Abstract
Background
The treatment of Hodgkin’s disease (HD; also called Hodgkin’s lymphoma) in children and adolescents with radiotherapy and chemotherapy leads to high survival rates but has a number of late effects. The most serious one is the development of a secondary malignant tumor, usually in the field that was irradiated. In women, breast cancer can arise in this way.
Methods
Data on the occurrence of secondary breast cancer (sBC) were collected from 590 women who were treated in five consecutive pediatric HD treatment studies in the years 1978–1995 and then re-evaluated in a late follow-up study after a median interval of 17.8 years (maximum, 33.7 years). Information was obtained from 1999 onward by written inquiry to the participants and their treating physicians. The cumulative incidence of sBC was calculated by the Gooley method.
Results
By July 2012, sBC had been diagnosed in 26 of 590 female HD patients; the breast cancer was in the irradiated field in 25 of these 26 patients. Their age at the time of treatment for HD was 9.9 to 16.2 years (the pubertal phase), and sBC was discovered with a median latency of 20.7 years after HD treatment (shortest latency, 14.3 years) and at a median age of 35.3 years (youngest age, 26.8 years). The radiation dose to the supradiaphragmatic fields ranged from 20 to 45 Gy. The cumulative incidence for sBC 30 years after treatment for HD was 19% (95% confidence interval, 12% to 29%). For women aged 25 to 45 in this series, the frequency of breast cancer was 24 times as high as in the corresponding normal population.
Conclusion
Women who were treated for HD in childhood or adolescence have an increased risk of developing breast cancer as young adults. The risk is associated with prior radiotherapy and with the age at which it was administered (the pubertal phase). Because of these findings, a structured breast cancer screening project for this high-risk group has been initiated in collaboration with the German Consortium for Hereditary Breast and Ovarian Cancer (Deutsches Konsortium für familiären Brust- und Eierstockkrebs).
Radiotherapy for Hodgkin’s disease (HD; also called Hodgkin’s lymphoma) during childhood or adolescence leaves female patients at considerably increased risk of developing secondary breast cancer (sBC) in early adulthood. Evidence for this finding has been accumulating in the international literature since the 1990s (1– 12). A number of European and North American working groups have already introduced structured breast cancer screening projects for female HD patients who underwent radiotherapy at an early age (13– 16).
Because at first only a few cases of sBC were identified in the long-term follow-up of pediatric HD trial patients (17), for a long time in Germany the available data were insufficient to justify including this risk group in an intensive screening program for early disease. A particular issue was convincing the health insurance companies to cover the costs of the screening examinations. However, the incidence of sBC among former HD trial patients has increased so sharply in the past few years that we pushed forward plans for screening measures in the form of a structured program of screening examinations for this new risk group, and eventually, in 2012, succeeded in establishing it as a collaborative project.
The present article aims to explain why we felt it necessary to push for an appropriate solution to the problem of early disease recognition. It does so by presenting our own, up-to-date data on the incidence of sBC in Germany from the long-term Late Effects of HD project of the Society of Paediatric Oncology and Hematology (GPOH, Gesellschaft für Pädiatrische Onkologie und Hämatologie).
Patients and methods
Patients
The results presented here are from the cohort of, originally, 1407 patients of both sexes who were treated for HD during the years 1978 to 1995 in the first five consecutive multicenter pediatric treatment trials HD-78 to HD-90 (Table 1), and were then observed over the long term in the GPOH Late Effects of HD project (working group under G. Schellong, Münster).
Table 1. Characteristics of patients in pediatric Hodgkin’s disease trials HD-78 to HD-90.
| Trials | HD-78, HD-82, HD-85, HD-87, HD-90 | ||
| Patient recruitment period | 1978–1995 | ||
| Male/female (total) | 817/590 (1407) | ||
| n | Median | Range | |
| Female | 590 | ||
| Age at diagnosis of HD (years) | 13.8 | 2.9–17.9 | |
| Radiation dose to the chest region (Gy) | 30 | 0–50 | |
| Alive at last follow-up | 534 | ||
| Age (years) | 31.1 | 6.7–47.0 | |
| Follow-up (years) | 17.8 | 0.1–33.7 | |
| Data as of 1 July 2012 | |||
HD, Hodgkin’s disease
The analyses relating to sBC are based on calculations regarding the 590 girls in the overall group, 95% of whom survived for more than 10 years (Figure 1). All trial patients were less than 18 years old at the time of onset of the primary disease (median age: 13.8 years [Table 1]).
