Abstract
Background
A 2008 expert consensus statement recommended an in-person follow-up visit between 2 and 12 weeks after new cardiovascular implantable electronic device (CIED) placement.
Objective
To assess outcomes associated with adherence to the experts’ recommendations.
Methods
Using data from the National Cardiovascular Data Registry’s (NCDR®) ICD Registry™ linked to Medicare claims, we studied the association between follow-up within 2–12 weeks after CIED placement between January 1, 2005, and September 30, 2008, and all-cause mortality and risk of readmission within 1 year.
Results
Compared with patients who did not receive the recommended follow-up (n=43,060), those who did (n=30,256) were more likely to be older, white, to have received a CRT-D device, to have more advanced heart failure symptoms, and to have non-ischemic dilated cardiomyopathy. In Cox proportional hazards models adjusted for patient demographic and clinical factors, mortality was lower (hazard ratio (HR) 0.93, 95% confidence interval (CI) 0.88–0.98; P=0.005) but cardiovascular readmission was higher (HR 1.04, 95% CI 1.01–1.08, P=0.012) among patients who received initial follow-up within 2–12 weeks after CIED placement compared with those who did not. There was no association between CIED follow-up and readmission for heart failure (HR 1.00, 95% CI 0.96–1.05; P=0.878) or device-related infection (HR 1.22, 95% CI 0.98–1.51; P=0.075).
Conclusions
Follow-up within 2–12 weeks after CIED placement was independently associated with improved survival but increased cardiovascular readmission. Quality improvement initiatives designed to increase adherence to experts’ recommendations may be warranted.
Keywords: cardioverter-defibrillator, implantable, outpatient, registry, Medicare, assessment, outcomes
Introduction
Cardiovascular implantable electronic device (CIED) indications have evolved and expanded over time.1–4 CIED implantation rates have correspondingly risen. US implantable cardioverter-defibrillator (ICD) placement increased from 92,897 in 2006 to 141,374 in 2009.5 The proportion of these procedures that included cardiac resynchronization therapy (CRT-D) grew from 37.4% to 41.7% during the same time period.5 The number of pacemaker procedures has also risen, particularly among older patients.6
CIED management is becoming increasingly complex. Consequently, the Heart Rhythm Society (HRS) and the European Heart Rhythm Association (EHRA) issued an expert consensus statement in 2008 detailing the minimum frequency of in-person and remote follow-up of CIEDs. The statement recommends an in-person follow-up visit 2 to 12 weeks after implantation for patients with a new pacemaker, ICD, or CRT device; in-person or remote monitoring every 3 to 12 months for patients with pacemakers, including CRT; and in-person or remote monitoring every 3 to 6 months for patients with an ICD with or without CRT.7 A 2012 HRS/EHRA expert consensus statement reinforced the importance of at least biannual in-person clinic visits for CRT recipients.8 Although CIED follow-up is a key element of cardiology and electrophysiology practice and adherence to HRS/EHRA recommendations may improve outcomes, this has not been demonstrated empirically. Using a United States national ICD registry and Medicare Claims data, we sought to examine the association between completion of an in-person follow-up visit 2 to 12 weeks after new ICD or CRT with ICD (CRT-D) placement and death, cardiovascular readmission, heart failure readmission, and readmission for a device-related infection.
Methods
Data source
Data for this study included clinical data from the National Cardiovascular Data Registry’s (NCDR®) ICD Registry™ and Medicare claims data from the US Centers for Medicare & Medicaid Services (CMS). The NCDR® ICD Registry™ was established in 2005 through a partnership of the Heart Rhythm Society and the American College of Cardiology Foundation, and became the sole repository of ICD implantation data for Medicare beneficiaries on April 1, 2006. Although the CMS mandates hospitals to enter data on Medicare patients receiving primary prevention ICDs into the database, about 78 percent of participating hospitals report data on all implantations regardless of payer or indication.9 The registry contains patient demographic characteristics, medical history, clinical and procedural information, discharge medications, and in-hospital outcomes. Audits suggest more than 90% of fields accurately represent the data from the medical charts.10
The Medicare data include the 100% Medicare inpatient, outpatient, and carrier claims, and the corresponding denominator files for 2005 through 2009. The inpatient files contain institutional claims for facility costs covered under Medicare Part A and encrypted beneficiary identifiers, admission and discharge dates, dates of service, diagnosis-related groups (DRGs), International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic codes and procedure codes, and beneficiary demographic information. The carrier files contain noninstitutional provider claims for services covered under Medicare Part B, Current Procedural Terminology (CPT) codes and dates of service. The outpatient files contain claims by institutional outpatient providers (e.g., hospital outpatient departments and ambulatory surgery centers), dates of service, CPT codes, ICD-9-CM diagnostic codes and procedure codes. The denominator files include encrypted beneficiary identifiers, dates of birth, sex, race/ethnicity, dates of death, and information about program eligibility and enrollment.
