Abstract
A growing set of observations points to mitochondrial dysfunction, iron accumulation, oxidative damage and chronic inflammation as common pathognomonic signs of a number of neurodegenerative diseases that includes Alzheimer’s disease, Huntington disease, amyotrophic lateral sclerosis, Friedrich’s ataxia and Parkinson’s disease. Particularly relevant for neurodegenerative processes is the relationship between mitochondria and iron. The mitochondrion upholds the synthesis of iron–sulfur clusters and heme, the most abundant iron-containing prosthetic groups in a large variety of proteins, so a fraction of incoming iron must go through this organelle before reaching its final destination. In turn, the mitochondrial respiratory chain is the source of reactive oxygen species (ROS) derived from leaks in the electron transport chain. The co-existence of both iron and ROS in the secluded space of the mitochondrion makes this organelle particularly prone to hydroxyl radical-mediated damage. In addition, a connection between the loss of iron homeostasis and inflammation is starting to emerge; thus, inflammatory cytokines like TNF-alpha and IL-6 induce the synthesis of the divalent metal transporter 1 and promote iron accumulation in neurons and microglia. Here, we review the recent literature on mitochondrial iron homeostasis and the role of inflammation on mitochondria dysfunction and iron accumulation on the neurodegenerative process that lead to cell death in Parkinson’s disease. We also put forward the hypothesis that mitochondrial dysfunction, iron accumulation and inflammation are part of a synergistic self-feeding cycle that ends in apoptotic cell death, once the antioxidant cellular defense systems are finally overwhelmed.
Keywords: inflammation, neurodegeneration, mitochondrial dysfunction, iron toxicity, Parkinson’s disease
INTRODUCTION
Iron is an essential element necessary for the normal development of brain functions. Enzymes involved in neurotransmitter synthesis that possess iron as a prosthetic group are recognized targets of iron deficiency: monoamine oxidases A and B involved in dopamine catabolism, tryptophan hydroxylase, required for serotonin synthesis, tyrosine hydroxylase, required for dopamine and norepinephrine synthesis, glutamate decarboxylase involved in GABA synthesis and glutamate transaminase involved in L-glutamate synthesis, all belong to this group.
Abundant evidence shows that iron accumulation in particular areas of the brain is a hallmark of several neurodegenerative disorders (Zecca et al., 2004; Andersen et al., 2013), although it is uncertain whether iron accumulation is a primary cause of the disorder or a consequence of a previous dysfunction. Increased levels of iron promote cell death via hydroxyl radical formation, which enhances lipid peroxidation, protein aggregation, glutathione consumption, and nucleic acid modification. We recently put forward the hypothesis that iron accumulation, a process initiated by mitochondrial dysfunction, and the ensuing oxidative damage is part of the execution step, i.e., the death process of affected neurons (Núñez et al., 2012).
Mitochondrial dysfunction has long been associated with several neurodegenerative diseases that include Alzheimer’s disease (AD), Huntington’s disease (HD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and Friedrich’s Ataxia (FA; Schapira and Cooper, 1992; Moreira et al., 2010; Grubman et al., 2013). Mitochondrial dysfunction results in decreased ATP synthesis, as well as in decreased synthesis of iron–sulfur clusters (ISCs) and heme prosthetic groups. An association between mitochondrial dysfunction and mitochondrial iron accumulation has been found only in FA (Delatycki et al., 1999; Huang et al., 2009), although evidence for mitochondrial iron accumulation has been reported in experimental models of PD (Liang and Patel, 2004; Lee et al., 2009; Mena et al., 2011).
Inflammation in the central nervous system (CNS) is a condition strongly associated with neuronal death in several neurodegenerative disorders including PD and AD (Hirsch and Hunot, 2009). Inflammation is characterized by the occurrence of reactive microglia and a massive production of pro-inflammatory cytokines. These inflammatory processes trigger a chain of events including increased production of ROS and reactive nitrogen species (RNS), disruption of iron metabolism and mitochondrial dysfunction, finally leading to neurodegeneration.
THE BASIS OF IRON TOXICITY
The ability of iron to exchange readily one electron underlies its insertion in numerous catalytic processes found in living matter. The iron atom has octahedral coordination chemistry; therefore, it has six possible coordination bonds. Seminal work by Graf and associates demonstrated that iron is redox-inactive only if all its six coordination sites are stably bound. If one of the sites is free or loosely bound, iron is redox-active and competent of undertaking one-electron exchange reactions (Graf et al., 1984). It is noteworthy that Fe3+ complexes with the chelators desferrioxamine, DTPA or phytate at 1:10 (mol:mol) ratio result in redox-inactive iron whereas Fe3+ chelation with NTA, EDTA, EGTA, ATP, CDTA or bleomycin results in redox-active iron at the same 1:10 molar ratio (Graf et al., 1984).
Iron is a paramagnetic element with two stable oxidation states: 2+ and 3+. As mentioned above, both Fe2+ and Fe3+ establish coordination complexes with a great variety of ligands. Iron complexes display a variety of reduction potentials, ranging from very positive to negative values because of a basic concept in coordination chemistry that establishes that the ligand modifies the electron cloud surrounding the metal, thus modifying its reduction potential. This versatility in reduction potential allows for fine-tuning between iron reduction potential and the electron transfer process it catalyzes. It is estimated that the predominant reduction potential for iron in the intracellular milieu of the cell is near zero V (Clark, 1960; Wood, 1988). Many in vitro experiments confirm iron-mediated production of the hydroxyl radical (•OH), which arises from the following reactions:
The thermodynamic sum of reactions 1–3 gives reaction 4:
The intracellular environment provides abundant reducing power in the form of GSH (mM) and Asc (μM), which reduces Fe3+ to Fe2+:
Changes in free energy were calculated applying the equation δG = -nFE0 (Joule/mol), in which n is the number of electrons exchanged and F the Faraday constant. Reaction 1 values were from (Pierre and Fontecave, 1999); Reaction 2, the half-cell potential for H2O2 dismutation was considerer 0.45 V (Pierre and Fontecave, 1999) and the reduction potential of the Fe3+/Fe2+ half-cell was considered 0 V (Wood, 1988); Reaction 3 (Fenton reaction): E0 half-cell values from (Buettner, 1993; Buettner and Schafer, 2000). Half-cell potentials for reaction 5 were obtained from (Millis et al., 1993; Pierre and Fontecave, 1999). GSH: reduced glutathione; GSSG: oxidized glutathione; Asc: ascorbate; Asc•: ascorbate free radical.
The hydroxyl radical is considered one of the most reactive species generated in biological systems, since its reaction rate is only limited by diffusion, with rate constants in the 109–1012 Mol-1 s-1 range (Davies, 2005). This molecule induces irreversible damage to DNA, RNA, proteins, and lipids. Indeed, the hydroxyl radical is believed to be the etiological agent for several diseases and may be involved in the natural process of aging (Lipinski, 2011).
The main components of cell iron homeostasis are the divalent metal transporter 1 (DMT1), a Fe2+ transporter that brings iron into the cell, the transferrin receptor 1 (TfR1) that brings iron in through the endocytosis of Ferro-transferrin, the iron export transporter ferroportin 1 (FPN1) and the cytosolic iron storage protein ferritin. The expression of these proteins is transductionally regulated by the iron responsive element/iron regulatory protein (IRE/IRP) system, which is activated when cells have low iron levels, resulting in increased DMT1 and TfR1 levels and decreased FPN1 and ferritin expression (Muckenthaler et al., 2008).
In cells, iron in the 0.2–1.5 μM range is weakly complexed to low-molecular weight substrates such as citrate, carboxylates, amines, phosphate, nucleotides, GSH, and other molecules conforming the “cytosolic labile iron pool” (cLIP; Epsztejn et al., 1997; Kakhlon and Cabantchik, 2002; Petrat et al., 2002; Hider and Kong, 2011). Iron in this pool is redox-active, cycling between the Fe+2 and Fe+3 forms, with prevalence of the reduced form because of the reductive cytosol environment. This redox-active pool is suitable to experimental detection by the fluorophore calcein, which has higher affinity for Fe3+ than for Fe2+ but since the reduction potential for iron in the Fe-calcein complex is low, Fe3+ bound to calcein is readily reduced in the intracellular environment, resulting in decreased calcein fluorescence (Petrat et al., 2002). In cultured neuroblastoma cells the LIP represents about 3% of total cellular iron under basal culture conditions, but this percentage increases 3–4 fold, to μM concentrations, after exposure of cells to high extracellular iron concentrations (Núñez-Millacura et al., 2002; Núñez et al., 2004). In cell models, iron overload generates increased lipid peroxidation, protein modifications and damage to DNA, consistent with the production of the hydroxyl radical (Mello-Filho and Meneghini, 1991; Núñez et al., 2001; Sochaski et al., 2002; Zoccarato et al., 2005).
INFLAMMATORY CYTOKINES INDUCE THE PRODUCTION OF RNS, ROS AND IRON ACUMULATION
Postmortem tissues from patients with AD, PD, HD, ALS or FA show oxidative damage in the affected brain regions (Nunomura et al., 1999; Barnham et al., 2004; Emerit et al., 2004). The association between inflammation and oxidative damage is mediated by the release of RNS and ROS during the inflammatory process. In particular, activated microglia have high levels of nitric oxide synthase (NOS) and NADPH oxidase (NOX), two enzyme systems that mediate the increase in the oxidative tone induced by inflammation.
Microglia, the brain-resident immune cells, are essential for the generation of the inflammatory response. They are activated by distress signals released from neighboring cells, initiating an innate response characterized by the production of pro-inflammatory cytokines and, incidentally, phagocytosis (McGeer et al., 1988; Colton and Wilcock, 2010). Indeed, many cases of AD and PD are accompanied by a dramatic proliferation of reactive amoeboid macrophages and activated microglia in the substantia nigra (SN) or frontal cortex (McGeer et al., 1988; Possel et al., 2000; Kiyota et al., 2009; Hewett and Hewett, 2012), together with high expression of pro-inflammatory cytokines (Bauer et al., 1991; Mogi et al., 1994; Muller et al., 1998; Nagatsu, 2002; Hewett and Hewett, 2012).
Inducible NOS (iNOS, also called NOS-2), which is scarcely expressed in the brain is induced during gliosis in pathological situations including AD (Aliev et al., 2009) and PD (Dawson and Dawson, 1998). Up-regulation of iNOS and of cyclo-oxygenase-1 and cyclo-oxygenase-2 in amoeboid microglia occurs in the SN of human PD patients (Knott et al., 2000). A study on the levels of iNOS mRNA in postmortem PD basal ganglia found a significant increase in iNOS expression in the dorsal two-thirds of the striatum and in the medial medullary lamina of the globus pallidus, accompanied by a reduction in iNOS mRNA expression in the putamen (Eve et al., 1998).
