | HC vs. SZ | Increased GM density in striatal regions | |
| Fornito (2009) | 1646 | FES and Chronic | HC vs. SZ | Reduced GM volume and concentration in frontal, temporal, thalamic and striatal regions | |
| Bora (2011) | 1999 | Chronic and FES | HC vs. SZ | Chronic illness associated with more severe GM abnormalities | |
| Chan (2011) | 466 | FES | HC vs. FES | Lower GM volume | |
| 808 | Chronic | Chronic vs. FES | More extensive GM volume reductions than FES group | ||
| Olabi (2011) | 928 | Chronic and FES | HC vs. SZ | SZ show significantly greater reductions in WB volume and WB GM volume over time | |
| De Peri (2012) | 45 studies | FES | FES vs. HC | Significant overall reduction in WB total GM | |
| Haijma (2012) | 8327 | Chronic and FES | HC vs. SZ | Larger GM reductions were associated with longer duration of illness | |
| Vita (2012) | 813 | Chronic and FES | HC vs. SZ | FES have more significant pattern of progressive loss of WB GM volume than Chronic SZ. GM volume modulated by percentage of patients taking atypical APDs | |
| Medication exposure | Navari (2009) | 33 Studies | NN and NT | NT vs. HC or NN | Antipsychotics, specifically typical, cause increased volume of structures within the basal ganglia in the NT group |
| 8327 | Medicated | HC vs. medicated | Total brain volume of grey matter structures were significantly decreased | ||
| Leung (2011) | 162 | NN-FES | HC vs. NN-FES | SZ showed GM deficits in subcortical regions including the striatum | |
| 336 | NT-FES | NN-FES vs. NT-FES | NT SZ showed GM deficits in caudate, cingulate, and inferior TL | ||
| Haijma (2013) | 771 | NN | NN vs. HC and NT | NN had more pronounced volume reductions in the caudate nucleus and thalamus than HC or NT | |
| Ebdrup (2013) | 13 studies | Medicated | Baseline vs. after medication | No studies found that specific FGAs induce basal ganglia volume increases. SGA therapy associated with volumetric increases and decreases in basal ganglia. |
Abbreviations: APD, antipsychotic; FES, first episode schizophrenia; FGA, first generation antipsychotic; GM, grey matter; HC, healthy controls; NN, neuroleptic naïve; NT, neuroleptic treated; SGA, second generation antipsychotic; STG, superior temporal gyrus; SZ, schizophrenia; TL, temporal lobe; WB, whole brain;
Articles are listed in the references section
Some of the articles only reported their total number of studies and not sample size.
The relationship between the basal ganglia volume and APD treatment response is supported by the high density of D2 dopamine (DA) receptors located in this region (Chua et al., 2009). The exact mechanism behind altered basal ganglia volume seen following APD therapy is unknown, but it has been suggested that it could be due to a combination of changes in synaptic plasticity (Konradi and Heckers, 2001) and possibly neurogenesis (Kippin et al., 2005). Alternations in synaptic plasticity, such as changes in synapse morphology and synapse number, have been seen with APD administration (Benes et al., 1985; Meshul and Casey, 1989; Uranova et al., 1991; Kerns et al., 1992; See et al., 1992; Konradi and Heckers, 2001), and these micro-level changes could sum up to produce macro-level changes in regional brain volume. With regard to neurogenesis, work in the rat has shown that APDs can cause significant increases in both the total number and density of newly generated neurons and glia in the dorsal striatum (Wang et al., 2004). Wang and colleagues acknowledge that the total number of these newly generated neurons, while significant, is small and may not be related to the therapeutic effects of APD treatment. The volume changes seen in the basal ganglia following APD therapy are likely downstream effects of D2 receptor blockade.
This study’s finding that the caudate volume explained an additional 7% of variance in treatment response was significant, but additional work is needed to determine how this finding can be translated into clinical relevance. Future studies that investigate basal ganglia volume in larger groups of subjects (containing both medication-naïve and chronic patients) by employing individual-level predictive modeling will be necessary before measures of caudate volume can be more definitively evaluated as a biomarker with clinical utility. Also, it is important to consider that future prediction of treatment response in schizophrenia will likely be based on multiple demographic factors and biomarkers (e.g., age, duration of illness, genetics, neuroimaging data, cerebrospinal fluid, and blood markers) and not one modality in isolation. Therefore, even though the finding in this article explained only a small amount of the variance in clinical response, it could still prove useful in future models that incorporate multiple pieces of data.
