Abstract
Importance
In the past decade, significant progress in genomic medicine and technological advances have revolutionized our approach to common complex disorders in many areas of medicine, including ophthalmology. A major disorder that still needs major genetic progress is diabetic retinopathy (DR), one of the leading causes of blindness in adults.
Objective
To perform a literature review, present the current findings, and highlight some key challenges.
Methods
Thorough literature review of the genetic factors for DR, including heritability scores, twin studies, family studies, candidate gene studies, linkage studies, and genome-wide association studies (GWAS).
Results
While there is clear demonstration of a genetic contribution in the development and progression of DR, the identification of susceptibility loci through candidate gene approaches, linkage studies, and GWAS is still in its infancy. The greatest obstacles remain a lack of power due to small sample size of available studies and a lack of phenotype standardization. In this review, we also discuss novel technologies and novel approaches, such as intermediate phenotypes for biomarkers, proteomics, metabolomics, exome chips, and next-generation sequencing that may facilitate future studies of DR.
Conclusions and Relevance
The field of the genetics of DR is still in its infancy and is a challenge due to the complexity of the disease itself. This review outlines some strategies and lessons for future investigation to improve our understanding of this most complex of genetic disorders.
INTRODUCTION
Diabetic retinopathy (DR), an important microvascular complication of diabetes mellitus (DM), is a leading cause of visual impairment in adults 20 to 74 years of age1. Over 93 million people worldwide have DR, 17 million of whom have proliferative diabetic retinopathy (PDR) and 28 million of whom have vision-threatening DR2. This number will continue to escalate with an aging population, increasing obesity, and a rapidly progressing diabetes epidemic. More individuals, especially Hispanics, people of African descent, and Asians, will be vulnerable to blinding DR in coming years3, 4. There is clearly a need to develop strategies to identify at-risk individuals for early interventions.
In comparison to other major causes of visual loss such as age-related macular degeneration (AMD)5, myopia6, 7 and glaucoma8, 9, the search for genetic clues to DR has not progressed as rapidly. To date, few studies which have reported on possible susceptibility genes for DR have yielded inconsistent results. There is clearly a familial relationship in DR, as twin and family studies indicate a genetic basis10–17. Several candidate gene studies have reported promising genes18–21 but few of them have been replicated, and the few positive findings show only weak genetic associations18–20, 22. In genome-wide approaches, three linkage studies performed in Pima Indians and Mexican Americans have identified regions on chromosomes 1, 3, and 12 to be suggestive with DR13, 23, 24.
In contrast to AMD, myopia and glaucoma, very few genome-wide association studies (GWAS) have been conducted thus far on DR. The few GWAS are of modest sample sizes in Hispanics, Chinese, and Caucasian populations and have reported borderline associations with DR in either type 1 or type 2 diabetes25–28.
In this review, we will highlight these key genetic studies of DR with an emphasis on the most recent developments. We will also discuss issues and challenges with elucidating the genetics of DR and indicate approaches that will provide the opportunity to advance our knowledge of this complex genetic disorder.
DEFINITION AND CLASSIFICATION OF DR
The diagnosis of DR is clinically defined by the presence of retinal microvascular lesions in subjects with diabetes; however these retinal lesions are not specific and may also be present in subjects without diabetes29, 30. The classification of DR is graded by severity and divided into non-proliferative diabetic retinopathy (NPDR) and PDR. Key retinal changes in NPDR include microaneurysms, hard exudates, cotton wool spots, intraretinal microvascular abnormalities, and venous beading; these further subdivide NPDR into mild, moderate, and severe forms. Key retinal changes in PDR include neovascularization of optic disc or elsewhere, preretinal hemorrhage, or vitreous hemorrhage. On the other hand, clinically significant macular edema (CSME), which is graded as its own entity, can develop at any stage of the DR spectrum. Thus, the various classifications in DR grading, resulting in heterogeneity of DR phenotype, pose a significant challenge in any genetic study like DR. The assessment of DR via a standardized stereoscopic photograph has been proposed to overcome this issue and more researchers have utilized this approach by adopting and grading DR using the Early Treatment Diabetic Retinopathy Study (ETDRS) severity scale or a similar modification. Recently, the assessment of DR and diabetic macular edema (DME) via optical coherence tomography (OCT) has been proposed as an imaging modality to better visualize the intra-retinal morphological changes in subjects with diabetes31; however, the classification of DR and DME via OCT has not been clearly defined nor adopted for use.
GENETICS OF DIABETES
DR occurs on the background of diabetes. That genetic factors play a major role in etiology of diabetes has long been appreciated due to ethnic differences in frequency, increased familial aggregation, and a dramatically higher concordance in monozygotic versus dizygotic twins. This is true for each of the major subforms of diabetes, type 1 and type 2.
To date, approximately 60 loci have been successfully identified for type 2 diabetes, of which only 3 were discovered prior to the GWAS era32, 33. Although most of these studies were carried out in individuals of European descent, more recent studies of Asians34, Hispanics35, and African-Americans35–37 have also demonstrated some level of associations for these signals, supporting the hypothesis that these signals (or the causal variants that are in high linkage disequilibrium with these signals) are likely common alleles that are widely distributed in the human population and each contributing a small effect on disease risk32.
These important discoveries through large collaborative efforts by GWAS approaches have led to substantial progress in the understanding of genetics in type 2 diabetes, leading to the identification of novel pathways, demonstrating mechanistic associations, and supporting prior epidemiological studies38. These findings have illustrated some important key lessons that are useful in other genetics studies like DR. One, joining forces by international collaborative efforts is necessary to increase statistical power by increasing sample size38. Two, are the analysis methods. Both analysis by treating the phenotype as a dichotomous trait and analysis of a related, quantitative trait are useful38. Three, connection of genetic findings with more defined physiological parameters increases understanding38. Elucidating the genetic basis of type 2 diabetes offers an ideal model to approach the genetic study of DR. However, it should be apparent that the phenotype of type 2 diabetes does have several advantages, such as the ease of classification, and readily available large samples of subjects even without detailed assessment.
ENVIRONMENTAL FACTORS FOR DR
The etiology of DR remains complex and poorly understood. Large epidemiological studies have consistently demonstrated that the duration of diabetes and adequacy of glycemic control are two of the major contributors to the development and progression of DR2, 39, 40. This was robustly documented in the Wisconsin Epidemiological Study of Diabetic Retinopathy (WESDR) study, which found that duration of diabetes was the strongest predictor for progression of DR, with prevalence of DR varied from 17% in type 1 DM to 29% in type 2 DM for subjects with diabetes for less than 5 years and the rate increasing dramatically to almost 100% for type 1 DM and 78% for type 2 DM in subjects with diabetes for more than 15 years41, 42. In other landmark studies, such as the Diabetes Control and Complications Trial (DCCT), UK Prospective Diabetes Study (UKPDS), the ACCORD Eye, and META-EYE study groups, intensive glycemic control was effective in reducing the rate of DR progression in both type 1 and type 2 diabetes2, 39, 40, 43. Other studies have also demonstrated that blood pressure control is another risk modifier2, 44, 45, although some studies did not support this finding43. Very recently, there is increasing evidence supporting an association between dyslipidemia and diabetic retinopathy46; the Fenofibrate Intervention and Event Lowering in Diabetes Study (FIELD)47 as well as the ACCORD eye study43 both demonstrated that reduction in lipids could also limit DR progression.
Despite strong evidence for DR susceptibility, these environmental risk modifiers by themselves do not account for the complete risk susceptibility. First, this is exemplified by clinical observations that some individuals develop DR despite good glycemic control and short duration of disease, while others do not develop DR, even with poor glycemic control and longer duration of diabetes48. Second, the strongest environmental factors (duration of diabetes and glycosylated hemoglobin) only explained about 11% of the variation in retinopathy risk in the DCCT trial49, 50. Similarly, a combination of glycosylated hemoglobin, blood pressure, and total cholesterol only explained about 10% of the variation in retinopathy risk in the WESDR study51, suggesting that the remaining ~90% of the variation in retinopathy risk is presumably explained by other risk factors. Finally, population studies show that retinopathy signs such as microaneurysms was detectable in 7~13% of non-diabetics as well as in subjects where glycosylated hemoglobin level was well below 5%29 and that SNPs associated with diabetes or hypertension were not associated with retinopathy in individuals without diabetes52, suggesting that other risk factors, independent of hyperglycemia and diabetes, contribute to the development and progression of retinopathy signs similar to DR.
GENETIC FACTORS FOR DR
Attempts to identify gene or genes in the development of DR have been conducted over the past few decades. To date, these studies have been limited to twin studies10, family studies11–17, candidate gene studies18–22, linkage studies13, 23, 24, and small-scale GWAS with modest sample sizes25–28.
In support of a genetic hypothesis of DR, several studies have shown a discrepant rate of the prevalence of DR among US populations, with a significantly higher prevalence observed in Hispanics, African-Americans, and Chinese-Americans3, 4. In comparison to whites, other risk modifiers such as duration of diabetes, glycemic control, and blood pressure appear to account for the higher prevalence of DR observed in African-Americans, but these factors do not explain the higher prevalence seen in the Hispanics53–56, suggesting that other factors, including genetic factors, may influence susceptibility to DR.