Inflammatory mediators, including LPS and some cytokines (TNF-α, IL-1β, and IFN-γ) induce the transcriptional activation of the iNOS gene in astrocytes and microglia via activation of the transcription factors STAT1 and NF-kB (Grzybicki et al., 1996; Possel et al., 2000; Hewett and Hewett, 2012). These factors translocate to the nucleus and bind to response elements present in the iNOS coding sequence. Upregulation of microglial iNOS expression is also observed after administration of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP; Liberatore et al., 1999; Tieu et al., 2003; Kokovay and Cunningham, 2005; Yokoyama et al., 2008). Interestingly, administration of MPTP produces significantly less neuronal loss in mice deficient in iNOS compared to their wild type counterparts (Dexter et al., 1986; Liberatore et al., 1999; Dehmer et al., 2000). In the 6-hydroxidopamine (6-OHDA) model, iNOS activity in the striatum induces neurodegeneration in rats. Pretreatment with the iNOS inhibitor L-NAME blocks amphetamine-induced rotations and significantly restores striatal dopamine (DA) levels in 6-OHDA treated rats (Barthwal et al., 2001). In neuroinflammatory models of PD, iNOS also participates in nigral neurodegeneration. Injection of LPS induces iNOS expression in the SN in a time- and dose-dependent manner; iNOS is present mainly in fully activated microglia with the characteristic amoeboid morphology. Furthermore, LPS-induced loss of dopaminergic neurons decreases significantly by administration of an iNOS inhibitor (Arimoto and Bing, 2003; Singh et al., 2005).
The iNOS enzyme is a relevant factor in the neurodegenerative process associated to AD. Early observations reported increased iNOS and nitrotyrosine protein modifications in AD brains, mainly in neurofibrillary tangle-bearing neurons and neuropil threads as well as in astrocytes (Vodovotz et al., 1996; Smith et al., 1997; Wallace et al., 1997). Studies in transgenic mice overexpressing amyloid beta precursor protein (APP) demonstrated that several pathological changes such as vessel lesions, amyloid deposition and mitochondrial DNA deletions, are associated with the degree of NOS overexpression (Seyidova et al., 2004). Nevertheless, the APPsw/iNOS(-/-) mice, which express human APP mutations on an iNOS knockout background, show increased appearance of tau pathology, neuronal death, neuroinflammation and behavioral deficits compared with the parental APPsw mice (Colton et al., 2008). This evidence indicates that in AD, the production of NO can be protective or damaging, depending on the levels of NO production.
The phagocyte NOX is the main regulated source of ROS generation. The catalytic component of the NOX complex is composed by a family of multiple-pass transmembrane proteins, named NOX1–4. The most studied, NOX2, also known as gp91phox or phagocyte oxidase (PHOX), is highly expressed in innate immune cells including microglia and it is most likely the predominant NOX isoform expressed in astrocytes, while neurons express both NOX2 and NOX4 (Skalnik et al., 1991; Noh and Koh, 2000; Lavigne et al., 2001; Abramov et al., 2004; Pawate et al., 2004). NOX2 forms a complex with p67phox, p47phox, p40phox, and p22phox subunits. Several stimuli induce NOX2 complex priming, including pro-inflammatory cytokines (TNF-α, IL-1β) and Toll-like receptor (TLR) agonists like LPS, peroxynitrite and proteases. The primed NOX2 complex requires yet additional activation to initiate substantial ROS production. PKC activators, growth factors, complement protein C5a and G protein-coupled receptor agonists generate a fully active NOX complex (Yang et al., 2009,2013; Sareila et al., 2011).
Activation of NOX also occurs in experimental models of PD and AD. Treatment with MPTP results in increased synthesis of the proinflammatory cytokine IL-1β and increased membrane translocation of p67phox that is prevented by minocycline, a tetracycline derivative that exerts multiple anti-inflammatory effects (Wu et al., 2002). In addition, aging mice treated with MPTP display an increase in gp91phox and 3-nitrotyrosine (L’Episcopo et al., 2010; Huh et al., 2011). In agreement, gp91phox-/- mice display decreased degeneration of dopaminergic neurons induced by MPTP compared to wild type mice (Wu et al., 2003; Zhang et al., 2004). The unilateral injection of 6-OHDA into the right striatum of rats induces an increase of NOX1 and NOX2 both in the striatum and the SN. In concordance, dopaminergic neuronal and TNF-α and IFN-γ induction triggered by 6-OHDA are abrogated in the gp91phox-/- or minocycline treated mice (Hernandes et al., 2013). Additionally, striatal injection of 6-OHDA increases NOX1 expression in dopaminergic neurons in rat SN, and also increases 8-oxo-dG content, a marker of DNA oxidative damage. Moreover, NOX1 knockdown reduces 6-OHDA-induced oxidative DNA damage and dopaminergic neuronal degeneration (Choi et al., 2012).
Microglia of AD subjects display activated NOX2, resulting in the formation of ROS that are toxic to neighboring neurons (Shimohama et al., 2000). In conjunction, an increment in NOX1 and NOX3 mRNA levels in the frontal lobe tissue from AD brains was reported, suggesting the participation of other NOX family members in AD neuropathology (de la Monte and Wands, 2006). Recently, increased NOX-dependent ROS production in the superior/middle temporal gyri at the earliest clinical manifestations of disease, but not in late-stage AD, was reported (Bruce-Keller et al., 2010). Genetic inactivation of NOX2 in 12- to 15-month-old mice overexpressing the APPsw mutation (Tg2576 mice) results in reduced oxidative damage and rescues both the vascular and behavioral alterations observed in Tg2576 mice (Park et al., 2008). Studies done in cell cultures replicated the postmortem and animal findings on oxidative damage driven by NOX activation. Experiments using co-cultures of neuronal and glial cells found that Aβ acts preferentially on astrocytes but causes neuronal death (Abramov et al., 2004; Abramov and Duchen, 2005). The Aβ peptide causes transient increases in cytoplasmic calcium in astrocytes, associated with increased ROS generation, glutathione depletion and mitochondrial depolarization. Neuronal death after Aβ exposure was reduced both by NOX inhibitors and in the gp91phox knockout mice. These data are consistent with a sequence of events in which Aβ activates NOX in astrocytes by increasing cytoplasmic calcium, generating an oxidative burst that causes the death of neighboring neurons (Abramov et al., 2004; Abramov and Duchen, 2005; Park et al., 2008).
Inflammatory conditions such as those found in neurodegenerative diseases also affect iron homeostasis through transcriptional modification of iron transporters. In this context, the observation that the transcription factor NFκB induces DMT1 expression is highly relevant for understanding the relationship between inflammation and iron homeostasis (Paradkar and Roth, 2006). We recently reported that the pro-inflammatory cytokines TNF-α, IL-6 and the TLR4 agonist LPS directly regulate DMT1mRNA and protein levels and induce a transient decrease in FPN1 protein, thus generating an increment of iron content in neurons and microglia (Urrutia et al., 2013). Supporting the results described above, a recent study using primary cultures of ventral mesencephalic neurons demonstrated that TNF-α or IL-1β induce an increment in DMT1 and TfR1 protein levels, together with a reduction of FPN1 levels, resulting in an increase in ferrous iron influx and decreased iron efflux in neurons (Wang et al., 2013). These findings were replicated in systemic tissues. Treatment of mouse splenocyte with LPS down-regulates the expression of FPN1 through a signaling mechanism mediated by TLR4 (Yang et al., 2002). Moreover, stimulation of macrophage cell lines with IFN-γ, TNF-α or LPS results in increased IRE-binding activity of IRP1 and IRP2, and increased DMT1 mRNA expression (Mulero and Brock, 1999; Wardrop and Richardson, 2000; Ludwiczek et al., 2003; Wang et al., 2005).
Considering that NFκB activation takes place downstream of TNF-α, IL-1 and LPS signaling pathways (Teeuwsen et al., 1991; Rothwell and Luheshi, 2000; Hanke and Kielian, 2011), inflammatory stimuli may induce DMT1 expression via NFκB activation. Indeed, TNF-α was detected in glial cells in the SN of PD patients but not in control subjects, together with immunoreactivity for TNF-α receptors in dopaminergic neurons of both control and PD patients (Boka et al., 1994). These findings are suggestive of a circuit in which activation of nigral microglia results in TNF-α secretion, which might increase iron uptake by dopaminergic neuron via NF-κB-induced DMT1 expression. Indeed, an increase in the nuclear immunoreactivity of NFκB has been observed in PD brains or in animal models for this disease (Hunot et al., 1997), so it is possible that activation of NF-κB via inflammatory stimuli contributes to iron accumulation in PD. Accordingly, inflammation could induce the production of hydroxyl radical trough the activation of two parallel pathways: (i) through DMT1-mediated increase of intracellular iron levels and (ii) through increased hydrogen peroxide levels mediated by NOX activation.
A positive feedback loop can be established between ROS/RNS and inflammatory cytokines. ROS induce intracellular signaling pathways that result in the activation of transcriptional factors like NF-kB, AP-1 and Nrf-2, which regulate the expression of pro-inflammatory mediators such as Cox-2, MCP-1, IL-6, TNF-α, IL-1α, and IL-1β (Hensley et al., 2000; Thannickal and Fanburg, 2000; Ueda et al., 2002; Ridder and Schwaninger, 2009; Kitazawa et al., 2011; Guo et al., 2012; Kawamoto et al., 2012; Phani et al., 2012; Song et al., 2012; Zhang et al., 2012; Tobon-Velasco et al., 2013). These cytokines and chemokines, in turn, stimulate a cascade of events leading to increased oxidative stress via iNOS and NOX activation.
INFLAMMATORY CONDITIONS INDUCE MITOCHONDRIAL DYSFUNCTION
The study of the relationship between inflammation and mitochondrial activity in the CNS is incipient. Intrastriatal injection of LPS induces mitochondrial dysfunction, microgliosis, iron accumulation and progressive degeneration of the dopamine nigro-striatal system (Zhang et al., 2005; Hunter et al., 2007, 2008; Choi et al., 2009), as observed in PD pathology. Similarly, cytokines such as IL-1β decrease mitochondrial activity through the production of NO in cardiomyocytes (Tatsumi et al., 2000).
Several reports indicate that TLRs regulate mitochondrial activity. Activation of TLR3 results in reduction of mitochondrial oxygen consumption mediated by opening of the permeability transition pore (Djafarzadeh et al., 2011). In co-cultures of cortical neurons with microglial cells, the TLR4 agonist LPS promotes decreased oxygen consumption and oxidative stress, with the subsequent nigral dopaminergic neuronal death in a rat model of inflammation (Xie et al., 2004; Hunter et al., 2007). Although these studies strongly suggest a link between TLRs and mitochondria dysfunction, further studies should clarify the molecular mechanisms involved and its relevance to particular neurodegenerative processes.