Our study was limited in that it consisted of a group of patients with schizophrenia who were heterogeneous in their medication exposure (65.2% had previously received antipsychotic medication). It is therefore unclear whether these results could be replicated in a group of medication-naïve patients.
In conclusion, we found that the volume of regions within the basal ganglia, measured when patients are unmedicated, is associated with treatment response to antipsychotic medication and that caudate volume, after controlling for baseline symptom severity and duration of illness, significantly explains a proportion of the observed variance in treatment response.
Acknowledgments
This work was supported by a National Institute of Mental Health Grant R01 MH081014 (ACL). We thank all of the volunteers with schizophrenia who took part in this project, as well as the staff of the Community Psychiatry Program at the University of Alabama at Birmingham. In addition, we thank Jacquelynn N. Copeland and Emily C McKinley for their advice on how to use the Freesurfer software. N.H. thanks the Howard Hughes Medical Institute’s Medical to Graduate Initiative for providing him with first year funding and ongoing training in translational research and acknowledges the UAB Ireland Travel Scholarship for enabling him to travel to Massachusetts General Hospital to be trained in the Freesurfer software.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biological Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkó G, Frecska E, Horváth S, Zádor G, Arató M. Predicting neuroleptic response from a combination of multilevel variables in acute schizophrenic patients. Acta Psychiatrica Scandinavica. 1990;82:408–412. doi: 10.1111/j.1600-0447.1990.tb03070.x. [DOI] [PubMed] [Google Scholar]
- Benes FM, Paskevich PA, Davidson J, Domesick VB. The effects of haloperidol on synaptic patterns in the rat striatum. Brain Research. 1985;329:265–273. doi: 10.1016/0006-8993(85)90532-3. [DOI] [PubMed] [Google Scholar]
- Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ, Yücel M, Velakoulis D, Pantelis C. Neuroanatomical abnormalities in schizophrenia: A multimodal voxelwise meta-analysis and meta-regression analysis. Schizophrenia Research. 2011;127:46–57. doi: 10.1016/j.schres.2010.12.020. [DOI] [PubMed] [Google Scholar]
- Breier A, Buchanan RW, Elkashef A, Munson RC, Kirkpatrick B, Gellad F. Brain morphology and schizophrenia: A magnetic resonance imaging study of limbic, prefrontal cortex, and caudate structures. Archives of General Psychiatry. 1992;49:921–926. doi: 10.1001/archpsyc.1992.01820120009003. [DOI] [PubMed] [Google Scholar]
- Buchsbaum MS, Shihabuddin L, Brickman AM, Miozzo R, Prikryl R, Shaw R, Davis K. Caudate and putamen volumes in good and poor outcome patients with schizophrenia. Schizophrenia Research. 2003;64:53–62. doi: 10.1016/s0920-9964(02)00526-1. [DOI] [PubMed] [Google Scholar]
- Chakos MH, Lieberman JA, Bilder RM, Borenstein M, Lerner G, Bogerts B, Wu H, Kinon B, Ashtari M. Increase in caudate nuclei volumes of first-episode schizophrenic patients taking antipsychotic drugs. American Journal of Psychiatry. 1994;151:1430–1436. doi: 10.1176/ajp.151.10.1430. [DOI] [PubMed] [Google Scholar]
- Chakos M, Lieberman J, Alvir J, Bilder R, Ashtari M. Caudate nuclei volumes in schizophrenic patients treated with typical antipsychotics or clozapine. The Lancet. 1995;345:456–457. doi: 10.1016/s0140-6736(95)90441-7. [DOI] [PubMed] [Google Scholar]
- Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophrenia Bulletin. 2011;37:177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua SE, Deng Y, Chen EY, Law CW, Chiu CP, Cheung C, Wong JC, Lienenkaemper N, Cheung V, Suckling J, McAlonan GM. Early striatal hypertrophy in first-episode psychosis within 3 weeks of initiating antipsychotic drug treatment. Psychological Medicine. 2009;39:793–800. doi: 10.1017/S0033291708004212. [DOI] [PubMed] [Google Scholar]
