Abstract
Introduction
Parenteral nutrition (PN) is a necessary therapy used to feed patients with gastrointestinal dysfunction. Unfortunately, PN results in intestinal atrophy and changes to host immune function. PN may also induce additional effects on gut motility that we hypothesized would result from changes in the enteric nervous system.
Methods
Mice received an IV catheter and were randomized to Chow (n =5), intravenous (IV) PN (n =6), or IV-PN+bombesin (BBS, 15 ug/kg TID)(n =6) for 5 days. Colons were removed and dissected to measure the length and circumference. Enteric neuronal density and neurotransmitter expression was determined by co-immunostaining whole-mount tissue with Hu and nNOS.
Results
The number of myenteric neurons expressing Hu and nNOS increased per unit length in the mid-colon during PN treatment compared to Chow. This increase was abrogated by the addition of BBS to the PN regimen. However, the percentage of nNOS expressing neurons was not significantly altered by PN. Morphometric analysis revealed a decrease in the length and circumference of the colon during PN administration that was partially normalized by supplementation of PN with BBS. A significant reduction in total fecal output was observed in PN animals compared to Chow and was increased by mice receiving BBS in addition to PN.
Conclusions
PN causes a constriction of the bowel wall reducing not only the length but also the circumference of the colon. These changes cause a condensation of enteric neurons but no difference in neurotransmitter expression. BBS supplementation partially restores the constriction and increases the fecal output during PN treatment compared to PN treatment alone.
Keywords: Enteric Nervous System, nNOS, Neurotransmitter, Motility, TPN, Parenteral Nutrition, Bombesin
1. Introduction
Parenteral Nutrition (PN) prevents progressive malnutrition and is life saving in patients with contraindications to enteral nutrition (EN). However, following long term PN with the lack of enteral stimulation, host defense mechanisms are altered and may result in an increased risk of systemic infections (1). The absence of oral feeding in rodents has been demonstrated to cause mucosal atrophy, intestinal epithelial barrier dysfunction, increased intestinal permeability, altered epithelial integrity and reductions in the mucous barrier (2–7).
In addition to mucosal immune defects in the small intestine, motility is also reduced during PN (8). Motility is regulated by the enteric nervous system (ENS) that is present along the length of the bowel wall (9). Several neurotransmitters, including nitric oxide (NO) that is produced by enteric neurons expressing the biosynthetic enzyme neuronal nitric oxide synthase (nNOS), mediate the relaxation of the smooth muscle to facilitate the movement of luminal contents. (10). Additionally, changes in ENS neuronal density and neurotransmitter expression underly dysmotility in other disease processes, including Hirschsprung’s disease (11–13).
The neuropeptide Bombesin (BBS) is an analogue of gastrin-releasing peptide that is secreted by the vagus nerve. BBS acts through the gastrin releasing peptide receptor to stimulate the release of a variety of gut peptides and hormones throughout the gastrointestinal tract, including somatostatin, neurotensin, motilin, gastrin, cholecystokinine, insulin and glucagon. BBS stimulates intestinal motility, improves intestinal integrity in models of gut barrier dysfunction, and acts trophically on the gastric and intestinal mucosa (14, 15). When administered during PN, BBS reverses some of the multiple defects in mucosal immune function (16, 17).
Since PN decreases intestinal motility, we hypothesized PN would affect nNOS expression in the ENS. Additionally, because of its restorative effect on gut mucosal immunity and trophic effects on the mucosa, we hypothesized that stimulation of the ENS with BBS would prevent some ENS neurotransmitter alterations during PN. To test these hypotheses, we measured: neuronal density, neurotransmitter expression, length and circumference of the colon and total fecal output of enterally fed and PN treated animals, both with and without addition of BBS to the PN regimen.
2. Methods
2.1 Animals
All animal procedures were approved by the Animal Care and Use Committee of the University of Wisconsin and Middleton Veterans Administration Hospital. Male Institute of Cancer Research (ICR) mice (aged 6–8 weeks) were housed in an American Association for Accreditation of Laboratory Animal Care-accredited conventional facility on the V.A Williamson Hospital Campus. Following an acclimatization period of one week in an environment controlled for temperature and humidity, ICR mice received intravenous catheters. Catherized mice were supplied ad libitum standard rodent chow and received 0.9% saline (IV, 4 mL/day) for two days before randomization to standard rodent Chow, intravenous PN, or intravenous PN + BBS injections (100 μL of 15 μg/kg BBS, TID) (n=minimum of 5/group) for 5 days (Figure 1). Chow and PN received saline as a vehicle control (100 μL saline, three times per day (TID)). Fecal material was collected from the animals and the total output was measured.
