Abstract
Scavenging rate constants of eight hydrophilic antioxidants, including caffeic acid, chlorogenic acid, genistein, glutathione, N-acetylcysteine, rutin, trolox, and uric acid against multiple ROS, namely superoxide anion, hydroxyl radical, singlet oxygen, and alkoxyl radical were determined with the electron spin resonance method. Direct flash photolysis measurement of the second-order rate constant in the reaction of alkoxyl radical plus the spin trap 5,5-dimethyl-pyrroline N-oxide made it possible to evaluate scavenging rate constants in antioxidants. The magnitudes of scavenging rate constants were notably dependent on the character of each ROS and the overall rate constants were highest in hydroxyl radical scavenging and the lowest in superoxide anion. The highest scavenging rate constant against superoxide anion was obtained by chlorogenic acid (2.9 × 105 M−1 s−1) and the lowest was by N-acetylcysteine (5.0 × 102 M−1 s−1). For singlet oxygen, the highest was by glutathione (9.4 × 108 M−1 s−1) and the lowest was by uric acid (2.3 × 106 M−1 s−1). All other numbers are listed and illustrated. Redox potential measurements of the antioxidants indicated that the antioxidants are likely to react with superoxide anion and singlet oxygen through electron transfer processes.
Keywords: reactive oxygen species (ROS), hydrophilic antioxidant, radical scavenging, spin trapping, redox potential
Introduction
Reactive oxygen species (ROS) such as superoxide anion (O2−•), hydroxyl radical (HO•), and singlet oxygen (1O2) are produced as a result of biochemical processes in vivo, and are considered as main causes of oxidative damage in cells and tissues.(1,2) Oxidative damage in biological systems is essentially the oxidation of lipids and proteins and can be inhibited or delayed by antioxidants(3); therefore, scavenging rate determination of antioxidants against ROS is meaningful. Previous studies evaluated the oxygen radical scavenging capacity (ORSC) of pure antioxidant compounds and foods.(4–6) Conventional ORSC method utilizes oxidative damage of certain fluorescent probe, but has a few experimental disadvantages.(6) We proposed a new method of ORSC evaluation (ORSC-ESR or ORSC-EPR), using an electron spin resonance (ESR) trapping technique.(7,8) Thus, we have reported ORSC-ESR measurement of the scavenging rates in various lipophilic antioxidant compounds.(9) However, the ORSC method uses a single free radical species that is generated from the azo-initiator 2,2'-azobis(2-amidino-propane) dihydrochloride (AAPH). This free radical species has been identified as alkoxyl radical (RO•).(10)
Antioxidants possess scavenging ability against multiple ROS. The scavenging rate certainly depends on the kind of ROS to be scavenged, while a majority of previous studies determined scavenging rates against a single ROS. Recently, Oowada et al.(11) proposed a method to determine scavenging capacity against multiple ROS in biological specimens such as human serum. ORSC-ESR method has been also utilized to evaluate antioxidant capacity of food such as mushrooms.(12) However, ROS scavenging data for pure antioxidant compounds are still lacking. In this study, we selected eight well-documented pure antioxidant compounds and generated data sets of relative scavenging rate constants against superoxide anion, hydroxyl radical and singlet oxygen.
Furthermore, the scavenging rate constant between the spin trap DMPO and RO• was directly evaluated with flash photolysis technique. By combining this rate constant with relative rate constants from ESR measurement, all scavenging rate constants were calculated. In addition, in order to verify the mechanism of scavenging reaction we performed redox potential measurements for the antioxidants.
Materials and Methods
Materials and equipment
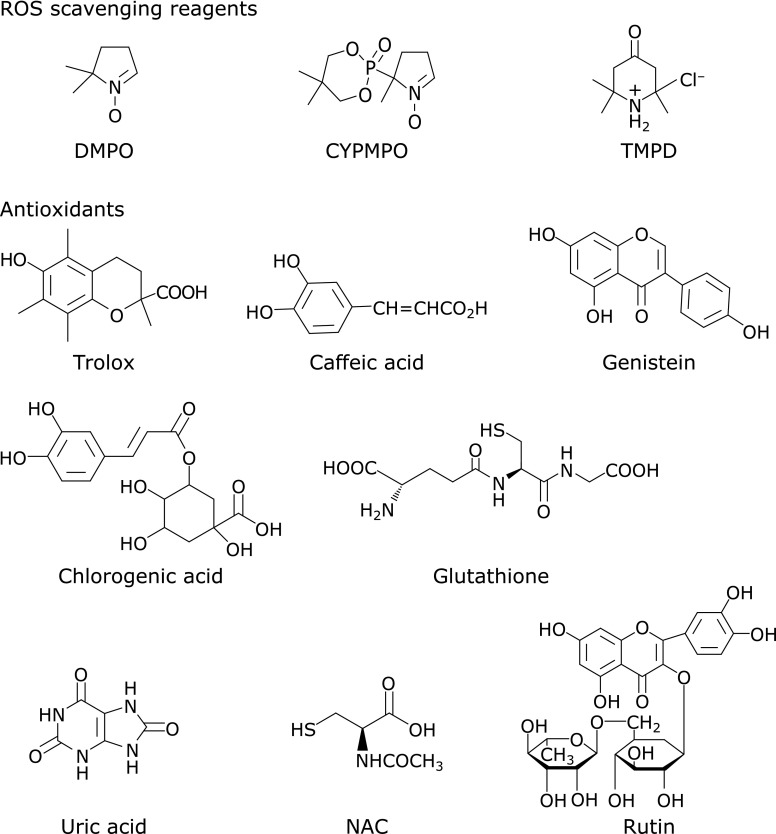
The spin traps used are shown in Fig. 1; i.e., 5,5-dimethyl-pyrroline N-oxide (DMPO) and 5-(2,2-dimethyl-1,3-propoxy cyclophosphoranyl)-5-methyl-1-pyrroline N-oxide (CYPMPO). These compounds were purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan) and Radical Research Inc. (Hino, Japan), respectively. 2,2,6,6-Tetramethyl-4-piperidone hydrochloride (TMPD) was obtained from Aldrich Chemical Company Inc. (Milwaukee, WI) and used as the detection reagent of singlet oxygen.(13,14) Rose bengal and riboflavin were obtained from Wako Pure Chem. Ind., Ltd. (Osaka, Japan) and used as photo-sensitizers to generate singlet oxygen and superoxide anion, respectively. Hydrogen peroxide and AAPH were purchased from Wako Pure Chem. and was used as a source of hydroxyl and alkoxyl radicals, respectively. Fig. 1 shows the eight natural hydrophilic antioxidants used in this study. Trolox, reduced glutathione, caffeic acid (3,4-dihydroxycinnamic acid), rutin, chlorogenic acid, and N-acetylcysteine (NAC) were obtained from Wako Pure Chem. Genistein and uric acid were purchased from Tokyo Chemical Industry and Nacalai Tesque, Inc. (Kyoto, Japan), respectively. Water was purified by distillation, and a 100 mM (M = mol dm−3) sodium phosphate buffer (pH 7.4) was used as a solvent.