Twin and Family Studies
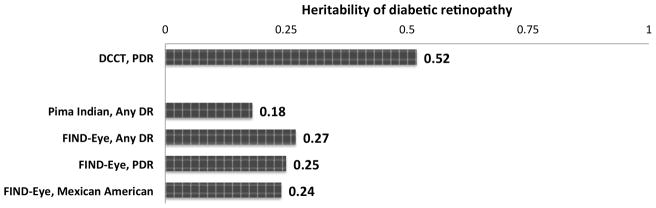
In twin studies, Leslie and Pyke found the same degree of severity in 95% (35 of 37) concordant type 2 diabetic twins, compared to only 68% (21 of 31) in concordant type 1 diabetic twins10. This early observation was extended by familial aggregation studies, with siblings and relatives of diabetics with DR having as high as a 3-fold increased risk for DR compared to siblings and relatives of diabetics without DR11–17 (Table 1). This trend was seen in either type 1 or type 2 diabetes and also across different ethnicities. Furthermore, evidence for a familial aggregation is more consistently seen in the presence of more severe retinopathy and less in the presence of any retinopathy, with heritability scores ranging from 18–27% for any DR13, 17 and 25–52% for PDR12, 17 in either type 1 or type 2 diabetes (Figure 1). Thus, as previously mentioned, it is important to not only provide a standardized assessment of DR, but also to compare the “no DRs” with the “more severe stages of DR”. The Family Investigation of Nephropathy and Diabetes (FIND)-Eye study, where nearly half of their 2368 diabetic subjects are Mexican-Americans, demonstrated the heritability of any DR in this population of type 2 diabetes is as high as 24%17.
Table 1.
Summary of familial aggregation studies in diabetic retinopathy
| Ethnicity (Study) | Type of Diabetes | Heritability | Evidence of familial aggregation | References | |
|---|---|---|---|---|---|
| Caucasians (DCCT) | 1 | Low evidence of increased risk for any DR. Strong evidence of familial clustering for severe DR; OR= 3.1 in relatives of diabetics with and without DR. | 11 | ||
| Caucasians (FinnDiane) | 1 | PDR | 0.52 | Strong evidence of familial clustering in PDR. | 12 |
| Pima Indians | 2 | Any DR | 0.18 | Modest evidence of familial clustering. | 13 |
| South Indian | 2 | Strong evidence of familial clustering; OR=3.37 in sibling of diabetic with and without DR. | 14 | ||
| Chinese | 2 | Strong evidence of familial clustering in any DR. DR diagnosed in 7.1% of sibling without DR versus 29.7% of siblings with DR. |
15 | ||
| Mexican American | 2 | Strong evidence of familial clustering in severe DR (OR=1.72), but not any DR. | 16 | ||
| Multi-Ethnic* (FIND-Eye) | 2 | Any DR PDR Mexican-American |
0.27 0.25 0.24 |
Strong evidence of familial clustering. | 17 |
Mexican-American, European American, African Americans, and American Indians.
Figure 1.
Candidate gene studies
Most genetic research in DR have utilized the candidate gene approach. Several pathways and processes have been proposed to play an important role in the pathogenesis of DR. This led to the testing of a number of hypothesized candidate genes. Though a number of candidate genes and genetic variants have been proposed in the literature, few of them have been replicated, and the few positive findings only showed weak genetic associations with DR18, 19.
In this approach, the analysis compares the frequency of a particular genetic variant in subjects with (cases) or without DR (controls). Several pathways and processes have been proposed, including the renin-angiotensin system, glucose-induced pathways, vascular endothelial dysfunction, tissue matrix remodeling, and angiogenesis18, 19. Potential candidate genes involved in these pathways and processes include angiotensin-I converting enzyme (ACE), angiotensin II type 1 receptor (AGTR1), angiotensinogen (AGT), vascular endothelial growth factor (VEGF), aldose reductase (AKR1B1), receptor for advanced glycation endproducts (RAGE), glucose transporter 1 (GLUT1), apolipoprotein E (APOE), methylenetetrahydrofolate reductase (MTHFR), plasminogen activator inhibitor-1 (PAI-1), α2β1 Integrin (ITGA2), peroxisome proliferator-activated receptor gamma (PPAR-γ), and nitric oxide synthases (NOS3). Their associations or lack thereof with DR have been extensively documented in prior reviews18–21. Here, we summarize the most important findings with a focus on aldose reductase (AKR1B1) and vascular endothelial growth factor (VEGF), because of their biological implications.
Aldose reductase (AKR1B1) is an enzyme which catalyzes the reduction of glucose to sorbitol during glucose metabolism. Increased activation of aldose reductase has been shown to induce metabolic and biochemical changes, leading to the development of early DR as well as PDR57, 58. For these reasons, AKR1B1 was proposed as a highly suspect candidate for genetic association studies in DR. While a great deal of prior work have shown inconsistent results, a recent meta-analysis by Abhary et al. examining 20 candidate genes in DR found that variants in aldose reductase had the most significant association with DR.20 In particular, the meta-analysis identified the z-2 microsatellite confers risk of DR (OR=2.33, 95% CI=1.49~3.64, p=0.0002) in either type 1 or type 2 diabetes. This trend was similar and significant in the subgroup analysis of NPDR (p=0.0075) and PDR (p=0.0023). On the other hand, the z+2 microsatellites conferred protection against overall DR (OR=0.58, 95% CI=0.36~0.93, p=0.02), but this association was only seen in subjects with type 1 diabetes, not type 2. It was independent of the studied ethnicity. In addition, a few studies examining the association of another AKR1B1 polymorphism at the promoter (rs759853) found that the T allele confers protection for DR (OR=0.49, 95% CI=0.36~0.68, p<0.0001) in type 1 diabetes, but was not significant in type 2 diabetes20.
Vascular endothelial growth factor (VEGF), a key player involved in angiogenesis and a potent mediator of vascular permeability, is activated by microvascular changes associated with diabetes due to hypoxia. This activation of VEGF leads to breakdown of the blood-retinal barrier and retinal neovascularization59, 60. Conversely, anti-VEGF therapies, such as bevacizumab (Avastin, Genentech), ranibizumab (Lucentis, Genentech), and aflibercept (Eyelea, Bayer and Regeneron) have been shown to ameliorate these changes59, 61, 62. A number of polymorphisms (rs201096320, 21, 63–71, rs2564820, 63, 68, rs157036020, 63, 72, rs309503920, 63, 70, rs3556939420, 73, rs69994720, 66, 74–77, rs1320735163, 72, 75, rs73528672, rs214632372, 77, rs83306163, 68, 69, 75, rs302502175, 76, rs1043476, rs83306876 and rs83307077) in VEGF have been analyzed either with DR or severe DR. The only conclusive finding from these efforts is that the C allele of rs2010963 (-634C/G), though insignificantly associated with DR or PDR, does confer risk for NPDR (OR=1.61, 95% CI=1.23~2.10, p=0.0005) in the meta-analyses20, 21.
A number of other individual candidate genes have been examined with DR20, 65, 78–94 and their findings are summarized (Table 2). However, it is difficult to draw any conclusions from these studies, since the sample sizes of individual studies were often quite small. The P-values obtained from these efforts are sometimes nominally significant, but cannot withstand corrections for multiple testing. In most cases, no replication has been attempted. Furthermore, there are also conflicting findings from multiple studies. Although meta-analysis techniques have been undertaken, findings remain largely inconclusive due to problems with analysis in multiple and different ethnicities (direction of effect and allele frequencies may be different), publication bias, and lack of standardization for DR phenotype.
Table 2.