The production of ROS and RNS affects mitochondrial activity through destabilization of the ISCs (Cassina and Radi, 1996; Brown and Borutaite, 2004). The free radical superoxide damages and/or oxidizes 4Fe-4S clusters, which results in the formation of the “null” 3Fe-4S center form (Flint et al., 1993; Hausladen and Fridovich, 1994; Gardner et al., 1995; Bouton et al., 1996). Additionally, NO reacts with 4Fe-4S clusters generating [(NO)2Fe(SR)2] type complexes that inactivate several mitochondrial iron–sulfur enzymes including proteins which compose the electron transport chain (Drapier, 1997; see below). The above data are consistent with the notion that inflammation, ROS/RNS production, and mitochondrial dysfunction are linked processes.
Additionally, recent evidence shows that under certain conditions mitochondria can modulate the immune response. The mitochondrial protein MARCH5 (an ubiquitin E3 ligase constitutively expressed in the mitochondrion outer membrane) positively regulates TLR7 and TLR4 signaling, resulting in NFκB activation and expression of the NFκB-responsive genes IL-6 and TNF-α (Shi et al., 2011). In addition, activation of TLR1, TLR2 and TLR4 results in augmented mitochondrial ROS production by inducing translocation to mitochondria of TRAF6 (TLR signaling adaptor, tumor necrosis factor receptor-associated factor 6), which leads to the engagement and ubiquitination of ECSIT (evolutionarily conserved signaling intermediate in Toll pathways), a protein required for efficient assembly of mitochondrial complex I (West et al., 2011). It remains to be demonstrated whether this mechanism is operative in CNS cells.
Interestingly, mitochondrial ROS (mtROS) could arguably activate the inflammatory response. In vascular endothelium, mtROS act as intermediate signaling molecules to trigger production of IL-6 (Lee et al., 2010). In addition, patients with the autoinflammatory disorder TRAPS (tumor necrosis factor receptor-associated periodic syndrome), exhibit altered mitochondrial function with enhanced mtROS generation and increased production of IL-6, TNFα, and IL-1β; decreasing mtROS levels by the general antioxidant N-acetylcysteine effectively reduces inflammatory cytokine production after LPS stimulation (Bulua et al., 2011). These results point to novel pathways that link inflammation to mtROS production.
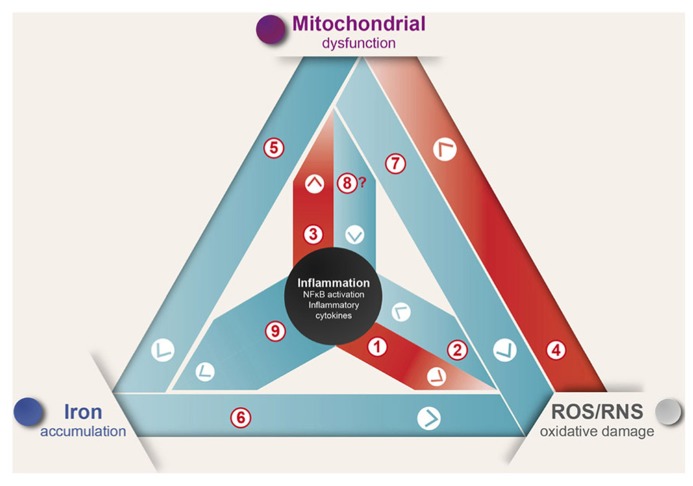
In summary, inflammation induces ROS production and mitochondrial dysfunction generating a self-feeding cycle that could lead to neurodegeneration in diseases where inflammation and oxidative damage are prevalent (Figure 1). In this cycle, [1] inflammation induces ROS and RNS generation by activation of the NOX and iNOS enzymes (Possel et al., 2000; Sareila et al., 2011; Hewett and Hewett, 2012); [2] in turn, ROS/RNS induce the expression of inflammatory cytokines (Baeuerle and Henkel, 1994; Sen and Packer, 1996). [3] Additionally, inflammation induces mitochondrial dysfunction through activation of TLR signaling (Xie et al., 2004; Djafarzadeh et al., 2011). [4] ROS in turn induce mitochondrial dysfunction by destabilizing ISCs, which results in the inactivation of several mitochondrial iron–sulfur enzymes (Cassina and Radi, 1996; Brown and Borutaite, 2004). [5] Mitochondrial dysfunction leads to IRP1 activation and increased iron uptake (Lee et al., 2009; Mena et al., 2011). [6] Iron increases oxidative damage by transforming mild oxidative molecules like superoxide and hydrogen peroxide into the hydroxyl radical (Graf et al., 1984). [7] Electron transport chain inhibition increases ROS production by electron leak (Drose and Brandt, 2012), and arguably could modulate the innate immune response by TLR signaling regulation (Shi et al., 2011) [8]. Finally, [9] inflammation is likely to cause iron accumulation through induction of DMT1 expression and transient ferroportin decrease (Urrutia et al., 2013; Wang et al., 2013).
FIGURE 1.
Inflammation causes ROS/RNS production, mitochondrial dysfunction, and iron accumulation. Inflammation, oxidative damage, and mitochondrial dysfunction are common features of neurodegenerative diseases. A complex net of relationships connect these features, which through feedback mechanisms contribute to the evolvement of neuronal death (see text for details).
MITOCHONDRIAL DYSFUNCTION, INFLAMMATION AND IRON ACCUMULATION IN THE DEATH OF NEURONS IN PD
Mitochondria have a key role in iron metabolism in association with the synthesis of ISCs and heme, prosthetic groups that are vital for cell function. Iron complexes are particularly relevant components of the electron transport chain: 12 proteins contain ISCs and eight proteins contain heme in their active centers (Rouault and Tong, 2005). Other proteins that have ISCs are the Krebs cycle enzymes aconitase and succinate dehydrogenase, ribonucleotide reductase, an enzyme that catalyzes the formation of deoxyribonucleotides from ribonucleotides, and ferrochelatase, involved in the addition of Fe to porphyrin IX during heme synthesis. We refer the reader to http://www.nlm.nih.gov/cgi/mesh/2011/MB_cgi?mode=&term=Iron–Sulfur+Proteins for a comprehensive listing of ISC-containing proteins. Particular attention should be given to cytoplasmic IRP1, which contain a 4Fe-4S cluster in its inactive form and becomes active in the clusterless form (Haile et al., 1992; Shand and Volz, 2013).
Mitochondria have a redox-active iron pool (Petrat et al., 2001); an increase in this pool directly associates with an increase in oxidative damage and with calcium-dependent changes in the mitochondrial permeability transition pore (Pelizzoni et al., 2011; Kumfu et al., 2012; Zhang and Lemasters, 2013). Thus, cells must regulate tightly their mitochondrial Fe levels because an iron shortage affects numerous processes that have iron as a co-factor, including the electron transport chain, whereas an excess of redox-active iron promotes the generation of the noxious hydroxyl radical. How mitochondria regulate their iron content and what, if any, is the interplay between cytoplasmic and mitochondrial iron are incipient but highly relevant subjects to understand the mechanisms of mitochondrial dysfunction in neurodegenerative diseases.
There is increasing evidence that mitochondrial dysfunction plays an important role in the development of neurodegenerative diseases such as AD, HD, FA, and PD (Enns, 2003; Mandemakers et al., 2007; Sas et al., 2007; Gogvadze et al., 2009; Jellinger, 2009). Imbalances in ROS and ATP levels derived from mitochondrial dysfunction affect neurons particularly, given their dependence on ATP to propagate electrical signals, maintain ionic gradients, and facilitate anterograde and retrograde transport along axons (Su et al., 2013). The involvement of mitochondrial dysfunction in the pathophysiology of PD was noted very early in the study of the disease. Evidence of mitochondrial dysfunction in PD began in the eighties, when, after an intravenous injection of illicit drugs, four college students developed marked Parkinsonism. Analysis of the substances injected revealed the presence of MPTP, a compound metabolized by astrocytes into 1-methyl-4-phenylpyridinium (MPP+), which is then released into the extracellular space. MPP+ is taken up selectively by dopaminergic (DA) neurons where it inhibits mitochondrial complex I (Heikkila et al., 1984; Langston et al., 1984; Nicklas et al., 1985; Gautier et al., 2013). Further evidence showed that complex I activity and the number of complex I subunits are decreased in postmortem tissue of idiopathic PD patients (Bindoff et al., 1989; Mizuno et al., 1989; Schapira et al., 1989). These results strongly suggest that mitochondrial dysfunction is a pathognomonic sign in the pathophysiology of PD. Reduced complex-I activity and an increased susceptibility to MPP+ were also observed in cybrids containing mitochondrial DNA from PD patients (Swerdlow et al., 1996, 2001; Gu et al., 1998a), suggesting the presence of mitochondrial DNA-encoded defects in PD (Chaturvedi and Flint Beal, 2013). Additionally, in the epidemiology field, the use in farming of the highly lipophilic pesticide rotenone, a potent inhibitor of mitochondrial complex I, has been linked to a higher incidence of PD in agricultural workers (Betarbet et al., 2000; Tanner et al., 2011; Pezzoli and Cereda, 2013).
Mitochondrial complex I is a major source of ROS. Complex I from mitochondria of PD patients contain 47% more protein carbonyls localized to catalytic subunits and a 34% decrease in complex I 8-kDa subunit. NADH-driven electron transfer rates through complex I inversely correlate with complex I protein oxidation status and with the reduction in the 8-kDa subunit protein levels (Keeney et al., 2006).
Knowledge on the mechanisms that associate mitochondrial dysfunction and iron dyshomeostasis in PD is incipient. Treatment of SH-SY5Y dopaminergic neuroblastoma cells with mitochondrial complex I inhibitors such as rotenone or MPP+ results in ROS production and increased mitochondrial iron uptake (Lee et al., 2009; Mena et al., 2011). Moreover, inhibition of complex I by rotenone decreases the activity of three ISC-containing enzymes: mitochondrial and cytoplasmic aconitases and xanthine oxidase, and decreases the ISC content of glutamine phosphoribosyl pyrophosphate amidotransferase (Mena et al., 2011). The reduction in cytoplasmic aconitase activity is associated with an increase in iron regulatory IRP1 mRNA binding activity and with an increase in the mitochondrial labile iron pool (Mena et al., 2011). Since IRP1 activity post-transcriptionally regulates the expression of iron import proteins, ISC synthesis inhibition may result in a false iron deficiency signal with the ensuing iron accumulation.