Fig. 1.
Structures of DMPO, CYPMPO, TMPD and various hydrophilic antioxidants.
A JEOL FA200 X-band spectrometer (Akishima, Japan) was used to record ESR spectra. Spectrometer settings were: sweep time of 30 s, time constant of 0.1 s, and microwave power of 5 mW. The ESR signal intensities of the spin adduct in the presence and absence of antioxidants were measured to calculate the scavenging rate constants according to the kinetic equation shown in the following section.
Scavenging of superoxide anion
The ESR spin trapping method was applied to evaluate free radical scavenging rates. The spin-trapping method has been recognized as a useful tool for the detection and identification of unstable free radicals.(15) Spin trapping compounds (spin traps) such as CYPMPO react with superoxide to form stable free radical called spin adducts (Scheme 1).
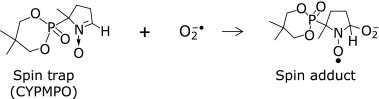 Scheme 1 Scheme 1
|
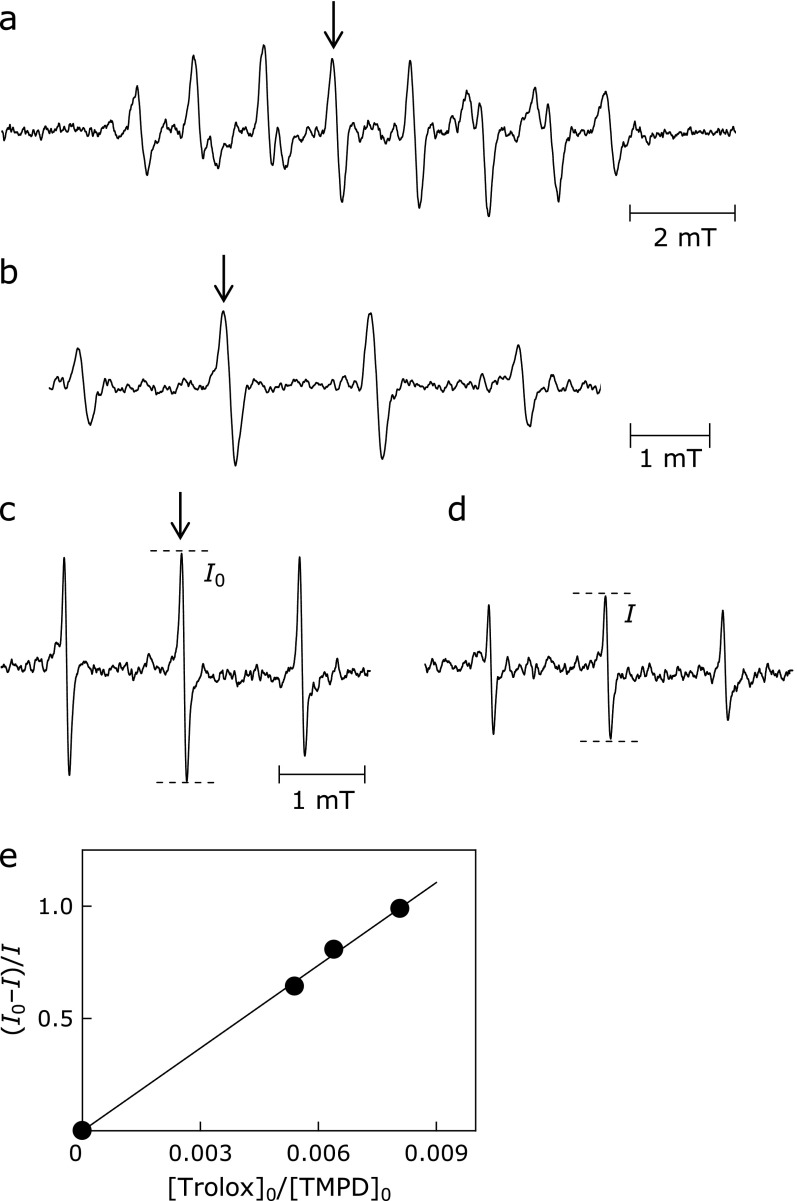
ESR signal pattern was used to identify the spin adduct and its intensity was used for the kinetic measurements of superoxide anion. The superoxide anion was generated from riboflavin (60 µM) and ethylendiaminetetraacetic acid disodium salt dehydrate (EDTA, 10 mM) with 30 s UV irradiation. UV source was a 200 W mercury arc with a band-path (500–600 nm) filter (RUF-203S, Radical Research Inc., Hino, Japan). After UV irradiation, the ESR signal of the spin adducts with CYPMPO (10 mM) was recorded (Fig. 2a).
Fig. 2.
ESR spectra of spin adducts produced after photolysis of precursors/sensitizers: (a) Superoxide anion adduct of CYPMPO (Isomer 1: AP = 5.3 mT, AN = 1.29 mT, AH = 1.15 mT; Isomer 2: AP = 5.10 mT, AN = 1.31 mT, AH = 1.03 mT). (b) Hydroxyl radical adduct of DMPO (AN = AH = 1.50 mT). (c and d) Tempol radical formed after the reaction of singlet oxygen with TMPD (AN = 1.60 mT): [trolox]0 = (c) 0 and (d) 480 µM. [TMPD]0 = 100 mM. Horizontal broken lines in the ESR spectra demonstrate the change in signal height by the addition of the antioxidant trolox. (e) A plot of (I0 – I)/I vs [trolox]0/[TMPD]0 according to Eq. (1). Peaks marked with arrows were used for the antioxidant capacity assay.