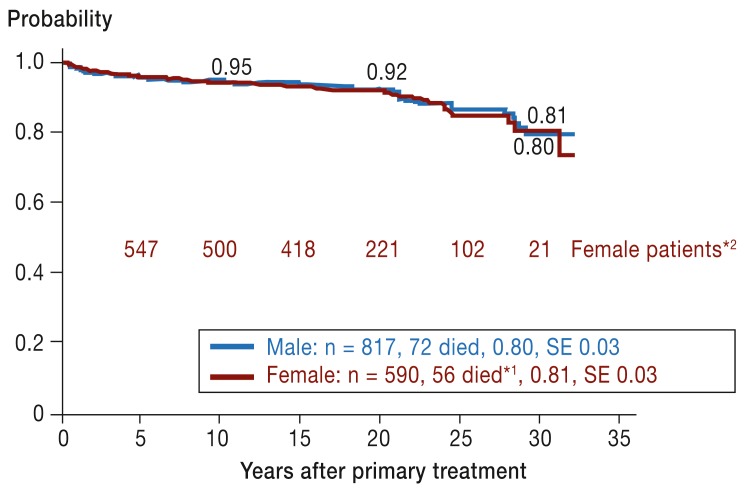
Figure 1.
Overall survival after 30 years in Hodgkin’s disease treatment trials HD-78 to HD-90 in boys and girls (as of 1 July 2012).
*1 Causes of death in female patients: Hodgkin’s disease (n = 18),
post-splenectomy sepsis (n = 7), secondary malignancy (n = 15, 3 of them breast cancer), heart disease (n = 6), other (n = 10, including accidents and suicide)
*2 With documentation of disease course
SE, standard error
Primary treatment of Hodgkin’s disease
The protocols of all five trials specified combined chemo- and radiotherapy (18– 20). The chemotherapy in the early, middle, and advanced disease stages consisted of 2, 4, or 6 to 8 treatment cycles respectively, using mainly the cytostatic drugs prednisone, vincristine, procarbazine, doxorubicin, etoposide, and cyclophosphamide in varying proportions. The cumulative dose of anthracycline was the same in all patients: 160 mg/m2 body surface area. The subsequent radiotherapy was carried out in the first trial, HD-78, as extended-field irradiation with total doses of 36 to 40 Gy for the regions involved, while the non-involved adjacent fields were randomized to receive either the same dose or 18–20 Gy. From the second trial (HD-82) onwards, the treatment was changed to a strict involved-field irradiation protocol, and from one trial to the next the total doses were reduced stepwise down to 20 to 25 Gy in the HD-90 trial. If defined residual lymphoma was present after chemotherapy, local boosts up to a total dose of 30 to 35 Gy were suggested.
We received follow-up information primarily from the participating hospitals. Later on the information came from the patients themselves, after the study center had switched in 1999 to sending the patients questionnaires at intervals of about 3 to 4 years. When any health problems were reported, we asked for additional information from physicians and patients. Thus, when breast cancer was diagnosed, they received written reports giving details of the diagnosis and treatment. These findings regarding late treatment effects, acquired in the course of long-term follow-up, were published under various aspects—e.g., as they related to impaired male fertility, to secondary malignant tumors, post-splenectomy infections, and sequelae of cardiac irradiation (17, 21– 24)—and were integrated where possible into the follow-up care of the long-term survivors.
Statistical methods
Overall survival was calculated according to the Kaplan–Meier method (25). The cumulative incidence was calculated using Gooley’s method (26), taking death as the competitive risk. The ratio of observed to expected number of cases of breast cancer gives the standardized incidence ratio (SIR) (27). The expected number was calculated based on data from the Robert Koch Institute (RKI) (www.rki.de/Krebs/DE/Home/Datenbankabfrage/c50_brust.xls). The date of the evaluations was 1 July 2012. Endpoints for the calculations were the date of breast cancer diagnosis, most recent follow-up information on disease course, death, or 1 July 2012, whichever came first.