We linked the registry data to Medicare claims data using the method developed by Hammill et al.11 For patients with multiple device implants in the registry, we selected the first implant for the analysis.
Study Cohort
From the linked data set, we selected patients who were 65 years or older; underwent CIED placement and discharged home alive between January 1, 2005, and September 30, 2008; were enrolled in fee-for-service Medicare for at least 12 weeks after the index discharge; and were discharged from hospitals that performed greater than 10 implantations between January 1, 2005, and September 30, 2008. We required that patients be alive and not hospitalized for 12 weeks after the index discharge, the period during which we looked for follow-up visits. We excluded patients who had their device removed within 12 weeks of implantation.
Exposure
The CIED follow-up status was ascertained within 2–12 weeks after the index discharge by searching for the CPT codes 93742, 93744, 93282, 93283, 93284, 93289 or 93741 or 93743 (with an evaluation and management visit on the same claim) in the Medicare carrier and outpatient files.
Outcomes
Outcomes of interest were all-cause mortality, cardiovascular readmission, heart failure readmission, and readmission for device-related infection at 1 year after the landmark time. Mortality was ascertained on the basis of death dates in the Medicare denominator files, and readmission was ascertained on the basis of Medicare inpatient claims. We defined cardiovascular readmission using DRGs 104–112, 115–118, 121–145, 479, 514–518, 525–527, 535, 536, and 547–558 before October 1, 2007, and codes 215–238, 242–254, 258–262, and 280– 316 on or after October 1, 2007. We defined heart failure readmission using ICD-9-CM diagnostic codes 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, and 428.x. when used as primary codes on an inpatient claim.12 We defined readmission for device-related infection using ICD-9-CM diagnosis code 996.6 when used as a primary or a secondary code on an inpatient claim. Transfers to or from another hospital and admissions for rehabilitation (DRG code 462 or ICD-9-CM diagnostic codes V57.xx) did not count as readmissions.
Follow-up time began at the conclusion of the 12-week ascertainment period (day 84). We considered day 84 (12 weeks) after the index discharge as a landmark time. The length of follow-up for all events was 1 year after the landmark time; days to events were calculated from the landmark time. For patients who did not experience an event, we defined a censoring date as the earliest of (a) 1 year following the landmark time, (b) the end of claims data availability, which was Dec. 31, 2009, or (c) the date of a patient’s enrollment in a Medicare Managed Care plan.
Statistical Analysis
We described the baseline characteristics of the study population using frequencies with percentages for categorical variables and means with standard deviations (SDs) for continuous variables. We tested for differences between the 2 groups using the chi-square tests for categorical variables and t tests for continuous variables.
We estimated the unadjusted cumulative incidence of each outcome at 1 year after the landmark time for each group. For mortality, estimates were based on the Kaplan-Meier estimator, and differences between groups were assessed using log-rank tests. For the readmission outcomes, estimates were based on the cumulative incidence function, which accounts for the competing risk of mortality, and Gray tests were used to assess differences between groups.13
We estimated the unadjusted and adjusted associations between adherence to initial follow-up within 2–12 weeks after CIED placement and each outcome using Cox proportional hazards models and covariates from the registry. In the unadjusted model, adherence to the initial follow-up visit was the only covariate. In the adjusted model, age, race, reason for admission, atrial fibrillation/flutter, type of device (ICD vs. CRT-D), prior myocardial infraction (MI), diabetes mellitus, hypertension, congestive heart failure, New York Heart Association (NYHA) class, ischemic heart disease, non-ischemic dilated cardiomyopathy, cerebrovascular disease, primary valvular disease, end stage renal disease (ESRD), left ventricular ejection fraction (LVEF), chronic lung disease, and systolic blood pressure also were included. For all models, significance tests and confidence intervals were based on robust standard errors to account for clustering of patients by hospital. We reported hazard ratios and 95% confidence intervals. We used = .05 to establish statistical significance of all tests. All tests were 2-sided.
We used SAS version 9.2 (SAS Institute Inc, Cary, North Carolina) for all analyses. The institutional review board of the Duke University Health System approved the study.