- Conley RR, Buchanan RW. Evaluation of treatment-resistant schizophrenia. Schizophrenia Bulletin. 1997;23:663–674. doi: 10.1093/schbul/23.4.663. [DOI] [PubMed] [Google Scholar]
- Crespo-Facorro B, Pelayo-Terán JM, Pérez-Iglesias R, Ramírez-Bonilla M, Martínez-García O, Pardo-García G, Vázquez-Barquero JL. Predictors of acute treatment response in patients with a first episode of non-affective psychosis: Sociodemographics, premorbid and clinical variables. Journal of Psychiatric Research. 2007;41:659–666. doi: 10.1016/j.jpsychires.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Cuesta MJ, Peralta V, De Leon J. Schizophrenic syndromes associated with treatment response. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 1994;18:87–99. doi: 10.1016/0278-5846(94)90026-4. [DOI] [PubMed] [Google Scholar]
- De Peri L, Crescini A, Deste G, Fusar-Poli P, Sacchetti E, Vita A. Brain structural abnormalities at the onset of schizophrenia and bipolar disorder: a meta-analysis of controlled magnetic resonance imaging studies. Current Pharmaceutical Design. 2012;18:486–494. doi: 10.2174/138161212799316253. [DOI] [PubMed] [Google Scholar]
- Ebdrup BH, Glenthøj B, Rasmussen H, Aggernaes B, Langkilde AR, Paulson O, Lublin H, Skimminge AB, Baaré W. Hippocampal and caudate volume reductions in antipsychotic-naive first-episode schizophrenia. Journal of Psychiatry and Neuroscience. 2010;35:95–104. doi: 10.1503/jpn.090049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebdrup BH, Norbak H, Borgwardt S, Glenthøj B. Volumetric changes in the basal ganglia after antipsychotic monotherapy: a systematic review. Current Medicinal Chemistry. 2013;20:438–447. doi: 10.2174/0929867311320030015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebdrup BH, Skimminge A, Rasmussen H, Aggernaes B, Oranje B, Lublin H, Baaré W, Glenthøj B. Progressive striatal and hippocampal volume loss in initially antipsychotic-naive, first-episode schizophrenia patients treated with quetiapine: relationship to dose and symptoms. The International Journal of Neuropsychopharmacology. 2011;14:69–82. doi: 10.1017/S1461145710000817. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. American Journal of Psychiatry. 2008;165:1015–1023. doi: 10.1176/appi.ajp.2008.07101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A, Yücel M, Patti J, Wood SJ, Pantelis C. Mapping grey matter reductions in schizophrenia: an anatomical likelihood estimation analysis of voxel-based morphometry studies. Schizophrenia Research. 2009;108:104–113. doi: 10.1016/j.schres.2008.12.011. [DOI] [PubMed] [Google Scholar]
- Fusar-Poli P, Radua J, McGuire P, Borgwardt S. Neuroanatomical maps of psychosis onset: voxel-wise meta-analysis of antipsychotic-naive VBM Studies. Schizophrenia Bulletin. 2012;38:1297–1307. doi: 10.1093/schbul/sbr134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts H, Spiros A, Roberts P, Twyman R, Alphs L, Grace AA. Blinded prospective evaluation of computer-based mechanistic schizophrenia disease model for predicting drug response. PLoS ONE. 2012;7:e49732. doi: 10.1371/journal.pone.0049732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghafari E, Fararouie M, Shirazi HG, Farhangfar A, Ghaderi F, Mohammadi A. Combination of estrogen and antipsychotics in the treatment of women with chronic schizophrenia. Clinical Schizophrenia & Related Psychoses. 2013;6:172–176. doi: 10.3371/CSRP.GHFA.01062013. [DOI] [PubMed] [Google Scholar]
- Glahn DC, Laird AR, Ellison-Wright I, Thelen SM, Robinson JL, Lancaster JL, Bullmore E, Fox PT. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biological Psychiatry. 2008;64:774–781. doi: 10.1016/j.biopsych.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haijma SV, Van Haren N, Cahn W, Koolschijn PCMP, Hulshoff Pol HE, Kahn RS. Brain volumes in schizophrenia: a meta-analysis in over 18,000 subjects. Schizophrenia Bulletin. 2013;39 (5):1129–1138. doi: 10.1093/schbul/sbs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablensky A, Sartorius N, Ernberg G, Anker M, Korten A, Cooper JE, Day R, Bertelsen A. Schizophrenia manifestations, incidence and course in different cultures. A World Health Organization ten-country study. Psychological Medicine. 1992;22:1–97. doi: 10.1017/s0264180100000904. [DOI] [PubMed] [Google Scholar]