Results
Data on incidence of breast cancer after treatment for HD in childhood or adolescence
The overall survival of boys and girls is shown by the two curves in Figure 1. There is no visible difference. For 20 years the survival rates were over 90% but dropped within the following 10 years to 80% and 81% as a result of deaths from various causes. Causes of death among the 56 female patients who died are given in the footnote to Figure 1. So far, breast cancer has been the cause of death in 3 female patients.
As of 1 July 2012, 26 young women in the follow-up cohort had been diagnosed with sBC between July 1997 and March 2011 (for data see Table 2). So far no case of sBC has been diagnosed in male patients. The median age of the affected women at the time of HD treatment was 13.3 years. The range was quite small, 9.9 to 16.2 years. The median radiation dose to mediastinum, axillae, and/or the clavicular region was 35.5 Gy, and was in the range of 20 to 45 Gy if the second course of radiation in the 7 patients with recurrence is included. In 96% of the affected women, the tumor was located in or at the margin of the radiation field. The median interval between primary and secondary malignancy was 20.7 years, with the shortest being 14.3 years. Median age at breast cancer diagnosis was 35.3 years; the youngest woman at diagnosis was 26.8 years old.
Table 2. Characteristics of the 26 women with breast cancer (BC) after Hodgkin’s disease (HD) in childhood or adolescence.
| n | Median | Range | |
|---|---|---|---|
| Age at HD (years) | 13.3 | 9.9–16.2 | |
| HD recurrence | 7 | ||
| Radiation dose for HD (Gy) (infraclavicular region/mediastinum/axilla) | 35 | 20–41 (1 pat. 0) | |
| incl. radiotherapy for recurrence | 35.5 | 20–45 (1 pat. 0) | |
| Interval between HD and BC (years) | 20.7 | 14.3–31.3 | |
| Age at diagnosis of breast cancer (years) | 35.3 | 26.8–44.6 | |
| Hormone receptor status ER/PR+ | 24 | ||
| Grading G2 | 16 | ||
| Ductal histology | 20 | ||
| Advanced stage | 13 | ||
| Bilateral (synchronous or metachronous) | 6 | ||
| BC recurrence | 8 | ||
| Death due to BC | 3 |
Particularly noteworthy is the fact that in half of the affected women (n = 13) the breast cancer was diagnosed at a locally and/or generally advanced stage. Six patients developed synchronous or metachronous bilateral disease.
Figure 2 shows the curves for cumulative sBC incidence for the whole group of 590 patients (whether or not they received radiation to the chest) in relation to follow-up time and to age. At 30 years’ follow-up the curves reach a cumulative incidence of 16% (95% confidence interval [CI] 10% to 26%), and at the age of 45 years, 10% [95% CI: 7% to 16%). Compared to the age-matched general population (Robert Koch Institute), the median SIR calculated for 25- to 45-year-old women is 24 (range: 18 to 49): that is, the incidence of breast cancer in the female patient cohort is 24 times that in the age-matched population.
Figure 2.
Cumulative incidence (Cum. inc.) of breast cancer (BC) in the total group of female patients in pediatric treatment trials HD-78 to HD-90 in relation to time since primary therapy (blue line) and age reached (red broken line), with 95% confidence intervals, as of 1 July 2012
The cumulative sBC incidences of the young women who had received radiation to the chest region are shown in Figure 3, which also takes account of age at the time of HD treatment (<9 years versus 9 years or older). The incidence in the 74 women who had undergone irradiation at less than 9 years was 0 up to the age of 32 for the entire follow-up period, whereas the incidence among those who were older at the time of treatment (trial limit <18 years) was up to 30% (95% CI: 15% to 62%). The difference is statistically significant (p = 0.04).
Figure 3.
Cumulative incidence (Cum. inc.) of breast cancer (BC) in the group of female patients in pediatric treatment trials HD-78 to HD-90 who received radiation to the chest region, with 95% confidence intervals, as of 1 July 2012
So far it has not been possible to analyze the influence of radiation dose on breast cancer incidence, because the number of female patients who received low-dose irradiation (<20 to 25 Gy) and have a long enough post-irradiation follow-up time (>20 years) is still too low for statistical evaluation.