Summary of individual candidate gene studies of diabetic retinopathy†
| Gene | Polymorphism | Comments | References |
|---|---|---|---|
| AKR1B1/ALR2 | (CA)n dinucleotide repeats | z-2 microsatellite confers risk in all DR, NPDR, and PDR of type 1 and type 2 DM * | 20 |
| z+2 and z microsatellite protective against all DR in type 2 DM; no ethnic difference * | |||
| rs759853 | T allele protective against DR in type 1 DM * | ||
| rs35839483 | not associated with DR | 85 | |
| VEGF | rs2010963 (−634 C/G) | insignificant finding for DR or PDR, but C allele confers risk for NPDR in meta-analysis * | 20, 21, 63–71 |
| rs25648 | T allele increases risk of DR but finding insignificant | 20, 63, 68 | |
| rs1570360 (−116A>G) | inconsistent and insignificant finding | 20, 63, 72 | |
| rs3095039 | T allele increases risk, but finding inconsistent and insignificant | 20, 63, 70 | |
| rs35569394 | −2549DEL increases risk but finding insignificant | 20, 73 | |
| rs699947 (−2578 A/C) | A allele increases risk but finding insignificant | 20, 66, 74–77 | |
| rs13207351 (−152A) | associated with PDR but insignificant in other studies | 63, 72, 75 | |
| rs735286 (+4618) | Haplotype-tagged SNP associated with severity of DR | 72 | |
| rs2146323 (+5092) | Haplotype-tagged SNP associated with severity of DR, associated with early progression of DR | 72, 77 | |
| rs833061 (−1498 C/T) | inconsistent and insignificant finding | 63, 68, 69, 75 | |
| rs3025021 | inconclusive | 75,76 | |
| rs10434 | G allele associated with blinding DR. | 76 | |
| rs833068 | G allele confers risk in DR | 76 | |
| rs833070 | associated with early progression of DR but weak association | 77 | |
| ITGA2 (α2β1 integrin) | rs2910964 | A allele increases risk * but one study in Chinese was not significant | 20, 78, 79 |
| AGTR1 | rs5186 | C allele confers protection, but finding insignificant | 20 |
| ACE | INS/DEL at intron 16 | inconsistent and insignificant finding | 20 |
| rs4343 | associated with DR in Chinese | 122 | |
| ADRB3 | rs4994 | inconsistent and insignificant finding | 20 |
| AGT | rs4762 | C allele confers protection * | 20 |
| APOE | E2/E3/E4 | inconsistent and insignificant finding | 20, 81, 82 |
| FGF2 | rs41456044 | A allele increases risk, but finding insignificant | 20 |
| rs308395 | G allele increases risk, but finding insignificant | 20 | |
| NOS3 | rs1799983 | G allele increases risk, but finding insignificant | 20, 79 |
| rs41322052 | inconsistent and insignificant finding | 20, 83 | |
| rs3138808 | inconsistent and insignificant finding | 20 | |
| SLC2A1 | rs841853 | insignificant finding | 20 |
| HLA | DR1-8 | insignificant finding | 20 |
| SDH (sorbitol dehydrogenase) | rs2055858 and rs3759890 | Weak association | 84 |
| ICAM1 | rs13306430 | G allele confers protection * | 20 |
| rs5498 | not associated with DR | 85 | |
| MTHFR | rs1801133 | T allele increases risk but finding insignificant | 20 |
| NPY | rs16139 | C allele increases risk but finding insignificant | 20 |
| PAI-1 | rs1799768 | 4G/5G allele increases risk but finding insignificant | 20, 86 |
| PON1 | rs662 | inconsistent and insignificant finding | 20 |
| PPARG | rs1801282 | G allele confers protection but finding insignificant | 20 |
| AGER/RAGE | rs1800624 (−374T/A) | inconsistent and insignificant finding | 20, 85, 87, 88 |
| rs1800625 (−429T/C) | inconsistent and insignificant finding | 20, 85, 89 | |
| rs2070600 | associated with DR of Indian ethnicity | 85 | |
| VDR | rs10735810 | T allele increases risk, but finding insignificant | 20 |
| EDN1 (endothelin-1) | rs5370 (Lys198Asn) | Reduced risk in Chinese | 79 |
| ROCK2 | Thr431Asn and Arg83Lys | no association | 90 |
| CPVL/CHN2 | rs39059 | increases risk of DR, significant in meta-analysis * | 91 |
| FRMD3 | rs10868025 | significant in stage 1, but insignificant in stage 2. | 91 |
| CARS | rs451041 | not significant | 91 |
| IRS2 | rs1411766 | not significant | 91 |
| SOD2 | rs4880 | C allele reduced risk | 92 |
| MnSOD | Ala16Val | Significant with DR | 65 |
| CA (Carbonic anhydrase) | rs2403104, rs17741410, rs1496533, rs17814594, rs12544332, rs1496529, rs725605, rs2645050, rs2645049, rs13278559 | not associated with DR | 93 |
| PEDF | rs12150053, rs12948385, rs8697961, rs1136287 | not associated with DR | 85 |
| EPO | rs1617640 | not associated with DR | 85 |
| HTRA1/ARMS2 | rs11200638, rs10490924 | not associated with DR | 85 |
| CFH | rs1061170, rs3753394 | not associated with DR | 85 |
| PSMD9 | rs74421874, rs3825172, rs14259 | associated with DR | 94 |
genome-wide approaches not included;
significant and consistent direction in more than 1 study
Thus, alternatively, two studies with a larger scale have examined candidate genes and DR using an approach that mimics a genome-wide approach. This methodology is useful when the effect sizes of individual variants, such as DR, are small and the study population is limited. The first study, the Candidate gene Association Resource (CARe) did not find genes previously associated with type 2 diabetes, diabetic nephropathy, and diabetic retinopathy to be associated with DR22. The most interesting finding from this study is that variants in the P-selectin (SELP), after adjusting for known DR risk factors, remained significantly associated with DR in the European Americans, but was not seen in the African-Americans, Hispanic Americans, or Asian Americans22. The second study, examining 193 candidate genes with DR of type 1 diabetic African-Americans, found nominal associations in 13 genes with progression of DR95. A number of these genes are involved in pathways related to glucose metabolism, inflammatory processes, angiogenesis/vascular permeability, insulin signaling, retinal development, or blood pressure regulation, highlighting not only the implications of these genes but suggesting that a number of biological pathways are simultaneously involved in DR. Even with these large-scale attempts of examining candidate genes in DR, no definite conclusion can be drawn at this time without replication efforts in larger cohorts.
Linkage studies
A potential problem with the candidate gene approach is its basis depends on an a priori hypothesis implicating that a particular gene of interest plays a functional importance in the pathophysiology of DR. If the hypothesis is wrong, then the genetic association will be negative or inconsistent. This has led to hypothesis-free approaches (also known as agnostic approaches), first by linkage, and recently, by GWAS. In these two approaches, no initial biochemical or pathophysiological induction is proposed; the results are instead, driven by chromosomal location.
Linkage analysis is based on the principles of genetic recombination to map genomic regions by the observations seen in family members. It is based on the assumption of co-segregation of genetic marker with DR susceptibility loci within the family. If linkage is present, the marker is inherited together with the causal variant. If it is not present, the marker is inherited independently. As a result, the closer the physical distance of the marker to DR susceptibility loci, the stronger the evidence is for linkage.
Linkage analysis has been the mainstay approach for studying Mendelian disorders, and has succeeded for a handful of common complex disorders such as Crohn’s disease; the latter success with the identification of NOD2/CARD15 on chromosome 1696. However, certain presentations of DR pose significant challenges in family studies. For example, the late-onset of DR, especially for those with type 2 diabetes, suggests that the parents of the proband are often deceased, leaving only one generation of family members available to study. Thus, other study designs, such as sib-pair analysis, have been the dominant model used for linkage studies in DR.
Three linkage studies performed in Pima Indians and Mexican Americans have implicated regions on chromosomes 1, 3, and 12 for DR (Table 3)13, 23, 24. However, with the possible exception of 1p36, none of these regions reached genome wide statistical linkage significance of a LOD (logarithm of odds) score > 3.3. One study demonstrated a LOD of 3.01 for single-point and 2.58 for multiple point analysis at 1p36 in the Pima Indians, indicating suggestive evidence of linkage for DR in this population13. There are several limitations with this approach. One, linkage studies only offer rough estimates of the genomic region as the mapping resolution is generally low, literally a test of millions of basepairs. Much more extensive efforts are required to pinpoint specific causal variants responsible for DR. Second, linkage studies often benefit from large families. Linkage studies on DR have thus far been conducted in Pima Indians and Mexican Americans, where large families are available for study. It has not been reported in other ethnicities. Third, the effect size (penetrance) of individual variants may be of sufficiently small magnitude that most study would be underpowered to detect genomic locations via co-segregation expected for complex multifactorial disorders like DR.
Table 3.
Summary of linkage studies of diabetic retinopathy
| Ethnicity | Type of Diabetes | Sample size | Definition of DR | Region | LOD score | Nearest markers | References |
|---|---|---|---|---|---|---|---|
| Pima Indians | 2 | 103 sibpairs | Any DR | 3q26 | 1.36 | D3S3053, D3S2427 | 24 |
| 9q21 | 1.46 | D9S1120, D9S910 | |||||
| Pima Indians | 2 | 211 sibships | Retinopathy score in worst eye | 1p36 | 3.01 and 2.58 * | D1S3669 | 13 |
| Mexican Americans | 2 | 282 sibpairs | Any DR | 1p36 | 1.24 | GGAT2A07 | 23 |
| 3q12 | 2.41 | GATA68D03 | |||||
| 7p15 | 1.02 | GATA41G07 | |||||
| 12p13 | 2.47 | GATA49D12 | |||||
| 15q25 | 1.07 | ATA28G05 | |||||
| 15q26 | 1.16 | GATA22F01 | |||||
| Severe NPDR/PDR | 2q37 | 1.11 | AFM112yd4 | ||||
| 3p26 | 1.29 | GATA22G12 | |||||
| 3q12 | 1.40 | GATA68D03 | |||||
| 12q23 | 1.03 | GATA85A04 |
for single and multi-point analysis, respectively.
Genome-Wide Association Studies (GWAS)
More recent technological advances have revolutionized the field toward the second hypothesis-free generating approach, GWAS, in which hundreds of thousand and even millions of single-nucleotide polymorphisms (SNPs) can be tested against traits such as DR. These developments include microarray-based technology with tag SNPs, utilizing the concept of linkage disequilibrium where adjacent or correlated SNPs co-segregate together in populations. Data from publicly available database such as the HapMap and the 1000 Genome Project have been instrumental in developing such arrays.