Figure 1.
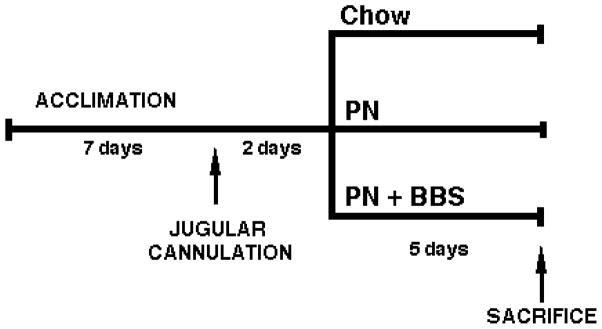
Schematic representation of the experimental protocol utilized. Mice were catherized IV, allowed two days of ad libitum standard rodent chow before being randomized to Chow, PN or PN+BBS (15μg/kg, TID) for five days.
2.2 Immunohistochemistry
Following humane euthanasia, the colon was removed, dissected, opened longitudinally along the mesentery and fixed in 4% paraformaldehyde (PFA) overnight at 4°C pinned in a sylgard dish without stretching mucosa side down. The circumference of the mid-colon was measured and recorded for each animal. The mucosa was removed from the tissue prior to immmunostaining to reduce autofluorescence. Colonic whole-mount tissues were incubated in phosphate buffered saline (PBS) containing 0.25–1.0% Triton X-100 for 4–6 hours at room temperature (RT). Primary antibodies were then applied to the tissues in PBS (anti-human Hu; 1:1000 (patient serum, WI) and anti-rabbit nNOS; 1:300 (BD transduction laboratories, CA)) and rocked overnight at 4°C. The following day, the tissues were rinsed in PBS 3 × 1 hour at RT before the secondary antibodies were added overnight at 4°C (donkey anti-human Dylight 488 and donkey anti-rabbit Cy3 (1:500, Jackson Immuno Research)).
2.3 Image Analysis
Fluorescent images were acquired on a Nikon A1 confocal microscope (20x magnification). Images were captured, processed, and analyzed with Metamorph software (Molecular Devices, Palo Alto, CA, USA) or Nikon Elements (Nikon, USA). The images were processed using the photo editing software Paint.NET (dotPDN LLC) and Photoshop (Adobe, USA). The neuronal density within the myenteric plexus was determined in the mid-colon by counting the number of cells immunostained with the pan neuronal marker, Hu (300–1500 neurons/image, 3 images/animal). Expression of the neurotransmitter nNOS was also determined and calculated as a proportion of the Hu+ neurons (300–1500 neurons/image, 3 images/animal).
2.4 Statistical Analysis
Data are expressed as mean ± standard deviation (SD). Statistical analysis was performed using analysis of variance (ANOVA) and Bonferroni/Dunn corrected for multiple comparisons with α = 0.05 (Statview 5.0.1, SAS, Cary, NC).
3. Results
3.1 Neuronal and nNOS density increase in the mid-colon of PN treated animals compared to Chow and are normalized by administration of BBS
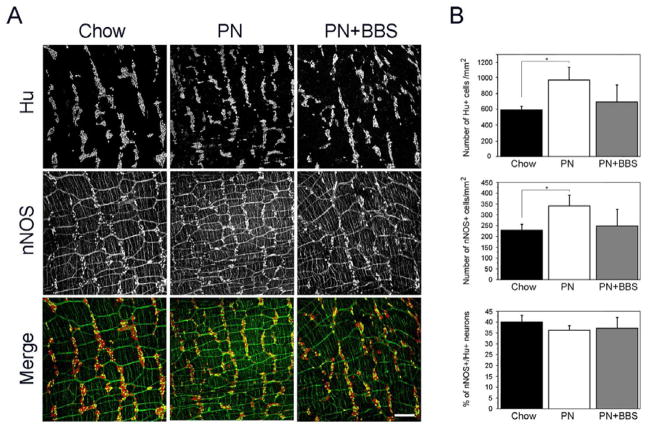
In order to determine the neuronal density, colonic tissues isolated from animals that had recieved Chow, PN or PN+BBS were immunostained with the pan neuronal marker Hu (Figure 2A) and the number of Hu+ cells were counted within the myenteric plexus of the mid-colon (Figure 2B). PN animals demonstrated increased neuronal density compared to Chow (967± 164 mm2 versus 587 ± 52 mm2, p=0.002) (Figure 2B), while addition of BBS to PN abrogated the changes to neuronal density (686 ± 222 mm2 versus 587 ± 52 mm2, p=0.34) (Figure 2B).