Implementing an intensive breast cancer screening program for women at high risk after HD
As already mentioned, because of the increased risk of breast cancer, some European and North American countries have already established multimodal breast cancer screening programs especially for young women who underwent radiotherapy in childhood or adolescence, although even in these countries the programs are often not universally available (13– 16). Also, the way in which these projects—most of which use breast MRI as an important part of the examination—are financed is not entirely transparent.
In Germany, an intensive multimodal breast cancer screening project for young women at high risk after radiotherapy in childhood or adolescence was initiated on a much broader basis and implemented in 2012. This project was able to make use of existing structures set up by the German Consortium for Hereditary Breast and Ovarian Cancer (Deutsches Konsortium für familiären Brust- und Eierstockkrebs), consisting of 15 specialized centers (for addresses, see www.krebshilfe.de/brustkrebszentren.html).
Women carrying a mutation in the BRCA-1 or BRCA-2 gene also have an increased risk of breast cancer in early adulthood (28– 30). A study recently published in the USA that compared cumulative breast cancer incidences in long-term survivors after radiotherapy for HD at the age of <21 years with those in a group of BRCA-1 mutation carriers showed that the increases in incidence up to the age of 50 years were very similar; at the age of 50 years, these cumulative incidences reached 30% and 31% (data in [11]). Therefore, the consortium has already developed a multimodal screening program for women with a genetically increased risk of breast cancer in early adulthood, which has been implemented in collaboration with the statutory health insurance companies and has been documented and reviewed by the study center at the University of Leipzig (Prof. Löffler) (29, 30). These high-risk patients from the pediatric HD therapy trials from 1978 on were enrolled in a separate project (HD-BRCA-12). The screening program, adapted for the female HD patients, is shown in Table 3. Details of the entry criteria for the patients and the overall study procedure were defined in writing and consented to by all 15 centers, the principal investigators of the HD trials, and the national associations of the health insurance companies (Table 3).
Table 3. Entry criteria and plan for screening examinations for women at high risk of breast cancer after radiotherapy for Hodgkin’s disease in childhood or adolescence.
Entry criteria for screening examinations
| |
| Plan for screening examinations | |
|
|
Every 6 months:
|
From age 25 years |
| In case of unclear or suspicious findings, or every 1–2 years depending on individual risk–benefit ratio: –Mammography | Optional from age 40 years |
| Transfer into regular healthcare (i.e., mammography screening) | From age 50 years |
Women treated outside of the pediatric HD trials can also be enrolled in the screening project so long as they fulfill the other criteria (see Table 3). The age range at the time of HD therapy can be expanded to 9 to 20 years. Enquiries regarding such women should be directed to one of the Leaders of the Late Effects of HD project, Prof. G. Schellong or Dr. W. Dörffel, or to one of the 15 consortium centers. The consortium’s multimodal examination program for young women at high risk of breast cancer specifies four modes of examination for use in the screening program from the age of 25 years onwards (Table 3).
This collaborative project is embedded in a follow-up program so that its effectiveness in the particular subgroup of long-term survivors of HD can be tested.
Discussion
The findings of the present study document a marked increase in the incidence of breast cancer during early adulthood in the cohort of female patients treated in the German–Austrian HD treatment trials during the years 1978 to 1995. This is shown both by the comparison with the age-matched general population (SIR 24) and by the marked rise in cumulative incidence of sBC up to the end of the follow-up period so far (30 to 35 years) and/or up to the maximum age attained (40 to 50 years).
For comparison, we have drawn on other studies in the literature. These have reported long-term follow-up of patient groups that were treated in childhood and adolescence for HD and fulfilled the following criteria: the majority of patients received radiotherapy to the supradiaphragmatic region, and data were available on cumulative sBC incidences up to the age of at least 40 years.
Among 480 female patients in the Late Effects Study Group (LESG) (4), the cumulative incidence up to the age of 40 years was 13.9% (95% CI: 8.9% to 19.1%), while up to the age of 45 years it was 20.1% (95% CI: 11.1% to 29.0%). Among 806 female patients in the Childhood Cancer Survival Study (CCSS), the incidence up to the age of 40 years was 12.9% (95% CI: 9.3% to 16.6%), and up to the age of 50 years it was 30% (95% CI: 25% to 35%) (11). The cumulative incidence in the overall cohort of 590 female patients observed up to the age of 40 years is 10% (95% CI: 7% to 16%). Thus, the differences between the three groups of patients are small, and the results therefore support each other. The markedly higher incidences at the ages of 45 and 50 years in the LESG and CCSS groups respectively (4, 11) indicate that there is no sign that the steep curve is plateauing out, and the increased risk of breast cancer will probably continue past the age of 50 years.