Since the first reported success of a well-designed GWAS just six years ago, more than 2000 loci have demonstrated significant and often replicated associations with one or more common complex disorders32. While this field has received a number of criticisms, the reality is that the use of GWAS has been the most successful approach in the genetics of common diseases to date. The utilization of this technology in the study of DR is relatively recent. Four small-scale GWAS with modest sample sizes conducted in Mexican-American, Chinese, and Caucasian populations have found borderline or weak associations with DR in either type 1 or type 2 diabetes25–28. One study, conducted in 103 cases (subjects with moderate-to-severe NPDR and PDR) versus 183 controls (subjects with normal to early NPDR) of Mexican-Americans, found borderline significance with DR at 6 loci25 (Table 4). The second study conducted in 174 cases (subjects with NPDR and PDR) versus 675 controls (subjects who are diabetics with no DR and non-diabetics) in the Chinese found several SNPs that have appeared to attain genome-wide statistical significance with DR26 (Table 4). The main problem in this latter study was the utilization of all 6 genetic models (genotype, allele, trend, additive, dominant, and recessive) simultaneously in their analysis to determine the most significant P-value. Had proper corrections for multiple testing been used, the stringent cut-off for P-value should have been multiplied by 6, due to 6 different genetic models run on each SNP. In this way, none of the SNP or loci reached typical genome-wide “statistical significance” of P-value 10−8 after correction for multiple comparisons. The third study conducted in 973 cases (subjects with PDR and diabetic macular edema) versus 1856 controls (all others including NPDR) of Caucasian type 1 diabetics found borderline significance at several SNPs/loci with DR in a combined meta-analysis27 (Table 4). However, a replication analysis conducted on the top signals in the WESDR type 1 diabetic did not confirm these associations97. The most recent DR GWAS study, conducted by the authors and colleagues, compared 1007 Chinese type 2 diabetic subjects with “extreme DR phenotype”, defined as 570 individuals with diabetes ≥ 8 years duration without DR (controls) versus 437 individuals with PDR (cases) (Table 4). Both groups had similar levels of HbA1c and duration of diabetes, two of the most important epidemiological confounders in the study of DR. Association analysis resulted in 3 top loci. Though borderline significant, the authors hypothesized that if the detected loci are true associations with DR, then subjects with the clinically intermediate eye phenotype (NPDR) would have intermediate frequencies of the risk allele. They then extended these top findings to 479 subjects with NPDR and did observe that the risk allele of the top 3 SNPs had an intermediate frequency in the NPDR group, suggesting potential DR susceptibility genes in the Chinese that are independent of the level of HbA1c and diabetes duration28. To summarize the GWAS of DR to date, none of the regions reached genome-wide statistical significance. Some of the limitations in these studies include very modest sample size by GWAS standard, combining heterogeneous phenotypes (subjects with PDR, NPDR, diabetic macular edema) as cases, poor characterization of normal subjects (subjects with no DR) as these subjects are often only assessed one point in time, and poor DR standardization 25–28.
Table 4.
Summary of Genome-wide association studies of diabetic retinopathy
| Ethnicity | Type of Diabetes | Sample Size | Platform and Number of SNPs | Covariates | Top SNPs | Region | Risk Allele | Odds Ratio | P-value | Gene(s) | References | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases (defined) | Controls (defined) | |||||||||||
| Mexican-Americans | 2 | 103 (moderate-to-severe NPDR and PDR) | 183 (Normal to early NPDR) | Affymetrix GeneChip 100K; 421,010 SNPs (imputed) | age, gender, diabetes duration and HbA1c | rs6909083 | 6p12 | NA | NA | 2 × 10-5 | TINAG | 25 |
|
| ||||||||||||
| rs17083119 | 6q22 | NA | NA | 3 × 10-5 | C6orf170 | |||||||
|
| ||||||||||||
| rs2300782 | 5q22 | A | 2.64 | 6 × 10-5 | CAMK4 | |||||||
|
| ||||||||||||
| rs10519765 | 15q13 | G | 3.33 | 6 × 10-5 | FMN1 | |||||||
|
| ||||||||||||
| rs899036 | 11p12 | A | 3.13 | 3 × 10-4 | API5 | |||||||
|
| ||||||||||||
| rs10501943 | 11q22 | C | 3.04 | 3 × 10-4 | CNTN5 | |||||||
|
| ||||||||||||
| Taiwanese (Chinese) | 2 | 174 (NPDR and PDR) | 575 diabetics (no DR) and 100 nondiabetics | Illumina HumanHap550; 550K SNPs | diabetes duration and HbA1c | rs17376456 | 5q15 | A | 3.63* | 3 × 10-15† | C5orf36 | 26 |
|
| ||||||||||||
| rs2038823 | 13q32 | C | 2.33* | 5 × 10-11† | HS6ST3 | |||||||
|
| ||||||||||||
| rs4838605 | 10q11 | C | 1.58* | 2 × 10-9† | ARHGAP22 | |||||||
|
| ||||||||||||
| rs12219125 | 10p12 | T | 1.62* | 9 × 10-9† | PLXDC2-NEBL | |||||||
|
| ||||||||||||
| rs4462262 | 10q21 | C | 1.54* | 9 × 10-8† | ZWINT-MRPS35P3 | |||||||
|
| ||||||||||||
| rs2811893 | 1p32 | T | 1.50* | 3 × 10-7† | MYSM1 | |||||||
|
| ||||||||||||
| rs1571942 | 10p12 | C | 1.67* | 3 × 10-7† | PLXDC2 | |||||||
|
| ||||||||||||
| rs4470583 | 4q32 | A | 1.16* | 4 × 10-7† | RPS14P7 -FSTL5 | |||||||
|
| ||||||||||||
| Caucasians (GoKinD and EDIC) | 1 | 973 (PDR and DME) | 1856 (all others, including NPDR) | Affy 5.0 and Illumina HumanHap550; 2,543,887 SNPs (imputed) | no adjustments | rs476141 | 1q44 | A | 1.37 | 1 × 10-7 | AKT3 and ZNF238 | 27 |
|
| ||||||||||||
| rs4787008 | 16p13 | G | 1.47 | 6 × 10-7 | RBFOX1 | |||||||
|
| ||||||||||||
| rs13064954 | 3q25 | G | 1.02 | 7 × 10-7 | LEKR1-CCNL1 | |||||||
|
| ||||||||||||
| rs9866141 | 3q25 | T | 1.02 | 9 × 10-7 | KRT18P34-VEPH1 | |||||||
|
| ||||||||||||
| rs17404956 | 5q34 | A | 1.16 | 1 × 10-6 | RPLP0P9-ODZ2 | |||||||
|
| ||||||||||||
| rs10403021 | 19q12 | C | 1.01 | 2 × 10-6 | VSTM2B-POP4 | |||||||
|
| ||||||||||||
| rs10927101 | 1q44 | A | 1.33 | 2 × 10-6 | AKT3-ZNF238 | |||||||
|
| ||||||||||||
| rs2696835 | 16q24 | C | 2.27 | 3 × 10-6 | IRF8-FOXF1 | |||||||
|
| ||||||||||||
| rs1970671 | 18q21 | G | 1.37 | 3 × 10-6 | MAP1LC3P-TCF4 | |||||||
|
| ||||||||||||
| rs2115386 | 19p13 | C | 1.12 | 3 × 10-6 | INSR | |||||||
|
| ||||||||||||
| rs10199521 | 2p25 | T | 1.46 | 3 × 10-6 | MYT1L-TSSC1 | |||||||
|
| ||||||||||||
| rs7772697 | 6q25 | T | 1.35 | 3 × 10-6 | UST-TAB2 | |||||||
|
| ||||||||||||
| rs6702784 | 1p34 | C | 1.08 | 4 × 10-6 | OSCP1 | |||||||
|
| ||||||||||||
| rs1342038 | 1q25 | G | 1.49 | 4 × 10-6 | TNFSF4-RPL26P11 | |||||||
|
| ||||||||||||
| rs3007729 | 1p36 | T | 1.35 | 5 × 10-6 | IGSF21-KLHDC7A | |||||||
|
| ||||||||||||
| rs10910200 | 1q42 | G | 1.35 | 6 × 10-6 | KIAA1804-KCNK1 | |||||||
|
| ||||||||||||
| rs11867934 | 17p11 | C | 1.43 | 7 × 10-6 | TNFRSF13B-MPRIP | |||||||
|
| ||||||||||||
| rs11765845 | 7p15 | A | 1.02 | 7 × 10-6 | CREB5 | |||||||
|
| ||||||||||||
| rs1073203 | 5q23 | G | 1.54 | 9 × 10-6 | RPSAP37-GRAMD3 | |||||||
|
| ||||||||||||
| Taiwanese (Chinese) | 2 | 437 (PDR) | 570 diabetics ≥8 years (no DR) | Illlumina OmniExpress; 2,166,765 (imputed) | no adjustments; age and gender | rs9565164 | 13q22.2 | C | 1.7 | 1.3 × 10-7 | TBC1D4-COMMD6-UCHL3 | 28 |
|
| ||||||||||||
| rs1399634 | 2q31.1 | A | 1.5 | 2.0× 10-6 | LRP2-BBS5 | |||||||
|
| ||||||||||||
| rs2380261 | 2q37.2 | T | 1.5 | 2.1× 10-6 | ARL4C-SH3BP4 | |||||||
Dominant model;
Determined by the most significant P-value from 6 genetic models (genotype, allele, trend, additive, dominant, and recessive)
FUTURE DIRECTIONS
Biomarkers, Proteomics, and Metabolomics
An interesting approach to find genetic susceptibility genes in DR is through the intermediate associations with biomarkers (also known as intermediate phenotypes), an approach analogous to that of the investigations seen in lipids with myocardial infarctions98 and glucose related traits/obesity with type 2 diabetes99, 100. A number of systemic biomarkers have been a subject of investigation for association with DR. Many of these biomarkers are related to markers of systemic inflammation101–106, angiogenesis106, endothelial dysfunction102, insulin resistance101, hemostatic disturbance103, and homocysteinemia102, 103, suggesting that one or more of these processes are involved in the pathogenesis of DR. Analyzing the genetic associations of these biomarkers (genes for the quantitative assessments of biomarkers) might shed some important knowledge about the genetic interplays that are responsible for the development and progressions of DR.