Kinetic formulation of the competitive reaction between the spin trap CYPMPO and antioxidant has been published elsewhere.(7) Briefly, in the presence of antioxidants (AOx) and the spin trap CYPMPO, the superoxide anion scavenging reaction should occur as follows:
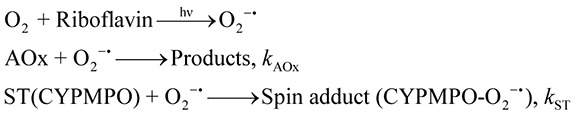 |
where ST denotes the spin trap reagent. kAOx and kST are rate constants. A simple formula for the superoxide scavenging capacity calculation can be derived from the above reactions:(7)
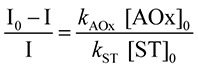 (1) (1)
|
where I and I0 are ESR signal heights of the spin adduct in the presence and absence of antioxidants, respectively. The symbol [ ]0 denotes the initial concentration of the component. The relative superoxide scavenging rate was determined from the slope (kAOx/kST) of a plot of (I0 – I)/I against [AOx]0/[ST]0. The hydrophilic antioxidant trolox has been conventionally adopted as a standard.(6) The relative superoxide scavenging rate is expressed as ORSC values in the trolox equivalent unit (TEU).
Hydroxyl radical and alkoxyl radical scavenging
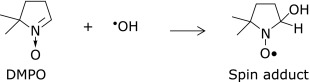
The spin trap DMPO was employed for hydroxyl radical and alkoxyl radical because DMPO provides unique ESR spectral patterns for these radicals and the stability of the spin adducts is excellent (Scheme 2).
 Scheme 2 Scheme 2
|
Hydroxyl radical was generated by UV irradiation of a H2O2 solution (4 mM) and the UV photolysis of AAPH was adopted to produce alkoxyl radical. After UV irradiation, the ESR signal of the hydroxyl radical adduct with DMPO (5 mM) was recorded by an ESR spectrometer (Fig. 2b). Equation 1 for the hydroxyl radical scavenging assay was formulated using the competitive reaction system described below:(7)
 |
where R• represents either HO• or RO•. Kinetic formulation to calculate relative scavenging rate constants is exactly the same as Eq. (1), but ST should be equal to DMPO in this case.
Singlet oxygen scavenging
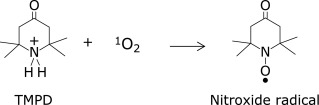
The reaction between singlet oxygen and TMPD to form a stable nitroxide radical was utilized to quantify singlet oxygen (Scheme 3).(13,14)
 Scheme 3 Scheme 3
|
Exactly speaking, this reaction is not classified as spin trapping, but for the sake of convenience we treat this as spin trapping reaction.
Singlet oxygen was generated via VIS irradiation (5 s, band-path of 500–600 nm) of aqueous rose bengal solution (30 µM). In the presence of TMPD and antioxidants, the following competitive reaction should occur:
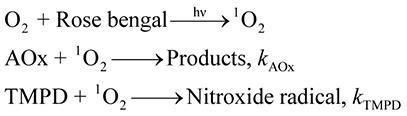 |
As shown in Fig. 2c and d, when TMPD and AOx (e.g., trolox) were mixed, the ESR peak intensity decreased, compared with that in the absence of AOx, indicating that part of 1O2 was scavenged or deactivated by the antioxidant. According to the above reactions, the relative 1O2 scavenging rates can be evaluated from Eq. (1) for ESR signal intensity. A typical plot of (I0 – I)/I against [trolox]0/[TMPD]0 for the trolox/TMPD system is shown in Fig. 2e with a straight line passing through the origin, suggesting that the above competitive mechanism is justifiable.
Flash photolysis determination of RO• trapping rate constant
A laser flash photolysis system (TSP-1000, UNISOKU, Hirakata, Japan) equipped with a Nd-YAG laser (Continuum Surelight-I, FWHM<5 ns, 2 Hz) was employed to determine the trapping rate constant of DMPO against RO• radical. Excitation light source [λex (excitation wavelength) = 355 nm] was used to generate RO• radical from AAPH in a phosphate buffer (0.1 M).
The RO• adduct of DMPO has one absorption band at 260 nm and time course of this band was followed after the flash photolysis. The reaction mechanism for the AAPH decomposition leading to the formation of ROO• and RO• is well documented.(16,17) In excess DMPO, the pseudo-first-order kinetics would be held for the DMPO trapping reaction, thus the reaction mechanism for the DMPO-OR adduct formation can be expressed as follows:
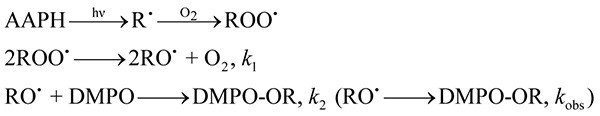 |
where kobs (= k2[DMPO]0) denotes the pseudo-first-order rate constant.
The kinetic formulation for the above reaction has been made,(18) and the concentration of DMPO-OR can be expressed as:
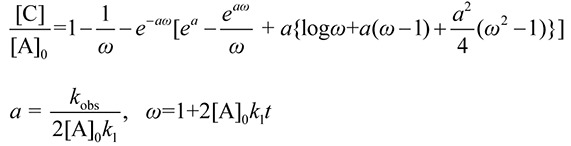 (2) (2)
|
where [C] and [A] denote the concentrations of the DMPO-OR and ROO•. Appropriate curve fitting was performed for the time course curve and kobs was calculated according to Eq. (2).
Redox potential measurements of various antioxidants
An RRDE-3A rotating ring disk electrode (BAS) and an electrochemical analyzer model 701 D (BAS) were used to measure the electrochemical properties (redox potentials) of antioxidants in a phosphate buffer at 298 ± 1 K with pH = 7.4, [AOx] = 1.0 × 10−3 M, and [NaClO4] = 1 M. A glassy carbon rotating disk (2,000 rpm) electrode, platinum wire electrode, and Ag/Ag+ electrode were used as the working, counter, and reference electrodes, respectively. The oxidation wave of K3Fe(CN)6 was observed at +210 mV vs Ag/Ag+.