Results
The initial data set included 87,134 patients at 991 hospitals who underwent new transvenous CIED placement between January 1, 2005, and September 30, 2008, and were discharged alive. During the 12-week ascertainment period, 439 patients died, 13,125 had a cardiovascular readmission, 5,475 were readmitted for heart failure, and 648 were readmitted with a device-related infection. The final study cohort included 73,316 patients, of which 30,256 (41.3%) received an initial follow-up visit 2 to 12 weeks after discharge.
Table 1 shows the baseline characteristics of the study population. In comparison to patients who did not have an initial in-person CIED follow-up visit, those who had such a visit received an ICD less frequently (49.4% v. 57.3%) and CRT-D more frequently (50.7% v. 42.7%); had a higher prevalence of a number of comorbidities including atrial fibrillation or atrial flutter, more advanced heart failure symptoms, and nonischemic cardiomyopathy; and were more frequently prescribed an antiarrhythmic agent and warfarin at discharge.
Table 1.
Baseline characteristics of patients according to whether or not an initial CIED follow-up visit between 2 and 12 weeks occurred
| Variable | CIED Follow-up (n=30,256) |
No CIED follow-up (n=43,060) |
P |
|---|---|---|---|
| Age, y (mean, SD) | 75.1 (6.1) | 74.9 (6.2) | <.0001 |
| 65–79 | 20,872 (69.0%) | 30,389 (70.6%) | <.0001 |
| >=80 | 9,384 (31.0%) | 12,671 (29.4%) | |
| Male sex | 22,767 (75.2%) | 32,339 (75.1%) | 0.6531 |
| Race | <.0001 | ||
| White | 27,330 (90.3%) | 38,393 (89.2%) | |
| Black | 1,796 (5.9%) | 2,989 (6.9%) | |
| Other/unknown | 1,130 (3.7%) | 1,678 (3.9%) | |
| Type of device | <.0001 | ||
| ICD | 14,904 (49.3%) | 24,658 (57.3%) | |
| CRT-D | 20,872 (50.7%) | 18.402 (42.7%) | |
| Reason of admission | 0.2607 | ||
| ICD | 19,357 (64.0%) | 27,528 (63.9%) | |
| Heart Failure | 3,618 (12.0%) | 5,034 (11.7%) | |
| Cardiac, other | 6,481 (21.4%) | 9,269 (21.5%) | |
| Non-Cardiac | 800 (2.6%) | 1,229 (2.9%) | |
| Medical history | |||
| Atrial fibrillation or flutter | 12,512 (41.4%) | 16,597 (38.5%) | <.0001 |
| Hypertension | 23,305 (77.0%) | 33,636 (78.1%) | 0.0005 |
| Congestive Heart Failure | 24,336 (80.4%) | 34,337 (79.7%) | 0.0211 |
| NYHA Functional class | <.0001 | ||
| Class I | 3,199 (10.6%) | 4,486 (10.4%) | |
| Class II | 9,682 (32.0%) | 14,974 (34.8%) | |