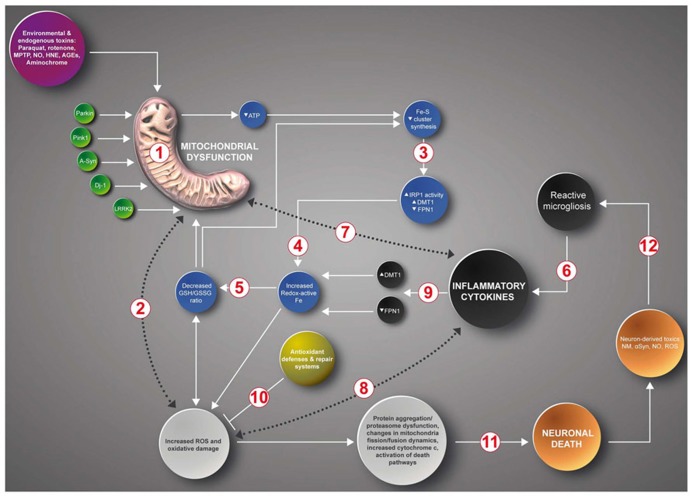
Considering the evidence discussed, we propose that inhibition of mitochondrial complex I by endogenous and/or exogenous toxins or by inflammatory processes resulting from trauma or other causes, engage a vicious cycle of increased oxidative stress and increased iron accumulation (Figure 2). In this scheme, inhibition of mitochondrial complex I by endogenous or exogenous toxins, or because of mutations in PD genes Parkin, Pink 1, alpha-synuclein, DJ-1 or LRRK2 (Langston and Ballard, 1983; Schapira et al., 1990; Hsu et al., 2000; Silvestri et al., 2005; Martin et al., 2006; Junn et al., 2009; Angeles et al., 2011; Mena et al., 2011), results in decreased electron transport chain activity [1] and the ensuing ATP synthesis decrease and ROS increase [2]. Decreased ATP levels impairs ISC synthesis that results in decreased activity of ISC-containing proteins and increased mRNA binding activity of the iron homeostasis protein IRP1. IRP1 activation leads to increased DMT1 and TfR1 expression (Lee et al., 2009; Mena et al., 2011) [3] and the ensuing iron accumulation (Asenjo, 1968; Dexter et al., 1987; Faucheux et al., 2003; Michaeli et al., 2007) [4]. Increased ROS and increased redox-active iron promotes the consumption of intracellular reductants such as GSH and ascorbate (Perry et al., 1982; Ehrhart and Zeevalk, 2003; Núñez et al., 2004; Jomova et al., 2010) [5], resulting in a further decrease in mitochondrial activity and ISC synthesis (Harley et al., 1993; Gu et al., 1998b; Jha et al., 2000; Chinta et al., 2007; Danielson et al., 2011). Another input to this cycle is contributed by inflammatory cytokines liberated by activated microglia and astrocytes (Mogi et al., 1994) [6], which enhance mitochondrial dysfunction (Tatsumi et al., 2000; Xie et al., 2004; Hunter et al., 2007; Djafarzadeh et al., 2011) [7], increase ROS production (Grzybicki et al., 1996) [8] and increase iron accumulation by modifying the expression of the iron transporters DMT1 and FPN1 (Urrutia et al., 2013; Wang et al., 2013) [9]. As discussed in the text, increased ROS back-feed the production of cytokines. Increased ROS levels, in particular increased hydroxyl radical generation, produces increased oxidative damage, which is counteracted by antioxidant defenses [10]. In time, the positive feedback loop of mitochondrial dysfunction, iron dyshomeostasis and inflammation results in alpha-synuclein aggregation, proteasomal dysfunction, changes in mitochondrial fission/fusion dynamics, opening of the mitochondrion PTP, increased cytoplasmic cytochrome c and activation of death pathways [11]. Debris and toxins from dying neurons enhance the activation of glial cells, which contributes to the inflammatory network (Zecca et al., 2008; Hirsch and Hunot, 2009; Gao et al., 2011) [12].
FIGURE 2.
A positive feedback loop in the death of neurons in PD. Inhibition of mitochondrial complex I by endogenous or exogenous toxins or mutations in PD genes Parkin, Pink 1, Alpha-synuclein, DJ-1 or LRRK2 generates a multifactorial positive feedback loop. In this loop, complex I inhibition results in iron accumulation driven by decreased Fe-S cluster synthesis, IRP1 activation, increased DMT1 and TfR1 expression and decreased FPN1 expression, increased ROS levels and decreased glutathione levels. Both increased oxidative stress and low GSH levels further inhibit complex I activity. Another input to this cycle is contributed by inflammatory cytokines that through self-feeding cycles induce mitochondrial dysfunction, increased ROS/RNS production and iron accumulation mediated by the transcriptional regulation of DMT1 and FPN1 (see text). The cumulative oxidative damage finally results in apoptotic death (see text for details).
In summary, because of the innate interconnectivity of mitochondrial complex I dysfunction, iron accumulation, oxidative stress, and inflammation, probably the initiation of any one of these factors will induce or enhance the others through the generation of a positive feedback loop that in time will end in apoptotic neuronal death. Still unanswered is the question of why neurons of the SNc are so particularly prone to carry-on this cycle. On examination of this cycle, several therapeutic targets come to mind. Its intervention should result in prolonged life of the affected neurons.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was financed by grant 1130068 from FONDECYT, and grant ACT1114 from PIA-CONICYT.
REFERENCES
- Abramov A. Y., Canevari L., Duchen M. R. (2004). Calcium signals induced by amyloid beta peptide and their consequences in neurons and astrocytes in culture. Biochim. Biophys. Acta 1742 81–87 10.1016/j.bbamcr.2004.09.006 [DOI] [PubMed] [Google Scholar]
- Abramov A. Y., Duchen M. R. (2005). The role of an astrocytic NADPH oxidase in the neurotoxicity of amyloid beta peptides. Philos. Trans. R. Soc. Lond. B Biol. Sci. 360 2309–2314 10.1098/rstb.2005.1766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliev G., Palacios H. H., Lipsitt A. E., Fischbach K., Lamb B. T., Obrenovich M. E., et al. (2009). Nitric oxide as an initiator of brain lesions during the development of Alzheimer disease. Neurotox. Res. 16 293–305 10.1007/s12640-009-9066-5 [DOI] [PubMed] [Google Scholar]
- Andersen H. H., Johnsen K. B., Moos T. (2013). Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell. Mol. Life Sci. 10.1007/s00018-013-1509-8 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angeles D. C., Gan B. H., Onstead L., Zhao Y., Lim K. L., Dachsel J., et al. (2011). Mutations in LRRK2 increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Hum. Mutat. 32 1390–1397 10.1002/humu.21582 [DOI] [PubMed] [Google Scholar]
- Arimoto T., Bing G. (2003). Up-regulation of inducible nitric oxide synthase in the substantia nigra by lipopolysaccharide causes microglial activation and neurodegeneration. Neurobiol. Dis. 12 35–45 10.1016/S0969-9961(02)00017-7 [DOI] [PubMed] [Google Scholar]
- Asenjo A. (1968). Cytosiderosis and iron deposits in ventrolateral nucleus of the thalamus in Parkinson’s disease. Clinical and experimental study. Johns Hopkins Med. J. 122 284–294 [PubMed] [Google Scholar]
- Baeuerle P. A., Henkel T. (1994). Function and activation of NF-kappa B in the immune system. Annu. Rev. Immunol. 12 141–179 10.1146/annurev.iy.12.040194.001041 [DOI] [PubMed] [Google Scholar]
- Barnham K. J., Masters C. L., Bush A. I. (2004). Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 3 205–214 10.1038/nrd1330 [DOI] [PubMed] [Google Scholar]
- Barthwal M. K., Srivastava N., Dikshit M. (2001). Role of nitric oxide in a progressive neurodegeneration model of Parkinson’s disease in the rat. Redox Rep. 6 297–302 10.1179/135100001101536436 [DOI] [PubMed] [Google Scholar]
- Bauer J., Strauss S., Schreiter-Gasser U., Ganter U., Schlegel P., Witt I., et al. (1991). Interleukin-6 and alpha-2-macroglobulin indicate an acute-phase state in Alzheimer’s disease cortices. FEBS Lett. 285 111–114 10.1016/0014-5793(91)80737-N [DOI] [PubMed] [Google Scholar]
- Betarbet R., Sherer T. B., MacKenzie G., Garcia-Osuna M., Panov A. V., Greenamyre J. T. (2000). Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat. Neurosci. 3 1301–1306 10.1038/81834 [DOI] [PubMed] [Google Scholar]
- Bindoff L. A., Birch-Machin M., Cartlidge N. E., Parker W. D., Jr., Turnbull D. M. (1989). Mitochondrial function in Parkinson’s disease. Lancet 2 49 10.1016/S0140-6736(89)90291-2 [DOI] [PubMed] [Google Scholar]
- Boka G., Anglade P., Wallach D., Javoy-Agid F., Agid Y., Hirsch E. C. (1994). Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson’s disease. Neurosci. Lett. 172 151–154 10.1016/0304-3940(94)90684-X [DOI] [PubMed] [Google Scholar]
- Bouton C., Raveau M., Drapier J. C. (1996). Modulation of iron regulatory protein functions. Further insights into the role of nitrogen- and oxygen-derived reactive species. J. Biol. Chem. 271 2300–2306 10.1074/jbc.271.4.2300 [DOI] [PubMed] [Google Scholar]
- Brown G. C., Borutaite V. (2004). Inhibition of mitochondrial respiratory complex I by nitric oxide, peroxynitrite and S-nitrosothiols. Biochim. Biophys. Acta 1658 44–49 10.1016/j.bbabio.2004.03.016 [DOI] [PubMed] [Google Scholar]
- Bruce-Keller A. J., Gupta S., Parrino T. E., Knight A. G., Ebenezer P. J., Weidner A. M., et al. (2010). NOX activity is increased in mild cognitive impairment. Antioxid. Redox Signal. 12 1371–1382 10.1089/ars.2009.2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner G. R. (1993). The pecking order of free radicals and antioxidants: lipid peroxidation, alpha-tocopherol, and ascorbate. Arch. Biochem. Biophys. 300 535–543 10.1006/abbi.1993.1074 [DOI] [PubMed] [Google Scholar]
- Buettner G. R., Schafer F. Q. (2000). Free radicals, oxidants, and antioxidants. Teratology 62 234 [DOI] [PubMed] [Google Scholar]
- Bulua A. C., Simon A., Maddipati R., Pelletier M., Park H., Kim K. Y., et al. (2011). Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in TNFR1-associated periodic syndrome (TRAPS). J. Exp. Med. 208 519–533 10.1084/jem.20102049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassina A., Radi R. (1996). Differential inhibitory action of nitric oxide and peroxynitrite on mitochondrial electron transport. Arch. Biochem. Biophys. 328 309–316 10.1006/abbi.1996.0178 [DOI] [PubMed] [Google Scholar]