Results and Discussion
ESR spin trapping determination of relative scavenging rates
Table 1 lists the relative scavenging rate constants of eight hydrophilic antioxidants for superoxide anion, hydroxyl radical, and singlet oxygen. In the same table, relative scavenging rate constants were expressed in the trolox equivalent unit (TEU). Trolox is a vitamin E analog that has been employed as a standard antioxidant in the ORSC method, where ORSC values are expressed relative to those of trolox, i.e., trolox equivalent unit.(6,7)
Table 1.
Relative scavenging rate constants and values in TEU for ROS scavenging of various hydrophilic antioxidants
| Antioxidants | O2−• |
HO• |
1O2 |
RO• |
E1/2(Ox) (eV) | E – E1/2(Ox) (eV) | |||
|---|---|---|---|---|---|---|---|---|---|
| kAOx/kCYPMPO | TEU | kAOx/kDMPO | TEU | kAOx/kTMPD | TEU | TEU | |||
| Chlorogenic acid | 5,980 ± 319 | 137 ± 9 | 3.43 ± 0.03 | 0.39 ± 0.01 | 758 ± 11 | 6.2 ± 0.2 | 2.3 | 0.36 | 3.45 |
| Uric acid | 222 ± 8 | 5.1 ± 0.7 | 3.40 ± 0.04 | 0.38 ± 0.01 | 4.57 ± 0.50 | 0.037 ± 0.004 | 2.2* | 0.47 | 3.79 |
| Caffeic acid | 4,780 ± 219 | 109 ± 6 | 2.88 ± 0.01 | 0.33 ± 0.01 | 1,080 ± 25 | 8.8 ± 0.3 | 1.6* | 0.31 | 3.68 |
| Rutin | 322 ± 12 | 7.3 ± 0.4 | 2.28 ± 0.05 | 0.26 ± 0.01 | 685 ± 11 | 5.6 ± 0.2 | 1.4* | 0.236 | 3.19 |
| Trolox | 43.8 ± 1.5 | 1 | 8.85 ± 0.03 | 1 | 123 ± 3 | 1 | 1 | 0.23 | 4.08 |
| Glutathione | 23.3 ± 0.8 | 0.53 ± 0.01 | 5.87 ± 0.01 | 0.66 ± 0.01 | 1,856 ± 14 | 15.1 ± 0.3 | 0.57* | –0.01 | 6.06 |
| Genistein | 57.3 ± 2.0 | 1.3 ± 0.6 | 4.23 ± 0.05 | 0.48 ± 0.01 | 384 ± 15 | 3.1 ± 0.2 | 0.43* | 0.195 | 3.64 |
| NAC | 10.3 ± 0.4 | 0.24 ± 0.01 | 4.87 ± 0.03 | 0.55 ± 0.01 | 404 ± 6 | 3.3 ± 0.1 | 0.40* | <–0.010 | >6.06 |
*Cited from Ref. (7).
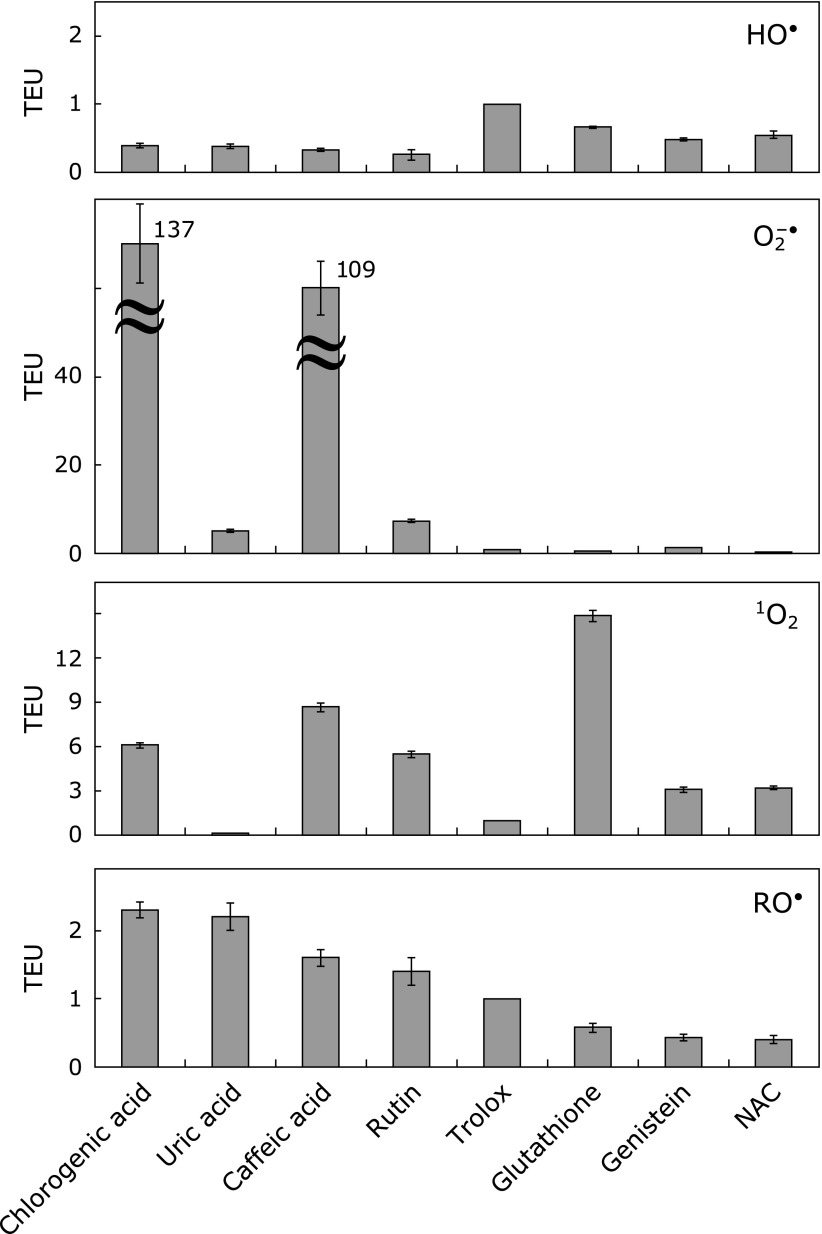
Relative scavenging rates against RO• radical (ORSC values) are also given in Table 1. Results indicated that: (1) superoxide anion: the magnitude in TEU ranged from 0.24 to 137, and chlorogenic acid and caffeic acid showed the highest scavenging rates, and the order of superoxide scavenging rate is; chlorogenic acid > caffeic acid > rutin > uric acid > genistein ≈ trolox > glutathione > NAC, (2) hydroxyl radical: the magnitude in TEU ranged from 0.26 to 1, and the rates are not sensitive to the structure of antioxidants, and the order of antioxidant ability is trolox > glutathione > genistein > chlorogenic acid ≈ uric acid ≈ caffeic acid ≈ rutin, and (3) singlet oxygen: the magnitude in TEU ranged from 0.037 to 15.1, and glutathione shows the highest antioxidant capacity, and the order of the rates is glutathione > caffeic acid > chlorogenic acid ≈ rutin > NAC ≈ genistein > trolox > uric acid. The order is not in agreement with the data for superoxide anion. The magnitude in TEU can be compared only within the same ROS.