| Class III | 16,063 (53.1%) | 21,864 (50.8%) | |
| Class IV | 1,312 (4.3%) | 1,736 (4.0%) | |
| Non-ischemic dilated cardiomyopathy | 8,014 (26.5%) | 10,800 (25.1%) | <.0001 |
| Ischemic heart disease | 22,004 (72.7%) | 31,742 (73.7%) | 0.0029 |
| Prior heart transplant | 53 (0.2%) | 63 (0.1%) | 0.3330 |
| Ventricular tachycardia | 11,540 (38.1%) | 16,196 (37.6%) | 0.1463 |
| Cardiac arrest | 2,403 (7.9%) | 3,365 (7.8%) | 0.5277 |
| Prior ICD device | 3,219 (10.6%) | 4,561 (10.6%) | 0.8387 |
| Prior CRT device | 967 (3.2%) | 1,548 (3.6%) | 0.0035 |
| Cerebrovascular disease | 5,034 (16.6%) | 7,234 (16.8%) | 0.5634 |
| Prior MI | 17,429 (57.6%) | 25,555 (59.3%) | <.0001 |
| Prior PCI | 10,146 (33.5%) | 14,752 (34.3%) | 0.0412 |
| Prior CABG | 12,897 (42.6%) | 18,610 (43.2%) | 0.1106 |
| Primary valvular disease | 2,899 (9.6%) | 3,701 (8.6%) | <.0001 |
| Renal insufficiency (creatinine >2.0) | 2,484 (8.2%) | 3,635 (8.4%) | 0.2640 |
| ESRD | 958 (3.2%) | 1,434 (3.3%) | 0.2187 |
| Diabetes mellitus | 10,539 (34.8%) | 15,544 (36.1%) | 0.0004 |
| Chronic lung disease | 6,783 (22.4%) | 10,106 (23.5%) | 0.0009 |
| Prior hospitalizations | 12,979 (42.9%) | 18,388 (42.7%) | 0.6011 |
| Adverse event | 1,164 (3.8%) | 1,498 (3.5%) | 0.0087 |
| Lab tests (mean, SD) | |||
| Systolic blood pressure, mm Hg | 132 (22.1) | 133 (22.5) | <.0001 |
| Left ventricular ejection fraction, % | 27.6 (9.9) | 27.6 (9.9) | 0.8495 |
| BUN, mg/dL | 25.8 (13.4) | 25.6 (13.5) | 0.0429 |
| Creatinine, mg/dL | 1.4 (1.0) | 1.4 (1.0) | 0.5400 |
| Sodium, mmol/L | 139 (3.5) | 139 (3.5) | 0.2531 |
| Medications at discharge | |||
| Angiotensin converting enzyme inhibitor | 17,598 (58.2%) | 25,505 (59.2%) | 0.0038 |
| Antiarrhythmic agent | 5,740 (19.0%) | 7,697 (17.9%) | 0.0002 |
| Antihypertensive | 1,146 (3.8%) | 1,677 (3.9%) | 0.4590 |
| Angiotensin Receptor Blocker | 5,604 (18.5%) | 7,725 (17.9%) | 0.0443 |
| Aspirin | 20,128 (66.5%) | 28,816 (66.9%) | 0.2638 |
| Beta blocker | 25,564 (84.5%) | 36,240 (84.2%) | 0.2256 |
| Calcium channel blocker | 2,486 (8.2%) | 3,742 (8.7%) | 0.0235 |
| Digoxin | 8,361 (27.6%) | 11,700 (27.2%) | 0.1664 |
| Diuretic | 19,749 (65.3%) | 27,788 (64.5%) | 0.0389 |
| Nitrate | 6,535 (21.6%) | 9,171 (21.3%) | 0.3284 |
| Platelet aggregation inhibitor | 6,774 (22.4%) | 10,167 (23.6%) | 0.0001 |
| Statin | 20,041 (66.2%) | 28,485 (66.2%) | 0.8080 |
| Warfarin | 10,336 (34.2%) | 13,490 (31.3%) | <.0001 |