- Chaturvedi R. K, Flint Beal M. (2013). Mitochondrial diseases of the brain. Free Radic. Biol. Med. 63 1–29 10.1016/j.freeradbiomed.2013.03.018 [DOI] [PubMed] [Google Scholar]
- Chinta S. J., Kumar M. J., Hsu M., Rajagopalan S., Kaur D., Rane A., et al. (2007). Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J. Neurosci. 27 13997–14006 10.1523/JNEUROSCI.3885-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. H., Cristovao A. C., Guhathakurta S., Lee J., Joh T. H., Beal M. F., et al. (2012). NADPH oxidase 1-mediated oxidative stress leads to dopamine neuron death in Parkinson’s disease. Antioxid. Redox Signal. 16 1033–1045 10.1089/ars.2011.3960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi D. Y., Liu M., Hunter R. L., Cass W. A., Pandya J. D., Sullivan P. G., et al. (2009). Striatal neuroinflammation promotes Parkinsonism in rats. PLoS ONE 4:e5482 10.1371/journal.pone.0005482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. M. (1960). Oxidation-Reduction Potentials of Organic Systems. Baltimore: Williams & Wilkins [Google Scholar]
- Colton C., Wilcock D. M. (2010). Assessing activation states in microglia. CNS Neurol. Disord. Drug Targets 9 174–191 10.2174/187152710791012053 [DOI] [PubMed] [Google Scholar]
- Colton C. A., Wilcock D. M., Wink D. A., Davis J., Van Nostrand W. E., Vitek M. P. (2008). The effects of NOS2 gene deletion on mice expressing mutated human AbetaPP. J. Alzheimers Dis. 15 571–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson S. R., Held J. M., Oo M., Riley R., Gibson B. W., Andersen J. K. (2011). Quantitative mapping of reversible mitochondrial Complex I cysteine oxidation in a Parkinson disease mouse model. J. Biol. Chem. 286 7601–7608 10.1074/jbc.M110.190108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies M. J. (2005). The oxidative environment and protein damage. Biochim. Biophys. Acta 1703 93–109 10.1016/j.bbapap.2004.08.007 [DOI] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M. (1998). Nitric oxide in neurodegeneration. Prog. Brain Res. 118 215–229 10.1016/S0079-6123(08)63210-0 [DOI] [PubMed] [Google Scholar]
- Dehmer T., Lindenau J., Haid S., Dichgans J., Schulz J. B. (2000). Deficiency of inducible nitric oxide synthase protects against MPTP toxicity in vivo. J. Neurochem. 74 2213–2216 10.1046/j.1471-4159.2000.0742213.x [DOI] [PubMed] [Google Scholar]
- de la Monte S. M., Wands J. R. (2006). Molecular indices of oxidative stress and mitochondrial dysfunction occur early and often progress with severity of Alzheimer’s disease. J. Alzheimers Dis. 9 167–181 [DOI] [PubMed] [Google Scholar]
- Delatycki M. B., Camakaris J., Brooks H., Evans-Whipp T., Thorburn D. R., Williamson R., et al. (1999). Direct evidence that mitochondrial iron accumulation occurs in Friedreich ataxia. Ann. Neurol. 45 673–675 [DOI] [PubMed] [Google Scholar]
- Dexter D., Carter C., Agid F., Agid Y., Lees A. J., Jenner P., et al. (1986). Lipid peroxidation as cause of nigral cell death in Parkinson’s disease. Lancet 2 639–640 10.1016/S0140-6736(86)92471-2 [DOI] [PubMed] [Google Scholar]
- Dexter D. T., Wells F. R., Agid F., Agid Y., Lees A. J., Jenner P., et al. (1987). Increased nigral iron content in postmortem parkinsonian brain. Lancet 2 1219–1220 10.1016/S0140-6736(87)91361-4 [DOI] [PubMed] [Google Scholar]
- Djafarzadeh S., Vuda M., Takala J., Ochs M., Jakob S. M. (2011). Toll-like receptor-3-induced mitochondrial dysfunction in cultured human hepatocytes. Mitochondrion 11 83–88 10.1016/j.mito.2010.07.010 [DOI] [PubMed] [Google Scholar]
- Drapier J. C. (1997). Interplay between NO and [Fe-S] clusters: relevance to biological systems. Methods 11 319–329 10.1006/meth.1996.0426 [DOI] [PubMed] [Google Scholar]
- Drose S., Brandt U. (2012). Molecular mechanisms of superoxide production by the mitochondrial respiratory chain. Adv. Exp. Med. Biol. 748 145–169 10.1007/978-1-4614-3573-0_6 [DOI] [PubMed] [Google Scholar]
- Ehrhart J., Zeevalk G. D. (2003). Cooperative interaction between ascorbate and glutathione during mitochondrial impairment in mesencephalic cultures. J. Neurochem. 86 1487–1497 10.1046/j.1471-4159.2003.01954.x [DOI] [PubMed] [Google Scholar]
- Emerit J., Edeas M., Bricaire F. (2004). Neurodegenerative diseases and oxidative stress. Biomed. Pharmacother. 58: 39–46 10.1016/j.biopha.2003.11.004 [DOI] [PubMed] [Google Scholar]
- Enns G. M. (2003). The contribution of mitochondria to common disorders. Mol. Genet. Metab. 80 11–26 10.1016/j.ymgme.2003.08.009 [DOI] [PubMed] [Google Scholar]
- Epsztejn S., Kakhlon O., Glickstein H., Breuer W., Cabantchik I. (1997). Fluorescence analysis of the labile iron pool of mammalian cells. Anal. Biochem. 248 31–40 10.1006/abio.1997.2126 [DOI] [PubMed] [Google Scholar]
- Eve D. J., Nisbet A. P., Kingsbury A. E., Hewson E. L., Daniel S. E., Lees A. J., et al. (1998). Basal ganglia neuronal nitric oxide synthase mRNA expression in Parkinson’s disease. Brain Res. Mol. Brain Res. 63 62–71 10.1016/S0169-328X(98)00259-9 [DOI] [PubMed] [Google Scholar]
- Faucheux B. A., Martin M. E., Beaumont C., Hauw J. J., Agid Y., Hirsch E. C. (2003). Neuromelanin associated redox-active iron is increased in the substantia nigra of patients with Parkinson’s disease. J. Neurochem. 86 1142–1148 10.1046/j.1471-4159.2003.01923.x [DOI] [PubMed] [Google Scholar]
- Flint D. H., Tuminello J. F., Emptage M. H. (1993). The inactivation of Fe-S cluster containing hydro-lyases by superoxide. J. Biol. Chem. 268 22369–22376 [PubMed] [Google Scholar]
- Gao H. M., Zhang F., Zhou H., Kam W., Wilson B., Hong J. S. (2011). Neuroinflammation and alpha-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ. Health Perspect. 119 807–814 10.1289/ehp.1003013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner P. R., Raineri I., Epstein L. B., White C. W. (1995). Superoxide radical and iron modulate aconitase activity in mammalian cells. J. Biol. Chem. 270 13399–13405 10.1074/jbc.270.22.13399 [DOI] [PubMed] [Google Scholar]
- Gautier C. A., Corti O., Brice A. (2013). Mitochondrial dysfunctions in Parkinson’s disease. Rev. Neurol. (Paris) 10.1016/j.neurol.2013.06.003 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Gogvadze V., Orrenius S., Zhivotovsky B. (2009). Mitochondria as targets for chemotherapy. Apoptosis 14 624–640 10.1007/s10495-009-0323-0 [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. (1984). Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J. Biol. Chem. 259 3620–3624 [PubMed] [Google Scholar]
- Grubman A., White A. R., Liddell J. R. (2013). Mitochondrial metals as a potential therapeutic target in neurodegeneration. Br. J. Pharmacol. 10.1111/bph.12513 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzybicki D., Gebhart G. F., Murphy S. (1996). Expression of nitric oxide synthase type II in the spinal cord under conditions producing thermal hyperalgesia. J. Chem. Neuroanat. 10 221–229 10.1016/0891-0618(96)00139-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M., Cooper J. M., Taanman J. W., Schapira A. H. (1998a). Mitochondrial DNA transmission of the mitochondrial defect in Parkinson’s disease. Ann. Neurol. 44 177–186 10.1002/ana.410440207 [DOI] [PubMed] [Google Scholar]
- Gu M., Owen A. D., Toffa S. E., Cooper J. M., Dexter D. T., Jenner P., et al. (1998b). Mitochondrial function, GSH and iron in neurodegeneration and Lewy body diseases. J. Neurol. Sci. 158 24–29 10.1016/S0022-510X(98)00095-1 [DOI] [PubMed] [Google Scholar]
- Guo R. B., Wang G. F., Zhao A. P., Gu J., Sun X. L., Hu G. (2012). Paeoniflorin protects against ischemia-induced brain damages in rats via inhibiting MAPKs/NF-kappaB-mediated inflammatory responses. PLoS ONE 7:e49701 10.1371/journal.pone.0049701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Tang C. K., Chin J., Harford J. B., Klausner R. D. (1992). Reciprocal control of RNA-binding and aconitase activity in the regulation of the iron-responsive element binding protein: role of the iron-sulfur cluster. Proc. Natl. Acad. Sci. U.S.A. 89 7536–7540 10.1073/pnas.89.16.7536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke M. L., Kielian T. (2011). Toll-like receptors in health and disease in the brain: mechanisms and therapeutic potential. Clin. Sci. 121 367–387 10.1042/CS20110164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley A., Cooper J. M., Schapira A. H. (1993). Iron induced oxidative stress and mitochondrial dysfunction: relevance to Parkinson’s disease. Brain Res. 627 349–353 10.1016/0006-8993(93)90341-J [DOI] [PubMed] [Google Scholar]
- Hausladen A., Fridovich I. (1994). Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. J. Biol. Chem. 269 29405–29408 [PubMed] [Google Scholar]
- Heikkila R. E., Hess A., Duvoisin R. C. (1984). Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine in mice. Science 224 1451–1453 10.1126/science.6610213 [DOI] [PubMed] [Google Scholar]
- Hensley K., Robinson K. A., Gabbita S. P., Salsman S., Floyd R. A. (2000). Reactive oxygen species, cell signaling, and cell injury. Free Radic. Biol. Med. 28 1456–1462 10.1016/S0891-5849(00)00252-5 [DOI] [PubMed] [Google Scholar]