Figure 2.
Increased colonic myenteric neuronal density and number of nNOS expressing cells during PN treatment is abrogated by supplementation of PN with BBS. (A) Immunostaining of the myenteric plexus within the colon of Chow, PN and PN+BBS treated animals with Hu (upper panel), nNOS (middle panel) and Hu/nNOS (lower panel). Scale bar equals 200 μm. (B) Bar graphs showing the neuronal density (mm2), number of nNOS expressing cells (mm2) and the proportion of nNOS cells within the Hu+ population of neurons.
Neuronal nitric oxide synthase (nNOS) is the primary relaxation neurotransmitter involved in intestinal motility (9). Colonic tissues from animals receiving Chow, PN or PN+BBS were stained for nNOS (Figure 2A) and the number of nNOS+ cells was counted (Figure 2B). Similar to the findings in neuronal density, an increase in the number of cells expressing the neurotransmitter, nNOS was also detected in PN animals compared to Chow (348 ± 51 mm2 versus 235 ± 27 mm2, p=0.006) (Figure 2B). The addition of BBS to PN resulted in return of nNOS staining to the level observed in Chow animals (252 ± 80 mm2 versus 235 ± 27 mm2, p=0.64) (Figure 2B).
3.2 Morphometric analysis of the colon of Chow, PN and PN+BBS animals
Enteral deprivation causes mucosal atrophy, while BBS acts trophically on the gastric and intestinal mucosa (14, 18). To understand the effect of PN and PN+BBS in our model, we measured the length and circumference of the colon in Chow, PN and PN+BBS animals. We noted a small but statistically significant reduction in the length of the colon in both PN and PN+BBS treatment animals when compared to Chow (9± 0cm versus 10± 0cm, p=0.008 and 9± 0.5cm versus 10± 0cm, p=0.004 respectively) (Figure 3A). A reduction in the circumference of the mid-colon was also observed in PN treated animals compared to Chow (0.51 ± 0.05cm versus 0.67 ± 0.1cm, p=0.0007) (Figure 3B). While there was some restoration in circumference of the mid-colon by BBS administration, it was not statistically significant from PN treatment alone (0.56 ± 0.08cm versus 0.51 ± 0.05cm, p=0.2).
Figure 3.
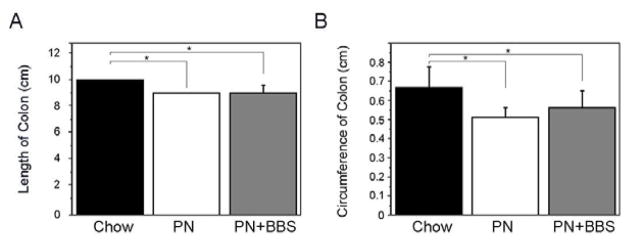
PN reduces the length and circumference of the colon, which is partially restored by the addition of BBS. (A) Length and (B) circumference of the colon (cm) measured in Chow, PN and PN+BBS animals.
3.3 Neurotransmitter density in the mid-colon of Chow, PN and PN+BBS animals
Due to the changes we noted in the morphometry of the colon in enterally deprived animals, we considered the possibility that the observed increase in neuronal and neurotransmitter density may be an artifact of the altered morphometry. Therefore, we performed co-localization of Hu (neurons) and nNOS (relaxation neurotransmitter) and measured nNOS density as the proportion of neurons within the Hu+ population (Figure 2A). We noted similar percentages of Hu+ neurons expressing nNOS within the Chow (40 ± 3%), PN (36 ± 2%) and PN+BBS (37 ± 5%) animals (Figure 2B).
3.4 Fecal output as a determinant of gut motility
To determine if there is a functional defect in motility in our model of enteral deprivation, fecal material was collected from each animal on a daily basis and the total fecal output was weighed. PN animals produced significantly less fecal material than the Chow animals (0.033 ± 0.017gm versus 1.5 ± 0.34gm, p<0.0001) (Figure 4). Addition of BBS resulted in increased fecal output compared to PN alone which was statistically significant when the two treatments were compared directly in the absence of Chow data (0.049 ± 0.007gm versus 0.033 ± 0.017gm, p=0.044) (Figure 4).