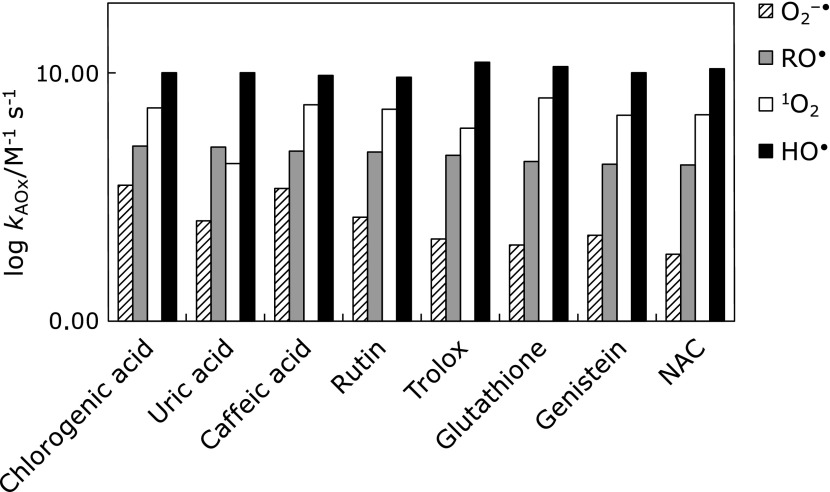
Fig. 3 shows the relative ROS scavenging rates of the eight hydrophilic antioxidants. These are relative values as compared with the relative RO• radical scavenging rates (ORSC-ESR values). Also, Fig. 3 clearly indicates that the present relative ROS scavenging rates do not correlate with the relative RO• radical scavenging rate.
Fig. 3.
A bar graph that represents the relative ROS scavenging abilities (TEU) for various hydrophilic antioxidants: (HO•) hydroxyl radical, (O2−•) superoxide anion, (1O2) singlet oxygen, and (RO•) AAPH-derived alkoxyl radical.
Evaluation of DMPO trapping rate constants against RO•
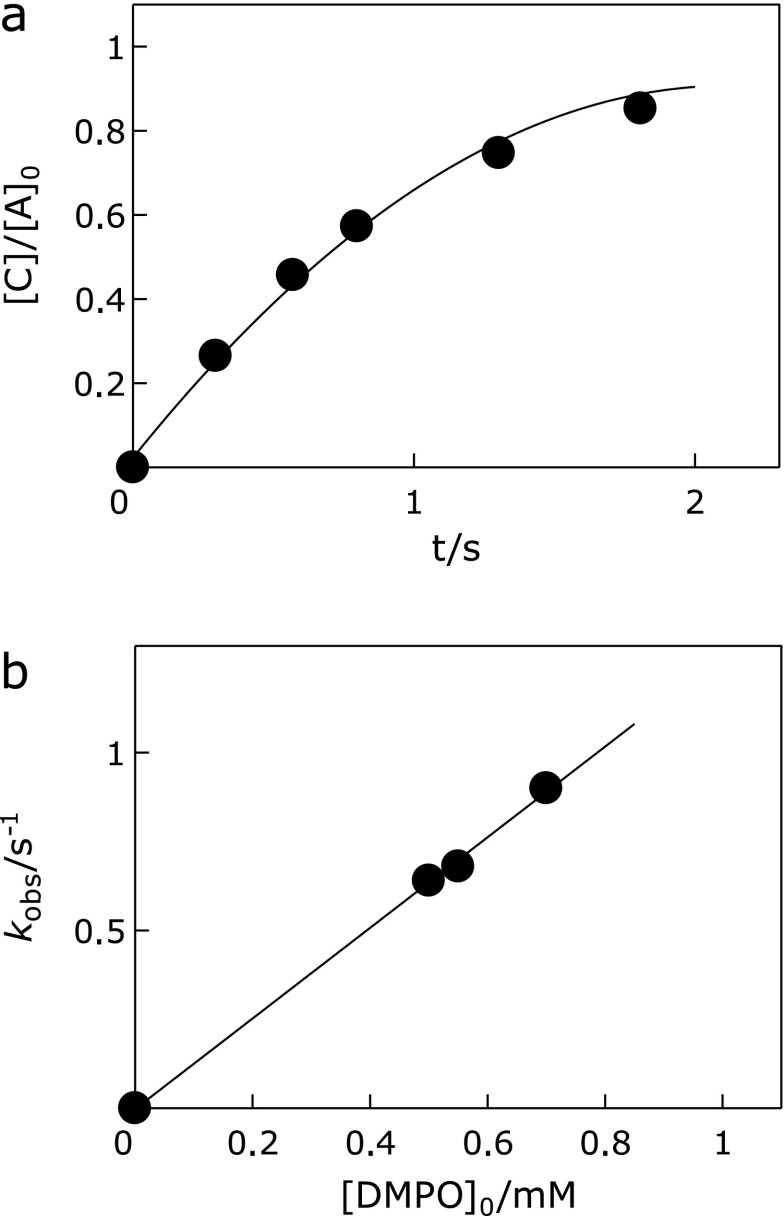
The rate constant for DMPO + RO• reaction was measured using laser flash photolysis method. Fig. 4a shows the time dependence ([C]/[A]0) of the DMPO-OR product. Using the kinetic parameters (k1 = 1.7 × 104 M−1 s−1)(16,17) in Eq. (2), the kobs and [A]0 values were determined from fitting to the experimental data: [A]0 = 1.0 × 10−5 M and kobs = 0.64, 0.68, and 0.92 s−1 at [DMPO]0 = 0.50, 0.55, and 0.70 mM, respectively.