- Hernandes M. S., Santos G. D., Cafe-Mendes C. C., Lima L. S., Scavone C., Munhoz C. D., et al. (2013). Microglial cells are involved in the susceptibility of NADPH oxidase knockout mice to 6-hydroxy-dopamine-induced neurodegeneration. PLoS ONE 8:e75532 10.1371/journal.pone.0075532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewett J. A., Hewett S. J. (2012). Induction of nitric oxide synthase-2 expression and measurement of nitric oxide production in enriched primary cortical astrocyte cultures. Methods Mol. Biol. 814 251–263 10.1007/978-1-61779-452-0_17 [DOI] [PubMed] [Google Scholar]
- Hider R. C., Kong X. L. (2011). Glutathione: a key component of the cytoplasmic labile iron pool. Biometals 24 1179–1187 10.1007/s10534-011-9476-8 [DOI] [PubMed] [Google Scholar]
- Hirsch E. C., Hunot S. (2009). Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 8 382–397 10.1016/S1474-4422(09)70062-6 [DOI] [PubMed] [Google Scholar]
- Hsu L. J., Sagara Y., Arroyo A., Rockenstein E., Sisk A., Mallory M., et al. (2000). alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 157 401–410 10.1016/S0002-9440(10)64553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. L., Becker E. M., Whitnall M., Suryo Rahmanto Y., Ponka P., Richardson D. R. (2009). Elucidation of the mechanism of mitochondrial iron loading in Friedreich’s ataxia by analysis of a mouse mutant. Proc. Natl. Acad. Sci. U.S.A. 106 16381–16386 10.1073/pnas.0906784106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh S. H., Chung Y. C., Piao Y., Jin M. Y., Son H. J., Yoon N. S., et al. (2011). Ethyl pyruvate rescues nigrostriatal dopaminergic neurons by regulating glial activation in a mouse model of Parkinson’s disease. J. Immunol. 187 960–969 10.4049/jimmunol.1100009 [DOI] [PubMed] [Google Scholar]
- Hunot S., Brugg B., Ricard D., Michel P. P., Muriel M. P., Ruberg M., et al. (1997). Nuclear translocation of NF-kappaB is increased in dopaminergic neurons of patients with parkinson disease. Proc. Natl. Acad. Sci. U.S.A. 94 7531–7536 10.1073/pnas.94.14.7531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R. L., Dragicevic N., Seifert K., Choi D. Y., Liu M., Kim H. C., et al. (2007). Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J. Neurochem. 100 1375–1386 10.1111/j.1471-4159.2006.04327.x [DOI] [PubMed] [Google Scholar]
- Hunter R. L., Liu M., Choi D. Y., Cass W. A., Bing G. (2008). Inflammation and age-related iron accumulation in F344 rats. Curr. Aging Sci. 1 112–121 10.2174/1874609810801020112 [DOI] [PubMed] [Google Scholar]
- Jellinger K. A. (2009). Recent advances in our understanding of neurodegeneration. J. Neural Transm. 116 1111–1162 10.1007/s00702-009-0240-y [DOI] [PubMed] [Google Scholar]
- Jha N., Jurma O., Lalli G., Liu Y., Pettus E. H., Greenamyre J. T., et al. (2000). Glutathione depletion in PC12 results in selective inhibition of mitochondrial complex I activity. Implications for Parkinson’s disease. J. Biol. Chem. 275 26096–26101 10.1074/jbc.M000120200 [DOI] [PubMed] [Google Scholar]
- Jomova K., Vondrakova D., Lawson M., Valko M. (2010). Metals, oxidative stress and neurodegenerative disorders. Mol. Cell. Biochem. 345 91–104 10.1007/s11010-010-0563-x [DOI] [PubMed] [Google Scholar]
- Junn E., Jang W. H., Zhao X., Jeong B. S., Mouradian M. M. (2009). Mitochondrial localization of DJ-1 leads to enhanced neuroprotection. J. Neurosci. Res. 87 123–129 10.1002/jnr.21831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakhlon O., Cabantchik Z. I. (2002). The labile iron pool: characterization, measurement, and participation in cellular processes(1). Free Radic. Biol. Med. 33 1037–1046 10.1016/S0891-5849(02)01006-7 [DOI] [PubMed] [Google Scholar]
- Kawamoto E. M., Lima L. S., Munhoz C. D., Yshii L. M., Kinoshita P. F., Amara F. G., et al. (2012). Influence of N-methyl-D-aspartate receptors on ouabain activation of nuclear factor-kappaB in the rat hippocampus. J. Neurosci. Res. 90 213–228 10.1002/jnr.22745 [DOI] [PubMed] [Google Scholar]
- Keeney P. M., Xie J., Capaldi R. A., Bennett J. P. , Jr (2006). Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J. Neurosci. 26 5256–5264 10.1523/JNEUROSCI.0984-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa M., Cheng D., Tsukamoto M. R., Koike M. A., Wes P. D., Vasilevko V., et al. (2011). Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal beta-catenin pathway function in an Alzheimer’s disease model. J. Immunol. 187 6539–6549 10.4049/jimmunol.1100620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T., Yamamoto M., Xiong H., Lambert M. P., Klein W. L., Gendelman H. E., et al. (2009). CCL2 accelerates microglia-mediated Abeta oligomer formation and progression of neurocognitive dysfunction. PLoS ONE 4:e6197 10.1371/journal.pone.0006197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knott C., Stern G., Wilkin G. P. (2000). Inflammatory regulators in Parkinson’s disease: iNOS, lipocortin-1, and cyclooxygenases-1 and -2. Mol. Cell. Neurosci. 16 724–739 10.1006/mcne.2000.0914 [DOI] [PubMed] [Google Scholar]
- Kokovay E., Cunningham L. A. (2005). Bone marrow-derived microglia contribute to the neuroinflammatory response and express iNOS in the MPTP mouse model of Parkinson’s disease. Neurobiol. Dis. 19 471–478 10.1016/j.nbd.2005.01.023 [DOI] [PubMed] [Google Scholar]
- Kumfu S., Chattipakorn S., Fucharoen S., Chattipakorn N. (2012). Mitochondrial calcium uniporter blocker prevents cardiac mitochondrial dysfunction induced by iron overload in thalassemic mice. Biometals 25 1167–1175 10.1007/s10534-012-9579-x [DOI] [PubMed] [Google Scholar]
- Langston J. W., Ballard P. A. , Jr (1983). Parkinson’s disease in a chemist working with 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. N. Engl. J. Med. 309 310 10.1056/NEJM198308043090511 [DOI] [PubMed] [Google Scholar]
- Langston J. W., Irwin I., Langston E. B., Forno L. S. (1984). Pargyline prevents MPTP-induced parkinsonism in primates. Science 225 1480–1482 10.2307/1693552 [DOI] [PubMed] [Google Scholar]
- Lavigne M. C., Malech H. L., Holland S. M., Leto T. L. (2001). Genetic requirement of p47phox for superoxide production by murine microglia. FASEB J. 15 285–287 10.1096/fj.00-0608fje [DOI] [PubMed] [Google Scholar]
- Lee D. W., Kaur D., Chinta S. J., Rajagopalan S., Andersen J. K. (2009). A disruption in iron-sulfur center biogenesis via inhibition of mitochondrial dithiol glutaredoxin 2 may contribute to mitochondrial and cellular iron dysregulation in mammalian glutathione-depleted dopaminergic cells: implications for Parkinson’s disease. Antioxid. Redox Signal. 11 2083–2094 10.1089/ARS.2009.2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. W., Lee W. H., Kim P. H. (2010). Oxidative mechanisms of IL-4-induced IL-6 expression in vascular endothelium. Cytokine 49 73–79 10.1016/j.cyto.2009.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- L’Episcopo F., Tirolo C., Caniglia S., Testa N., Serra P. A., Impagnatiello F., et al. (2010). Combining nitric oxide release with anti-inflammatory activity preserves nigrostriatal dopaminergic innervation and prevents motor impairment in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J. Neuroinflammation 7 83 10.1186/1742-2094-7-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L. P., Patel M. (2004). Iron-sulfur enzyme mediated mitochondrial superoxide toxicity in experimental Parkinson’s disease. J. Neurochem. 90 1076–1084 10.1111/j.1471-4159.2004.02567.x [DOI] [PubMed] [Google Scholar]
- Liberatore G. T., Jackson-Lewis V., Vukosavic S., Mandir A. S., Vila M., Mcauliffe W. G., et al. (1999). Inducible nitric oxide synthase stimulates dopaminergic neurodegeneration in the MPTP model of Parkinson disease. Nat. Med. 5 1403–1409 10.1038/70978 [DOI] [PubMed] [Google Scholar]
- Lipinski B. (2011). Hydroxyl radical and its scavengers in health and disease. Oxid. Med. Cell. Longev. 2011 809696 10.1155/2011/809696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwiczek S., Aigner E., Theurl I., Weiss G. (2003). Cytokine-mediated regulation of iron transport in human monocytic cells. Blood 101 4148–4154 10.1182/blood-2002-08-2459 [DOI] [PubMed] [Google Scholar]
- Mandemakers W., Morais V. A, De Strooper B. (2007). A cell biological perspective on mitochondrial dysfunction in Parkinson disease and other neurodegenerative diseases. J. Cell Sci. 120 1707–1716 10.1242/jcs.03443 [DOI] [PubMed] [Google Scholar]
- Martin L. J., Pan Y., Price A. C., Sterling W., Copeland N. G., Jenkins N. A., et al. (2006). Parkinson’s disease alpha-synuclein transgenic mice develop neuronal mitochondrial degeneration and cell death. J. Neurosci. 26 41–50 10.1523/JNEUROSCI.4308-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer P. L., Itagaki S., Mcgeer E. G. (1988). Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 76 550–557 10.1007/BF00689592 [DOI] [PubMed] [Google Scholar]
- Mello-Filho A. C., Meneghini R. (1991). Iron is the intracellular metal involved in the production of DNA damage by oxygen radicals. Mutat. Res. 251 109–113 10.1016/0027-5107(91)90220-I [DOI] [PubMed] [Google Scholar]
- Mena N. P., Bulteau A. L., Salazar J., Hirsch E. C., Núñez M. T. (2011). Effect of mitochondrial complex I inhibition on Fe-S cluster protein activity. Biochem. Biophys. Res. Commun. 409 241–246 10.1016/j.bbrc.2011.04.137 [DOI] [PubMed] [Google Scholar]
- Michaeli S., Oz G., Sorce D. J., Garwood M., Ugurbil K., Majestic S., et al. (2007). Assessment of brain iron and neuronal integrity in patients with Parkinson’s disease using novel MRI contrasts. Mov. Disord. 22 334–340 10.1002/mds.21227 [DOI] [PubMed] [Google Scholar]
