Abstract
Women exposed to diethylstilbestrol (DES) in utero frequently develop vaginal adenosis, from which clear cell adenocarcinoma can arise. Despite decades of extensive investigation, the molecular pathogenesis of DES-associated vaginal adenosis remains elusive. Here we report that DES induces vaginal adenosis by inhibiting the BMP4/Activin A-regulated vaginal cell fate decision through a downregulation of RUNX1. BMP4 and Activin A produced by vaginal mesenchyme synergistically activated the expression of ΔNp63, thus deciding vaginal epithelial cell fate in the Müllerian duct epithelial cells (MDECs) via direct binding of SMADs on the highly conserved 5′sequence of ΔNp63. Therefore, mice in which Smad4 was deleted in MDECs failed to express ΔNp63 in vaginal epithelium and developed adenosis. This SMAD-dependent ΔNp63 activation required RUNX1, a binding partner of SMADs. Conditional deletion of Runx1 in the MDECs induced adenosis in the cranial portion of vagina, which mimicked the effect of developmental DES-exposure. Furthermore, neonatal DES exposure downregulated RUNX1 in the fornix of the vagina, where DES-associated adenosis is frequently found. This observation strongly suggests that the downregulation of RUNX1 is the cause of vaginal adenosis. However, once cell fate was determined, the BMP/Activin-SMAD/RUNX1 signaling pathway became dispensable for the maintenance of ΔNp63 expression in vaginal epithelium. Instead, the activity of the ΔNp63 locus in vaginal epithelium was maintained by a ΔNp63-dependent mechanism. This is the first demonstration of a molecular mechanism through which developmental chemical exposure causes precancerous lesions by altering cell fate.
Keywords: DES daughter, Vaginal clear cell adenocarcinoma, Endocrine disruptor, p63
Introduction
Diethylstilbestrol (DES) is a non-steroidal estrogen that was administered to pregnant women from the 1940s to the 1970s mainly for the prevention of miscarriages (FDA, 1972; Smith, 1948). It has been estimated that as many as 90% of women exposed to DES in utero develop cervical and vaginal adenoses, compared to less than 4% in the general population (Antonioli and Burke, 1975; Bibbo et al., 1975; Herbst et al., 1975; Johnson et al., 1979; Kaufman et al., 1977; Ng et al., 1975; O’Brien et al., 1979). Adenosis of the cervix and vagina is defined as the presence of columnar cells within the stratified squamous epithelium of the ectocervix and vagina, and is considered as a congenital anomaly that arises via a disruption of normal development. Vaginal adenosis itself is benign, but is believed to be a precursor of vaginal clear cell adenocarcinoma, which is 40 times more likely to occur in women exposed to DES in utero (DES daughters) (Hoover et al., 2011; Troisi et al., 2007). For this reason, the pathogenesis of DES-associated vaginal adenosis has been an important issue of public health. However, despite decades of extensive research, the molecular mechanism underlying the development of DES-associated vaginal adenosis remains elusive.
The epithelium of the female reproductive tract, including the uterus, cervix and vagina develops from columnar epithelium of the Müllerian duct (MD) (Kurita, 2010). As the MDs differentiate into distinctive organs, initially undifferentiated Müllerian duct epithelial cells (MDECs) are induced to express organ specific phenotypes by factors secreted from adjacent mesenchyme (Boutin et al., 1991; Cunha, 1976; Kurita et al., 2001a). For example, at birth, epithelial cells in the mouse uterus and vagina are mostly undifferentiated (Kurita et al., 2001a), and uterine and vaginal mesenchyme can program the epithelium of the neonatal uterus and vagina to differentiate into specialized uterine and vaginal epithelia, respectively. In mice, the developmental plasticity of the MDECs is gradually lost around postnatal day 5 (P5) and thereafter. Accordingly, epithelial cells from adult uterus and vagina cannot be reprogrammed by factors secreted from vaginal or uterine mesenchyme (Cunha, 1976; Kurita et al., 2001a, 2004). The molecular nature of the mesenchymal factors that induce stratified squamous differentiation of MDECs in the vagina and the downstream signaling pathways that mediate cell fate decision are yet to be determined.
The transformation related protein 63 gene (Trp63/TP63 in mouse/humans), an ortholog of the transformation related protein p53 (Trp53/TP53), plays a critical role in the cell fate decision of vaginal epithelium (Kurita et al., 2004). Trp63 can be transcribed from two distinct promoters generating two isoforms with alternative N-terminal sequences, the transactivating (TA) and the N-terminally truncated (ΔN) p63 (Yang et al., 1998). Its essential role in the formation of stratified squamous epithelium was clearly demonstrated by the phenotypes of Trp63 null mice, which completely lacked stratified squamous epithelium (Mills et al., 1999; Yang et al., 1999). In the vagina, Trp63 controls not only stratified squamous differentiation but also determines vaginal epithelial identity, thus allowing full expression of the various differentiation states characteristic of vaginal epithelium (e.g. cornification and mucification). Prior to squamous differentiation, undifferentiated MDECs express p63, and p63 determines the cell fate of MDECs to be vaginal epithelium. Hence, Trp63 null vaginal epithelium differentiates into simple columnar uterine epithelium within the vagina (Kurita et al., 2004). We have also demonstrated that DES action via ERα induces vaginal adenosis by blocking the p63 induction in MDECs (Kurita et al., 2004). However, the molecular mechanism of Trp63 induction as well as the mechanism through which DES/ERα disrupts this process is unknown.
In our current study, we demonstrated that the differentiation program of vaginal epithelium is regulated by ΔNp63. Hence, we investigated the molecular mechanism that controls ΔNp63 expression in MDECs. This report demonstrates that two transforming growth factor β (TGFβ) family ligands, activin A (ActA) and bone morphogenetic protein 4 (BMP4), induce the vaginal epithelial cell fate in undifferentiated MDECs via an activation of the ΔNp63 locus; that SMAD4 and RUNX1 are essential to mediate the action of ActA and BMP4 in the activation of ΔNp63; and that DES inhibits ΔNp63 expression and vaginal differentiation in MDECs by reducing RUNX1 expression. Furthermore, we demonstrate that the BMP4/ActA-SMAD/RUNX1 signaling pathway is required for activation of ΔNp63 locus but not for the maintenance of ΔNp63 expression in the vaginal epithelium. Data presented in this study describes the molecular mechanism through which MDECs are specialized to become vaginal epithelium, and by doing so, this study gives the first insight on how in utero DES exposure causes pre-malignant lesions in the female genital tract.
Materials and methods
Mouse models
All procedures involving mice were approved by the Northwestern University Animal Care and Use Committee. The following mice were used in this study: Trp63floxN (Mills et al., 2002), ΔNp63-EGFP knock-in (Trp63 ΔNp63-EGFP-KI) (Romano et al., 2012), Wnt7a-Cre (Winuthayanon et al., 2010), Smad4flox (Yang et al., 2002), Pax2-Cre (MMRRC) (Ohyama and Groves, 2004), Pax8-rtTA (Traykova-Brauch et al., 2008), tetO-cre (Perl et al., 2002), RosaTE [Gt(ROSA)26Sortm4 (ACTB-tdTomato,–EGFP)Luo/J] (Muzumdar et al., 2007), Runx1flox (Taniuchi et al., 2002), NSG (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ), C56BL/6 (Jackson Laboratory, Bar Harbor, ME, USA), CD-1 (Charles River Laboratories, Roanoke, IL, USA). Lines originally in the mixed genetic background were crossed to wild-type C57BL/6J at least 3 times before experiments. All Cre strains were maintained by breeding with C57BL/6J. With some exceptions, conditional knockout lines were crossed with the RosaTE to verify appropriate Cre expression.
Drug treatments
Dorsomorphin
Dorsomorphin (Sigma-Aldrich, St. Louis, MO, USA), a kinase inhibitor of type I receptors for BMPs (ALK2, 3 and 6), was dissolved in dimethyl sulfoxide (DMSO) (Life Technologies, Carlsbad, CA, USA) at 10 mg/ml and mixed with peanut oil (Sigma-Aldrich) to 1 mg/ml before injection. The first injection started within 8 h after birth, and newborn mice received two daily injections of 5 mg/kg (5 ml/kg) dorsomorphin subcutaneously for 5 days (N=6). The control group (N=5) received the equal amount of DMSO+peanut oil, and the DES group (N=6), the positive control for ΔNp63 disruption, received 1 mg/kg DES in the same vehicle subcutaneously in the same schedule. Doxycycline (DOX): Mice were fed with water containing 500 μg/ml doxycy-cline hydrochloride hemiethanolate hemihydrate (Sigma-Aldrich) and librium. 17β-Estradiol: Six-months-old cKO mice received daily i.p. injection of 100 ng 17β-estradiol for 3 days. Diethylstilbestrol (DES): A 100 μg DES slow-release pellet was prepared as follows. Silastic tubing (Dow Corning, Elizabethtown, KY, USA) filled with DES powder (Sigma-Aldrich) at ~0.04 mg/mm was prepared by filling the tubing with 10 mg/ml DES solution in ethanol and allowing ethanol to evaporate through the wall of the tubing. The DES filled tubing was cut into 2.5 mm length and subcutaneously injected into newborn mice. As assessed by the degree of ΔNp63 repression at P3-5, the Silastic pellet technique was more effective and consistent than the standard protocol with daily injections of 5 μg DES in 20 μl peanut oil (Forsberg, 1972; Newbold and McLachlan, 1982; Plapinger and Bern, 1979).