One observation worth mentioning is that in 25 of the 26 women affected, the sBC occurred within the former radiation field. In many analyses of series of secondary malignancies (1– 6, 8), the location of the secondary tumors in relation to the former radiation fields is an important indicator in the assessment and recognition of the causative effect of the radiation burden. There is general consensus that this kind of association indicates a high probability of a causal relationship.
Another important finding of the analyses is the confirmation of existing suggestions from other authors that the age of the young female patients at the time of diagnosis and treatment for Hodgkin’s disease is an important risk factor for later development of sBC. There is wide consensus in the literature that supradiaphragmatic radiotherapy involving parts of the chest in girls aged 10 to 20 years is associated with the highest risk of sBC in comparison to the adjacent age groups (4, 9, 12). Among our own patients, all radiotherapy-associated cases of sBC occurred in women who had undergone radiotherapy between the ages of 9 and 16 years, whereas not a single case of sBC was recorded among the 74 women irradiated before the age of 9 years. The observed critical age range of 9 to 16 years corresponds to the variation range of puberty in girls, which starts with development of the breast buds (Tanner stage B2) (31, 32) and is associated with rapid proliferation of the glandular cells. The glandular cells rapidly extend in all directions beyond the areola into the breast tissue, and are thus especially vulnerable to radiation exposure (33).
To what extent the radiation dose affects the incidence of sBC cannot be judged on the basis of existing published evidence. Whether it can be confirmed that the risk increases linearly with the dose, as published in several studies (5, 8, 34), remains to be shown in further studies with longer follow-up times. However, a rise in sBC incidence after higher doses in the region of up to 40 Gy has already been proven (3, 8, 9, 12, 34, 35).
Because of the high prospective breast cancer incidence rates, a quality-controlled intensive screening program has been established through close collaboration between the pediatric HD register and the German Consortium for Hereditary Breast and Ovarian Cancer, which is being tested for its effectiveness in a prospective long-term observation study.
Because of the density of pediatric oncology and hematology trials in Germany (36), women at high risk of disease throughout the country are identified through collaboration with the leaders of the HD trials and informed about the early screening on offer. The decision about whether or not to take part is of course made by the women themselves after counseling.
One additional question arises from a few indications in the literature which suggest that radiotherapy for other cancers during the critical years (second decade of life) can also induce sBC if parts of the chest are included (15). It would therefore be desirable for these women also to be included in the intensive early recognition project.
In the more recent HD therapy trials of the GPOH and the EuroNet-PHL groups, radiotherapy is no longer carried out if the lymphoma has responded very well to the chemotherapy (in various percentages of the patients, depending on stage and therapy group; the percentages are growing rapidly from one trial to the next) (37, 38) (http://clinicaltrials.gov/ct/show/NCT00433459). The incidence of sBC will therefore probably gradually drop in the near future. At the present time, however, radiotherapy still has to be part of the treatment for a large percentage of pediatric HD patients. In particular, when supradiaphragmatic radiotherapy is necessary in girls over the age of 9, the extent of irradiated parts of the breast should be kept as small as can be medically justified.
Key Messages.
Supradiaphragmatic radiotherapy that irradiates areas of the chest as part of treatment for Hodgkin’s disease in childhood or adolescence leaves women with a high risk of developing secondary breast cancer (sBC) in early adulthood.
The documented incidence of sBC is based on long-term follow-up data of (originally) 590 patients treated between 1978 and 1995 in the in German–Austrian therapy trials.
The incidence of breast cancer in female patients aged 25 to 45 years who have previously been treated for Hodgkin’s disease is 24 times higher than that in the age-matched general population.
The age dependence indicates a particular vulnerability of the glandular cells in the proliferation phase during puberty: among the women who had been irradiated in the supradiaphragmatic area at less than 9 years of age, not one later developed breast cancer, whereas women who received supradiaphragmatic radiation between the ages of 9 to 16 years, including to areas of the chest, had a cumulative incidence of 19% at 30 years’ follow-up (95% confidence interval: 12% to 29%).