Data are presented as n (%) unless otherwise specified.
Abbreviations: BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CRT, cardiac resynchronization therapy; CRT-D, cardiac resynchronization therapy-defibrillator; ESRD, end-stage renal disease; ICD, implantable cardioverter-defibrillator; MI, myocardial infarction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SD, standard deviation.
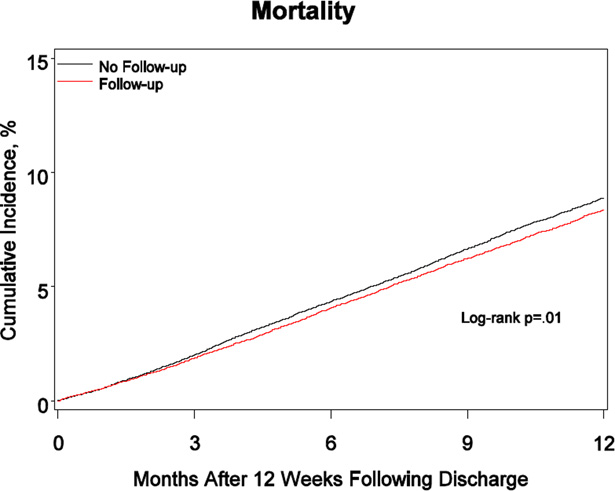
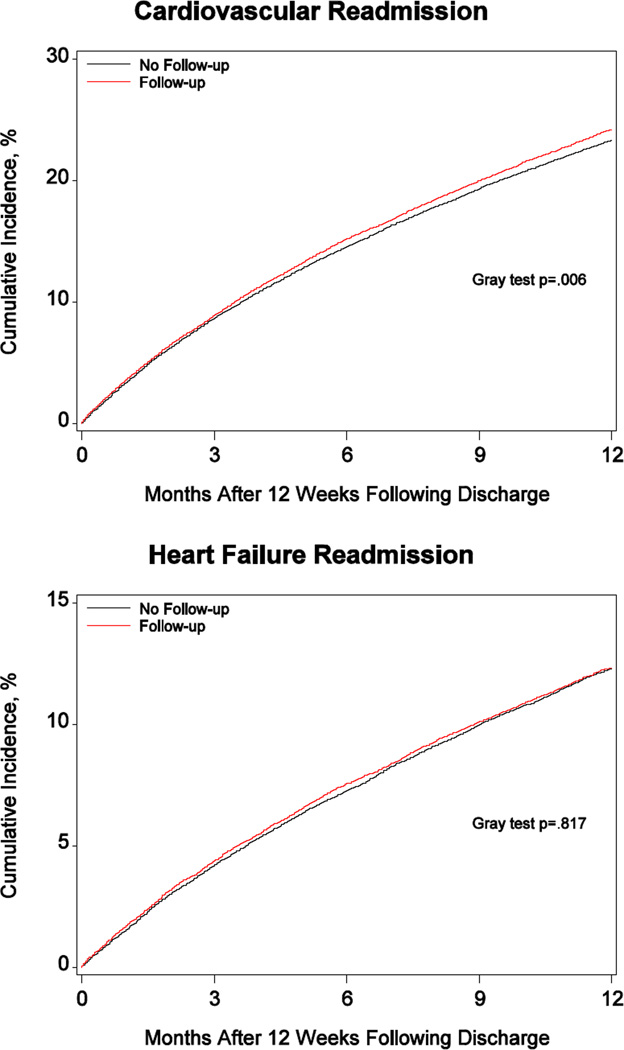
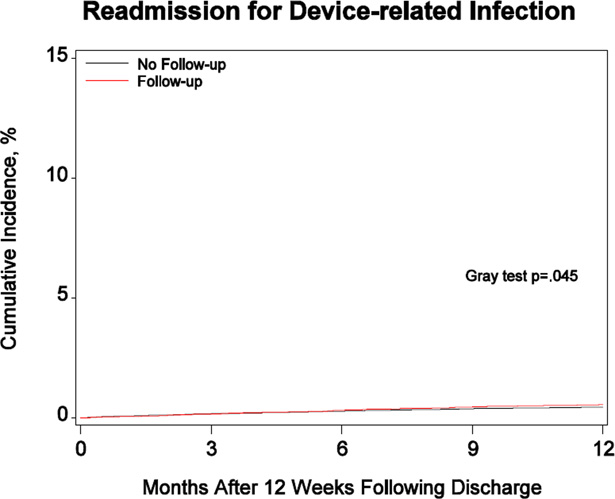
Table 2 shows the observed 1-year cumulative incidence of study outcomes. Compared with patients who did not have the first in-person follow-up visit, those who did had a lower incidence of all-cause mortality (8.5% v. 9.0%; P = 0.0095), higher incidence of cardiovascular readmission (24.4% v. 23.5%; P = 0.0060), and comparable rates of heart failure readmission (12.5% v. 12.4%; P = 0.8171) and device-related infection (0.6% v. 0.5%; P = 0.0452). Figure 1, Panels A–D show the corresponding cumulative incidence curves.
Table 2.
Cumulative incidence of events at 1 year by whether or not an initial CIED follow-up between 2 and 12 weeks occurred
| Event | CIED Follow-up (n=30,256) |
No CIED follow-up (n=43,060) |
P |
|---|---|---|---|
| Death | 2,561 (8.5) | 3,881 (9.0) | 0.0095 |
| Cardiovascular readmission | 7,385 (24.4) | 10,130 (23.5) | 0.0060 |
| Heart failure readmission | 3,768 (12.5) | 5,345 (12.4) | 0.8171 |
| Readmission for device-related infection | 169 (0.6) | 195 (0.5) | 0.0452 |
Values are expressed as number of events (cumulative incidence per 100 patients at risk).
Abbreviation: CIED, cardiovascular implantable electronic device.
Figure.
Cumulative incidence of mortality, cardiovascular readmission, heart failure readmission, and readmission for device-related infection
Table 3 shows associations between completion of the initial CIED follow-up visit and study outcomes using Cox proportional hazards models. A follow-up visit within 12 weeks was associated with a lower relative risk of death (adjusted HR 0.93, 95% CI 0.88–0.98; P = 0.005). Initial CIED follow-up was also associated with a higher risk of cardiovascular readmission (adjusted HR 1.04, 95% CI 1.01–1.08, P = 0.012). However, initial CIED follow-up was not associated with either heart failure readmission or readmission for device-related infection.