- Keck PE, Wilson DR, Strakowski SM, McElroy SL, Kizer DL, Balistreri TM, Holtman HM, DePriest M. Clinical predictors of acute risperidone response in schizophrenia, schizoaffective disorder, and psychotic mood disorders. Journal of Clinical Psychiatry. 1995;56:466–470. [PubMed] [Google Scholar]
- Kerns JM, Sierens DK, Kao LC, Klawans HL, Carvey PM. Synaptic plasticity in the rat striatum following chronic haloperidol treatment. Clinical Neuropharmacology. 1992;15:488–500. doi: 10.1097/00002826-199212000-00006. [DOI] [PubMed] [Google Scholar]
- Keshavan M, Bagwell W, Haas G, Sweeney J, Schooler N, Pettegrew J. Changes in caudate volume with neuroleptic treatment. Lancet. 1994;344:1434–1434. doi: 10.1016/s0140-6736(94)90599-1. [DOI] [PubMed] [Google Scholar]
- Kippin TE, Kapur S, van der Kooy D. Dopamine specifically inhibits forebrain neural stem cell proliferation, suggesting a novel effect of antipsychotic drugs. Journal of Neuroscience. 2005;25:5815–5823. doi: 10.1523/JNEUROSCI.1120-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konradi C, Heckers S. Antipsychotic drugs and neuroplasticity: insights into the treatment and neurobiology of schizophrenia. Biological Psychiatry. 2001;50:729–742. doi: 10.1016/s0006-3223(01)01267-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung M, Cheung C, Yu K, Yip B, Sham P, Li Q, Chua S, McAlonan G. Gray matter in first-episode schizophrenia before and after antipsychotic drug treatment. Anatomical likelihood estimation meta-analyses with sample size weighting. Schizophrenia Bulletin. 2011;37:199–211. doi: 10.1093/schbul/sbp099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen Z, Deng W, He Z, Wang Q, Jiang L, Ma X, Wang Y, Chua SE, Cheung C, McAloan GM, Sham PC, Collier DA, Gong Q, Li T. Volume increases in putamen associated with positive symptom reduction in previously drug-naive schizophrenia after 6 weeks antipsychotic treatment. Psychological Medicine. 2011;42:1475–1483. doi: 10.1017/S0033291711002157. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Schooler NR, Wilson WH. Predictors of therapeutic response to haloperidol in acute schizophrenia. Psychopharmacology Bulletin. 1991;27:97–101. [PubMed] [Google Scholar]
- Meshul CK, Casey DE. Regional, reversible ultrastructural changes in rat brain with chronic neuroleptic treatment. Brain Research. 1989;489:338–346. doi: 10.1016/0006-8993(89)90867-6. [DOI] [PubMed] [Google Scholar]
- Molina V, Hernández JA, Sanz J, Paniagua JC, Hernández AI, Martín C, Matías J, Calama J, Bote B. Subcortical and cortical gray matter differences between Kraepelinian and non-Kraepelinian schizophrenia patients identified using voxel-based morphometry. Psychiatry Research: Neuroimaging. 2010;184:16–22. doi: 10.1016/j.pscychresns.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Molina V, Martín C, Ballesteros A, Herrera AS, Hernández-Tamames J. Optimized voxel brain morphometry: association between brain volumes and the response to atypical antipsychotics. European Archives of Psychiatry and Clinical Neuroscience. 2011;261:407–416. doi: 10.1007/s00406-010-0182-2. [DOI] [PubMed] [Google Scholar]
- Molina V, Reig S, Pascau J, Sanz J, Sarramea F, Gispert JD, Luque R, Benito C, Palomo T, Desco M. Anatomical and functional cerebral variables associated with basal symptoms but not risperidone response in minimally treated schizophrenia. Psychiatry Research: Neuroimaging. 2003;124:163–175. doi: 10.1016/s0925-4927(03)00107-0. [DOI] [PubMed] [Google Scholar]