Microarray
Total RNA was extracted from the vaginae of P2 Trp63 cHet and cKO, and uteri of P2 Trp63 cHet females. Twelve samples (3 groups of 4 replicates each taken from 4 to 6 mice) were hybridized to two Illumina MouseWG-6 version2 slides. The array data were average normalized (GSE44697), and then further analyzed at the Genomic Core at Northwestern University (Table S1).
Hanging droplet culture of uterine piece
Uterine horns dissected from P1 mice were cleaned in cold DMEM/Hams F-12 50:50 medium supplemented with antibiotic-antimycotic (Life Technologies). Each uterine horn was cut into 3 pieces. Then, each piece was placed in the top of 100 μl PCR tube, and the PCR tube top was filled with 80 μl of serum free culture medium (DMEM/Hams F-12 50:50 medium with Insulin-Transferrin-Selenium-G Supplement) (Life Technologies) containing 0.1 or 0.2 μg/ml recombinant mouse BMP4 and/or Activin A (R&D system, Minneapolis, MN, USA). The tube-tops containing the uterine piece and medium were placed on a 96 well plate to cover wells. The wells were filled with the culture medium to maintain the humidity, the plate was covered with the lid, placed in a CO2 incubator, and cultured for 4 days with twice daily medium changes. After 4 days, uterine pieces were fixed and processed for histological analysis.
Immunostaining
Tissue collected for immunohistochemistry was fixed in 4% paraformaldehyde or Modified Davidson’s fixative solution (Electron Microscopy Sciences, Hatfield, PA, USA) overnight. Tissue was processed, embedded in paraffin and sectioned at 5 μm. Slides were deparaffinized by soaking into pre-warmed 1.3% Palmolive Ultra Original Concentrated (Colgate, New York, NY, USA), immersed into antigen-retrieval buffer (10 mM Sodium Citrate, 0.05% Tween-20, pH 6.0) and heated in the Deni Electric Pressure Cooker (Keystone Manufacturing Co, Buffalo, NY, USA) for 45 min. For the detection of both ΔN and TAp63 isoforms (labeled as p63 in figures), anti-p63 mouse IgG (clone 4A4) (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) was used. In addition, the following primary antibodies were used at the indicated concentration: anti-ΔNp63 (PC373) (1:2000); anti-pSMAD2/3 (anti-pSMAD2, cross reacts with pSMAD3) (1:400, Millipore, Billerica, MA, USA); anti-Cytokeratin 14 (LL001, 1:50), anti-GFP (1:100), (Santa Cruz Biotechnology); anti-p(S463/465) SMAD1/5/8 (1:100, Cell Signaling Technology, Danvers, MA, USA); anti-PR (1:100, DAKO); anti-RUNX1 (1:100, Epitomics, Burlingame, CA, USA); anti-SMAD4 (1:25); anti-Ki-67/MKI67 (1:100, Abcam, Cambridge, MA, USA); anti-ERα (1:100, Lab Vision Co., Fremont, CA, USA). Secondary antibodies (AlexaFluor594-anti-mouse IgG, AlexaFluor488-anti-rabbit IgG, and biotinylated anti-mouse or anti-rabbit IgG) and Streptavidin-HRP were purchased from Jackson ImmunoResearch (West Grove, PA, USA). Micrographs were captured using a BZ-9000 microscope (Keyence, Itasca, IL, USA). Images were captured on the same day using the same exposure settings. The quantitative analysis on the squamous transformation of uterine epithelium was performed on immunostained slides for K14 using ImageJ (NIH, MD, USA) as previously reported (Kurita et al., 2001b). Briefly, all images were captured under the same lighting conditions. The epithelial areas were manually selected from the images. Then, K14-positive epithelial areas were selected in the combination of yellow and black channels in CMYK mode as the area with optical density higher than that of the negative control. The ratio of squamous epithelial area was calculated by dividing the K14-positive area by the total epithelial area.
5′ Rapid amplification of cDNA ends (5′ RACE)
Vaginal epithelium was collected from 10 P21 CD-1 mice as previously described (Kurita et al., 2001a). 5′ RACE of separated vaginal epithelium was performed using the GeneRacer Kit (Life Technologies) by following the manufacturer’s instructions. The following primers were used: p63 reverse GACCAGCACGCTCTGCCTTCCCGTGATA; p63 nested reverse AGTACAGCTTCTTCAGTTCGGTGGAATA.
Chromatin immunoprecipitation (ChIP) assay
Epithelia isolated from P1.5-3 C57BL/6J mice were fixed in DMEM with 27 μl/ml of 37% formaldehyde at 37 °C for 10 min. Pelleted samples were flash frozen and stored at −80 °C. Samples were thawed in 400 μl of 50 mM Tris–HCl pH 8.0, 10 mM EDTA, 10% SDS with a proteinase inhibitor cocktail (Millipore), digested using 20 units of Micrococcal Nuclease (New England Biolabs, Ipswich, MA, USA) at 37 °C for 10 min. Samples were diluted to 2 μg/ml DNA with ChIP Dilution Buffer (Millipore) and rotated at 4°C overnight with anti-SMAD4 (Santa Cruz Biotechnology) or Rabbit IgG overnight. For the visualization of PCR products on electrophoresis gels, anti-histone H3 rabbit IgG (Abcam) was used as the positive control for immunoprecipitation. The protein-chromatin complexes were isolated by Dynabeads (Life Technologies). A 10% Chelex 100 bead (BioRad, Des Plaines, IL, USA) solution was added and boiled for 10 min. The samples were treated with proteinase K for 30 min at 55 °C and boiled again for 10 min. DNA was eluted into ~100 μl H2O. Real-time PCR was performed on an ABI Prism 7000 using Power SYBR Green PCR mastermix (Life Technologies) with the following primers: Forward; AGAGTGCACTTTCTTATGAAAGAGA; Reverse CCCAAACAAACCTACCACCC.
Subrenal capsule grafting
The procedure for subrenal grafting of mouse embryonic vaginae has been described previously (Kurita et al., 2004). Briefly, dissected embryonic vaginae were transplanted under the subrenal capsule of adult females NSG mice. Two weeks after grafting, grafts on kidney were collected, photographed under the Zeiss Discovery dissecting microscope with fluorescence, fixed and processed for histological analyses.
Immunoblotting analysis
Approximately one third of the cranial portion of the vaginae from P3 mice with DES or control pellets were homogenized in lysis buffer with a protease inhibitor cocktail (Roche, Indianapolis, IN, USA). Proteins from 2 vaginae were run per well with 4 replicates for each group. Standard Western blot protocol was performed with ECL Prime Blocking and Prime Western Blotting Detection agents (GE Healthcare Biosciences, Pittsburgh, PA, USA). Since DES reduces the epithelial proliferation rate, the relative yield of epithelial tissue within the vagina was expectedly reduced. Therefore, the level of RUNX1 was normalized to E-cadherin, an epithelial marker. After detection with anti-RUNX1 antibody (1:5000), blots were stripped and reprobed with anti-E-cadherin antibody (1:10,000, Abcam). Chemiluminescence signal was detected and quantified using the FluorChem HD2 System (Proteinsimple, Santa Clara, CA, USA).
Results
ΔNp63 is required for vaginal epithelial cell fate decision Müllerian duct epithelial cells (MDECs)
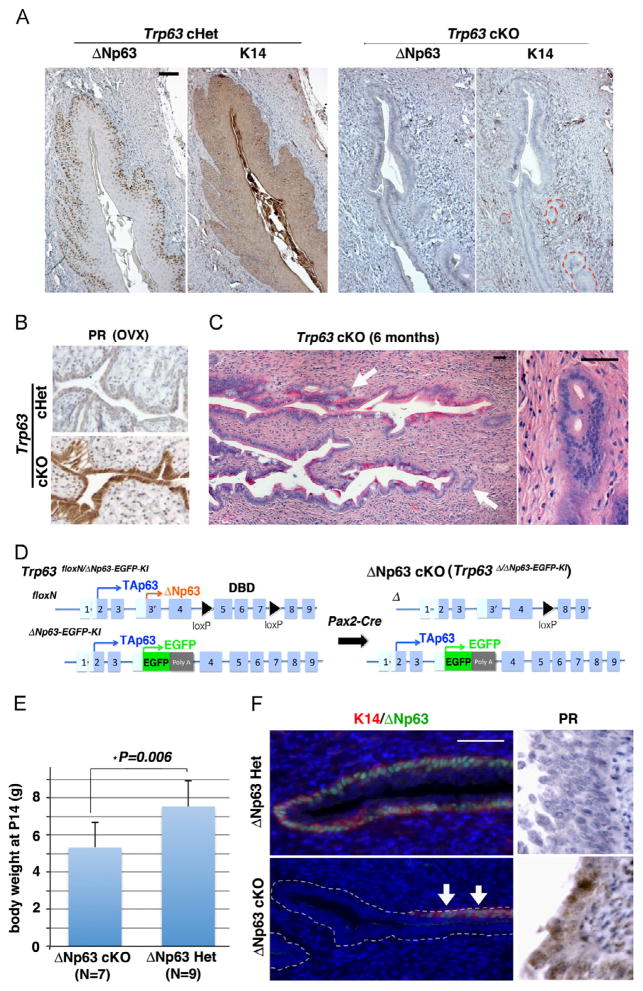
To confirm the essential role of Trp63 in the vaginal epithelial cell fate decision, Trp63 was conditionally deleted in MDECs by crossing Trp63floxN/floxN (Mills et al., 2002) and Pax2-Cre (Kurita, 2010; Ohyama and Groves, 2004) mice. Female Trp63floxN/floxN; Pax2-Cre+ (Trp63 conditional knockout, cKO) mice demonstrated extensive surface adenoses (Johnson et al., 1979) in the cervix and vagina (Fig. 1A and B). Gland-like structures of occult adenoses were also detected focally in fully mature female cKO mice. The vaginal epithelia of Trp63 cKO mice did not express cytokeratin 14 (K14), a marker for squamous epithelial cells (Fig. 1A), but expressed progesterone receptor (PR) in the absence of estrogen (Fig. 1B), which is a unique marker for murine uterine epithelium (Kurita et al., 2000). When the adult Trp63 cKO mice were treated with estradiol, the occult adenosis became easily detectable in H&E stained sections (Fig. 1C, arrows). The phenotypes of Trp63 cKO confirmed the critical role of Trp63 in the cell fate decision to be vaginal or uterine epithelium.