Similarly, the investigation of proteomics and metabolomics and its relationship to the genome (also called functional genomics) will be another area of investigation in the study of DR. Proteomics is a large-scale study of the structure and function of proteins. A prior study examining the vitreous proteome in non-diabetic, diabetic without DR, and PDR subjects using label-free mass spectrometry-based spectral counting approaches found a number of proteins associated with key biological pathways in the kallikrein-kinin, coagulation, and complement systems to exhibit protein alterations in subjects with PDR compared to the other groups107. A review of key findings of proteomics in diabetic retinopathy of both animal and human studies concluded that multiple proteins such as apolipoprotein A-I and apolipoprotein H are more likely to contribute to retinal pathology than single proteins alone108.
Metabolomics is a global measurement of the immediate cellular state within a given biological system, taking into account the genetic profiles, altered enzymatic activities, environmental and lifestyle factors. A recent small study, examining the metabolomics in DR of 89 Chinese patients, found disturbances in fatty acid (stearic acid, linoleic acid, arachidonic acid), amino acids (aspartic acid) and glucose alterations to vary differently among diabetics without DR, NPDR, and PDR subjects109. Though the study of metabolomics in ophthalmology is rather new, its applications in other fields such as oncology has demonstrated successful clinical utility, ranging from quantitative assessment of metabolomic biomarkers for cancer diagnosis, optimization of therapeutic agents, evaluation of treatment efficacy and response, and prediction of treatment toxicity or resistance110. In the future, the application of proteomics and metabolomics to the study of DR may facilitate in the discovery, identification, or quantification of biomarkers to aid in early disease detection, diagnosis, and treatment response.
Next-Generation Sequencing and Exome Chip studies
Massively parallel sequencing technology has been a breakthrough in the transformation of genomic medicine for Mendelian disorders. With high-throughput sequencing, scientists have been able to utilize large amounts of sequenced data with lower-cost reads to address a range of biological diseases111–113, the origin of human protein-coding variants114, as well as determine population-specific whole-genome sequencing databases115. Although current exome sequencing studies are well powered to discover functional variants, current exome sequencing studies are not as well powered to establish an association. Thus, the exome chip was design to provide a cost effective way to examine large number of samples. The exome chip array was designed to test ~250,000 SNPs covering putative functional exonic variants (non-synonymous variants, splice variants, stop altering variants, etc.) from a range of diseases and populations116. This approach has been successfully applied to the identification of low-frequency and rare nonsynonymous variants that contribute to processes such as fasting insulin processing and secretion in non-diabetic subjects117. It is without a doubt that the future directions in the genetics of DR will encompass a number of these novel technologies.
KEY POINTS & STRATEGIES
Thus, to approach the genetics of DR in a systematic way would require large collaborative efforts as well as several methodological improvements. First, the establishment of large scale consortia has been successful for a number of disorders, such as diabetes99, 118, lipids98, blood pressure and cardiovascular diseases119, and can be organized based on disease phenotypes or cohort120. Second, an important aim is the standardization of a DR phenotype, classified either with the ETDRS severity scale or a similar modification. A third aim is the standardization of associated phenotypes (diabetes duration, glycemic controls, blood pressure, lipid profiles, and medications), in order to minimize heterogeneity in the comparison. Fourth, the genetic effect of each variant on DR is likely to be modest and larger sample cohorts are required to find modest associations. Fifth, large meta-analyses between different cohorts and different ethnicities have proven difficult to conduct to date due to technical challenges. Standardization of study protocols between different studies could be improved upon to increase power. Sixth, novel statistical approaches such as utilizing a combination of GWA and genome-wide linkage studies to first prioritize the genome could be a more efficient means to identify candidate genes for DR121, though this approach would first require strong evidence of linkage peaks in families. Furthermore, studies of different ethnicities need to be conducted to find population-specific signals in DR, given the different prevalence rate observed in different populations. Lastly, novel approaches such as biomarkers as intermediate phenotypes, proteomics, metabolomics, exome array, and next-generation sequencing may integrate systematic information in the field of functional genomics or systems biology to better our understanding of the complexity of DR.
CONCLUSION
DR remains as one of the most complex, heterogeneous, multifactorial disorders in any genetic studies. The identification of genetic susceptibility loci for DR through candidate gene approaches, linkage studies, and GWAS has not proven markedly successful to date, given the often conflicting and inconclusive results. It is clear that the study of the genetics of DR is still in its infancy and faces many challenges due to the complexity of the disease itself. A number of challenges and strategies are detailed in this review. Only when we achieve these important milestones may it be possible to understand the genetic contributions in DR, identify true genetic variants, and subsequently develop early screening assays for at-risk individuals and novel therapies to combat this common cause of blindness in adults.
Acknowledgments
The authors thank Dr. Kent D. Taylor for his assistance in language and structure of manuscript. This study was supported by the National Institutes of Health (EY014684) and ARRA Supplement (EY014684-03S1, -04S1), the National Institute of Diabetes and Digestive and Kidney Disease grant DK063491 to the Southern California Diabetes Endocrinology Research Center, the Eye Birth Defects Foundation Inc., the Cedars-Sinai Board of Governor’s Chair in Medical Genetics. The Clinical and Translational Science Institute (CTSI) was supported by the National Center for Research Resources, Grant UL1RR033176, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000124. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. JIR had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Conflict of Interest Statement: Each author declares no conflict of interest.
References
- 1.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology. 1998;105(10):1801–1815. doi: 10.1016/S0161-6420(98)91020-X. [DOI] [PubMed] [Google Scholar]
- 2.Yau JW, Rogers SL, Kawasaki R, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care. 2012;35(3):556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang X, Saaddine JB, Chou CF, et al. Prevalence of diabetic retinopathy in the United States, 2005–2008. JAMA. 2010;304(6):649–656. doi: 10.1001/jama.2010.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong TY, Klein R, Islam FM, et al. Diabetic retinopathy in a multi-ethnic cohort in the United States. Am J Ophthalmol. 2006;141(3):446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein RJ, Zeiss C, Chew EY, et al. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308(5720):385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solouki AM, Verhoeven VJ, van Duijn CM, et al. A genome-wide association study identifies a susceptibility locus for refractive errors and myopia at 15q14. Nat Genet. 2010;42(10):897–901. doi: 10.1038/ng.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hysi PG, Young TL, Mackey DA, et al. A genome-wide association study for myopia and refractive error identifies a susceptibility locus at 15q25. Nat Genet. 2010;42(10):902–905. doi: 10.1038/ng.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burdon KP, Macgregor S, Hewitt AW, et al. Genome-wide association study identifies susceptibility loci for open angle glaucoma at TMCO1 and CDKN2B-AS1. Nat Genet. 2011;43(6):574–578. doi: 10.1038/ng.824. [DOI] [PubMed] [Google Scholar]
- 9.Thorleifsson G, Walters GB, Hewitt AW, et al. Common variants near CAV1 and CAV2 are associated with primary open-angle glaucoma. Nat Genet. 2010;42(10):906–909. doi: 10.1038/ng.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leslie RD, Pyke DA. Diabetic retinopathy in identical twins. Diabetes. 1982;31(1):19–21. doi: 10.2337/diab.31.1.19. [DOI] [PubMed] [Google Scholar]