Table 3.
Associations between adherence to an initial CIED follow-up visit 2 to 12 weeks after device implantation and study outcomes
| Event | Unadjusted | Adjusted* | ||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Death | 0.94 (0.89–0.99) | .017 | 0.93 (0.88–0.98) | .005 |
| Cardiovascular readmission | 1.04 (1.01–1.08) | .020 | 1.04 (1.01–1.08) | .012 |
| Heart failure readmission | 1.00 (0.96–1.05) | .941 | 1.00 (0.95–1.04) | .843 |
| Device-related infection | 1.23 (1.00–1.52) | .053 | 1.21 (0.98–1.49) | .083 |
Adjusted for the following covariates: age, race, insurance payor, reason for admission, atrial fibrillation/flutter, type of device (ICD vs. CRT-D), prior myocardial infarction, diabetes mellitus, hypertension, heart failure, New York Heart Association class, ischemic/nonischemic cardiomyopathy, cerebrovascular disease, primary valvular disease, end-stage renal disease, left ventricular ejection fraction, chronic lung disease, and systolic blood pressure.
Time to events were censored at the earliest of (a) 1 year following the time of 12 weeks after the index hospital discharge, (b) the end of claims data availability (December 31, 2009), or (c) the date when the patient enrolled in a Medicare Managed Care plan.
Abbreviations: CIED, cardiovascular implantable electronic device; CI, confidence interval; HR, hazard ratio.
Table 4 shows reasons for cardiovascular readmission. Compared with their counterparts who did not have an in-person initial follow-up visit, patients who did were more often admitted for cardiac arrhythmias, including atrial tachycardia and atrial flutter, as well as procedural complications.
Table 4.
Reasons for cardiovascular hospitalization
| Reason | Total (n=17,515) |
Follow-up (n=7,385) |
No follow-up (n=10,130) |
P-value |
|---|---|---|---|---|
| Heart failure | 7,799 (44.5%) | 3,207 (43.4%) | 4,592 (45.3%) | 0.0123 |
| Coronary heart disease | 2,379 (13.6%) | 981 (13.3%) | 1,398 (13.8%) | 0.3241 |
| Peripheral vascular disease | 711 (4.1%) | 286 (3.9%) | 425 (4.2%) | 0.2852 |
| Cardiac dysrhythmias | 2,409 (13.8%) | 1,060 (14.4%) | 1,349 (13.3%) | 0.0492 |
| Paroxysmal tachycardia | 996 (41.3%) | 446 (42.1%) | 550 (40.8%) | 0.5187 |
| Atrial fibrillation and flutter | 1,056 (43.8%) | 466 (44.0%) | 590 (43.7%) | 0.9116 |
| Other | 357 (14.8%) | 148 (14.0%) | 209 (15.5%) | 0.2939 |
| Hypertension | 476 (2.7%) | 208 (2.8%) | 268 (2.6%) | 0.4921 |
| Complications peculiar to certain procedures | 1,434 (8.2%) | 651 (8.8%) | 783 (7.7%) | 0.0097 |
| Symptoms involving respiratory system and other chest symptoms |
676 (3.9%) | 310 (4.2%) | 366 (3.6%) | 0.0473 |
| Other | 1,631 (9.3%) | 682 (9.2%) | 949 (9.4%) | 0.7644 |
Discussion
To our knowledge, this study is the first to examine the association between adherence to the HRS/EHRA recommendations regarding CIED follow-up and clinical outcomes. There are 3 main findings. First, after accounting for patient demographics and comorbid conditions, completion of an initial, in-person follow-up 2–12 weeks after new ICD or CRT-D placement was associated with a significant survival benefit. Second, initial CIED follow-up was also associated with increased cardiovascular readmissions. Third, there was no evidence that initial CIED follow-up was associated with reduced readmission for heart failure or a device-related infection.
Our study is the first study to demonstrate survival benefit in association with an in-person follow-up visit 2–12 weeks after new ICD or CRT-D placement. Although the absolute survival benefit seems modest, it is likely clinically significant in view of the large number of CIED recipients. Reasons underlying the survival benefit associated with initial CIED follow-up are likely multifactorial. It is plausible that clinic visits lead to prompt identification and correction of problems. Previously subclinical life-threatening ventricular arrhythmias captured by newly-implanted devices may lead to the initiation or up-titration of life-saving therapies like beta-blockers. Stroke-related death may be reduced by the detection of atrial fibrillation and the initiation of an anticoagulant. CRT survival benefit may be maximized by ensuring >95%, ideally 100%, biventricular pacing via pharmacologic nodal blockade. Other CIED-related problems such as inadequate pacing outputs or diaphragmatic pacing can also be identified and addressed. Some of these problems may result in hospitalization that may partially account for the significantly increased cardiovascular readmissions observed in the group with adequate CIED follow-up.