Fig. 1.
ΔN63 is required for commitment of MEDCs to vaginal epithelial cell fate. (A) Immunohistochemical detection of K14 squamous differentiation markers in the vagina of adult Trp63 cHet and cKO mice (P60). While cHet vaginal epithelium demonstrated stratified squamous differentiation with the expression of K14, vaginal epithelial cells in cKO remained simple columnar and K14-negative. The gland-like structures are outlined. (B) Uterine epithelial differentiation marker (PR). Trp63 cHet and cKO females were ovariectomized (OVX) at age of 8 weeks, and the expression of PR was analyzed 2 weeks later. Due to the absence of ovarian steroids, vaginal epithelium became thin in both ovariectomized cHet and cKO mice. The expression of PR in the absence of estrogen indicates uterine epithelial differentiation of MDECs in the cKO vagina. (C) Adenosis lesion in 6 month-old Trp63 cKO vagina (H&E). Adult female mice (6-month-old) were given 17β-estradiol 100 ng/day for 3 days to demonstrate the absence of squamous epithelial cells in the fornix. The fornix was completely lined by columnar epithelium (surface adenosis), and gland-like structures of occult adenosis were often found (arrows). (D) Strategy for conditional ablation of ΔNp63 in MDECs. The ΔNp63 cKO mice carry a copy of the Trp63floxN allele and the ΔNp63-EGFP knock-in allele. Activation of Cre generates a null allele for the floxed allele, thus TAp63 from the ΔNp63-EGFP knock-in allele is the only p63 protein expressed in MDECs. (E) Body weight of ΔNp63 Het and cKO female mice at P14. The body weight of female ΔNp63 cKO mice was significantly lower than that of ΔNp63 Het (Student’s t-test, *p=0.006). Bars indicate average ± SD. (F) Immunodetection of K14 and PR in the vaginal fornix of P14 ΔNp63 Het and cKO mice. The ΔNp63 protein (green) was detected in a subset of epithelial cells within the vagina of ΔNp63 cKO (arrows), indicating an incomplete gene deletion. These ΔNp63 positive cells were also positive for K14 (red), indicating normal vaginal differentiation. The K14-negative vaginal epithelial cells in ΔNp63 cKO mice expressed PR at P14. Thus, the cell fate of MDECs to be vaginal or uterine epithelium is defined by the presence or absence of ΔNp63 expression. Bar=50 μm.
To identify the promoter of Trp63 in vaginal epithelium, 5′ RACE analysis was performed on P21 vaginal epithelium and generated cDNA clones only for the ΔNp63 isoform (10/10) (Fig. S1), thus verifying the predominant expression of ΔNp63 in vaginal epithelium as we previously reported (Kim et al., 2013; Kurita et al., 2005). To assess the necessity of ΔNp63 in vaginal epithelial differentiation, we generated ΔNp63 cKO mice (Fig. 1D) (Kim et al., 2013). While Trp63 cKO mice demonstrated no health problems, ΔNp63 cKO mice were significantly smaller than heterozygous control (Fig. 1E) and often die before weaning. Since Pax2-Cre was active in multiple tissues including the kidney, endothelium, and brain, the loss of balance between TA and ΔN forms in these vital tissues is the likely cause of premature death. The vagina of ΔNp63 cKO mice appeared to be underdeveloped and frequently demonstrated the expression of ΔNp63/K14 due to incomplete gene deletion (Fig. 1F, arrows). In addition, the ΔNp63 cKO mice died before they reached the appropriate age to develop the occult adenosis. Nonetheless, as assessed at P14, ΔNp63 null vaginal epithelium showed identical phenotypes to that of Trp63 cKO mice, such as expressing differentiation markers for uterine epithelium (Fig. 1F, PR). These observations confirmed that the presence and the absence of ΔNp63 expression determine the cell fate of MDECs within the vagina to become vaginal (presence) or uterine (absence) epithelium.
BMP4 and Activin A synergically activate ΔNp63 and induce vaginal epithelial cell fate in MDECs
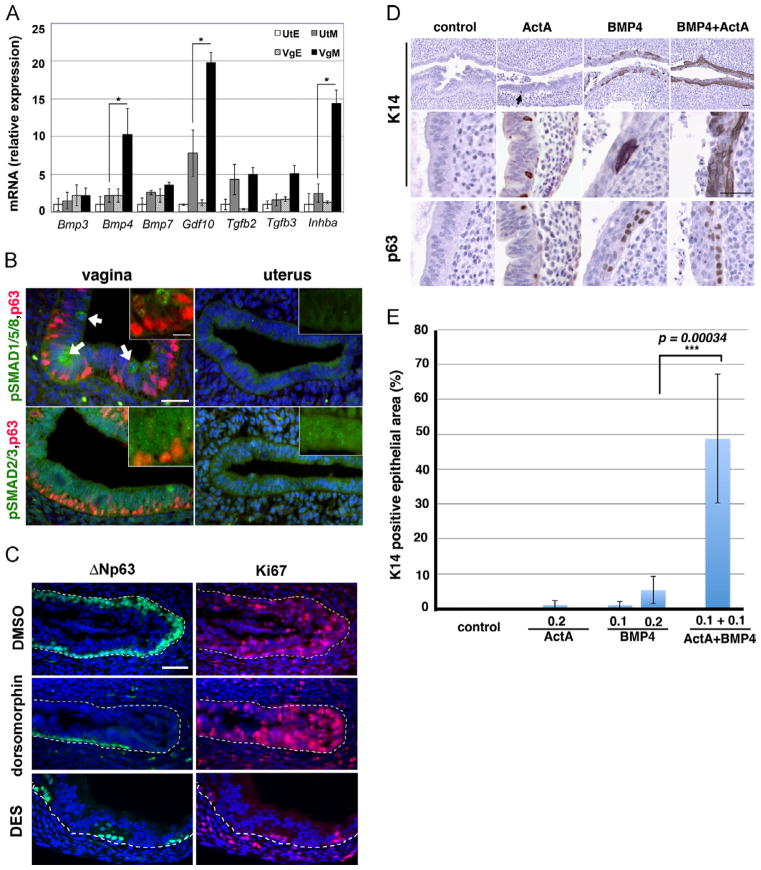
To identify the mesenchymal factors that induce ΔNp63 in MDECs, we performed a microarray analysis on the uterus and vagina isolated from postnatal day 2 (P2) Trp63 cKO and cHet mice. Differential gene expression between vaginal and uterine tissues should reflect both the upstream and downstream of Trp63 expression, whereas the comparison between cKO and cHet vaginae should identify what is downstream of Trp63 as differences. Thus, the three-way comparison should identify what is upstream of Trp63. By this method two TGFβ family ligands were identified: bone morphogenetic protein 4 (Bmp4) and inhibin βA (Inhba) as potentially upstream of ΔNp63 (Table S1), while the levels of SMADs and receptors for TGFβ family ligands were similar in both uterus and vagina (Fig. S2). The expression levels of TGFβ family ligands (Bmp3, Bmp4, Bmp7, Gdf10, Tgfb2, Tgfb3 and Inhba), which were positively detected by microarray in either the uterus or vagina, were further examined by quantitative RT-PCR using mRNA extracted from epithelium and mesenchyme of P2 uteri and vaginae (Fig. 2A). Bmp4 and Inhba, the activin A (ActA) subunit, were more robustly expressed in vaginal compared to the uterine mesenchyme. Although the expression level of Gdf10 was significantly higher in vaginal than uterine mesenchyme, the uterine mesenchyme still expressed a substantial level of Gdf10, suggesting that GDF10 does not induce vaginal epithelial differentiation. In agreement with the expression pattern of ligands, receptor-mediated phosphorylation of BMP-regulated SMADs (pSMAD1/5/8) was detected only in vaginal epithelium (Fig. 2B, white arrows). Interestingly, at P2-3, pSMAD1/5/8 was detected only in a small subset of vaginal epithelial cells with a minimum level of p63 expression, indicating that BMP4 action occurs before activation of the ΔNp63 locus. Unlike pSMAD1/5/8, activation of ActA-regulated SMADs (pSMAD2/3) was uniformly detected in both uterine and vaginal epithelia (Fig. 2B). Since GDF10 signaling is mediated by SMAD2/3 (Miyazono et al., 2010), the activation of SMAD2/3 in the uterus may reflect the activity of GDF10 produced by uterine mesenchyme. The expression pattern of phospho-R-SMADs suggested the critical role of BMP4 in the activation of the ΔNp63 locus in MDECs. This was further confirmed as the injection of neonatal mice with dorsomorphin, a kinase inhibitor of type I receptors for BMPs (ALK2, 3 and 6) (Yu et al., 2008), inhibited the induction of ΔNp63 in the vaginal epithelium. Whereas a continuous layer of ΔNp63 positive cells formed in the cervix and vagina of control mice (DMSO) by P6, P6 dorsomorphin treated mice showed patches of ΔNp63-negative cells within the vaginal epithelium (N=6 each, Fig. 2C). However, the effect of BMP-inhibitor was not identical to that of DES. Whereas DES inhibited both the epithelial proliferation and the expression of ΔNp63, inhibition of BMP signaling did not affect proliferation of vaginal epithelium as assessed by Ki-67/MKI67 (Fig. 2C).