- 11.The Diabetes Control and Complications Trial Research Group. Clustering of long-term complications in families with diabetes in the diabetes control and complications trial. Diabetes. 1997;46(11):1829–1839. [PubMed] [Google Scholar]
- 12.Hietala K, Forsblom C, Summanen P, Groop PH. Heritability of proliferative diabetic retinopathy. Diabetes. 2008;57(8):2176–2180. doi: 10.2337/db07-1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Looker HC, Nelson RG, Chew E, et al. Genome-wide linkage analyses to identify loci for diabetic retinopathy. Diabetes. 2007;56(4):1160–1166. doi: 10.2337/db06-1299. [DOI] [PubMed] [Google Scholar]
- 14.Rema M, Saravanan G, Deepa R, Mohan V. Familial clustering of diabetic retinopathy in South Indian Type 2 diabetic patients. Diabetic Medicine : A Journal of the British Diabetic Association. 2002;19(11):910–916. doi: 10.1046/j.1464-5491.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Gao Y, Zhou Z, Wang J, Zhou Q, Li Q. Familial clustering of diabetic retinopathy in Chongqing, China, type 2 diabetic patients. Eur J Ophthalmol. 2010;20(5):911–918. doi: 10.1177/112067211002000516. [DOI] [PubMed] [Google Scholar]
- 16.Hallman DM, Huber JC, Jr, Gonzalez VH, Klein BE, Klein R, Hanis CL. Familial aggregation of severity of diabetic retinopathy in Mexican Americans from Starr County, Texas. Diabetes Care. 2005;28(5):1163–1168. doi: 10.2337/diacare.28.5.1163. [DOI] [PubMed] [Google Scholar]
- 17.Arar NH, Freedman BI, Adler SG, et al. Heritability of the severity of diabetic retinopathy: the FIND-Eye study. Invest Ophthalmol Vis Sci. 2008;49(9):3839–3845. doi: 10.1167/iovs.07-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liew G, Klein R, Wong TY. The role of genetics in susceptibility to diabetic retinopathy. Int Ophthalmol Clin. 2009;49(2):35–52. doi: 10.1097/IIO.0b013e31819fd5d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng DP. Human genetics of diabetic retinopathy: current perspectives. Journal of Ophthalmology. 2010:6. doi: 10.1155/2010/172593. Article ID 172593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Abhary S, Hewitt AW, Burdon KP, Craig JE. A systematic meta-analysis of genetic association studies for diabetic retinopathy. Diabetes. 2009;58(9):2137–2147. doi: 10.2337/db09-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao T, Zhao J. Association between the -634C/G polymorphisms of the vascular endothelial growth factor and retinopathy in type 2 diabetes: a meta-analysis. Diabetes Res Clin Pract. 2010;90(1):45–53. doi: 10.1016/j.diabres.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Sobrin L, Green T, Sim X, et al. Candidate gene association study for diabetic retinopathy in persons with Type 2 Diabetes: The Candidate Gene Association Resource (CARe) Invest Ophthalmol Vis Sci. 2011;52(10):7593–7602. doi: 10.1167/iovs.11-7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hallman DM, Boerwinkle E, Gonzalez VH, Klein BE, Klein R, Hanis CL. A genome-wide linkage scan for diabetic retinopathy susceptibility genes in Mexican Americans with type 2 diabetes from Starr County, Texas. Diabetes. 2007;56(4):1167–1173. doi: 10.2337/db06-1373. [DOI] [PubMed] [Google Scholar]
- 24.Imperatore G, Hanson RL, Pettitt DJ, Kobes S, Bennett PH, Knowler WC. Sib-pair linkage analysis for susceptibility genes for microvascular complications among Pima Indians with type 2 diabetes. Pima Diabetes Genes Group. Diabetes. 1998;47(5):821–830. doi: 10.2337/diabetes.47.5.821. [DOI] [PubMed] [Google Scholar]
- 25.Fu YP, Hallman DM, Gonzalez VH, et al. Identification of diabetic retinopathy genes through a genome-wide association study among Mexican-Americans from Starr County, Texas. Journal of Ophthalmology. 2010:9. doi: 10.1155/2010/861291. Article ID 861291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang YC, Lin JM, Lin HJ, et al. Genome-wide association study of diabetic retinopathy in a Taiwanese population. Ophthalmology. 2011;118(4):642–648. doi: 10.1016/j.ophtha.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 27.Grassi MA, Tikhomirov A, Ramalingam S, Below JE, Cox NJ, Nicolae DL. Genome-wide meta-analysis for severe diabetic retinopathy. Hum Mol Genet. 2011;20(12):2472–2481. doi: 10.1093/hmg/ddr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sheu WH, Kuo JZ, Lee IT, et al. Genome-wide association study in a Chinese population with diabetic retinopathy. Hum Mol Genet. 2013 doi: 10.1093/hmg/ddt161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong TY, Liew G, Tapp RJ, et al. Relation between fasting glucose and retinopathy for diagnosis of diabetes: three population-based cross-sectional studies. Lancet. 2008;371(9614):736–743. doi: 10.1016/S0140-6736(08)60343-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Klein R, Klein BE, Moss SE, Wong TY. The relationship of retinopathy in persons without diabetes to the 15-year incidence of diabetes and hypertension: Beaver Dam Eye Study. Trans Am Ophthalmol Soc. 2006;104:98–107. [PMC free article] [PubMed] [Google Scholar]
- 31.Buabbud JC, Al-latayfeh MM, Sun JK. Optical coherence tomography imaging for diabetic retinopathy and macular edema. Current diabetes reports. 2010;10(4):264–269. doi: 10.1007/s11892-010-0129-z. [DOI] [PubMed] [Google Scholar]
- 32.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90(1):7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morris AP, Voight BF, Teslovich TM, et al. Large-scale association analysis provides insights into the genetic architecture and pathophysiology of type 2 diabetes. Nat Genet. 2012;44(9):981–990. doi: 10.1038/ng.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sim X, Ong RT, Suo C, et al. Transferability of type 2 diabetes implicated loci in multi-ethnic cohorts from Southeast Asia. PLoS Genetics. 2011;7(4):e1001363. doi: 10.1371/journal.pgen.1001363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer ND, Goodarzi MO, Langefeld CD, et al. Quantitative trait analysis of type 2 diabetes susceptibility loci identified from whole genome association studies in the Insulin Resistance Atherosclerosis Family Study. Diabetes. 2008;57(4):1093–1100. doi: 10.2337/db07-1169. [DOI] [PubMed] [Google Scholar]
- 36.Ng MC, Saxena R, Li J, et al. Transferability and fine mapping of type 2 diabetes loci in African Americans: the Candidate Gene Association Resource Plus Study. Diabetes. 2013;62(3):965–976. doi: 10.2337/db12-0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu CT, Ng MC, Rybin D, et al. Transferability and fine-mapping of glucose and insulin quantitative trait loci across populations: CARe, the Candidate Gene Association Resource. Diabetologia. 2012;55(11):2970–2984. doi: 10.1007/s00125-012-2656-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Billings LK, Florez JC. The genetics of type 2 diabetes: what have we learned from GWAS? Ann N Y Acad Sci. 2010;1212:59–77. doi: 10.1111/j.1749-6632.2010.05838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 40.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- 41.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch Ophthalmol. 1984;102(4):520–526. doi: 10.1001/archopht.1984.01040030398010. [DOI] [PubMed] [Google Scholar]
- 42.Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. III. Prevalence and risk of diabetic retinopathy when age at diagnosis is 30 or more years. Arch Ophthalmol. 1984;102(4):527–532. doi: 10.1001/archopht.1984.01040030405011. [DOI] [PubMed] [Google Scholar]
- 43.Chew EY, Ambrosius WT, Davis MD, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matthews DR, Stratton IM, Aldington SJ, Holman RR, Kohner EM. Risks of progression of retinopathy and vision loss related to tight blood pressure control in type 2 diabetes mellitus: UKPDS 69. Arch Ophthalmol. 2004;122(11):1631–1640. doi: 10.1001/archopht.122.11.1631. [DOI] [PubMed] [Google Scholar]
- 45.UK Prospective Diabetes Study (UKPDS) Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 46.Lim LS, Wong TY. Lipids and diabetic retinopathy. Expert opinion on biological therapy. 2012;12(1):93–105. doi: 10.1517/14712598.2012.641531. [DOI] [PubMed] [Google Scholar]
- 47.Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 48.Sun JK, Keenan HA, Cavallerano JD, et al. Protection from retinopathy and other complications in patients with type 1 diabetes of extreme duration: the joslin 50-year medalist study. Diabetes Care. 2011;34(4):968–974. doi: 10.2337/dc10-1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hirsch IB, Brownlee M. Beyond hemoglobin A1c--need for additional markers of risk for diabetic microvascular complications. JAMA. 2010;303(22):2291–2292. doi: 10.1001/jama.2010.785. [DOI] [PubMed] [Google Scholar]