Follow-up visits present an opportunity for device optimization that may reduce heart failure readmissions. However, no association was observed, suggesting device optimization may not have occurred often enough during the study period to make an appreciable impact. This deserves further study. Absolute device infection rates were low, largely driven by the sterile technique of the implanting team and periprocedural antibiotic use,14,15 and this may have limited our ability to detect an association with CIED follow-up.
In a prior study, the majority of Medicare beneficiaries did not have an initial in-person CIED follow-up visit between 2004 and 2009.16 The observed mortality benefit in the current study may provide clinical justification for addressing these gaps in care via quality improvement initiatives. Such initiatives may include educational efforts targeting patients and providers and registries focused on quality improvement. The development of performance measures relating to adequate CIED follow-up may also be warranted. When implemented, these metrics should be valid, reproducible, and feasible.
The disproportionate number of clinical events occurring in the subacute period after pacemaker placement has been observed previously.17 Further study is required to ascertain optimal timing and mode of CIED follow-up, whether it be in-person or transtelephonic. Future studies should also replicate the findings of the current analysis. They should include non-Medicare patients as well as those with pacemakers, implantable loop recorders, and the subcutaneous ICD if the technology gains traction. Finally, in view of rising healthcare costs, the cost and cost-effectiveness of CIED follow-up also requires further study.
Limitations
Data from the NCDR® ICD Registry™ are limited to ICDs and CRT-Ds. Thus, our study may not be generalizable to pacemakers or implantable loop recorders, which are less complex and may require less frequent follow-up visits. Similarly, because only Medicare beneficiaries were included in this analysis, the findings may not be generalizable to non-Medicare patients. Our findings are dependent on accurate coding and identification of relevant codes in the Medicare Claims Database. Inconsistent coding, more common among codes unrelated to billing, may impact the validity of our findings. In view of the observational study design, the observed survival advantage may reflect residual confounding in that patients who do not attend a follow-up visit may be frail or sick in unmeasured ways. Alternatively, initial outpatient follow-up after CIED placement may be linked to physicians’ adherence to other guideline recommendations, patient compliance, or even healthcare-seeking behavior. It is possible that the same patients who completed an initial follow-up visit were also more likely to receive other evidence-based therapies with proven survival benefit unrelated to CIED follow-up and as such received higher quality overall care. A prospective trial would offer more rigorous data, but randomizing patients with a new CIED to a follow-up clinic visit or not is fraught with ethical ambiguity and may not be feasible in the current regulatory environment.
Conclusions
Completion of an in-person CIED follow-up visit 2–12 weeks after new ICD or CRT-D placement was associated with a significant survival benefit and increased cardiovascular readmissions. In contrast, there was no evidence of a link between CIED follow-up and readmission for heart failure or device-related infection. The observed significant survival benefit suggests quality improvement initiatives designed to increase adherence to experts’ recommendations regarding adequate follow-up of CIEDs may be considered.
Acknowledgments
Sources of Funding: Primary funding was provided by the National Heart, Lung, and Blood Institute 5R01HL093071-04. P.L.H. was funded by NIH T-32 training grant HL069749-09. The funding sources had no role in the design, analysis, or interpretation of the data or in the decision to submit the manuscript for publication.
Partners and Sponsors
ICD Registry™ is an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society. The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com.