In Germany, as in some other countries, a structured breast cancer screening program has been set up for patients in this high-risk group. The program operates in collaboration between the German Consortium for Hereditary Breast and Ovarian Cancer and the previous chairmen of the HD trials in consensus with the national associations of the statutory health insurance companies. It came into effect in 2012.
Acknowledgments
Translated from the original German by Kersti Wagstaff, MA.
The authors in both working groups are grateful for ongoing and effective support, both material and non-material, from the following funding institutions: Deutsche Kinderkrebsstiftung (German Childhood Cancer Foundation), Kinderkrebshilfe Münster e.V. (Children‘s Cancer Aid Münster), Jens-Brunken-Stiftung Varel (Jens Brunken Foundation, Varel), Deutsche Krebshilfe (German Cancer Aid).
Footnotes
Conflict of interest statement
Dr. Dörffel works in an honorary capacity as leader of the HD Late Effects study group and is a member of the Helios Clinic in Berlin-Buch. The remaining authors declare that no conflict of interest exists.
References
- 1.Hancock SL, Tucker MA, Hoppe RT. Breast cancer after treatment of Hodgkin‘s disease. J Natl Cancer Inst. 1993;85:25–31. doi: 10.1093/jnci/85.1.25. [DOI] [PubMed] [Google Scholar]
- 2.Bhatia S, Robison LL, Oberlin O, et al. Breast cancer and other -second neoplasms after childhood Hodgkin’s disease. New Engl J Med. 1996;334:745–751. doi: 10.1056/NEJM199603213341201. [DOI] [PubMed] [Google Scholar]
- 3.Sankila R, Garwicz S, Olsen JH, et al. Risk of subsequent malignant neoplasms among 1641 Hodgkin’s disease patients diagnosed in childhood and adolescence: a population-based cohort study in the five Nordic countries. J Clin Oncol. 1996;14:1442–1446. doi: 10.1200/JCO.1996.14.5.1442. [DOI] [PubMed] [Google Scholar]
- 4.Bhatia S, Yasni Y, Robison LL, et al. High risk of subsequent neoplasms continues with extended follow-up of childhood Hodgkin’s disease; Report from the Late Effects Study Group. J Clin Oncol. 2003;21:4386–4394. doi: 10.1200/JCO.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 5.Travis LB, Hill DA, Dores GM, et al. Breast cancer following radiotherapy and chemotherapy among young women with Hodgkin -disease. JAMA. 2003;290:465–475. doi: 10.1001/jama.290.4.465. [DOI] [PubMed] [Google Scholar]
- 6.Kenney LB, Yasui Y, Inskip PD, et al. Breast cancer after childhood cancer: A report from the Childhood Cancer Survivor Study. Ann -Intern Med. 2004;141:590–597. doi: 10.7326/0003-4819-141-8-200410190-00006. [DOI] [PubMed] [Google Scholar]
- 7.Basu SK, Schwarz C, Fisher SG, et al. Unilateral and bilateral breast cancer in women surviving pediatric Hodgkin’s disease. Int J Radiation Oncol Biol Phys. 2008;72:34–40. doi: 10.1016/j.ijrobp.2008.04.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inskip PD, Robison LL, Stovall M, et al. Radiation dose and breast cancer risk in the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:3901–3907. doi: 10.1200/JCO.2008.20.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alm El-Din MA, Hughes KS, Finkelstein DM, et al. Breast cancer -after treatment of Hodgkin’s lymphoma: Risk factors that really -matter. Int J Radiation Oncol Biol Phys. 2009;73:69–74. doi: 10.1016/j.ijrobp.2008.03.066. [DOI] [PubMed] [Google Scholar]
- 10.O’Brien MM, Donaldson SS, Balise RR, Whittemore AS, Link MP. Second malignant neoplasms in survivors of pediatric Hodgkin’s lymphoma treated with low-dose radiation and chemotherapy. J Clin Oncol. 2010;28:1232–1239. doi: 10.1200/JCO.2009.24.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moskowitz CS, Chou JF, Wolden SL, et al. New insights into the risk of breast cancer in childhood cancer survivors treated with chest radiation: A report from the Childhood Cancer Survivor Study (CCSS) and the Women‘s Environmental Cancer and Radiation -Epidemiology (WECARE) Study. J Clin Oncol. 2012;30 CRA9513. [Google Scholar]