Fig. 4.
(a) Time course curve of the DMPO-OR adduct formation. [C] is the adduct concentration under the conditions of: [DMPO]0 = 0.5 mM, [AAPH]0 = 10 mM, and [A]0 = 10 µM. Solid line represents the [C]/[A]0 values calculated from Eq. (2). (b) Plots of the kobs values against [DMPO]0.
The second order rate constant k2 for the DMPO trapping reaction was evaluated to be k2 = 1.27 × 103 M−1 s−1 from the slope of the plots of kobs against [DMPO]0 (Fig. 4b). A straight line that passes through the origin was obtained, indicating that the reaction scheme and calculation procedures using Eq. (2) are justifiable.
Direct measurement of the reaction rate constant has made it possible to directly compare the scavenging rate constants in multiple ROS. In the present experiment, using a competitive trapping method, we have determined the relative RO• spin trapping rates for CYPMPO and DMPO to be kCYPMPO/kDMPO = 17.8, from which the rate constant of the RO• + CYPMPO reaction was calculated to be kCYPMPO (RO•) = 2.26 × 104 M−1 s−1. The rate constants of traps (DMPO and CYPMPO) for HO• and O2−• were determined to be kDMPO(HO•) = 2.8 × 109 M−1 s−1 and kCYPMPO(O2−•) = 48 M−1 s−1, respectively.(19,20) Rate constant data for 1O2 scavenging was not available in the present experiments; therefore, we utilized the rate constants [ktrolox(1O2) = 6.22 × 107 M−1 s−1 (21)] between trolox and 1O2 to calculate the rate constants for other antioxidants. Rate constant data are listed in Table 2 and these numbers are illustrated in a logarithmic scale (Fig. 5).
Table 2.
Rate constants (kAOx/M−1 s−1) for ROS scavenging of various hydrophilic antioxidants
| Antioxidants | O2−• | HO• | 1O2 | RO• |
|---|---|---|---|---|
| Chlorogenic acid | (288 ± 15) × 103 | (9.60 ± 0.08) × 109 | (38.6 ± 0.5) × 107 | (111 ± 7) × 105 |
| Uric acid | (10.6 ± 0.4) × 103 | (9.52 ± 0.11) × 109 | (2.30 ± 0.25) × 106 | (106 ± 6) × 105 |
| Caffeic acid | (229 ± 10) × 103 | (8.06 ± 0.03) × 109 | (54.7 ± 1.2) × 107 | (75.9 ± 0.9) × 105 |
| Rutin | (15.4 ± 0.6) × 103 | (6.38 ± 0.14) × 109 | (34.8 ± 0.5) × 107 | (69.0 ± 8.5) × 105 |
| Trolox | (2.10 ± 0.07) × 103 | (24.8 ± 0.1) × 109 | (6.22 ± 0.15) × 107 | (48.3 ± 3.7) × 105 |
| Glutathione | (1.11 ± 0.04) × 103 | (16.4 ± 0.1) × 109 | (93.9 ± 0.7) × 107 | (27.6 ± 1.5) × 105 |
| Genistein | (2.75 ± 0.10) × 103 | (11.8 ± 0.2) × 109 | (19.1 ± 0.7) × 107 | (20.7 ± 1.6) × 105 |
| NAC | (0.504 ± 0.020) × 103 | (13.6 ± 0.1) × 109 | (20.5 ± 0.3) × 107 | (19.3 ± 2.5) × 105 |
Fig. 5.
A logarithmic representation of scavenging rate constants against various ROS in eight hydrophilic antioxidants.
It should be pointed out that scavenging rate constants against HO• radical is insensitive to the structure of antioxidants. The magnitude of the HO• rate constants is close to diffusion control, suggesting that HO• may react with any group of initial encounter. We also show that antioxidants scavenging rates against superoxide anion, hydroxyl radical, and singlet oxygen does not correlate with that for AAPH-derived RO• radical (ORSC value), indicating that ORSC value may not be representative antioxidant parameter. The logarithmic plot of the antioxidant’s reaction rates in Fig. 5 indicates that the ROS scavenging rates increase in the order of O2−•< RO•<1O2<HO•.