- Navari S, Dazzan P. Do antipsychotic drugs affect brain structure? A systematic and critical review of MRI findings. Psychological Medicine. 2009;39:1763–1777. doi: 10.1017/S0033291709005315. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Blehar MC, Kaufmann CA, York-Cooler C, et al. Diagnostic Interview for Genetic Studies: rationale, unique features, and training. Archives of General Psychiatry. 1994;51:849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Okugawa G, Nobuhara K, Takase K, Saito Y, Yoshimura M, Kinoshita T. Olanzapine increases grey and white matter volumes in the caudate nucleus of patients with schizophrenia. Neuropsychobiology. 2007;55:43–46. doi: 10.1159/000103575. [DOI] [PubMed] [Google Scholar]
- Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biological Psychiatry. 2011;70:88–96. doi: 10.1016/j.biopsych.2011.01.032. [DOI] [PubMed] [Google Scholar]
- Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Randolph C. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): utility as an outcome measure in clinical trials for MCI and mild dementia. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2010;6:486–487. [Google Scholar]
- See RE, Chapman MA, Meshul CK. Comparison of chronic intermittent haloperidol and raclopride effects on striatal dopamine release and synaptic ultrastructure in rats. Synapse. 1992;12:147–154. doi: 10.1002/syn.890120208. [DOI] [PubMed] [Google Scholar]
- Seeman MV. Psychopathology in women and men: focus on female hormones. American Journal of Psychiatry. 1997;154:1641–1647. doi: 10.1176/ajp.154.12.1641. [DOI] [PubMed] [Google Scholar]
- Steen RG, Mull C, McClure R, Hamer RM, Lieberman JA. Brain volume in first-episode schizophrenia: systematic review and meta-analysis of magnetic resonance imaging studies. British Journal of Psychiatry. 2006;188:510–518. doi: 10.1192/bjp.188.6.510. [DOI] [PubMed] [Google Scholar]
- Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, Wahlund LO, Westman E, Kinsey A, Güntert A, Proitsi P, Powell J, Causevic M, Killick R, Lunnon K, Lynham S, Broadstock M, Choudhry F, Howlett DR, Williams RJ, Sharp SI, Mitchelmore C, Tunnard C, Leung R, Foy C, O’Brien D, Breen G, Furney SJ, Ward M, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Hodges A, Murphy DG, Parkins S, Richardson JC, Resnick SM, Ferrucci L, Wong DF, Zhou Y, Muehlboeck S, Evans A, Francis PT, Spenger C, Lovestone S. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer disease. Archives of General Psychiatry. 2010;67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tost H, Braus DF, Hakimi S, Ruf M, Vollmert C, Hohn F, Meyer-Lindenberg A. Acute D2 receptor blockade induces rapid, reversible remodeling in human cortical-striatal circuits. Nature Neuroscience. 2010;13:920–922. doi: 10.1038/nn.2572. [DOI] [PubMed] [Google Scholar]
- Uranova NA, Orlovskaya DD, Apel K, Klintsova AJ, Haselhorst U, Schenk H. Morphometric study of synaptic patterns in the rat caudate nucleus and hippocampus under haloperidol treatment. Synapse. 1991;7:253–259. doi: 10.1002/syn.890070402. [DOI] [PubMed] [Google Scholar]
- van den Oord EJCG, Adkins DE, McClay J, Lieberman J, Sullivan PF. A systematic method for estimating individual responses to treatment with antipsychotics in CATIE. Schizophrenia Research. 2009;107:13–21. doi: 10.1016/j.schres.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HD, Dunnavant FD, Jarman T, Deutch AY. Effects of antipsychotic drugs on neurogenesis in the forebrain of the adult rat. Neuropsychopharmacology. 2004;29:1230–1238. doi: 10.1038/sj.npp.1300449. [DOI] [PubMed] [Google Scholar]
- Westman E, Muehlboeck JS, Simmons A. Combining MRI and CSF measures for classification of Alzheimer’s disease and prediction of mild cognitive impairment conversion. NeuroImage. 2012;62:229–238. doi: 10.1016/j.neuroimage.2012.04.056. [DOI] [PubMed] [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage. 2010;53:1135–1146. doi: 10.1016/j.neuroimage.2009.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods BT, Ward KE, Johnson EH. Meta-analysis of the time-course of brain volume reduction in schizophrenia: implications for pathogenesis and early treatment. Schizophrenia Research. 2005;73:221–228. doi: 10.1016/j.schres.2004.05.014. [DOI] [PubMed] [Google Scholar]