- 12.Swerdlow AJ, Cooke R, Bates A, et al. Breast cancer risk after supra-diaphragmatic radiotherapy for Hodgkin’s lymphoma in England and Wales: A national cohort study. J Clin Oncol. 2012;30:2745–2752. doi: 10.1200/JCO.2011.38.8835. [DOI] [PubMed] [Google Scholar]
- 13.Oeffinger KC, Ford JS, Moskowitz CS, et al. Breast cancer surveillance practices among women previously treated with chest radia-tion for a childhood cancer. JAMA. 2009;301:404–414. doi: 10.1001/jama.2008.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howell SJ, Searle C, Goode V, et al. The UK national breast cancer screening programme for survivors of Hodgkin lymphoma detects breast cancer at an early stage. Br J Cancer. 2009;101:582–588. doi: 10.1038/sj.bjc.6605215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Terenziani M, Casalini P, Scaperrotta G, et al. Occurrence of breast cancer after chest wall irradiation for pediatric cancer, as detected by a multimodal screening program. J Radiation Oncol Biol Phys. 2013;85:35–39. doi: 10.1016/j.ijrobp.2012.03.043. [DOI] [PubMed] [Google Scholar]
- 16.Colin C, de Vathaire F, Noël A, et al. Updated Relevance of Mammographic Screening Modalities in Women Previously Treated with Chest Irradiation for Hodgkin Disease. Radiology. 2012;265:669–676. doi: 10.1148/radiol.12120794. [DOI] [PubMed] [Google Scholar]
- 17.Schellong G, Riepenhausen M. Late effects after therapy of Hodgkin’s disease: Update 2003/04 on overwhelming post -splen-ectomy infections and secondary malignancies. Klin Pädiatr. 2004;216:364–369. doi: 10.1055/s-2004-832340. [DOI] [PubMed] [Google Scholar]
- 18.Bramswig JH, Hörnig-Franz I, Riepenhausen M, et al. The chal-lenge of pediatric Hodgkin’s disease: Where is the balance between cure and long-term toxicity? A report of the West German multicenter studies DAL-HD-78, DAL-HD-82 and DAL-HD-85. Leuk Lymphoma A. 1990;3:183–193. doi: 10.3109/10428199009050994. [DOI] [PubMed] [Google Scholar]
- 19.Schellong G, Hörnig-Franz I, Rath B, et al. Reducing radiation dos-age to 20-30 Gy in combined chemo-/radiotherapy of Hodgkin’s disease in childhood A report of the cooperative DAL-HD-87 -therapy study. Klin Pädiatr. 1994;206:253–262. doi: 10.1055/s-2008-1046611. [DOI] [PubMed] [Google Scholar]
- 20.Schellong G, Potter R, Bramswig JH, et al. High cure rates and -reduced long-term toxicity in pediatric Hodgkin’s disease: The -German-Austrian multicenter trial DAL-HD-90 The German–Austrian Pediatric Hodgkin’s Disease Study Group. J Clin Oncol. 1999;17:3736–3744. doi: 10.1200/JCO.1999.17.12.3736. [DOI] [PubMed] [Google Scholar]
- 21.Bramswig JH, Heimes U, Heiermann E, et al. The effects of differ-ent cumulative doses of chemotherapy on testicular function Results in 75 patients treated for Hodgkin’s disease during childhood or adolescence. Cancer. 1990;65:1298–1302. doi: 10.1002/1097-0142(19900315)65:6<1298::aid-cncr2820650607>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 22.Schellong G, Riepenhausen M, Creutzig U, et al. Low risk of secondary leukemias after chemotherapy without mechlorethamine in childhood Hodgkin’s disease. J Clin Oncol. 1997;15:2247–2253. doi: 10.1200/JCO.1997.15.6.2247. [DOI] [PubMed] [Google Scholar]