Reaction mechanism of ROS scavenging reaction
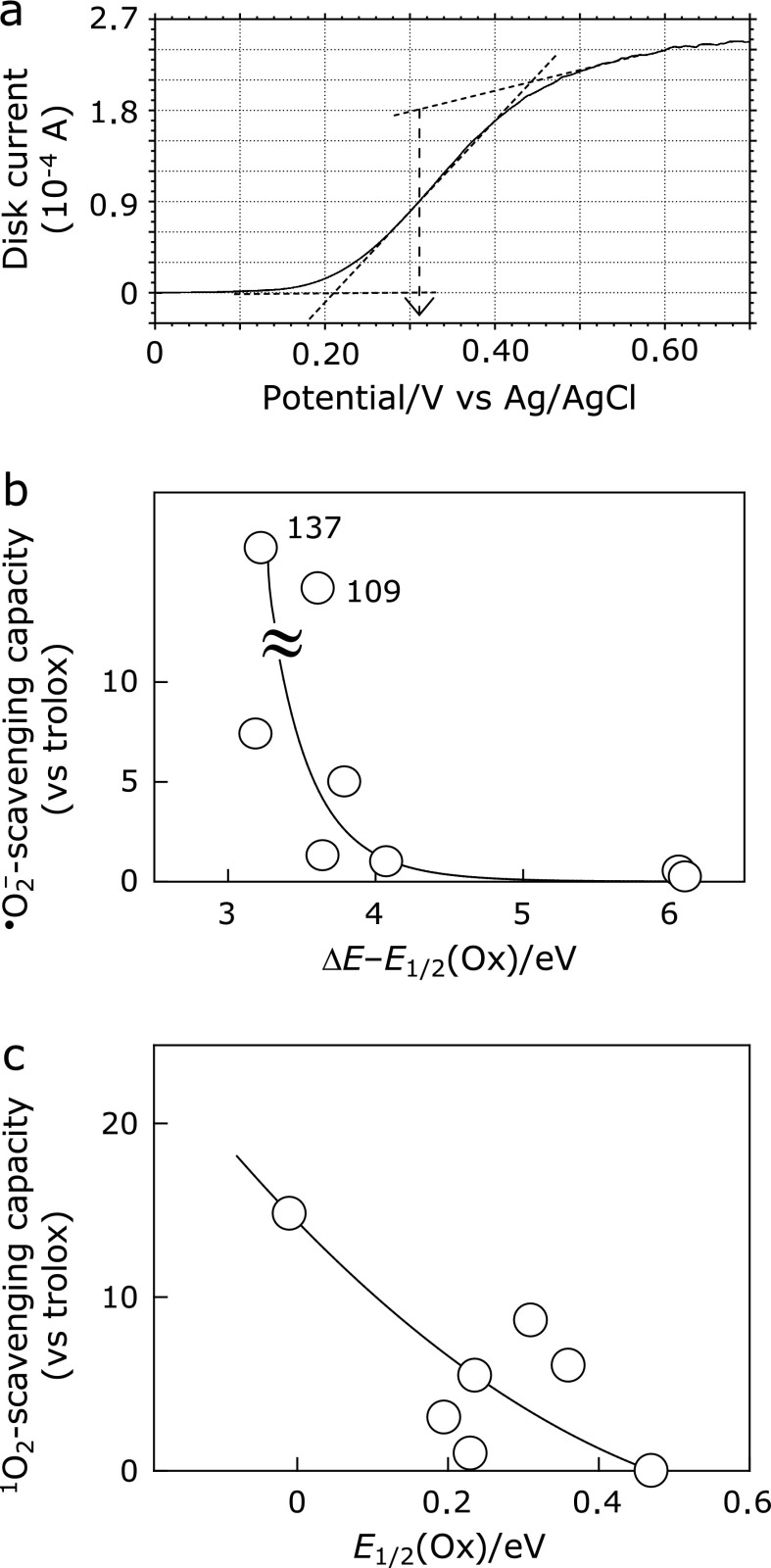
Based on the redox potentials of the antioxidants, the mechanism of the antioxidant’s reaction with ROS was elucidated. It is generally difficult to determine the half-wave potential values (E1/2) of redox reactions for phenolic antioxidants because of the potential window and follow-up chemical reaction. The rotating ring-disk electrode (RRDE) is a powerful technique for detecting the products, side-products or even short-lived intermediates of electrode reactions.(22) We have determined the E1/2(ox) values of antioxidants from the RRDE measurements. Fig. 6a shows the representative voltammogram of disk current in RRDE measurement for caffeic acid. As shown in Fig. 6a, the E1/2(ox) value of caffeic acid was determined with the aid of an attached computer program (an electrochemical analyzer model 701 D) and the data of antioxidants are listed in Table 1. The antioxidants’ capacity for electron donation and acceptance can be expressed in terms of E1/2(ox) and ΔE – E1/2(ox), respectively, where ΔE is the transition energy obtained from the absorption band in the UV region.(22,23) Smaller values of E1/2(ox) mean easier electron transfer from the antioxidant. In contrast, the antioxidants having a small value of ΔE – E1/2(ox) are good electron acceptors.
Fig. 6.
Electrochemical properties of the eight antioxidants: (a) Voltammogram of disk current in RRDE measurement (2,000 rpm) for caffeic acid. (b) The plot of superoxide anion scavenging rate constants against ΔE – E1/2(ox) of each antioxidant. (c) The plot of singlet oxygen scavenging rate constants against the oxidation potential E1/2(ox) of each antioxidant.
Fig. 6b shows that the superoxide anion scavenging rates increase with the decrease in the ΔE – E1/2(ox) value in antioxidants, suggesting that the primary process in the reaction of superoxide anion with antioxidants is characterized as reduction of antioxidants as follows(24):
For the singlet oxygen scavenging, as shown in Fig. 6c, the antioxidant that has smaller E1/2(ox) shows higher scavenging rates, suggesting that the antioxidants were operative as electron donors, that is in agreement with the previous results reported by Foley et al.(25) and Mukai et al.(26)
Conclusion
In eight hydrophilic antioxidants, we obtained scavenging rate constants against four ROS. The antioxidant that has high scavenging rate constant does not necessarily mean that it exercises high antioxidant activity in vivo because scavenging capacity (scavenging rate) value is calculated by multiplying the rate constant with the antioxidant concentration. It is highly likely that the local concentration of antioxidant varies a great deal in vivo. Therefore, one should be very cautious in judging the effectiveness of the antioxidant.