Figure 4.
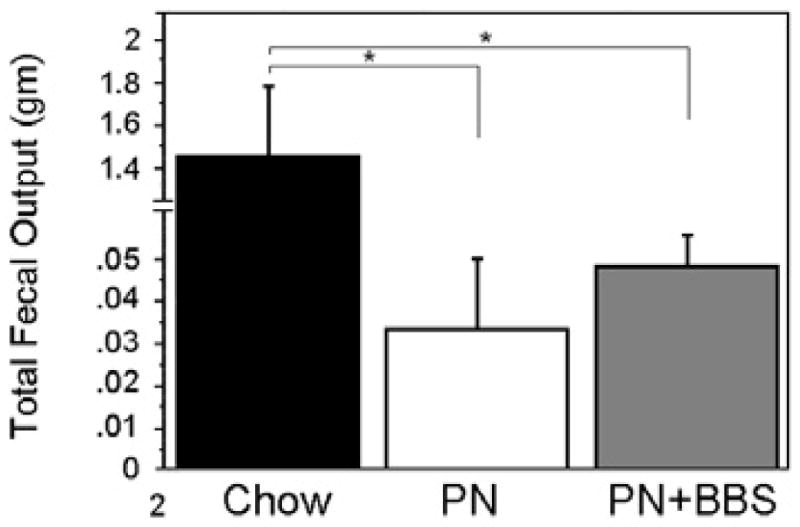
Total fecal ouput is reduced during PN treatment compared to Chow and is increased with BBS supplementation of PN. Bar graph showing the total fecal output (gm) measured from Chow, PN and PN+BBS animals.
4. Discussion
Parenteral nutrition has previously been shown to affect gastrointestinal mucosal immunity through local atrophy of the mucosal barrier as well as changes in systemic immune function (6). The majority of studies that have analyzed the effects of PN focused on the small intestine. Since the colon has the largest bacterial load, increased morbidity following long term PN may be related to bacterial translocation (19), This study examined the effects of PN with and without BBS supplementation on myenteric neuronal density and expression of a relaxation neurotransmitter, nNOS, within the colon. We additionally measured colon morphometry and fecal output.
We found increased neuronal density and number of cells expressing nNOS in animals undergoing PN treatment compared to Chow. We also observed a reduction in both the length and circumference of the colon. However, since number of nNOS expressing cells was measured as a proportion of the total neuronal population, we found no significant alterations in neurotransmitter expression in PN animals compared to Chow. These results are similar to the observations reported for treatment of rats with PN, where increases in neuronal density were documented in the small intestine (20) and suggest that PN does not affect gut function by changing enteric neuronal density or neurotransmitter expression.
The reduction in the circumference of the colon during PN treatment could result from muscular constriction or contraction. The addition of BBS to the PN regimen partially restored the decrease in colonic circumference suggesting that BBS is capable of exerting some degree of muscular relaxation within the colon. This relaxation may be mediated by nitric oxide since the action of BBS can be reversed by NOS inhibition (21), thereby returning the neuronal density and number of nNOS expressing cells to levels observed in Chow animals. Interestingly, the length of the colon was not altered by the supplementation of BBS to the PN regimen. This finding suggests that BBS may have a differential effect on the circular and longitudinal muscle layers and is consistent with previous in vitro smooth muscle studies (22). However, the exact mechanism by which PN affects the circumference and length of the colon is unclear and indeed the depth of the mucosa, submucosa and muscle layers were reported to be unchanged in rats undergoing PN treatment (23). Further investigations are required to determine how PN and BBS alter colonic morphometry.
Since PN is often utilized in patients that are critically ill, require the use of opiates, or have pre-existing gastrointestinal dysfunction, it is difficult to discern whether PN alone has a direct effect on gut motility. Experiments in the rat and dog have shown no change in motor activity in the jejunum and ileum during PN treatment (20, 24), however defects were observed in the duodenum and stomach of dogs (24). In our studies, we observed a dramatic decrease in the fecal output between PN and Chow animals, although this would be expected since the PN treated animals only received solid food for the first two days of the experiment. Supplementation of PN with BBS increased fecal output suggesting BBS changes motility. The effects of BBS on gut motility could be mediated by motilin secretion since BBS stimulates Motilin release that functions to initiate migrating motor complexes within the gastrointestinal tract (25, 26).