- Millis K. K., Weaver K. H., Rabenstein D. L. (1993). Oxidation/reduction potential of glutathione. J. Org. Chem. 58 4144–4146 10.1021/jo00067a060 [DOI] [Google Scholar]
- Mizuno Y., Ohta S., Tanaka M., Takamiya S., Suzuki K., Sato T., et al. (1989). Deficiencies in complex I subunits of the respiratory chain in Parkinson’s disease. Biochem. Biophys. Res. Commun. 163 1450–1455 10.1016/0006-291X(89)91141-8 [DOI] [PubMed] [Google Scholar]
- Mogi M., Harada M., Kondo T., Riederer P., Inagaki H., Minami M., et al. (1994). Interleukin-1 beta, interleukin-6, epidermal growth factor and transforming growth factor-alpha are elevated in the brain from parkinsonian patients. Neurosci. Lett. 180 147–150 10.1016/0304-3940(94)90508-8 [DOI] [PubMed] [Google Scholar]
- Moreira P. I., Zhu X., Wang X., Lee H. G., Nunomura A., Petersen R. B., et al. (2010). Mitochondria: a therapeutic target in neurodegeneration. Biochim. Biophys. Acta 1802 212–220 10.1016/j.bbadis.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muckenthaler M. U., Galy B., Hentze M. W. (2008). Systemic iron homeostasis and the iron-responsive element/iron-regulatory protein (IRE/IRP) regulatory network. Annu. Rev. Nutr. 28 197–213 10.1146/annurev.nutr.28.061807.155521 [DOI] [PubMed] [Google Scholar]
- Mulero V., Brock J. H. (1999). Regulation of iron metabolism in murine J774 macrophages: role of nitric oxide-dependent and -independent pathways following activation with gamma interferon and lipopolysaccharide. Blood 94 2383–2389 10.1074/jbc.275.9.6220 [DOI] [PubMed] [Google Scholar]
- Muller T., Blum-Degen D., Przuntek H., Kuhn W. (1998). Interleukin-6 levels in cerebrospinal fluid inversely correlate to severity of Parkinson’s disease. Acta Neurol Scand. 98 142–144 10.1111/j.1600-0404.1998.tb01736.x [DOI] [PubMed] [Google Scholar]
- Nagatsu T. (2002). Parkinson’s disease: changes in apoptosis-related factors suggesting possible gene therapy. J. Neural Transm. 109 731–745 10.1007/s007020200061 [DOI] [PubMed] [Google Scholar]
- Nicklas W. J., Vyas I., Heikkila R. E. (1985). Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 36 2503–2508 10.1016/0024-3205(85)90146-8 [DOI] [PubMed] [Google Scholar]
- Noh K. M., Koh J. Y. (2000). Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes. J. Neurosci. 20 RC111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez M. T., Gallardo V., Muñoz P., Tapia V., Esparza A., Salazar J., et al. (2004). Progressive iron accumulation induces a biphasic change in the glutathione content of neuroblastoma cells. Free Radic. Biol. Med. 37 953–960 10.1016/j.freeradbiomed.2004.06.005 [DOI] [PubMed] [Google Scholar]
- Núñez M. T., Tapia V., Toyokuni S., Okada S. (2001). Iron-induced oxidative damage in colon carcinoma (Caco-2) cells. Free Radic. Res. 34 57–68 10.1080/10715760100300061 [DOI] [PubMed] [Google Scholar]
- Núñez M. T., Urrutia P., Mena N., Aguirre P., Tapia V., Salazar J. (2012). Iron toxicity in neurodegeneration. Biometals 25 761–776 10.1007/s10534-012-9523-0 [DOI] [PubMed] [Google Scholar]
- Núñez-Millacura C., Tapia V., Muñoz P., Maccioni R. B., Núñez M. T. (2002). An oxidative stress-mediated positive-feedback iron uptake loop in neuronal cells. J. Neurochem. 82 240–248 10.1046/j.1471-4159.2002.00971.x [DOI] [PubMed] [Google Scholar]
- Nunomura A., Perry G., Pappolla M. A., Wade R., Hirai K., Chiba S., et al. (1999). RNA oxidation is a prominent feature of vulnerable neurons in Alzheimer’s disease. J. Neurosci. 19 1959–1964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradkar P. N., Roth J. A. (2006). Nitric oxide transcriptionally down-regulates specific isoforms of divalent metal transporter (DMT1) via NF-kappaB. J. Neurochem. 96 1768–1777 10.1111/j.1471-4159.2006.03702.x [DOI] [PubMed] [Google Scholar]
- Park L., Zhou P., Pitstick R., Capone C., Anrather J., Norris E. H., et al. (2008). Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. U.S.A. 105 1347–1352 10.1073/pnas.0711568105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawate S., Shen Q., Fan F., Bhat N. R. (2004). Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J. Neurosci. Res. 77 540–551 10.1002/jnr.20180 [DOI] [PubMed] [Google Scholar]
- Pelizzoni I., Macco R., Morini M. F., Zacchetti D., Grohovaz F., Codazzi F. (2011). Iron handling in hippocampal neurons: activity-dependent iron entry and mitochondria-mediated neurotoxicity. Aging Cell 10 172–183 10.1111/j.1474-9726.2010.00652.x [DOI] [PubMed] [Google Scholar]
- Perry T. L., Godin D. V., Hansen S. (1982). Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci. Lett. 33 305–310 10.1016/0304-3940(82)90390-1 [DOI] [PubMed] [Google Scholar]
- Petrat F., De Groot H., Rauen U. (2001). Subcellular distribution of chelatable iron: a laser scanning microscopic study in isolated hepatocytes and liver endothelial cells. Biochem. J. 356 61–69 10.1042/0264-6021:3560061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrat F., De Groot H., Sustmann R., Rauen U. (2002). The chelatable iron pool in living cells: a methodically defined quantity. Biol. Chem. 383 489–502 10.1515/BC.2002.051 [DOI] [PubMed] [Google Scholar]
- Pezzoli G., Cereda E. (2013). Exposure to pesticides or solvents and risk of Parkinson disease. Neurology 80 2035–2041 10.1212/WNL.0b013e318294b3c8 [DOI] [PubMed] [Google Scholar]
- Phani S., Loike J. D., Przedborski S. (2012). Neurodegeneration and inflammation in Parkinson’s disease. Parkinsonism Relat. Disord. 18(Suppl 1) S207–S209 10.1016/S1353-8020(11)70064-5 [DOI] [PubMed] [Google Scholar]
- Pierre J. L., Fontecave M. (1999). Iron and activated oxygen species in biology: the basic chemistry. Biometals 12 195–199 10.1023/A:1009252919854 [DOI] [PubMed] [Google Scholar]
- Possel H., Noack H., Putzke J., Wolf G., Sies H. (2000). Selective upregulation of inducible nitric oxide synthase (iNOS) by lipopolysaccharide (LPS) and cytokines in microglia: in vitro and in vivo studies. Glia 32 51–59 [DOI] [PubMed] [Google Scholar]
- Ridder D. A., Schwaninger M. (2009). NF-kappaB signaling in cerebral ischemia. Neuroscience 158 995–1006 10.1016/j.neuroscience.2008.07.007 [DOI] [PubMed] [Google Scholar]
- Rothwell N. J., Luheshi G. N. (2000). Interleukin 1 in the brain: biology, pathology and therapeutic target. Trends Neurosci. 23 618–625 10.1016/S0166-2236(00)01661-1 [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Tong W. H. (2005). Iron-sulphur cluster biogenesis and mitochondrial iron homeostasis. Nat. Rev. Mol. Cell Biol. 6 345–351 10.1038/nrm1620 [DOI] [PubMed] [Google Scholar]
- Sareila O., Kelkka T., Pizzolla A., Hultqvist M., Holmdahl R. (2011). NOX2 complex-derived ROS as immune regulators. Antioxid. Redox Signal. 15 2197–2208 10.1089/ars.2010.3635 [DOI] [PubMed] [Google Scholar]
- Sas K., Robotka H., Toldi J., Vecsei L. (2007). Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 257 221–239 10.1016/j.jns.2007.01.033 [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M. (1992). Mitochondrial function in neurodegeneration and ageing. Mutat. Res. 275 133–143 10.1016/0921-8734(92)90018-K [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Clark J. B., Jenner P., Marsden C. D. (1990). Mitochondrial complex I deficiency in Parkinson’s disease. J. Neurochem. 54 823–827 10.1111/j.1471-4159.1990.tb02325.x [DOI] [PubMed] [Google Scholar]
- Schapira A. H., Cooper J. M., Dexter D., Jenner P., Clark J. B., Marsden C. D. (1989). Mitochondrial complex I deficiency in Parkinson’s disease. Lancet 1 1269 10.1016/S0140-6736(89)92366-0 [DOI] [PubMed] [Google Scholar]
- Sen C. K., Packer L. (1996). Antioxidant and redox regulation of gene transcription. FASEB J. 10 709–720 [DOI] [PubMed] [Google Scholar]
- Seyidova D., Aliyev A., Rzayev N., Obrenovich M., Lamb B. T., Smith M. A., et al. (2004). The role of nitric oxide in the pathogenesis of brain lesions during the development of Alzheimer’s disease. In Vivo 18 325–333 [PubMed] [Google Scholar]
- Shand O., Volz K. (2013). The solution structure of apo-iron regulatory protein 1. Gene 524 341–346 10.1016/j.gene.2013.03.112 [DOI] [PubMed] [Google Scholar]
- Shi H. X., Liu X., Wang Q., Tang P. P., Liu X. Y., Shan Y. F., et al. (2011). Mitochondrial ubiquitin ligase MARCH5 promotes TLR7 signaling by attenuating TANK action. PLoS Pathog. 7:e1002057 10.1371/journal.ppat.1002057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimohama S., Tanino H., Kawakami N., Okamura N., Kodama H., Yamaguchi T., et al. (2000). Activation of NADPH oxidase in Alzheimer’s disease brains. Biochem. Biophys. Res. Commun. 273 5–9 10.1006/bbrc.2000.2897 [DOI] [PubMed] [Google Scholar]
- Silvestri L., Caputo V., Bellacchio E., Atorino L., Dallapiccola B., Valente E. M., et al. (2005). Mitochondrial import and enzymatic activity of PINK1 mutants associated to recessive parkinsonism. Hum. Mol. Genet. 14 3477–3492 10.1093/hmg/ddi377 [DOI] [PubMed] [Google Scholar]
- Singh S., Das T., Ravindran A., Chaturvedi R. K., Shukla Y., Agarwal A. K., et al. (2005). Involvement of nitric oxide in neurodegeneration: a study on the experimental models of Parkinson’s disease. Redox Rep. 10 103–109 10.1179/135100005X38842 [DOI] [PubMed] [Google Scholar]