- 23.Schellong G, Riepenhausen M, Bruch C, et al. Late valvular and -other cardiac diseases after different doses of mediastinal radio-therapy for Hodgkin disease in children and adolescents: Report from the longitudinal GPOH follow-up project of the German-Austrian DAL-HD Studies. Pediatr Blood Cancer. 2010;55:1145–1152. doi: 10.1002/pbc.22664. [DOI] [PubMed] [Google Scholar]
- 24.Dörffel W, Riepenhausen M, Ludwig WD, Schellong G. Langzeit-folgen nach Therapie eines Hodgkin-Lymphoms bei Kindern und Jugendlichen. Journal Onkologie. 2010;09:449–456. [Google Scholar]
- 25.Kaplan EL, Meier P. Nonparametric estimation from incomplete -observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Gooley TA, Leisenring W, Crowley J, et al. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Yasui Y, Liu Y, Neglia JP, et al. A methodological issue in the analysis of second-primary cancer incidence in long-term survivors of childhood cancers. Am J Epidemiol. 2003;158:1108–1113. doi: 10.1093/aje/kwg278. [DOI] [PubMed] [Google Scholar]
- 28.Antoniou AC, Cunningham AP, Peto J, et al. The BOADICEA model of genetic susceptibility to breast and ovarian cancers: updates and extensions. Br J Cancer. 2008;98:1457–1466. doi: 10.1038/sj.bjc.6604305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmutzler RK, Rhiem K, Breuer P, et al. Outcome of a structured surveillance programme in women with a familial predisposition for breast cancer. Eur J Cancer Prev. 2006;15:483–489. doi: 10.1097/01.cej.0000220624.70234.14. [DOI] [PubMed] [Google Scholar]
- 30.Meindl A, Ditsch N, Kast K, Rhiem K, Schmutzler RK. Hereditary -breast and ovarian cancer—new genes, new treatments, new -concepts. Dtsch Arztebl Int. 2011;108(19):323–330. doi: 10.3238/arztebl.2011.0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Largo RH, Prader A. Pubertal development in Swiss girls. Helv -Paediatr Acta. 1983;38:229–243. [PubMed] [Google Scholar]
- 32.Brämswig JH, Dübbers A. Disorders of pubertal development. Dtsch Arztebl Int. 2009;106(17):295–304. doi: 10.3238/arztebl.2009.0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demoor-Goldschmidt C, Supiot S, Mahé MA. Breast cancer after radiotherapy: risk factors and suggestion for breast delineation as an organ at risk in the prepuberal girl. Cancer Radiother. 2012;16:140–151. doi: 10.1016/j.canrad.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 34.Henderson TO, Amsterdam A, Bhatia S, et al. Systematic Review: Surveillance for breast cancer in women treated with chest radia-tion for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152:444–455. doi: 10.1059/0003-4819-152-7-201004060-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Leeuwen FE, Klokman WJ, Stovall M, et al. Roles of radiation dose, chemotherapy, and hormonal factors in breast cancer follow-ing Hodgkin’s disease. J Natl Cancer Inst. 2003;95:971–980. doi: 10.1093/jnci/95.13.971. [DOI] [PubMed] [Google Scholar]
- 36.Rössig C, Jürgens H, Schrappe M, et al. Effective Childhood Cancer Treatment: The Impact of Large Scale Clinical Trials in Germany and Austria. Pediatr Blood Cancer. 2013;60:1574–1581. doi: 10.1002/pbc.24598. [DOI] [PubMed] [Google Scholar]
- 37.Dörffel W, Rühl U, Lüders H, et al. Treatment of children and adolescents with Hodgkin lymphoma without radiotherapy for patients in complete remission after chemotherapy: Final results of the multinational trial GPOH-HD95. J Clin Oncol. 2013;31:1562–1568. doi: 10.1200/JCO.2012.45.3266. [DOI] [PubMed] [Google Scholar]
- 38.Mauz-Körholz C, Hasenclever D, Dörffel W, et al. Procarbazine-free OEPA-COPDAC chemotherapy in boys and standard OPPA-COPP in girls have comparable effectiveness in pediatric hodgkin’s lymphoma: The GPOH-HD-2002 Study. J Clin Oncol. 2010;28:3680–3686. doi: 10.1200/JCO.2009.26.9381. [DOI] [PubMed] [Google Scholar]