Abstract
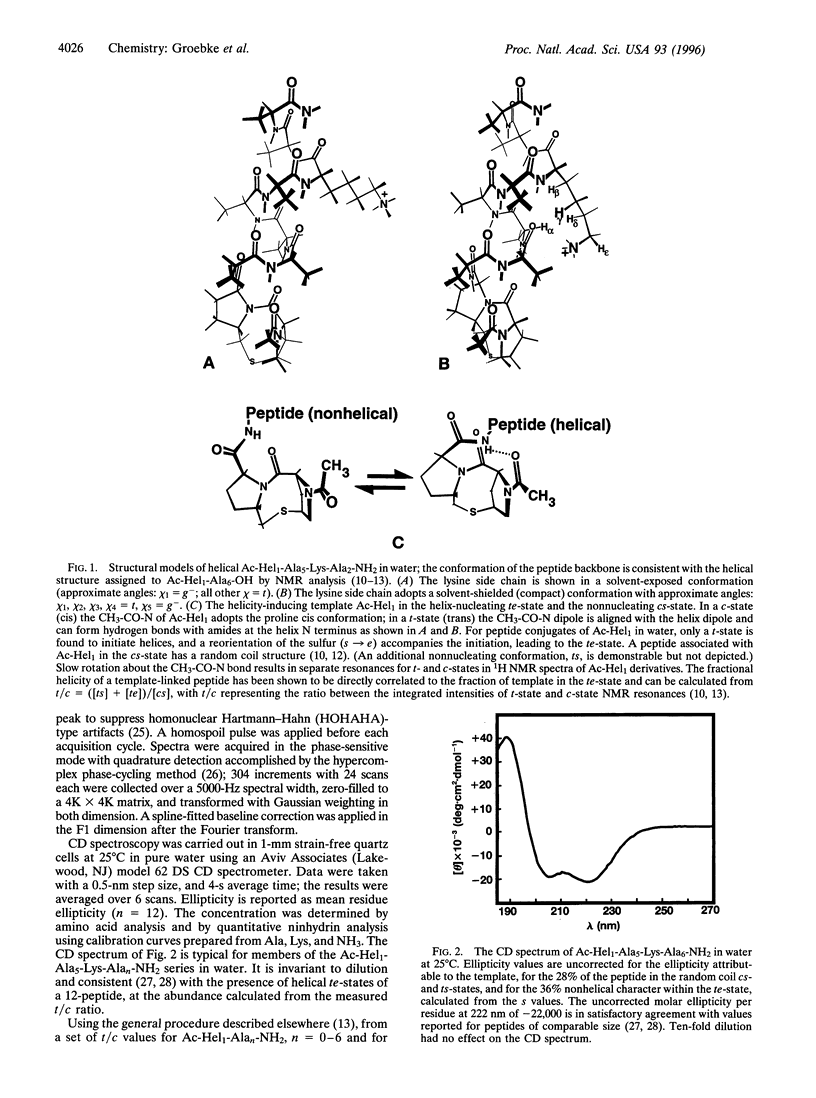
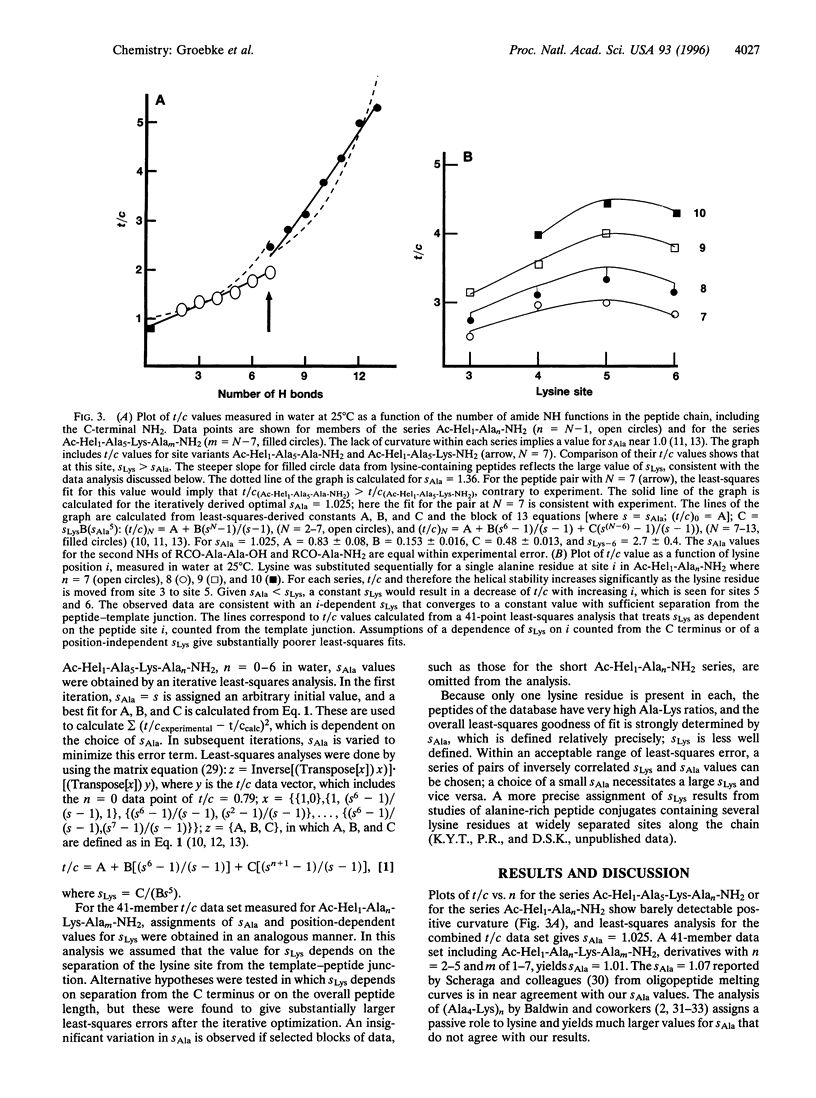
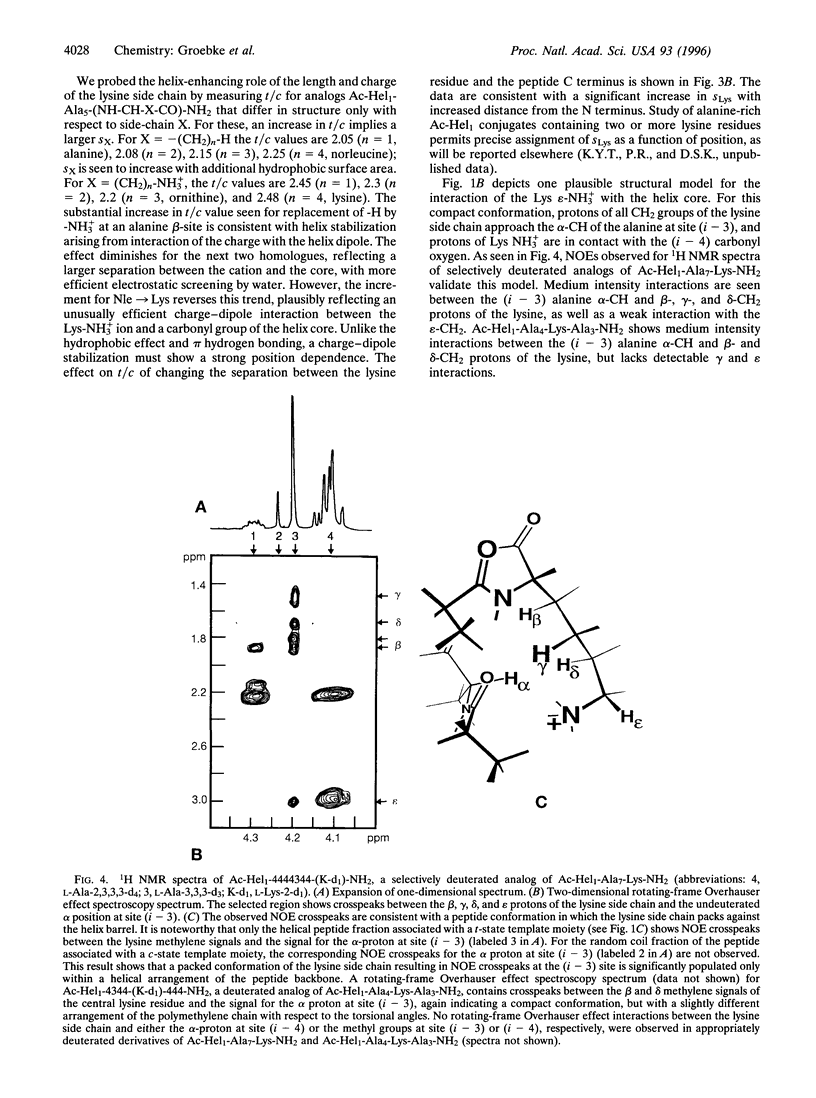
The helicity in water has been determined for several series of alanine-rich peptides that contain single lysine residues and that are N-terminally linked to a helix-inducing and reporting template termed Ac-Hel1. The helix-propagating constant for alanine (sAla value) that best fits the properties of these peptides lies in the range of 1.01-1.02, close to the value reported by Scheraga and coworkers [Wojcik, J., Altmann, K.-H. & Scheraga, H.A. (1990) Biopolymers 30, 121-134], but significantly lower than the value assigned by Baldwin and coworkers [Chakrabartty, A., Kortemme, T. & Baldwin, R.L. (1994) Protein Sci. 3,843-852]. From a study of conjugates Ac-Hel1-Ala(n)-Lys-Ala(m)-NH2 and analogs in which the methylene portion of the lysine side chain is truncated, we find that the unusual helical stability of Ala(n)Lys peptides is controlled primarily by interactions of the lysine side chain with the helix barrel, and only passively by the alanine matrix. Using 1H NMR spectroscopy, we observe nuclear Overhauser effect crosspeaks consistent with proton-proton contacts expected for these interactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chakrabartty A., Kortemme T., Baldwin R. L. Helix propensities of the amino acids measured in alanine-based peptides without helix-stabilizing side-chain interactions. Protein Sci. 1994 May;3(5):843–852. doi: 