Together, these data show that enteral deprivation affects the length and circumference of the colon but does not cause structural changes in the enteric nervous system. The addition of BBS to PN restores colonic morphometry to that observed in Chow animals. Additionally, fecal ouput increases possibly by stimulating gastrointestinal motility. Further studies investigating the temporal development of alterations to the enteric nervous system during PN or following restoration of enteral feeding will greatly enhance our understanding of the mechanisms underlying the functional impact of PN and PN+BBS on gut motility.
Acknowledgments
This research is supported by: Central Surgical Association Foundation Turcotte Award (AG), NIH-NIDDK RO1DK081634 grant (MLE) and I01BX001672 grant from the Biomedical Laboratory Research & Development Service of the VA Office of Research and Development (KAK). The contents of this article do not represent the views of the Veterans Affairs or the United States Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Minard G, Kudsk KA. Nutritional support and infection: does the route matter? World J Surg. 1998 Feb;22(2):213–9. doi: 10.1007/s002689900372. [DOI] [PubMed] [Google Scholar]
- 2.Yang H, Kiristioglu I, Fan Y, Forbush B, Bishop DK, Antony PA, et al. Interferon-gamma expression by intraepithelial lymphocytes results in a loss of epithelial barrier function in a mouse model of total parenteral nutrition. Ann Surg. 2002 Aug;236(2):226–34. doi: 10.1097/00000658-200208000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang H, Finaly R, Teitelbaum DH. Alteration in epithelial permeability and ion transport in a mouse model of total parenteral nutrition. Crit Care Med. 2003 Apr;31(4):1118–25. doi: 10.1097/01.CCM.0000053523.73064.8A. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Kudsk KA, Gocinski B, Dent D, Glezer J, Langkamp-Henken B. Effects of parenteral and enteral nutrition on gut-associated lymphoid tissue. J Trauma. 1995 Jul 1;39(1):44–51. doi: 10.1097/00005373-199507000-00006. discussion -2. [DOI] [PubMed] [Google Scholar]
- 5.Feng Y, McDunn JE, Teitelbaum DH. Decreased phospho-Akt signaling in a mouse model of total parenteral nutrition: a potential mechanism for the development of intestinal mucosal atrophy. Am J Physiol Gastrointest Liver Physiol. 2010 Jun;298(6):G833–41. doi: 10.1152/ajpgi.00030.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feng Y, Ralls MW, Xiao W, Miyasaka E, Herman RS, Teitelbaum DH. Loss of enteral nutrition in a mouse model results in intestinal epithelial barrier dysfunction. Ann N Y Acad Sci. 2012 Jul;1258:71–7. doi: 10.1111/j.1749-6632.2012.06572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun X, Yang H, Nose K, Nose S, Haxhija EQ, Koga H, et al. Decline in intestinal mucosal IL-10 expression and decreased intestinal barrier function in a mouse model of total parenteral nutrition. Am J Physiol Gastrointest Liver Physiol. 2008 Jan;294(1):G139–47. doi: 10.1152/ajpgi.00386.2007. [DOI] [PubMed] [Google Scholar]
- 8.Masclee AA, Gielkens HA, Lam WF, de Boer SY, Lamers CB. Effects of parenteral nutrients on gastrointestinal motility and secretion. Scand J Gastroenterol Suppl. 1996;218:50–5. doi: 10.3109/00365529609094731. [DOI] [PubMed] [Google Scholar]
- 9.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012 May;9(5):286–94. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 10.Grundy D, Schemann M. Enteric nervous system. Curr Opin Gastroenterol. 2006 Mar;22(2):102–10. doi: 10.1097/01.mog.0000208459.46395.16. [DOI] [PubMed] [Google Scholar]
- 11.Sandgren K, Larsson LT, Ekblad E. Widespread changes in neurotransmitter expression and number of enteric neurons and interstitial cells of Cajal in lethal spotted mice: an explanation for persisting dysmotility after operation for Hirschsprung’s disease? Dig Dis Sci. 2002 May;47(5):1049–64. doi: 10.1023/a:1015085923245. [DOI] [PubMed] [Google Scholar]