Acknowledgments
We thank Dr. Yashige Kotake for helpful discussion and critical reading of the manuscript. This work was supported in part by a Grant-in-Aid for Science Research (C) (No. 25450169) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Abbreviations
- AAPH
2,2'-azobis(2-amidinopropane) dihydrochloride
- AOx
antioxidant
- CYPMPO
5-(2,2-dimethyl-1,3-propoxy cyclophosphoranyl)-5-methyl-1-pyrroline N-oxide
- DMPO
5,5-dimethyl-pyrroline N-oxide
- ESR
electron spin resonance
- NAC
N-acetylcysteine
- ORSC
oxygen radical scavenging capacity
- ORAC-ESR
ESR spin trapping-based ORAC method
- RO•
alkoxyl radical
- ROS
reactive oxygen species
- ST
spin trap agent
- TEU
trolox equivalent unit
- TMPD
2,2,6,6-tetramethyl-4-piperidone hydrochloride
- trolox
6-hydroxy-2,5,7,8-tetra-methylchroman-2-carboxylic acid
- VIS
visible light
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Foley S, Navaratnam S, McGarvey DJ, Land EJ, Truscott TG, Rice-Evans A. Singlet oxygen quenching and redox properties of hydroxycinnamic acids. Free Radic Biol Med. 1999;26:1202–1208. doi: 10.1016/s0891-5849(98)00313-x. [DOI] [PubMed] [Google Scholar]
- 2.Jiménez A, Romojaro F, Gómez JM, Llanos MR, Sevilla F. Antioxidant systems and their relationship with the response of pepper fruits to storage at 20°C. J Agric Food Chem. 2003;51:6293–6299. doi: 10.1021/jf030052i. [DOI] [PubMed] [Google Scholar]
- 3.Wang SY, Chen H, Ehlenfeldt MK. Antioxidant capacities vary substantially among cultivars of rabbiteye blueberry (Vaccinium ashei Reade) Int J Food Sci Technol. 2011;46:2482–2490. [Google Scholar]
- 4.Glazer AN. Phycoerythrin fluorescence-based assay for reactive oxygen species. Methods Enzymol. 1990;186:161–168. doi: 10.1016/0076-6879(90)86106-6. [DOI] [PubMed] [Google Scholar]
- 5.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Rad Biol Med. 1993;14:303–311. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 6.Huang D, Ou B, Hampsch-Woodill M, Flanagan JA, Prior RL. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J Agric Food Chem. 2002;50:4437–4444. doi: 10.1021/jf0201529. [DOI] [PubMed] [Google Scholar]
- 7.Kohri S, Fujii H, Oowada S, et al. An oxygen radical absorbance capacity-like assay that directly quantifies the antioxidant’s scavenging capacity against AAPH-derived free radicals. Anal Biochem. 2009;386:167–171. doi: 10.1016/j.ab.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 8.Sueishi Y, Ishikawa M, Yoshioka D, et al. Oxygen radical absorbance capacity (ORAC) of cyclodextrin-solubilized flavonoids, resveratrol and astaxanthin as measured with the ORAC-EPR method. J Clin Biochem Nutr. 2012;50:127–132. doi: 10.3164/jcbn.11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ishikawa M, Sueishi Y, Endo N, et al. Cyclodextrin encapsulation of the functional group diminishes antioxidant’s free radical scavenging rates. Int J Chem Kinet. 2012;44:598–603. [Google Scholar]
- 10.Sueishi Y, Yoshioka D, Oowada S, et al. Is the oxygen radical absorbance capacity (ORAC) method a peroxyl-radical scavenging assay? Z Phys Chem. 2010;224:921–928. [Google Scholar]
- 11.Oowada S, Endo N, Kameya H, Shimmei M, Kotake Y. Multiple free-radical scavenging capacity in serum. J Clin Biochem Nutr. 2012;51:117–121. doi: 10.3164/jcbn.11-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanno T, Kawamura S, Harada E, Kameya H, Ukai M, Osawa T. Radical scavenging ability by ESR spin trapping and oxygen radical absorbance capacity of hot water extracts from mushrooms. Nippon Shokuhin Kagaku Kaishi. 2013;60:173–178. [Google Scholar]
- 13.Rabek JF, Ranby B, Arct J, Liu R. Singlet oxygen and free-radical oxidation of polydienes and related problems with stabilization: synergistic and antagonistic effects. J Photochem. 1984;25:519–536. [Google Scholar]
- 14.Tokuoka Y, Niitsu A, Watabe N, Murakami TN, Kawashima N. ESR spectroscopy of singlet oxygen generated by protoporphyrin IX in aqueous surfactant solutions. J Oleo Sci. 2003;52:135–140. [Google Scholar]
- 15.Janzen EG. Spin trapping. Methods Enzymol. 1984;105:188–198. doi: 10.1016/s0076-6879(84)05025-4. [DOI] [PubMed] [Google Scholar]
- 16.Rojas Wahl RU, Zeng L, Madison SA, DePinto RL, Shay BJ. Mechanistic studies on the decomposition of water soluble azo-radical-initiators. J Chem Soc Perkin Trans 2. 1998:2009–2018. [Google Scholar]
- 17.Krainev AG, Bigelow DJ. Comparison of 2,2'-azobis(2-amidinopropane) hydrochloride (AAPH) and 2,2'-azobis(2,4-dimethylvaleronitrile) (AMVN) as free radical initiators: a spin-trapping study. J Chem Soc Perkin Trans 2. 1996:747–754. [Google Scholar]
- 18.Capellos C, Bielski BHJ. Kinetic Systems: Mathematical Description of Chemical Kinetics in Solution. John Wiley & Sons Ltd.; New York and London: 1972. [Google Scholar]
- 19.Taniguchi H, Madden KP. An in situ radiolysis time-resolved ESR study of the kinetics of spin trapping by 5,5-dimethyl-1-pyrroline-N-oxide. J Am Chem Soc. 1999;121:11875–11879. [Google Scholar]
- 20.Kamibayashi M, Oowada S, Kameda H, et al. Synthesis and characterization of a practically better DEPMPO-type spin trap, 5-(2,2-dimethyl-1,3-propoxy cyclophosphoryl)-5-methyl-1-pyrroline N-oxide (CYPMPO) Free Rad Res. 2006;40:1166–1172. doi: 10.1080/10715760600883254. [DOI] [PubMed] [Google Scholar]
- 21.Ohara K, Kikuchi K, Origuchi T, Nagaoka S. Singlet oxygen quenching by trolox C in aqueous micelle solutions. J Photochem Photobiol B. 2009;97:132–137. doi: 10.1016/j.jphotobiol.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Okuno M, Kita M, Kashiwabara K, Fujita J. Redox potentials of cobalt(III) mixed-ligand complexes with sulfur, phosphorus, and nitrogen donor atoms and a correlation with their electronic spectra. Chem Lett. 1989;18:1643–1646. [Google Scholar]
- 23.Dodsworth ES, Lever ABP. Correlations between electrochemical potentials and optical charge transfer energies in ruthenium bipyridine derivatives. Chem Phys Lett. 1986;124:152–158. [Google Scholar]
- 24.Galano A, Vargas R, Martínez A. Carotenoids can act as anitioxidants by oxidizing the superoxide radical anion. Phys Chem Chem Phys. 2010;12:193–200. doi: 10.1039/b917636e. [DOI] [PubMed] [Google Scholar]
- 25.Foyer CH, Lelandais M, Kunert KJ. Photooxidative stress in plants. Physiol Plant. 1994;92:696–717. [Google Scholar]
- 26.Mukai K, Nagai S, Ohara K. Kinetic study of the quenching reaction of singlet oxygen by tea catechins in ethanol solution. Free Radic Biol Med. 2005;39:752–761. doi: 10.1016/j.freeradbiomed.2005.04.027. [DOI] [PubMed] [Google Scholar]