10.1002/pro.5560030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabartty A., Kortemme T., Padmanabhan S., Baldwin R. L. Aromatic side-chain contribution to far-ultraviolet circular dichroism of helical peptides and its effect on measurement of helix propensities. Biochemistry. 1993 Jun 1;32(21):5560–5565. doi: 10.1021/bi00072a010. [DOI] [PubMed] [Google Scholar]
- Chakrabartty A., Schellman J. A., Baldwin R. L. Large differences in the helix propensities of alanine and glycine. Nature. 1991 Jun 13;351(6327):586–588. doi: 10.1038/351586a0. [DOI] [PubMed] [Google Scholar]
- Dyson H. J., Rance M., Houghten R. A., Wright P. E., Lerner R. A. Folding of immunogenic peptide fragments of proteins in water solution. II. The nascent helix. J Mol Biol. 1988 May 5;201(1):201–217. doi: 10.1016/0022-2836(88)90447-0. [DOI] [PubMed] [Google Scholar]
- Fairman R., Shoemaker K. R., York E. J., Stewart J. M., Baldwin R. L. Further studies of the helix dipole model: effects of a free alpha-NH3+ or alpha-COO- group on helix stability. Proteins. 1989;5(1):1–7. doi: 10.1002/prot.340050102. [DOI] [PubMed] [Google Scholar]
- Fiori W. R., Miick S. M., Millhauser G. L. Increasing sequence length favors alpha-helix over 3(10)-helix in alanine-based peptides: evidence for a length-dependent structural transition. Biochemistry. 1993 Nov 16;32(45):11957–11962. doi: 10.1021/bi00096a003. [DOI] [PubMed] [Google Scholar]