- 50.Lachin JM, Genuth S, Nathan DM, Zinman B, Rutledge BN. Effect of glycemic exposure on the risk of microvascular complications in the diabetes control and complications trial--revisited. Diabetes. 2008;57(4):995–1001. doi: 10.2337/db07-1618. [DOI] [PubMed] [Google Scholar]
- 51.Antonetti DA, Klein R, Gardner TW. Diabetic retinopathy. N Engl J Med. 2012;366(13):1227–1239. doi: 10.1056/NEJMra1005073. [DOI] [PubMed] [Google Scholar]
- 52.Jensen RA, Sim X, Li X, et al. Genome-Wide Association Study of Retinopathy in Individuals without Diabetes. PLoS One. 2013;8(2):e54232. doi: 10.1371/journal.pone.0054232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris MI, Klein R, Cowie CC, Rowland M, Byrd-Holt DD. Is the risk of diabetic retinopathy greater in non-Hispanic blacks and Mexican Americans than in non-Hispanic whites with type 2 diabetes? A U.S. population study. Diabetes Care. 1998;21(8):1230–1235. doi: 10.2337/diacare.21.8.1230. [DOI] [PubMed] [Google Scholar]
- 54.Klein R, Sharrett AR, Klein BE, et al. The association of atherosclerosis, vascular risk factors, and retinopathy in adults with diabetes : the atherosclerosis risk in communities study. Ophthalmology. 2002;109(7):1225–1234. doi: 10.1016/s0161-6420(02)01074-6. [DOI] [PubMed] [Google Scholar]
- 55.Haffner SM, Fong D, Stern MP, et al. Diabetic retinopathy in Mexican Americans and non-Hispanic whites. Diabetes. 1988;37(7):878–884. doi: 10.2337/diab.37.7.878. [DOI] [PubMed] [Google Scholar]
- 56.Haffner SM, Hazuda HP, Stern MP, Patterson JK, Van Heuven WA, Fong D. Effects of socioeconomic status on hyperglycemia and retinopathy levels in Mexican Americans with NIDDM. Diabetes Care. 1989;12(2):128–134. doi: 10.2337/diacare.12.2.128. [DOI] [PubMed] [Google Scholar]
- 57.Greene DA, Lattimer SA, Sima AA. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N Engl J Med. 1987;316(10):599–606. doi: 10.1056/NEJM198703053161007. [DOI] [PubMed] [Google Scholar]
- 58.Obrosova IG, Kador PF. Aldose reductase / polyol inhibitors for diabetic retinopathy. Current pharmaceutical biotechnology. 2011;12(3):373–385. doi: 10.2174/138920111794480642. [DOI] [PubMed] [Google Scholar]
- 59.Aiello LP. Angiogenic pathways in diabetic retinopathy. N Engl J Med. 2005;353(8):839–841. doi: 10.1056/NEJMe058142. [DOI] [PubMed] [Google Scholar]
- 60.Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N Engl J Med. 1994;331(22):1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- 61.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 62.Stewart MW. Aflibercept (VEGF Trap-eye): the newest anti-VEGF drug. Br J Ophthalmol. 2012;96(9):1157–1158. doi: 10.1136/bjophthalmol-2011-300654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Awata T, Inoue K, Kurihara S, et al. A common polymorphism in the 5′-untranslated region of the VEGF gene is associated with diabetic retinopathy in type 2 diabetes. Diabetes. 2002;51(5):1635–1639. doi: 10.2337/diabetes.51.5.1635. [DOI] [PubMed] [Google Scholar]
- 64.Buraczynska M, Ksiazek P, Baranowicz-Gaszczyk I, Jozwiak L. Association of the VEGF gene polymorphism with diabetic retinopathy in type 2 diabetes patients. Nephrol Dial Transplant. 2007;22(3):827–832. doi: 10.1093/ndt/gfl641. [DOI] [PubMed] [Google Scholar]
- 65.Kangas-Kontio T, Vavuli S, Kakko SJ, et al. Polymorphism of the manganese superoxide dismutase gene but not of vascular endothelial growth factor gene is a risk factor for diabetic retinopathy. Br J Ophthalmol. 2009;93(10):1401–1406. doi: 10.1136/bjo.2009.159012. [DOI] [PubMed] [Google Scholar]
- 66.Nakamura S, Iwasaki N, Funatsu H, Kitano S, Iwamoto Y. Impact of variants in the VEGF gene on progression of proliferative diabetic retinopathy. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2009;247(1):21–26. doi: 10.1007/s00417-008-0915-3. [DOI] [PubMed] [Google Scholar]
- 67.Petrovic MG, Korosec P, Kosnik M, et al. Local and genetic determinants of vascular endothelial growth factor expression in advanced proliferative diabetic retinopathy. Mol Vis. 2008;14:1382–1387. [PMC free article] [PubMed] [Google Scholar]
- 68.Suganthalakshmi B, Anand R, Kim R, et al. Association of VEGF and eNOS gene polymorphisms in type 2 diabetic retinopathy. Mol Vis. 2006;12:336–341. [PubMed] [Google Scholar]
- 69.Szaflik JP, Wysocki T, Kowalski M, et al. An association between vascular endothelial growth factor gene promoter polymorphisms and diabetic retinopathy. Graefe’s archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2008;246(1):39–43. doi: 10.1007/s00417-007-0674-6. [DOI] [PubMed] [Google Scholar]
- 70.Uthra S, Raman R, Mukesh BN, et al. Association of VEGF gene polymorphisms with diabetic retinopathy in a south Indian cohort. Ophthalmic Genet. 2008;29(1):11–15. doi: 10.1080/13816810701663527. [DOI] [PubMed] [Google Scholar]
- 71.Yang Y, Andresen BT, Yang K, Zhang Y, Li X, Wang H. Association of vascular endothelial growth factor -634C/G polymorphism and diabetic retinopathy in type 2 diabetic Han Chinese. Exp Biol Med. 2010;235(10):1204–1211. doi: 10.1258/ebm.2010.010102. [DOI] [PubMed] [Google Scholar]
- 72.Churchill AJ, Carter JG, Ramsden C, et al. VEGF polymorphisms are associated with severity of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2008;49(8):3611–3616. doi: 10.1167/iovs.07-1383. [DOI] [PubMed] [Google Scholar]
- 73.Yang B, Cross DF, Ollerenshaw M, Millward BA, Demaine AG. Polymorphisms of the vascular endothelial growth factor and susceptibility to diabetic microvascular complications in patients with type 1 diabetes mellitus. J Diabetes Complications. 2003;17(1):1–6. doi: 10.1016/s1056-8727(02)00181-2. [DOI] [PubMed] [Google Scholar]
- 74.Chun MY, Hwang HS, Cho HY, et al. Association of vascular endothelial growth factor polymorphisms with nonproliferative and proliferative diabetic retinopathy. J Clin Endocrinol Metab. 2010;95(7):3547–3551. doi: 10.1210/jc.2009-2719. [DOI] [PubMed] [Google Scholar]
- 75.Yang X, Deng Y, Gu H, et al. Polymorphisms in the vascular endothelial growth factor gene and the risk of diabetic retinopathy in Chinese patients with type 2 diabetes. Mol Vis. 2011;17:3088–3096. [PMC free article] [PubMed] [Google Scholar]
- 76.Abhary S, Burdon KP, Gupta A, et al. Common sequence variation in the VEGFA gene predicts risk of diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50(12):5552–5558. doi: 10.1167/iovs.09-3694. [DOI] [PubMed] [Google Scholar]
- 77.Nakanishi K, Watanabe C. Single nucleotide polymorphisms of vascular endothelial growth factor gene intron 2 are markers for early progression of diabetic retinopathy in Japanese with type 1 diabetes. Clinica chimica acta; international journal of clinical chemistry. 2009;402(1–2):171–175. doi: 10.1016/j.cca.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 78.Uhlmann K, Kovacs P, Boettcher Y, Hammes HP, Paschke R. Genetics of diabetic retinopathy. Experimental and clinical endocrinology & diabetes : official journal, German Society of Endocrinology [and] German Diabetes Association. 2006;114(6):275–294. doi: 10.1055/s-2006-924260. [DOI] [PubMed] [Google Scholar]
- 79.Li H, Louey JW, Choy KW, et al. EDN1 Lys198Asn is associated with diabetic retinopathy in type 2 diabetes. Mol Vis. 2008;14:1698–1704. [PMC free article] [PubMed] [Google Scholar]
- 80.Liang S, Pan M, Hu N, et al. Association of angiotensin-converting enzyme gene 2350 G/A polymorphism with diabetic retinopathy in Chinese Han population. Mol Biol Rep. 2013;40(1):463–468. doi: 10.1007/s11033-012-2081-2. [DOI] [PubMed] [Google Scholar]
- 81.Santos A, Salguero ML, Gurrola C, Munoz F, Roig-Melo E, Panduro A. The epsilon4 allele of apolipoprotein E gene is a potential risk factor for the severity of macular edema in type 2 diabetic Mexican patients. Ophthalmic Genet. 2002;23(1):13–19. doi: 10.1076/opge.23.1.13.2203. [DOI] [PubMed] [Google Scholar]
- 82.Liew G, Shankar A, Wang JJ, et al. Apolipoprotein E gene polymorphisms are not associated with diabetic retinopathy: the atherosclerosis risk in communities study. Am J Ophthalmol. 2006;142(1):105–111. doi: 10.1016/j.ajo.2006.02.054. [DOI] [PubMed] [Google Scholar]
- 83.Bazzaz JT, Amoli MM, Pravica V, et al. eNOS gene polymorphism association with retinopathy in type 1 diabetes. Ophthalmic Genet. 2010;31(3):103–107. doi: 10.3109/13816810.2010.482553. [DOI] [PubMed] [Google Scholar]
- 84.Szaflik JP, Majsterek I, Kowalski M, et al. Association between sorbitol dehydrogenase gene polymorphisms and type 2 diabetic retinopathy. Exp Eye Res. 2008;86(4):647–652. doi: 10.1016/j.exer.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 85.Balasubbu S, Sundaresan P, Rajendran A, et al. Association analysis of nine candidate gene polymorphisms in Indian patients with type 2 diabetic retinopathy. BMC medical genetics. 2010;11:158. doi: 10.1186/1471-2350-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Nagi DK, McCormack LJ, Mohamed-Ali V, Yudkin JS, Knowler WC, Grant PJ. Diabetic retinopathy, promoter (4G/5G) polymorphism of PAI-1 gene, and PAI-1 activity in Pima Indians with type 2 diabetes. Diabetes Care. 1997;20(8):1304–1309. doi: 10.2337/diacare.20.8.1304. [DOI] [PubMed] [Google Scholar]