- Skalnik D. G., Strauss E. C., Orkin S. H. (1991). CCAAT displacement protein as a repressor of the myelomonocytic-specific gp91-phox gene promoter. J. Biol. Chem. 266 16736–16744 [PubMed] [Google Scholar]
- Smith M. A., Richey Harris P. L., Sayre L. M., Beckman J. S., Perry G. (1997). Widespread peroxynitrite-mediated damage in Alzheimer’s disease. J. Neurosci. 17 2653–2657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sochaski M. A., Bartfay W. J., Thorpe S. R., Baynes J. W., Bartfay E., Lehotay D. C., et al. (2002). Lipid peroxidation and protein modification in a mouse model of chronic iron overload. Metabolism 51 645–651 10.1053/meta.2002.30530 [DOI] [PubMed] [Google Scholar]
- Song Y., Qu R., Zhu S., Zhang R., Ma S. (2012). Rhynchophylline attenuates LPS-induced pro-inflammatory responses through down-regulation of MAPK/NF-kappaB signaling pathways in primary microglia. Phytother. Res. 26 1528–1533 10.1002/ptr.4614 [DOI] [PubMed] [Google Scholar]
- Su K., Bourdette D., Forte M. (2013). Mitochondrial dysfunction and neurodegeneration in multiple sclerosis. Front. Physiol. 4:169 10.3389/fphys.2013.00169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow R. H., Parks J. K., Cassarino D. S., Binder D. R., Bennett J. P., Jr., Di Iorio G., et al. (2001). Biochemical analysis of cybrids expressing mitochondrial DNA from Contursi kindred Parkinson’s subjects. Exp. Neurol. 169 479–485 10.1006/exnr.2001.7674 [DOI] [PubMed] [Google Scholar]
- Swerdlow R. H., Parks J. K., Miller S. W., Tuttle J. B., Trimmer P. A., Sheehan J. P., et al. (1996). Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann. Neurol. 40 663–671 10.1002/ana.410400417 [DOI] [PubMed] [Google Scholar]
- Tanner C. M., Kamel F., Ross G. W., Hoppin J. A., Goldman S. M., Korell M., et al. (2011). Rotenone, paraquat, and Parkinson’s disease. Environ. Health Perspect. 119 866–872 10.1289/ehp.1002839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatsumi T., Matoba S., Kawahara A., Keira N., Shiraishi J., Akashi K., et al. (2000). Cytokine-induced nitric oxide production inhibits mitochondrial energy production and impairs contractile function in rat cardiac myocytes. J. Am. Coll. Cardiol. 35 1338–1346 10.1016/S0735-1097(00)00526-X [DOI] [PubMed] [Google Scholar]
- Teeuwsen V. J., Schalken J. J., Van Der Groen G., Van De Akker R., Goudsmit J., Osterhaus A. D. (1991). An inhibition enzyme immunoassay using a human monoclonal antibody (K14) reactive with gp41 of HIV-1 for the serology of HIV-1 infections. AIDS 5 893–896 10.1097/00002030-199107000-00017 [DOI] [PubMed] [Google Scholar]
- Thannickal V. J., Fanburg B. L. (2000). Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 279 L1005–L1028 [DOI] [PubMed] [Google Scholar]
- Tieu K., Ischiropoulos H., Przedborski S. (2003). Nitric oxide and reactive oxygen species in Parkinson’s disease. IUBMB Life 55 329–335 10.1080/1521654032000114320 [DOI] [PubMed] [Google Scholar]
- Tobon-Velasco J. C., Limon-Pacheco J. H., Orozco-Ibarra M., Macias-Silva M., Vazquez-Victorio G., Cuevas E., et al. (2013). 6-OHDA-induced apoptosis and mitochondrial dysfunction are mediated by early modulation of intracellular signals and interaction of Nrf2 and NF-kappaB factors. Toxicology 304 109–119 10.1016/j.tox.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Ueda S., Masutani H., Nakamura H., Tanaka T., Ueno M., Yodoi J. (2002). Redox control of cell death. Antioxid. Redox Signal. 4 405–414 10.1089/15230860260196209 [DOI] [PubMed] [Google Scholar]
- Urrutia P., Aguirre P., Esparza A., Tapia V., Mena N. P., Arredondo M., et al. (2013). Inflammation alters the expression of DMT1, FPN1 and hepcidin, and it causes iron accumulation in central nervous system cells. J. Neurochem. 126 541–549 10.1111/jnc.12244 [DOI] [PubMed] [Google Scholar]
- Vodovotz Y., Lucia M. S., Flanders K. C., Chesler L., Xie Q. W., Smith T. W., et al. (1996). Inducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer’s disease. J. Exp. Med. 184 1425–1433 10.1084/jem.184.4.1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M. N., Geddes J. G., Farquhar D. A., Masson M. R. (1997). Nitric oxide synthase in reactive astrocytes adjacent to beta-amyloid plaques. Exp. Neurol. 144 266–272 10.1006/exnr.1996.6373 [DOI] [PubMed] [Google Scholar]
- Wang J., Song N., Jiang H., Xie J. (2013). Pro-inflammatory cytokines modulate iron regulatory protein 1 expression and iron transportation through reactive oxygen/nitrogen species production in ventral mesencephalic neurons. Biochim. Biophys. Acta 1832 618–625 10.1016/j.bbadis.2013.01.021 [DOI] [PubMed] [Google Scholar]
- Wang X., Garrick M. D., Yang F., Dailey L. A., Piantadosi C. A., Ghio A. J. (2005). TNF, IFN-gamma, and endotoxin increase expression of DMT1 in bronchial epithelial cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 289 L24–L33 10.1152/ajplung.00428.2003 [DOI] [PubMed] [Google Scholar]
- Wardrop S. L., Richardson D. R. (2000). Interferon-gamma and lipopolysaccharide regulate the expression of Nramp2 and increase the uptake of iron from low relative molecular mass complexes by macrophages. Eur. J. Biochem. 267 6586–6593 10.1046/j.1432-1327.2000.01752.x [DOI] [PubMed] [Google Scholar]
- West A. P., Brodsky I. E., Rahner C., Woo D. K., Erdjument-Bromage H., Tempst P., et al. (2011). TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature 472 476–480 10.1038/nature09973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood P. M. (1988). The potential diagram for oxygen at pH 7. Biochem. J. 253 287–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. C., Jackson-Lewis V., Vila M., Tieu K., Teismann P., Vadseth C., et al. (2002). Blockade of microglial activation is neuroprotective in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson disease. J. Neurosci. 22 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D. C., Teismann P., Tieu K., Vila M., Jackson-Lewis V., Ischiropoulos H., et al. (2003). NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc. Natl. Acad. Sci. U.S.A. 100 6145–6150 10.1073/pnas.0937239100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Smith C. J, Van Eldik L. J. (2004). Activated glia induce neuron death via MAP kinase signaling pathways involving JNK and p38. Glia 45 170–179 10.1002/glia.10314 [DOI] [PubMed] [Google Scholar]
- Yang C. S., Kim J. J., Lee S. J., Hwang J. H., Lee C. H., Lee M. S., et al. (2013). TLR3-triggered reactive oxygen species contribute to inflammatory responses by activating signal transducer and activator of transcription-1. J. Immunol. 190 6368–6377 10.4049/jimmunol.1202574 [DOI] [PubMed] [Google Scholar]
- Yang C. S., Shin D. M., Kim K. H., Lee Z. W., Lee C. H., Park S. G., et al. (2009). NADPH oxidase 2 interaction with TLR2 is required for efficient innate immune responses to mycobacteria via cathelicidin expression. J. Immunol. 182 3696–3705 10.4049/jimmunol.0802217 [DOI] [PubMed] [Google Scholar]
- Yang F., Liu X. B., Quinones M., Melby P. C., Ghio A., Haile D. J. (2002). Regulation of reticuloendothelial iron transporter MTP1 (Slc11a3) by inflammation. J. Biol. Chem. 277 39786–39791 10.1074/jbc.M201485200 [DOI] [PubMed] [Google Scholar]
- Yokoyama H., Takagi S., Watanabe Y., Kato H., Araki T. (2008). Role of reactive nitrogen and reactive oxygen species against MPTP neurotoxicity in mice. J. Neural Transm. 115 831–842 10.1007/s00702-008-0019-6 [DOI] [PubMed] [Google Scholar]
- Zecca L., Wilms H., Geick S., Claasen J. H., Brandenburg L. O., Holzknecht C., et al. (2008). Human neuromelanin induces neuroinflammation and neurodegeneration in the rat substantia nigra: implications for Parkinson’s disease. Acta Neuropathol. 116 47–55 10.1007/s00401-008-0361-7 [DOI] [PubMed] [Google Scholar]
- Zecca L., Youdim M. B., Riederer P., Connor J. R., Crichton R. R. (2004). Iron, brain ageing and neurodegenerative disorders. Nat. Rev. Neurosci. 5 863–873 10.1038/nrn1537 [DOI] [PubMed] [Google Scholar]
- Zhang F., Zhou H., Wilson B. C., Shi J. S., Hong J. S., Gao H. M. (2012). Fluoxetine protects neurons against microglial activation-mediated neurotoxicity. Parkinsonism Relat. Disord. 18(Suppl 1) S213–S217 10.1016/S1353-8020(11)70066-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Stanton D. M., Nguyen X. V., Liu M., Zhang Z., Gash D., et al. (2005). Intrapallidal lipopolysaccharide injection increases iron and ferritin levels in glia of the rat substantia nigra and induces locomotor deficits. Neuroscience 135 829–838 10.1016/j.neuroscience.2005.06.049 [DOI] [PubMed] [Google Scholar]
- Zhang W., Wang T., Qin L., Gao H. M., Wilson B., Ali S. F., et al. (2004). Neuroprotective effect of dextromethorphan in the MPTP Parkinson’s disease model: role of NADPH oxidase. FASEB J. 18 589–591 10.1096/fj.03-0983fje [DOI] [PubMed] [Google Scholar]
- Zhang X., Lemasters J. J. (2013). Translocation of iron from lysosomes to mitochondria during ischemia predisposes to injury after reperfusion in rat hepatocytes. Free Radic. Biol. Med. 63 243–253 10.1016/j.freeradbiomed.2013.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccarato F., Toscano P., Alexandre A. (2005). Dopamine-derived dopaminochrome promotes H(2)O(2) release at mitochondrial complex I: stimulation by rotenone, control by Ca(2+), and relevance to Parkinson disease. J. Biol. Chem. 280 15587–15594 10.1074/jbc.M500657200 [DOI] [PubMed] [Google Scholar]