- Hol W. G., Halie L. M., Sander C. Dipoles of the alpha-helix and beta-sheet: their role in protein folding. Nature. 1981 Dec 10;294(5841):532–536. doi: 10.1038/294532a0. [DOI] [PubMed] [Google Scholar]
- Hol W. G., van Duijnen P. T., Berendsen H. J. The alpha-helix dipole and the properties of proteins. Nature. 1978 Jun 8;273(5662):443–446. doi: 10.1038/273443a0. [DOI] [PubMed] [Google Scholar]
- Kemp D. S., Boyd J. G., Muendel C. C. The helical s constant for alanine in water derived from template-nucleated helices. Nature. 1991 Aug 1;352(6334):451–454. doi: 10.1038/352451a0. [DOI] [PubMed] [Google Scholar]
- Lehrman S. R., Tuls J. L., Lund M. Peptide alpha-helicity in aqueous trifluoroethanol: correlations with predicted alpha-helicity and the secondary structure of the corresponding regions of bovine growth hormone. Biochemistry. 1990 Jun 12;29(23):5590–5596. doi: 10.1021/bi00475a025. [DOI] [PubMed] [Google Scholar]
- Marqusee S., Robbins V. H., Baldwin R. L. Unusually stable helix formation in short alanine-based peptides. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5286–5290. doi: 10.1073/pnas.86.14.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merutka G., Shalongo W., Stellwagen E. A model peptide with enhanced helicity. Biochemistry. 1991 Apr 30;30(17):4245–4248. doi: 10.1021/bi00231a020. [DOI] [PubMed] [Google Scholar]
- Merutka G., Stellwagen E. Effect of amino acid ion pairs on peptide helicity. Biochemistry. 1991 Feb 12;30(6):1591–1594. doi: 10.1021/bi00220a021. [DOI] [PubMed] [Google Scholar]