Fig. 2.
BMP4 and Activin A instruct MDECs to express ΔNp63. (A) Quantitative RT-PCR analysis for TGFβ family ligands on the separated epithelium (E) and mesenchyme (M) of P2 uteri (Ut) and vaginae (Vg). In addition to Bmp4 and Inhba, Gdf10 was also significantly higher in the VgM than the UtM. Bars indicate average number ± SD (N=4). *p < 0.05 (Student’s t-test) for the comparisons indicated by the brackets. (B) Immunofluorescent analysis of P3 vagina and uteri for phosphorylation of BMP-activated (pSMAD1/5/8) and activin A-activated (pSMAD2/3) R-Smads (green). DAPI (blue), p63 (red). While pSMAD2/3 was uniformly expressed in both uterine and vaginal epithelial cells, pSMAD1/5/8 was detected only in a small subset of vaginal epithelial cells (arrows). These pSMAD1/5/8 positive cells co-expressed a minimum level of p63 (insert, bar=20 μm). (C) Dorsomorphin treatment inhibited expression of ΔNp63 in MDECs. Dotted lines indicate the boundary between epithelium and mesenchyme. Whereas a continuous layer of ΔNp63 (green)-positive basal cells formed throughout the vagina in the DMSO control group, the vaginal fornix of dorsomorphin- and DES-treated groups contained only a small number of ΔNp63 positive cells by P6. As assessed by proliferation marker Ki-67 (red), dorsomorphin did not affect epithelial proliferation. (D) BMP4 and ActA transformed uterine epithelium into vaginal epithelium. The treatment with 0.2 μg/ml BMP4 alone focally induced the squamous transformation of uterine epithelium as indicated by expression of p63 and K14. In contrast, 0.2 μg/ml ActA alone had no or minimum effect on the uterine epithelium. However, ActA synergistically enhanced the effect of BMP4, and 0.1 μg/ml BMP4+0.1 μg/ml ActA treatment induced p63/K14 positive squamous cells throughout the epithelium. Bar=50 μm. (E) Quantitative analysis on the degree of squamous transformation. Uterine explants incubated with 0.1 μg/ml ActA were negative for K14 and p63, thus excluded from the analysis. The ratio of K14 positive area was significantly higher in 0.2 μg/ml BMP4 group than control, 0.2 μg/ml ActA and 0.1 μg/ml BMP4 groups (Students’ t-test, p < 0.05). In addition, degree of squamous differentiation was higher in the 0.1 μg/ml ActA+0.1 μg/ml BMP4 group than any other groups (Student’s t-test, ***p < 0.0001).
To test whether BMP4 and/or ActA can be substitutions for the vaginal mesenchymal factors that activate the vaginal epithelial differentiation program in MDECs, uterine horns from P2 neonatal mice were cultured with BMP4 and/or ActA (Fig. 2D). The uterine epithelium in control medium maintained its simple columnar morphology and expressed neither p63 nor K14, whereas those treated with 0.2 μg/ml ActA showed a few cells that expressed p63 and K14 (Fig. 2D, arrow). BMP4 (0.2 μg/ml) exhibited a stronger effect than ActA in initiating vaginal epithelial differentiation in uterine epithelium as stratified layers of cells formed and more basal cells expressed p63 and K14 than the ActA-treated group. However, the most robust effect was observed when uterine pieces were cultured with 0.1 μg/ml BMP4 plus 0.1 μg/ml ActA. While each ligand alone exhibited minimum or no activity at this concentration (Fig. 2E), BMP4 and ActA synergistically amplified their activity to initiate the vaginal epithelial differentiation process as the uterine epithelium clearly differentiated into K14-positive stratified squamous epithelium with an extended p63-positive basal cell layer. These results established that differential expression of BMP4 and ActA between uterine and vaginal mesenchyme is the factor that determines the epithelial cell fate of MDECs in the uterus and vagina.
BMP4 and ActA induce ΔNp63 expression via direct binding of SMADs on the 5′ sequence of ΔNp63
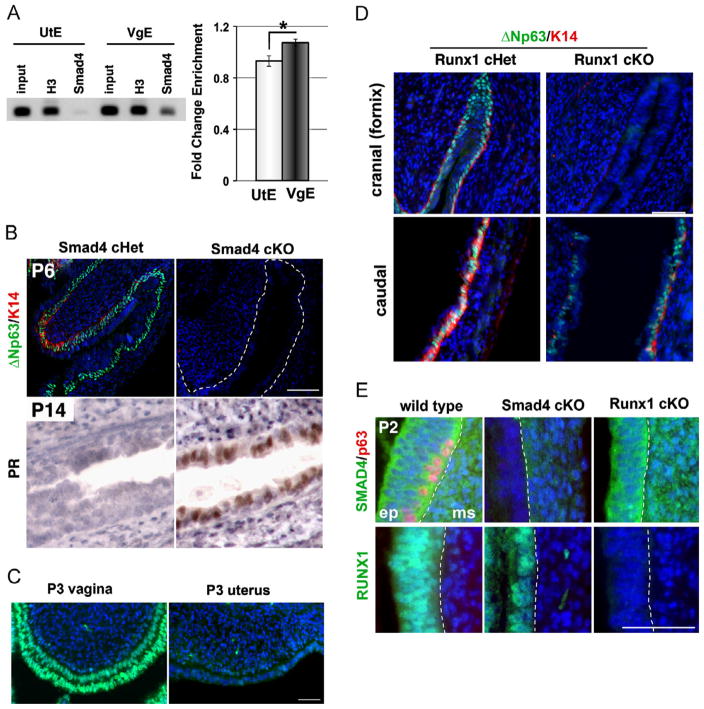
The essential role of BMP in the induction of ΔNp63 in MDEC was demonstrated by the dorsomorphin-treatment study (Fig. 2C). However, dorsomorphin inhibits the kinase activity of type I receptors for BMP, which can activate the SMAD-mediated transcription as well as other kinase pathways, such as mitogen-activated protein kinase. Thus, it was not clear if the BMP4 and ActA induced the expression of ΔNp63 through SMAD mediated signaling. ECR browser (Ovcharenko et al., 2004) and VISTA enhancer browser (http://enhancer.lbl.gov/) identified a highly conserved sequence on the 5′ region of ΔNp63 TSS. In silico analysis identified multiple SMAD binding elements conserved between the human and mouse within the region (Fig. S3). Furthermore, a SMAD5 binding element and SMAD3/4 binding sites within this sequence showed similar configurations to the Bmp2b/Smad5 regulated promoter of Zebrafish ΔNp63 (Bakkers et al., 2002). Therefore, a chromatin immunoprecipitation (ChIP) assay for SMAD4, the co-SMAD required for transcriptional activities of both BMP-regulated and Activin-regulated SMADs (Massague, 2012), was performed to test whether SMADs bind to the conserved 5′ region of ΔNp63 in vaginal and uterine epithelial cells isolated from P2-3 mice. By pull-down using anti-SMAD4 antibody, the chromatin fragments containing SMAD binding elements were significantly enriched in the vaginal epithelium in comparison to uterine epithelium (Fig. 3A, *p < 0.05). This agrees with our model in which BMP/Activin signaling activates expression of ΔNp63 via binding of SMADs on the 5′ proximal sequence of ΔNp63. Furthermore, the requirement of SMAD4 in the activation of ΔNp63 locus was tested by generating Smad4 cKO mice of MDECs. The Smad4flox/flox (Yang et al., 2002) mice were crossed with Wnt7a-Cre+ (Winuthayanon et al., 2010) mice, because the Smad4flox/flox; Pax2-Cre+ mice were embryonic lethal. The vaginae of Smad4flox/flox; Wnt7a-Cre+ (Smad4 cKO) were lined with ΔNp63- and K14- negative columnar epithelial cells (Fig. 3B). In addition, Smad4 cKO vaginal epithelium expressed PR in the absence of estrogen at P14, indicating uterine epithelial cell fate (Fig. 3B).
Fig. 3.