- 12.Roberts RR, Bornstein JC, Bergner AJ, Young HM. Disturbances of colonic motility in mouse models of Hirschsprung’s disease. Am J Physiol Gastrointest Liver Physiol. 2008 Apr 1;294(4):G996–G1008. doi: 10.1152/ajpgi.00558.2007. [DOI] [PubMed] [Google Scholar]
- 13.Zaitoun I, Erickson C, Barlow A, Klein T, Heneghan A, Pierre J, et al. Altered Neuronal Density and Neurotransmitter Expression in the Ganglionated Region of Ednrb Null Mice: Implications for Hirschsprung’s Disease. Neurogastroenterol Motil. 2013 doi: 10.1111/nmo.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chu KU, Evers BM, Ishizuka J, Townsend CM, Jr, Thompson JC. Role of bombesin on gut mucosal growth. Ann Surg. 1995 Jul;222(1):94–100. doi: 10.1097/00000658-199507000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gulluoglu BM, Kurtel H, Gulluoglu MG, Aktan AO, Yegen BC, Dizdaroglu F, et al. Bombesin ameliorates colonic damage in experimental colitis. Dig Dis Sci. 1999 Aug;44(8):1531–8. doi: 10.1023/a:1026646523284. [DOI] [PubMed] [Google Scholar]
- 16.DeWitt RC, Wu Y, Renegar KB, King BK, Li J, Kudsk KA. Bombesin recovers gut-associated lymphoid tissue and preserves immunity to bacterial pneumonia in mice receiving total parenteral nutrition. Ann Surg. 2000 Jan;231(1):1–8. doi: 10.1097/00000658-200001000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zarzaur BL, Ikeda S, Johnson CD, Le T, Sacks G, Kudsk KA. Mucosal immunity preservation with bombesin or glutamine is not dependent on mucosal addressin cell adhesion molecule-1 expression. JPEN J Parenter Enteral Nutr. 2002 Sep-Oct;26(5):265–70. doi: 10.1177/0148607102026005265. discussion 70. [DOI] [PubMed] [Google Scholar]
- 18.Assimakopoulos SF, Alexandris IH, Scopa CD, Mylonas PG, Thomopoulos KC, Georgiou CD, et al. Effect of bombesin and neurotensin on gut barrier function in partially hepatectomized rats. World J Gastroenterol. 2005 Nov 21;11(43):6757–64. doi: 10.3748/wjg.v11.i43.6757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacFie J, Reddy BS, Gatt M, Jain PK, Sowdi R, Mitchell CJ. Bacterial translocation studied in 927 patients over 13 years. Br J Surg. 2006 Jan;93(1):87–93. doi: 10.1002/bjs.5184. [DOI] [PubMed] [Google Scholar]
- 20.Ekelund M, Qader SS, Hallen M, Ekblad E. Effects of total parenteral nutrition on rat enteric nervous system, intestinal morphology, and motility. J Surg Res. 2005 Apr;124(2):187–93. doi: 10.1016/j.jss.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Castaneda AA, Kim YS, Chang LK, Cui Y, Mercer DW. Nitric oxide synthase inhibition negates bombesin-induced gastroprotection. Surgery. 2000 Sep;128(3):422–8. doi: 10.1067/msy.2000.107982. [DOI] [PubMed] [Google Scholar]
- 22.Vadokas B, Ludtke FE, Lepsien G, Golenhofen K, Mandrek K. Effects of gastrin-releasing peptide (GRP) on the mechanical activity of the human ileocaecal region in vitro. Neurogastroenterol Motil. 1997 Dec;9(4):265–70. doi: 10.1046/j.1365-2982.1997.d01-59.x. [DOI] [PubMed] [Google Scholar]
- 23.Ekelund M, Kristensson E, Ekblad E. Total parenteral nutrition causes circumferential intestinal atrophy, remodeling of the intestinal wall, and redistribution of eosinophils in the rat gastrointestinal tract. Dig Dis Sci. 2007 Aug;52(8):1833–9. doi: 10.1007/s10620-006-9678-z. [DOI] [PubMed] [Google Scholar]
- 24.Kaji T, Takamatsu H, Kajiya H. Motility of the gastrointestinal tract and gallbladder during long-term total parenteral nutrition in dogs. JPEN J Parenter Enteral Nutr. 2002 May-Jun;26(3):198–204. doi: 10.1177/0148607102026003198. [DOI] [PubMed] [Google Scholar]
- 25.Hashmonai M, Szurszewski JH. Effect of cerebroventricular perfusion of bombesin on gastrointestinal myoelectric activity. Am J Physiol. 1998 Apr;274(4 Pt 1):G677–86. doi: 10.1152/ajpgi.1998.274.4.G677. [DOI] [PubMed] [Google Scholar]
- 26.Chen CY, Tsai CY. Ghrelin and motilin in the gastrointestinal system. Curr Pharm Des. 2012;18(31):4755–65. doi: 10.2174/138161212803216915. [DOI] [PubMed] [Google Scholar]