- 87.Lindholm E, Bakhtadze E, Sjogren M, et al. The -374 T/A polymorphism in the gene encoding RAGE is associated with diabetic nephropathy and retinopathy in type 1 diabetic patients. Diabetologia. 2006;49(11):2745–2755. doi: 10.1007/s00125-006-0412-3. [DOI] [PubMed] [Google Scholar]
- 88.Ramprasad S, Radha V, Mathias RA, Majumder PP, Rao MR, Rema M. Rage gene promoter polymorphisms and diabetic retinopathy in a clinic-based population from South India. Eye. 2007;21(3):395–401. doi: 10.1038/sj.eye.6702239. [DOI] [PubMed] [Google Scholar]
- 89.Hudson BI, Stickland MH, Futers TS, Grant PJ. Effects of novel polymorphisms in the RAGE gene on transcriptional regulation and their association with diabetic retinopathy. Diabetes. 2001;50(6):1505–1511. doi: 10.2337/diabetes.50.6.1505. [DOI] [PubMed] [Google Scholar]
- 90.Demiryurek AT, Erbagci I, Oztuzcu S, et al. Lack of association between the Thr431Asn and Arg83Lys polymorphisms of the ROCK2 gene and diabetic retinopathy. Curr Eye Res. 2010;35(12):1128–1134. doi: 10.3109/02713683.2010.507903. [DOI] [PubMed] [Google Scholar]
- 91.Hu C, Zhang R, Yu W, et al. CPVL/CHN2 genetic variant is associated with diabetic retinopathy in Chinese type 2 diabetic patients. Diabetes. 2011;60(11):3085–3089. doi: 10.2337/db11-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tian C, Fang S, Du X, Jia C. Association of the C47T polymorphism in SOD2 with diabetes mellitus and diabetic microvascular complications: a meta-analysis. Diabetologia. 2011;54(4):803–811. doi: 10.1007/s00125-010-2004-5. [DOI] [PubMed] [Google Scholar]
- 93.Abhary S, Burdon KP, Gupta A, Petrovsky N, Craig JE. Diabetic retinopathy is not associated with carbonic anhydrase gene polymorphisms. Mol Vis. 2009;15:1179–1184. [PMC free article] [PubMed] [Google Scholar]
- 94.Gragnoli C. Proteasome modulator 9 gene is linked to diabetic and non-diabetic retinopathy in T2D. Ophthalmic Genet. 2011;32(4):228–230. doi: 10.3109/13816810.2011.592174. [DOI] [PubMed] [Google Scholar]
- 95.Roy MS, Hallman DM, Fu YP, Machado M, Hanis CL. Assessment of 193 candidate genes for retinopathy in African Americans with type 1 diabetes. Arch Ophthalmol. 2009;127(5):605–612. doi: 10.1001/archophthalmol.2009.48. [DOI] [PubMed] [Google Scholar]
- 96.Hugot JP, Chamaillard M, Zouali H, et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature. 2001;411(6837):599–603. doi: 10.1038/35079107. [DOI] [PubMed] [Google Scholar]
- 97.Grassi MA, Tikhomirov A, Ramalingam S, et al. Replication analysis for severe diabetic retinopathy. Invest Ophthalmol Vis Sci. 2012;53(4):2377–2381. doi: 10.1167/iovs.11-8068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dupuis J, Langenberg C, Prokopenko I, et al. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet. 2010;42(2):105–116. doi: 10.1038/ng.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McCarthy MI. Genomics, type 2 diabetes, and obesity. N Engl J Med. 2010;363(24):2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 101.Kuo JZ, Guo X, Klein R, et al. Systemic soluble tumor necrosis factor receptors 1 and 2 are associated with severity of diabetic retinopathy in Hispanics. Ophthalmology. 2012;119(5):1041–1046. doi: 10.1016/j.ophtha.2011.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Klein BE, Knudtson MD, Tsai MY, Klein R. The relation of markers of inflammation and endothelial dysfunction to the prevalence and progression of diabetic retinopathy: Wisconsin epidemiologic study of diabetic retinopathy. Arch Ophthalmol. 2009;127(9):1175–1182. doi: 10.1001/archophthalmol.2009.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nguyen TT, Alibrahim E, Islam FM, et al. Inflammatory, hemostatic, and other novel biomarkers for diabetic retinopathy: the multi-ethnic study of atherosclerosis. Diabetes Care. 2009;32(9):1704–1709. doi: 10.2337/dc09-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Limb GA, Hollifield RD, Webster L, Charteris DG, Chignell AH. Soluble TNF receptors in vitreoretinal proliferative disease. Invest Ophthalmol Vis Sci. 2001;42(7):1586–1591. [PubMed] [Google Scholar]
- 105.Limb GA, Soomro H, Janikoun S, Hollifield RD, Shilling J. Evidence for control of tumour necrosis factor-alpha (TNF-alpha) activity by TNF receptors in patients with proliferative diabetic retinopathy. Clin Exp Immunol. 1999;115(3):409–414. doi: 10.1046/j.1365-2249.1999.00839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Meleth AD, AgrÛn E, Chan CC, et al. Serum inflammatory markers in diabetic retinopathy. Invest Ophthalmol Vis Sci. 2005;46(11):4295–4301. doi: 10.1167/iovs.04-1057. [DOI] [PubMed] [Google Scholar]
- 107.Gao BB, Chen X, Timothy N, Aiello LP, Feener EP. Characterization of the vitreous proteome in diabetes without diabetic retinopathy and diabetes with proliferative diabetic retinopathy. Journal of proteome research. 2008;7(6):2516–2525. doi: 10.1021/pr800112g. [DOI] [PubMed] [Google Scholar]
- 108.Merchant ML, Klein JB. Proteomics and diabetic retinopathy. Clin Lab Med. 2009;29(1):139–149. doi: 10.1016/j.cll.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 109.Li X, Luo X, Lu X, Duan J, Xu G. Metabolomics study of diabetic retinopathy using gas chromatography-mass spectrometry: a comparison of stages and subtypes diagnosed by Western and Chinese medicine. Molecular bioSystems. 2011;7(7):2228–2237. doi: 10.1039/c0mb00341g. [DOI] [PubMed] [Google Scholar]
- 110.Spratlin JL, Serkova NJ, Eckhardt SG. Clinical applications of metabolomics in oncology: a review. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15(2):431–440. doi: 10.1158/1078-0432.CCR-08-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Emond MJ, Louie T, Emerson J, et al. Exome sequencing of extreme phenotypes identifies DCTN4 as a modifier of chronic Pseudomonas aeruginosa infection in cystic fibrosis. Nat Genet. 2012;44(8):886–889. doi: 10.1038/ng.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Boileau C, Guo DC, Hanna N, et al. TGFB2 mutations cause familial thoracic aortic aneurysms and dissections associated with mild systemic features of Marfan syndrome. Nat Genet. 2012;44(8):916–921. doi: 10.1038/ng.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Albrechtsen A, Grarup N, Li Y, et al. Exome sequencing-driven discovery of coding polymorphisms associated with common metabolic phenotypes. Diabetologia. 2013;56(2):298–310. doi: 10.1007/s00125-012-2756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fu W, O’Connor TD, Jun G, et al. Analysis of 6,515 exomes reveals the recent origin of most human protein-coding variants. Nature. 2013;493(7431):216–220. doi: 10.1038/nature11690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wong LP, Ong RT, Poh WT, et al. Deep whole-genome sequencing of 100 southeast Asian Malays. Am J Hum Genet. 2013;92(1):52–66. doi: 10.1016/j.ajhg.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Grove ML, Yu B, Cochran BJ, et al. Best practices and joint calling of the Human Exome Bead Chip: the CHARGE Consortium. PLoS ONE. 2013 doi: 10.1371/journal.pone.0068095. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Huyghe JR, Jackson AU, Fogarty MP, et al. Exome array analysis identifies new loci and low-frequency variants influencing insulin processing and secretion. Nat Genet. 2013;45(2):197–201. doi: 10.1038/ng.2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Saxena R, Hivert MF, Langenberg C, et al. Genetic variation in GIPR influences the glucose and insulin responses to an oral glucose challenge. Nat Genet. 2010;42(2):142–148. doi: 10.1038/ng.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ehret GB, Munroe PB, Rice KM, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478(7367):103–109. doi: 10.1038/nature10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Psaty BM, O’Donnell CJ, Gudnason V, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: Design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circulation Cardiovascular genetics. 2009;2(1):73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoo YJ, Bull SB, Paterson AD, Waggott D, Sun L. Were genome-wide linkage studies a waste of time? Exploiting candidate regions within genome-wide association studies. Genet Epidemiol. 2010;34(2):107–118. doi: 10.1002/gepi.20438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Liang S, Pan M, Hu N, et al. Association of angiotensin-converting enzyme gene 2350 G/A polymorphism with diabetic retinopathy in Chinese Han population. Mol Biol Rep. 2012 doi: 10.1007/s11033-012-2081-2. [DOI] [PubMed] [Google Scholar]