Vaginal cell fate induction by BMP4 and ActA is mediated by SMADs/RUNX1. (A) ChIP assay on 5′ conserved sequence of ΔNp63 with anti-SMAD4 antibody (Fig. S3). H3, anti-Histone H3 positive control. The fold change enrichment indicates a ratio of CT value of SMAD4 IP to that of rabbit IgG negative control. SMAD4 ChIP enriched the putative ΔNp63 regulatory sequence in vaginal (VgE) than uterine (UtE) epithelium (*p=0.02, Student’s t-test). Bars indicate average number ± SD (N=4). (B) Expression of vaginal and uterine epithelial markers in the vaginae of Smad4 cHet (control) and cKO mice. The boundary between epithelial and mesenchymal tissues is indicated with a white dotted line. VgE of Smad 4 cKO mice failed to express ΔNp63 (green) and K14 (red) (P6, upper panels). Instead, MDECs differentiated into UtE within vagina, expressing PR at P14 (brown). (C) RUNX1 expression in P3 vagina and uterus. At P3, RUNX1 (green) was highly expressed in VgE while UtE was mostly negative. Bar=50 μm. (D) Vaginal phenotypes of Runx1 cKO mice. The vaginal fornix of Runx1 cKO was totally negative for ΔNp63 (green) and K14 (red) at P7. However, the caudal portion of Runx1 cKO vagina contained ΔNp63/K14 positive stratified squamous epithelial cells. Therefore, requirement of RUNX1 for MDECs to express ΔNp63 was region specific. (E) SMAD4 and RUNX1 did not regulate expression of each other. Immunofluorescence assay for SMAD4 (green) and p63 (red) (the upper panels), and RUNX1 (green) (the lower panel) was performed on P2 vaginae. All panels are arranged as the epithelium (ep) on the left and the mesenchyme (ms) on the right side. The boundary between two tissues is indicated by a dotted line. At P2, wild-type vaginal tissues expressed SMAD4 in the epithelium and mesenchyme, and RUNX1 only in the epithelium. Vaginae of P2 Smad4 cKO mice expressed SMAD4 only in mesenchymal tissues, confirming the success of the epithelial-specific gene knockout strategy. Likewise, Runx1 cKO vaginal epithelium was totally negative for RUNX1. The expression of RUNX1 in Smad4 cKO as well as the expression of SMAD4 in Runx1 cKO was maintained. Bar=50 μm.
In summary, BMP4 and ActA produced by vaginal mesenchyme induce expression of ΔNp63, thus determining vaginal epithelial cell fate in MDECs, via direct binding of SMADs on the Smad binding elements within a highly conserved 5′ proximal sequence of ΔNp63.
RUNX1 is required for SMAD mediated activation of ΔNp63
The microarray analysis identified Runt-related transcription factor 1 (Runx1) as a gene highly present in vaginal tissues (Table S1). Immunofluorescence analysis agreed with this result; RUNX1 was more highly expressed in vaginal than uterine epithelium at P3 (Fig. 3C). RUNX family members have been shown to work as co-transcription factors in SMAD-mediated gene expression (Ito and Miyazono, 2003), and the RUNX1 expression pattern (Fig. 3C) strongly suggests an involvement of RUNX1 in the BMP4/ActA-regulated cell fate decision in MDECs. To test whether RUNX1 is required for the activation of ΔNp63 in MDECs, Runx1 cKO (Runx1flox/flox; Wnt7a-Cre+) mice were generated. Interestingly, the requirement of RUNX1 in ΔNp63 activation showed a regional specificity. In Runx1 cKO mice, the cranial-region of the vagina, where DES-associated adenoses usually develop, was lined with simple columnar cells that completely lacked expression of ΔNp63 and K14, establishing its essential role in vaginal epithelial differentiation (Fig. 3D, cranial). In contrast, the caudal region of vagina was lined with ΔNp63-positive squamous epithelium (Fig. 3D, caudal). Although the epithelial phenotypes of the upper vagina in Runx1 and Smad4 cKO mice were similar, the absence of one gene did not affect the expression of the other. Smad4 cKO mice still expressed RUNX1 in the vaginal epithelium, and Runx1 cKO mice expressed SMAD4 (Fig. 3E). Thus, the expression of RUNX1 and SMAD4 is independently regulated, but both transcription factors are essential for the activation of ΔNp63 in vaginal epithelium.
DES alters vaginal epithelial cell fate through downregulation of RUNX1
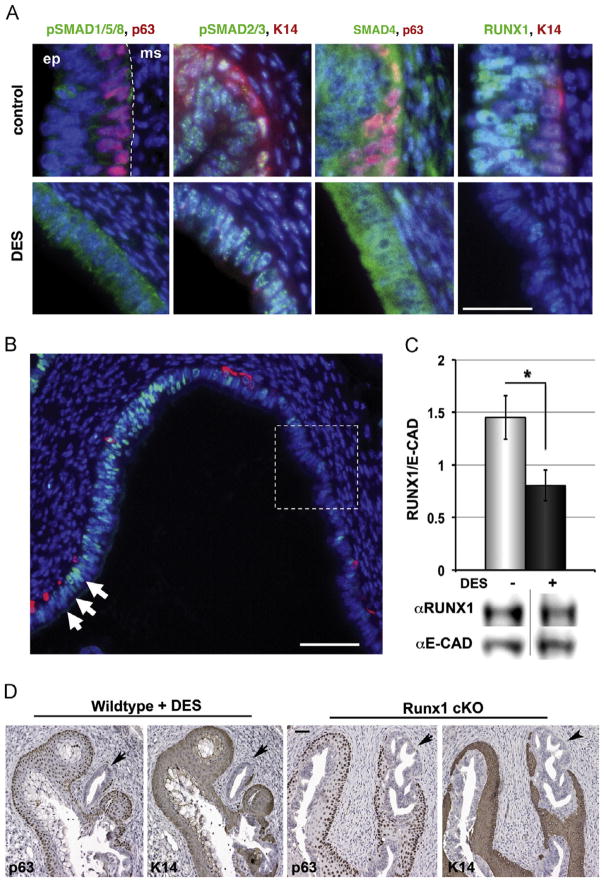
We have previously shown that neonatal DES exposure preferentially blocks expression of p63 in the fornix of the vagina and the common cervical canal in P5 mice, and these p63 negative cells persisted into adulthood and formed adenosis within these structures (Kurita et al., 2004). Thus, the disruption of the developmental signal by DES should be highly detected in the vaginal fornix and the common cervical canal. In order to determine if and at what level DES interrupts the BMP4/ActA-SMAD/RUNX1 signaling pathway, immunofluorescence analysis was performed focusing on the signal alterations in the vaginal fornix at P2-3. First, the effect of DES-exposure on the receptor-mediated phosphorylation of R-SMADs was examined. The signals for receptor-mediated phosphorylation of pSMAD2/3 showed minimum change (Fig. 4A). Surprisingly, the signals for receptor-mediated phosphorylation of pSMAD1/5/8 were higher in DES-treated than control vaginal epithelium (Fig. 4A). Therefore, DES does not interfere with the BMP4/ActA-SMAD/RUNX1 pathway at the upstream of R-SMADs. Additionally, overall expression of SMAD4 within the vaginal epithelium was unchanged with DES treatment (Fig. 4A). In contrast, the expression pattern of RUNX1 showed a dramatic change in response to DES-exposure, and the substantial number of epithelial cells in the vaginal fornices became almost negative for RUNX1 at P2-3 (Fig. 4A and B). Although RUNX1 was not uniformly downregulated throughout the vaginal epithelium, immunoblotting analysis of the upper vaginae detected a significant difference (*p < 0.05) in the level of RUNX1 between control and DES-treated mice (Fig. 4C). Having an essential role in ΔNp63 expression as demonstrated by the cKO study, the downregulation of RUNX1 in the fornix should block the BMP4/ActA action on the ΔNp63 locus. We propose that DES induces vaginal adenosis by inhibiting activation of vaginal epithelial cell fate via a down-regulation of RUNX1. This model is further supported by a striking phenotypic similarity between the vaginae of adult Runx1 cKO mice and adult wild type mice that were neonatally exposed to DES (Fig. 4D). In both Runx1 cKO and neonatally DES-exposed mice, unaffected squamous epithelial cells in the caudal portion of vagina grew upwards replacing the columnar epithelial cells in the surface adenosis during development. Thus, in the adult female, adenosis lesions remained mostly as the occult adenosis in the vaginal fornix. The region-specific requirement of RUNX1 in the activation of ΔNp63 may explain why adenosis occurs only in the upper part of vagina.
Fig. 4.