- Merutka G., Stellwagen E. Positional independence and additivity of amino acid replacements on helix stability in monomeric peptides. Biochemistry. 1990 Jan 30;29(4):894–898. doi: 10.1021/bi00456a007. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Fermi G. Stereochemistry of salt-bridge formation in alpha-helices and beta-strands. Proteins. 1988;4(4):294–295. doi: 10.1002/prot.340040408. [DOI] [PubMed] [Google Scholar]
- Rohl C. A., Scholtz J. M., York E. J., Stewart J. M., Baldwin R. L. Kinetics of amide proton exchange in helical peptides of varying chain lengths. Interpretation by the Lifson-Roig equation. Biochemistry. 1992 Feb 11;31(5):1263–1269. doi: 10.1021/bi00120a001. [DOI] [PubMed] [Google Scholar]
- Scholtz J. M., Qian H., York E. J., Stewart J. M., Baldwin R. L. Parameters of helix-coil transition theory for alanine-based peptides of varying chain lengths in water. Biopolymers. 1991 Nov;31(13):1463–1470. doi: 10.1002/bip.360311304. [DOI] [PubMed] [Google Scholar]
- Todd A. P., Millhauser G. L. ESR spectra reflect local and global mobility in a short spin-labeled peptide throughout the alpha-helix----coil transition. Biochemistry. 1991 Jun 4;30(22):5515–5523. doi: 10.1021/bi00236a026. [DOI] [PubMed] [Google Scholar]
- Vila J., Williams R. L., Grant J. A., Wójcik J., Scheraga H. A. The intrinsic helix-forming tendency of L-alanine. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7821–7825. doi: 10.1073/pnas.89.16.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]