Glossary of abbreviations
- CI
confidence interval
- CIED
cardiovascular implantable electronic device
- CMS
US Centers for Medicare &Medicaid Services
- CPT
current procedure terminology
- CRT
cardiac resynchronization therapy
- CRT-D
cardiac resynchronization therapy with ICD
- DRG
diagnosis-related group
- EHRA
European Heart Rhythm Association
- ESRD
end-stage renal disease
- HR
hazard ratio
- HRS
Heart Rhythm Society
- ICD
implantable cardioverter-defibrillator
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- LVEF
left ventricular ejection fraction
- NYHA
New York Heart Association
- NCDR
National Cardiovascular Data Registry
- SD
standard deviation
- US
United States
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Potential conflicts of interest (None unless otherwise noted below):
Lesley H. Curtis, PhD- MedTronic, Inc., research grants Bruce L. Wilkoff, MD- MedTronic, Inc., consulting fees/honoraria; St. Jude Medical, consulting fees/honoraria; SpectraNetics Corp., consulting fees/honoraria
References
- 1.Gregoratos G, Cheitlin MD, Conill A, et al. ACC/AHA guidelines for implantation of cardiac pacemakers and antiarrhythmia devices: A report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on pacemaker implantation) J Am Coll Cardiol. 1998;31:1175–1209. doi: 10.1016/s0735-1097(98)00024-2. [DOI] [PubMed] [Google Scholar]
- 2.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/ NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: Summary article. A report of the American College of Cardiology/American Heart Association task force on practice guidelines (ACC/AHA/NASPE committee to update the 1998 pacemaker guidelines) J Cardiovasc Electrophysiol. 2002;13:1183–1199. doi: 10.1046/j.1540-8167.2002.01183.x. [DOI] [PubMed] [Google Scholar]
- 3.Epstein AE, Dimarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities. Heart Rhythm. 2008;5:e1–e62. doi: 10.1016/j.hrthm.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Tracy CM, Epstein AE, Darbar D, et al. 2012 ACCF/AHA/HRS focused update of the 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines. Heart Rhythm. 2012;9:1737–1753. doi: 10.1016/j.hrthm.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 5.Hammill SC, Kremers MS, Stevenson LW, et al. Review of the registry's fourth year, incorporating lead data and pediatric icd procedures, and use as a national performance measure. Heart Rhythm. 2010;7:1340–1345. doi: 10.1016/j.hrthm.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 6.Brown DW, Croft JB, Giles WH, Anda RF, Mensah GA. Epidemiology of pacemaker procedures among Medicare enrollees in 1990, 1995, and 2000. Am J Cardiol. 2005;95:409–411. doi: 10.1016/j.amjcard.2004.09.046. [DOI] [PubMed] [Google Scholar]
- 7.Wilkoff BL, Auricchio A, Brugada J, et al. HRS/EHRA expert consensus on the monitoring of cardiovascular implantable electronic devices (CIEDs): Description of techniques, indications, personnel, frequency and ethical considerations. Heart Rhythm. 2008;5:907–925. doi: 10.1016/j.hrthm.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 8.Daubert JC, Saxon L, Adamson PB, et al. 2012 EHRA/HRS expert consensus statement on cardiac resynchronization therapy in heart failure: Implant and follow-up recommendations and management. Heart Rhythm. 2012;9:1524–1576. doi: 10.1016/j.hrthm.2012.07.025. [DOI] [PubMed] [Google Scholar]
- 9.Hammill SC, Kremers MS, Stevenson LW, et al. Review of the registry's second year, data collected, and plans to add lead and pediatric ICD procedures. Heart Rhythm. 2008;5:1359–1363. doi: 10.1016/j.hrthm.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Messenger JC, Ho KK, Young CH, et al. The National Cardiovascular Data Registry (NCDR) data quality brief: The NCDR data quality program in 2012. J Am Coll Cardiol. 2012;60:1484–1488. doi: 10.1016/j.jacc.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross JS, Chen J, Lin Z, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 14.de Oliveira JC, Martinelli M, Nishioka SA, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter-defibrillators: Results of a large, prospective, randomized, double-blinded, placebo-controlled trial. Circ Arrhythm Electrophysiol. 2009;2:29–34. doi: 10.1161/CIRCEP.108.795906. [DOI] [PubMed] [Google Scholar]
- 15.Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: A scientific statement from the American Heart Association. Circulation. 2010;121:458–477. doi: 10.1161/CIRCULATIONAHA.109.192665. [DOI] [PubMed] [Google Scholar]
- 16.Al-Khatib SM, Mi X, Wilkoff BL, et al. Follow-up of patients with new cardiovascular implantable electronic devices: Are experts' recommendations implemented in routine clinical practice? Circ Arrhythm Electrophysiol. 2013;6:108–116. doi: 10.1161/CIRCEP.112.974337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Udo EO, Zuithoff NP, van Hemel NM, et al. Incidence and predictors of short- and long-term complications in pacemaker therapy: The FOLLOWPACE study. Heart Rhythm. 2012;9:728–735. doi: 10.1016/j.hrthm.2011.12.014. [DOI] [PubMed] [Google Scholar]