DES alters vaginal epithelial cell fate through downregulation of RUNX1. (A) Effect of DES on BMP4/ActA-SMAD/RUNX1 signaling in the vaginal fornix. Immunofluorescence assay for phosphorylation of R-SMADs, SMAD4 and RUNX1 were performed on vaginae of P3 control and DES-treated mice. At P3, overall expression of SMAD4 as well as phosphorylation levels of BMP-regulated SMADs (pSMAD1/5/8) and Activin-regulated SMADs (pSMAD2/3) were unchanged or increased by DES in the VgE. In contrast, RUNX1 level was significantly reduced by DES in the fornix (corresponding to the area marked with a square in B). (B) The effect of DES on RUNX1 (green) expression was strongest in the outer wall of fornix, in which DES-associated adenosis most often develops (marked with a square). In contrast, RUNX1 expression was maintained relatively high in the lip of cervix (indicated by arrows), where DES usually shows minimum effect on the expression of ΔNp63 (Kurita et al., 2004). Bar=50 μm. (C) Immunoblotting analyses of DES-effect on the RUNX1 level in the cranial portion of vagina at P3. The RUNX1 level was lower in the DES-treated (+DES) than control (DES) vaginae (*p < 0.02, Student’s t-test). Bars indicate the average number ± SD for the relative signal intensity of RUNX1 over E-cadherin (E-CAD) (n=4). (D) Adult Runx1 cKO mice phenocopied neonatally DES-treated mice. Similar adenotic lesions negative for p63/K14 were detected in the vaginal fornices of Runx1 cKO and neonatally DES-exposed (WT+DES) female mice at P40 (arrows). Bar=50 μm.
The activation and the maintenance of ΔNp63 expression are controlled by different molecular mechanisms
Classic tissue recombination studies have demonstrated that the differentiation program in adult vaginal epithelium is maintained independently of vaginal mesenchymal factors (Cunha, 1976). Thus, adult vaginal epithelium combined with uterine mesenchyme maintains the expression of p63 (Kurita et al., 2004). These observations suggest that the transcriptional activity in the ΔNp63 locus is maintained by a BMP4/ActA-SMAD/RUNX1 independent mechanism. In agreement with this hypothesis, pSMAD1/5/8 (Fig. 2B) and RUNX1 expressions (Fig. 5A) were downregulated in K14-positive (fully differentiated) vaginal epithelial cells. In addition, SMAD4 became undetectable in vaginal epithelium by P6 (not shown), and Runx1 cKO vaginal epithelium, which was completely negative for RUNX1, maintained expression of p63 in the basal cells (Fig. 5B). To test the requirement of BMP4/ActA-SMAD/RUNX1 in the expression of ΔNp63 in adult vaginal epithelium, we developed doxycycline (Dox)-inducible, Smad4 knockout (Smad4flox/flox; tetO-cre +; Pax8-rtTA+; RosaTE/wt) mice for MDECs by crossing the Smad4flox/flox strain with Pax8-rtTA, tetO-cre and RosaTE mice. The RosaTE trans-gene allowed detection of Cre activation via EGFP expression, which indirectly indicated the deletion of floxed Smad4 alleles. When Smad4 was deleted in fetal/neonatal MDECs by DOX-administration to the mother throughout gestation and nursing, the EGFP reporter and p63 were exclusively expressed in the vaginal epithelium, confirming the essential role of Smad4 in ΔNp63 expression during vaginal development (Fig. 5A, embryo DOX). In contrast, when DOX was administered after ΔNp63 expression was established in vaginal epithelium, the deletion of Smad4 did not affect expression of ΔNp63, as revealed by co-expression of ΔNp63 and the EGFP reporter (Fig. 5A, adult DOX) in vaginal epithelial cells. Therefore, SMAD4 is dispensable in maintaining the expression of ΔNp63 in adult vaginal epithelium.
Fig. 5.
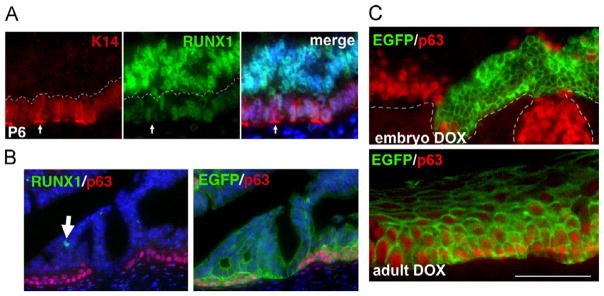
BMP4/ActA-SMAD/RUNX1 signaling is dispensable for expression of ΔNp63 in differentiated vaginal epithelial cells. (A) RUNX1 is downregulated in squamous basal cells in the vagina. At P6, basal cells with K14 expression (arrow) showed lower signals for RUNX1 than K14-negative cells (above the dotted line). (B) RUNX1 is dispensable for expression of ΔNp63 in the adult VgE. In the vaginae of adult Runx1 cKO mice (P42), RUNX1 was undetectable in epithelial cells that expressed p63 (red). White arrow indicates a rare RUNX1-positive cell. Furthermore, p63 (red) and EGFP for Cre-reporter (green) were co-expressed. Thus, RUNX1 is dispensable for expression of ΔNp63 in adult vaginal epithelium. (C) SMAD4 is dispensable for ΔNp63 expression in adult VgE. In DOX-inducible Smad4 KO mice for MDECs, the reverse tetracycline transactivator (rtTA) is expressed in embryonic MDECs as well as MDEC-derived adult epithelium including VgE. DOX administration induces the expression of the tetO-cre transgene, and the Cre recombinase generates the Smad4 null alleles. SMAD4 proteins were undetectable in the adult vaginal epithelial cells by immunostaining. Therefore, the efficacy of Cre-mediated recombination was indirectly monitored by expression of EGFP from RosaTE allele. When DOX was administered from embryo to neonate (E1.5-P5) through mother, p63 (red) was expressed only in small patches of MDECs lacking EGFP (green) expression. A 3 week-treatment of DOX starting at weaning (from P21 to P42) also effectively activated Cre recombinase in vaginal tissues as demonstrated by the expression of EGFP throughout the VgE. However, EGFP-positive basal cells expressed p63 (red), indicating that SMAD4 is dispensable for maintenance of ΔNp63 expression in the adult VgE. Bar=50 μm.
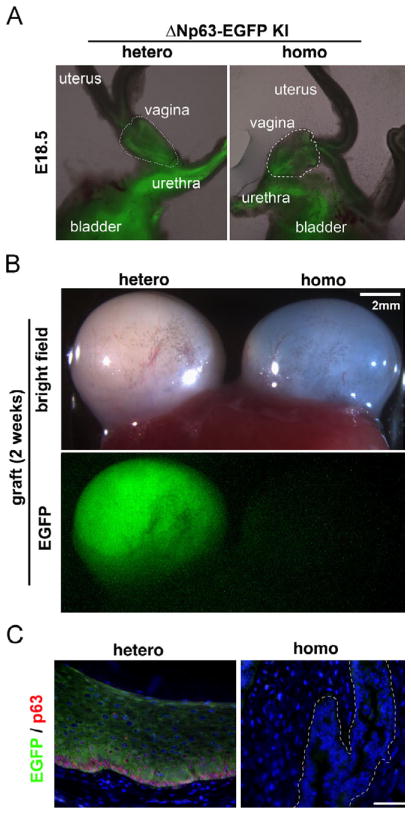
As we generated ΔNp63 cKO (Trp63Np63-EGFP-KI/floxN; Pax2-Cre+), we realized that EGFP was nearly undetectable in the vagina of the ΔNp63 cKO, suggesting that ΔNp63 was required to maintain the expression of ΔNp63 itself. To avoid complications caused by incomplete deletion of the floxed allele, we utilized homozygous and heterozygous mice for the ΔNp63-EGFP knock-in allele (ΔNKI). While heterozygous mice can express both TA and ΔN isoforms, homozygous mice only express TA forms. In both genotypes, transcriptional activity of the ΔNp63 locus was monitored by the expression of EGFP from the ΔNKI locus. At E18.5, EGFP was detected in the MDECs within the vagina and cervix of heterozygous and homozygous mice (N=3 each, Fig. 6A E18.5), indicating that the ΔNp63 locus is equally activated in the embryonic MDECs irrespective of ΔNp63 expression. The isolated embryonic vaginae were then transplanted under the subrenal capsule of NSG mice (Shultz et al., 2005) and grown for 2 weeks to develop into mature vaginal tissues. While the mature vaginae of ΔNKI heterozygous mice maintained EGFP expression, the originally EGFP-positive vaginae of ΔNKI homozygous mice lost EGFP expression after two weeks of in vivo growth, indicating that the ΔNp63 locus was transcriptionally inactive (Fig. 6B and C). These observations supported our model that ΔNp63 is required for the maintenance of its expression through an unidentified mechanism. Hence, there are two phases in the regulation of ΔNp63 expression in vaginal epithelium: its activation by the BMP4/ActA-SMAD/RUNX1 signaling pathway and the stabilization of the activated locus by a ΔNp63-dependent signaling pathway (Fig. 7). Because the first “activation of locus” phase is prerequisite for the second phase, disruption of the first phase by DES during development permanently affects the vaginal epithelial differentiation.
Fig. 6.
ΔNp63 stabilizes/maintains ΔNp63 expression in differentiated vaginal epithelium. (A) Activation of ΔNp63 locus is independent of ΔNp63 expression. In E18.5 urogenital tracts from ΔNKI heterozygous and homozygous mice, EGFP expression from ΔNKI locus was observed in the ΔNp63 positive epithelial tissues of the bladder and urethra in both genotypes. In the female reproductive tract, EGFP was detected in the vagina (outlined with dotted line) and cervix, but not in the uterus. (B and C) Stabilization of ΔNp63 activity requires ΔNp63 protein. Isolated vaginal anlages (A, outlined by dotted line) were grown as subrenal grafts for 2 weeks to develop into mature vaginae. Vaginal grafts of both genotypes grew equally (B, bright field). However, EGFP expression was detected only in the heterozygous vaginae (B, EGFP) (N=3 for each genotype). The absence of EGFP expression in the vaginal epithelium of homozygous mice was confirmed by a more sensitive immunofluorescence assay (C). While heterozygous vagina demonstrated normal morphology with EGFP (green) and p63 (red) expression, homozygous vagina was totally negative for EGFP and p63 (epithelium is outlined).
Fig. 7.
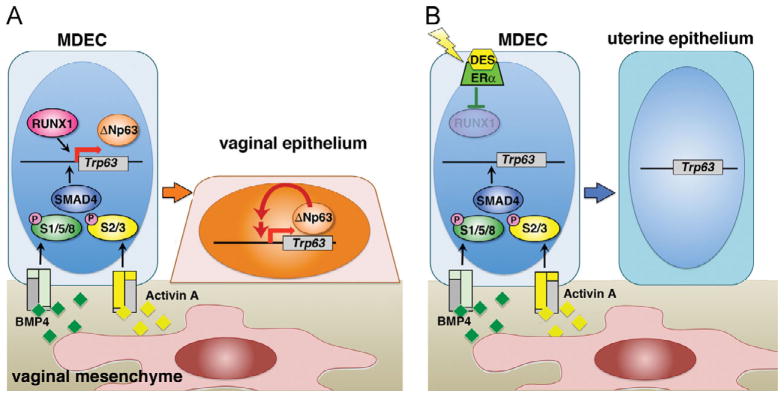
Models: Epithelial cell fate decision in developing vagina. (A) Normal development. Mesenchymal BMP4 and ActA instruct undifferentiated MDECs to become vaginal epithelium by activating transcription of ΔNp63 locus via SMAD/RUNX1-dependent mechanism. The BMP4/ActA action presumably induces chromatin remodeling to make the ΔNp63 locus accessible to transcriptional machineries. The transcriptional activity of ΔNp63 locus is then maintained/stabilized by a BMP/Activin-SMAD/RUNX-independent, ΔNp63-dependent mechanism (red arrow). (B) DES-associated vaginal adenosis. DES/ERα (Kurita et al., 2004) downregulates RUNX1, and inhibits activation of ΔNp63 locus and subsequent vaginal epithelial program. The ΔNp63-negative MDECs differentiate into columnar uterine epithelium within the vagina, forming vaginal adenosis.
Discussion
The DES incident is probably the best-documented case of chemical exposure in human history. The health conditions of DES daughters in the United States have been closely monitored for decades (Labarthe et al., 1978). The investigations presented here focused on the pathogenesis of vaginal adenosis, which shows a strikingly high incidence among DES daughters. Through a series of studies utilizing mouse models, we have successfully demonstrated for the first time that cell fate of vaginal epithelium is induced by BMP4 and ActA via SMAD mediated activation of the ΔNp63 locus, that the expression of RUNX1 is required for SMAD-mediated ΔNp63 activation in the cranial portion of the vaginal epithelium, and that developmental exposure to DES induces vaginal adenosis by altering epithelial cell fate via a down-regulation of RUNX1.
This study is particularly important for demonstrating that a chemical exposure during development can cause precancerous lesions by inducing metaplasia via an alteration of cell fate. Our findings are summarized in Fig. 7. As demonstrated by organ-culture study, we propose that BMP4 is the main player in activation of vaginal epithelial cell fate in MDECs and that ActA plays a supportive role. Recently, Nakajima et al. (2011) proposed that developmental exposure to DES induces vaginal adenosis by attenuating Activin-signaling. However, our study focusing on the fornix at P2-3 could not confirm their observation that DES reduces the signals for phospho-SMAD2 in neonatal vaginal epithelium. They also demonstrated that Inhibin blocked expression of p63 in the organ culture of neonatal vaginae. It should be noted that Inhibin blocks both ActA and BMP4 signaling (Wiater and Vale, 2003).
Whether SMADs and RUNX1 form a complex to activate ΔNp63 was not addressed in the current study due to technical difficulties. First, the yield of vaginal epithelium from a P2-3 mouse is very small. Second, the epithelial tissue is heterogeneous, and ΔNp63 is expressed only in a subpopulation of epithelial cells. Third, as clearly shown in the Runx1 cKO study, there is a regional difference in the function of RUNX1 within vaginal epithelium. The ECR browser identified a binding site for the RUNX/core binding factor β (CBFβ) heterodimer (TGTGGTT) conserved between human and mouse, ~500 bp downstream of the Smad binding sites. How SMADs and RUNX1 cooperate in the differentiation of MDECs is a subject for future investigation.
In DES daughters, vaginal adenosis is found in the cranial portion, particularly within the fornix of the vagina. Our previous study demonstrated that DES disrupts expression of p63 in vaginal epithelium only in the cranial portion of vagina (Kurita et al., 2004). These regional differences in the susceptibility to DES-induced adenosis may be explained by the regional difference in the role of RUNX1 in ΔNp63 expression. The mammalian RUNX family of transcription factors consists of three members: RUNX1, RUNX2 and RUNX3, and all 3 members have been shown to interact with SMADs to control the transcription of target genes (Ito and Miyazono, 2003). Since the levels of Runx2 and Runx3 mRNA in developing uteri and vaginae were below the detection level (GSE44697), the dispensability of RUNX1 in the lower vagina is unlikely due to the compensation by other RUNX family members. We hypothesize that RUNX1 facilitates SMADs mediated ΔNp63 activation by enhancing the recruitment of the chromatin-remodeling complex to the silent ΔNp63 locus (Lichtinger et al., 2010). In this context, ΔNp63 activation can be facilitated by other transcription factors that can recruit chromatin-remodeling complexes in the absence of RUNX1.
Utilizing the ΔNKI mice, we demonstrated that the ΔNp63 locus activated by BMP/Activin-SMAD/RUNX signaling is stabilized by a ΔNp63 dependent mechanism. Thus, fully differentiated vaginal epithelial cells maintain ΔNp63 expression independent of BMP4/ActA-SMAD/RUNX1. However, our study does not reveal if ΔNp63 self-regulates its expression in the adult vagina. Transcription activity of the ΔNp63 locus in adult vaginal epithelium may be maintained by ΔNp63. Alternatively, ΔNp63 may be required only for the stabilization of the locus during the development. To answer this question, conditional deletion of ΔNp63 in adult vaginal epithelium has to be performed.
How DES downregulates RUNX1 in MDECs also remains as the most important question of our next study. Since the response of MDECs to DES was uneven within the vagina, the experiment must be carefully designed to address this question. It should be noted that DES upregulated RUNX1 in the P3 uterus (Fig. S4), in agreement with recent report that estrogen induces RUNX1 in the adult uterine epithelium (Wall et al., 2013). This observation further emphasizes the importance of tissue microenvironment in the response of MDECs to DES.
Although some questions still remain, this study has opened the door to a new era of research on DES-induced malignancy by defining the cellular and molecular mechanisms through which DES induces vaginal adenosis. Given that there have been cases of vaginal adenoses and clear cell adenocarcinomas reported in women with no history of DES exposure (Laronda et al., 2012), this study suggests exposure to any compounds that disrupt the signaling pathway controlling the cell fate decision in vaginal epithelium could initiate these conditions.
Supplementary Material
Acknowledgments
The authors thank Dr. So-Youn Kim for technical help and Stacy A. Druschitz, Drs. So-Youn Kim, Tsutomu Kume and Gerald R. Cunha for editorial suggestions. This work was supported by the National Institutes of Health [HD30284 to R.R.B, RO1CA154358 and RO1HD064402 to T.K.].
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.ydbio.2013.06.024.
References
- Antonioli DA, Burke L. Vaginal adenosis. Analysis of 325 biopsy specimens from 100 patients. Am J Clin Pathol. 1975;64:625–638. doi: 10.1093/ajcp/64.5.625. [DOI] [PubMed] [Google Scholar]
- Bakkers J, Hild M, Kramer C, Furutani-Seiki M, Hammerschmidt M. Zebrafish DeltaNp63 is a direct target of Bmp signaling and encodes a transcriptional repressor blocking neural specification in the ventral ectoderm. Dev Cell. 2002;2:617–627. doi: 10.1016/s1534-5807(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bibbo M, Ali I, Al-Naqeeb M, Baccarini I, Climaco LA, Gill W, Sonek M, Wied GL. Cytologic findings in female and male offspring of DES treated mothers. Acta Cytol. 1975;19:568–572. [PubMed] [Google Scholar]
- Boutin EL, Sanderson RD, Bernfield M, Cunha GR. Epithelial-mesenchymal interactions in uterus and vagina alter the expression of the cell surface proteoglycan, syndecan. Dev Biol. 1991;148:63–74. doi: 10.1016/0012-1606(91)90317-v. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Müllerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- FDA. Selected item from the FDA drug bulletin-november 1971: diethylstil-bestrol contraindicated in pregnancy. Calif Med. 1972;116:85–86. [PMC free article] [PubMed] [Google Scholar]
- Forsberg JG. Estrogen, vaginal cancer, and vaginal development. Am J Obstet Gynecol. 1972;113:83–87. doi: 10.1016/0002-9378(72)90456-5. [DOI] [PubMed] [Google Scholar]
- Herbst AL, Poskanzer DC, Robboy SJ, Friedlander L, Scully RE. Prenatal exposure to stilbestrol. A prospective comparison of exposed female offspring with unexposed controls. N Engl J Med. 1975;292:334–339. doi: 10.1056/NEJM197502132920704. [DOI] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, Hartge P, Hatch EE, Herbst AL, Karlan BY, Kaufman R, Noller KL, Palmer JR, Robboy SJ, Saal RC, Strohsnitter W, Titus-Ernstoff L, Troisi R. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365:1304–1314. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- Ito Y, Miyazono K. RUNX transcription factors as key targets of TGF-beta superfamily signaling. Curr Opin Genet Dev. 2003;13:43–47. doi: 10.1016/s0959-437x(03)00007-8. [DOI] [PubMed] [Google Scholar]
- Johnson LD, Driscoll SG, Hertig AT, Cole PT, Nickerson RJ. Vaginal adenosis in stillborns and neonates exposed to diethylstilbestrol and steroidal estrogens and progestins. Obstetet Gynecol. 1979;53:671–679. [PubMed] [Google Scholar]
- Kaufman RH, Binder GL, Gray PM, Jr, Adam E. Upper genital tract changes associated with exposure in utero to diethylstilbestrol. Am J Obstet Gynecol. 1977;128:51–59. doi: 10.1016/0002-9378(77)90294-0. [DOI] [PubMed] [Google Scholar]
- Kim SY, Cordeiro MH, Serna VA, Ebbert K, Butler LM, Sinha S, Mills AA, Woodruff TK, Kurita T. Rescue of platinum-damaged oocytes from programmed cell death through inactivation of the p53 family signaling network. Cell Death Differ. 2013;20:987–997. doi: 10.1038/cdd.2013.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T. Developmental origin of vaginal epithelium. Differentiation. 2010;80:99–105. doi: 10.1016/j.diff.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Müllerian) epithelial differentiation. Dev Biol. 2001a;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- Kurita T, Cunha GR, Robboy SJ, Mills AA, Medina RT. Differential expression of p63 isoforms in female reproductive organs. Mech Dev. 2005;122:1043–1055. doi: 10.1016/j.mod.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee K, Cooke PS, Taylor JA, Lubahn DB, Cunha GR. Paracrine regulation of epithelial progesterone receptor by estradiol in the mouse female reproductive tract. Biol Reprod. 2000;62:821–830. doi: 10.1093/biolreprod/62.4.821. [DOI] [PubMed] [Google Scholar]
- Kurita T, Lee K, Saunders PTK, Cooke PS, Taylor JA, Lubahn DB, Zhao C, Mäkelä S, Gustafsson J-Å, Dahiya R, Cunha GR. Regulation of progesterone receptors and decidualization in uterine stroma of the estrogen receptor-a knockout mouse. Biol Reprod. 2001b;64:272–283. doi: 10.1095/biolreprod64.1.272. [DOI] [PubMed] [Google Scholar]
- Kurita T, Mills AA, Cunha GR. Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development. 2004;131:1639–1649. doi: 10.1242/dev.01038. [DOI] [PubMed] [Google Scholar]
- Labarthe D, Adam E, Noller KL, O’Brien PC, Robboy SJ, Tilley BC, Townsend D, Barnes AB, Kaufman RH, Decker DG, Fish CR, Herbst AL, Gundersen J, Kurland LT. Design and preliminary observations of National Cooperative Diethylstilbestrol Adenosis (DESAD) Project. Obstet Gynecol. 1978;51 (4):453–458. doi: 10.1097/00006250-197804000-00014. [DOI] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Butler LM, Kurita T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation. 2012;84:252–260. doi: 10.1016/j.diff.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtinger M, Hoogenkamp M, Krysinska H, Ingram R, Bonifer C. Chromatin regulation by RUNX1. Blood Cells Mol Dis. 2010;44:287–290. doi: 10.1016/j.bcmd.2010.02.009. [DOI] [PubMed] [Google Scholar]
- Massague J. TGFbeta signalling in context. Nat Rev. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills AA, Qi Y, Bradley A. Conditional inactivation of p63 by Cre-mediated excision. Genesis. 2002;32:138–141. doi: 10.1002/gene.10067. [DOI] [PubMed] [Google Scholar]
- Mills AA, Zheng B, Wang XJ, Vogel H, Roop DR, Bradley A. p63 is a p53 homologue required for limb and epidermal morphogenesis. Nature. 1999;398:708–713. doi: 10.1038/19531. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Kamiya Y, Morikawa M. Bone morphogenetic protein receptors and signal transduction. J Biochem. 2010;147:35–51. doi: 10.1093/jb/mvp148. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Nakajima T, Iguchi T, Sato T. Involvement of activin signaling in abnormalities of mouse vagina exposed neonatally to diethylstilbestrol. Cell Tissue Res. 2011;344:527–538. doi: 10.1007/s00441-011-1161-2. [DOI] [PubMed] [Google Scholar]
- Newbold RR, McLachlan JA. Vaginal adenosis and adenocarcinoma in mice exposed prenatally or neonatally to diethylstilbestrol. Cancer Res. 1982;42:2003–2011. [PubMed] [Google Scholar]
- Ng AB, Reagan JW, Hawliczek S, Wentz WB. Cellular detection of vaginal adenosis. Obstet Gynecol. 1975;46:323–328. [PubMed] [Google Scholar]
- O’Brien PC, Noller KL, Robboy SJ, Barnes AB, Kaufman RH, Tilley BC, Townsend DE. Vaginal epithelial changes in young women enrolled in the National Cooperative Diethylstilbestrol Adenosis (DESAD) project. Obstet Gynecol. 1979;53:300–308. [PubMed] [Google Scholar]
- Ohyama T, Groves AK. Generation of Pax2-Cre mice by modification of a Pax2 bacterial artificial chromosome. Genesis. 2004;38:195–199. doi: 10.1002/gene.20017. [DOI] [PubMed] [Google Scholar]
- Ovcharenko I, Nobrega MA, Loots GG, Stubbs L. ECR Browser: a tool for visualizing and accessing data from comparisons of multiple vertebrate genomes. Nucleic Acids Res. 2004;32:W280–286. doi: 10.1093/nar/gkh355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plapinger L, Bern HA. Adenosis-like lesions and other cervicovaginal abnormalities in mice treated perinatally with estrogen. J Natl Cancer Inst. 1979;63:507–518. [PubMed] [Google Scholar]
- Romano RA, Smalley K, Magraw C, Serna VA, Kurita T, Raghavan S, Sinha S. DeltaNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation. Development. 2012;139:772–782. doi: 10.1242/dev.071191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, Kotb M, Gillies SD, King M, Mangada J, Greiner DL, Handgretinger R. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- Smith OW. Diethylstilbestrol in the prevention and treatment of complications of pregnancy. Am J Obstet Gynecol. 1948;56:821–834. [PubMed] [Google Scholar]
- Taniuchi I, Osato M, Egawa T, Sunshine MJ, Bae SC, Komori T, Ito Y, Littman DR. Differential requirements for Runx proteins in CD4 repression and epigenetic silencing during T lymphocyte development. Cell. 2002;111:621–633. doi: 10.1016/s0092-8674(02)01111-x. [DOI] [PubMed] [Google Scholar]
- Traykova-Brauch M, Schonig K, Greiner O, Miloud T, Jauch A, Bode M, Felsher DW, Glick AB, Kwiatkowski DJ, Bujard H, Horst J, von Knebel Doeberitz M, Niggli FK, Kriz W, Grone HJ, Koesters R. An efficient and versatile system for acute and chronic modulation of renal tubular function in transgenic mice. Nat Med. 2008;14:979–984. doi: 10.1038/nm.1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troisi R, Hatch EE, Titus-Ernstoff L, Hyer M, Palmer JR, Robboy SJ, Strohsnitter WC, Kaufman R, Herbst AL, Hoover RN. Cancer risk in women prenatally exposed to diethylstilbestrol. Int J Cancer. 2007;121:356–360. doi: 10.1002/ijc.22631. [DOI] [PubMed] [Google Scholar]
- Wall EH, Hewitt SC, Liu L, Del Rio R, Case LK, Lin CY, Korach KS, Teuscher C. Genetic control of estrogen-regulated transcriptional and cellular responses in mouse uterus. FASEB J. 2013 doi: 10.1096/fj.12-213462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiater E, Vale W. Inhibin is an antagonist of bone morphogenetic protein signaling. J Biol Chem. 2003;278:7934–7941. doi: 10.1074/jbc.M209710200. [DOI] [PubMed] [Google Scholar]
- Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor alpha is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA. 2010;107:19272–19277. doi: 10.1073/pnas.1013226107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Kaghad M, Wang Y, Gillett E, Fleming MD, Dotsch V, Andrews NC, Caput D, McKeon F. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol Cell. 1998;2:305–316. doi: 10.1016/s1097-2765(00)80275-0. [DOI] [PubMed] [Google Scholar]
- Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson RT, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- Yang X, Li C, Herrera PL, Deng CX. Generation of Smad4/Dpc4 conditional knockout mice. Genesis. 2002;32:80–81. doi: 10.1002/gene.10029. [DOI] [PubMed] [Google Scholar]
- Yu PB, Hong CC, Sachidanandan C, Babitt JL, Deng DY, Hoyng SA, Lin HY, Bloch KD, Peterson RT. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.