Abstract
Nucleic acids have the potential to be used as therapies or vaccines for many different types of disease but delivery remains the most significant challenge to their clinical adoption. pH responsive peptides containing either histidine or derivatives of 2,3-diaminopropionic acid (Dap) can mediate effective DNA transfection in lung epithelial cells with the latter remaining effective even in the presence of lung surfactant containing bronchoalveolar fluid (BALF), making this class of peptides attractive candidates for delivering nucleic acids to lung tissues. To further assess the suitability of pH responsive peptides for pulmonary delivery by inhalation, dry powder formulations of pH responsive peptides and plasmid DNA, with mannitol as carrier, were produced by either spray drying (SD) or spray freeze drying (SFD). The properties of the two types of powders were characterised and compared using scanning electron microscopy (SEM), next generation impaction (NGI), gel retardation and in vitro transfection via a twin-stage impinger (TSI) following aerosolisation by a dry powder inhaler (Osmohaler™). Although the aerodynamic performance and transfection efficacy of both powders were good, the overall performance revealed SD powders to have a number of advantages over SFD powders and are the more effective formulation with potential for efficient nucleic acid delivery through inhalation.
Keywords: pulmonary delivery, gene delivery, non-viral vector, pH responsive, peptides, spray drying, spray freeze drying
1. INTRODUCTION
Nucleic acid therapy has the potential to treat a wide range of diseases affecting the airways including cystic fibrosis (CF) [1-3], lung cancer [4-6] and inflammatory diseases such as chronic obstructive respiratory disease (COPD) and asthma [7-10]. Inhalation is a desirable route of administration to deliver therapeutic nucleic acids to the lungs because of its non-invasive nature and lower endonuclease activity in the airways compared with the blood serum. In addition, direct application of therapeutic agents to the target site can minimize systemic adverse effects. Delivery however remains the biggest hurdle to nucleic acid therapy. Viral vectors are highly effective nucleic acid delivery agents, but the risk of insertional mutagenesis [11, 12] and high immunogenicity [13] have led researchers to seek safer alternatives. Non-viral vectors generally have a better safety profile compared with their viral counterparts but their transfection efficiency is often less than satisfactory for use in the clinic and more effective vectors for pulmonary delivery remain highly sought after.
Intracellular barriers pose a major challenge to nucleic acid delivery. Non-viral vectors usually enter cells through endocytosis [14] and, once inside cells, they are transported into the endosomes and eventually the lysosomes where acidification occurs and the degradative enzymes are activated. To ensure good transfection efficiency, therapeutic nucleic acids must be able to escape from the endosomes or lysosomes before degradation take place, or bypass the endosomal pathway completely. In our group, pH responsive peptides containing histidine or 2,3-diaminopropionic acid (Dap) as pH responsive elements are being investigated to deliver nucleic acids [15, 16]. These two structurally similar pH responsive peptides (LAH series and LADap series) are cationic amphipathic peptides and each peptide contains four or six pH responsive residues. They can form non-covalent complexes with nucleic acids and promote endosomal escape. In an acidic environment, peptides are released from the complexes and change their conformation enabling membrane destabilising activity. Subsequently, nucleic acids are released from the endosomal/lysosomal compartments into the cytoplasm [15, 17]. Our previous study has demonstrated that the pH responsive peptides are capable of mediating both highly efficient DNA or siRNA transfection in mammalian cell lines [15] (Abbate et al., unpublished). Others have shown that LAH4 peptides can effectively deliver nucleic acids to patient derived primary fibroblasts [18] and facilitate intracellular delivery in vivo by way of subcutaneous injection of protein-based vaccines adjuvanted with Toll-like receptor 9 agonist CpG oligonucleotide (CpG) to generate enhanced CD8+ T cell immune responses and antitumor effects [19]. With proven efficacy in delivery to a variety of cultured, primary cells and tumour cells together with the first example of in vivo tolerance and efficacy, we were interested to develop a pH responsive peptide-based formulation that is suitable for effective nucleic acid delivery by pulmonary administration.
Besides intracellular barriers, a further obstacle to pulmonary DNA delivery is the presence of airway surface liquid (ASL) which covers the epithelial cells along the respiratory tract [20]. This layer of liquid consists mainly of phospholipids and surfactant-associated proteins [21] which may affect the stability of the DNA complexes, and hence the delivery efficiency. Still more barriers to pulmonary delivery include the mucociliary clearance action of epithelial cells, the activity of macrophages [20, 22] and the high degree of branching of the respiratory tract, which prevents inhaled particles from entering the lower airways. In general, aerosol particles with aerodynamic diameters between 1-5 μm can achieve good lung deposition [22] and the choice of inhaler device therefore plays an important role in determining the success of aerosol delivery. Pulmonary delivery could be achieved by using nebulizers, metered dose inhalers (MDIs) and dry powder inhalers (DPIs). Dry powder aerosol formulation are preferred for macromolecules due to their higher stability during storage, better sterility, easy transportation compared with liquid formulations, and have been successfully used in formulation of other therapeutic macromolecules [23-26]. To prepare inhalable powder formulations of nucleic acids, either spray drying (SD) or spray freeze drying (SFD) are employed [27-30]. In the spray drying process, liquids are atomized into sprays which are rapidly evaporated by hot air-stream to give dry particles. The entire process can be completed as a single step in a spray dryer machine. On the contrary, spray freeze drying involves multiple steps; liquids are first sprayed into a cryogen such as liquid nitrogen and the droplets, which are frozen immediately, are transferred into a freeze dryer to allow the sublimation of ice, resulting in the formation of highly porous particles. Although both methods can produce inhalable dry powders and have been investigated to produce nucleic acid formulations for pulmonary delivery, the majority of these formulations used polymers and lipids as non-viral vectors [27-32] while much less work has been done to investigate the preparation of inhalable powders of nucleic acids with a peptide-based delivery vector.
In this study, the DNA delivery efficiency of LAH and LADap pH responsive peptides were evaluated on human lung epithelial cells (A549) with bronchoalveolar lavage fluid (BALF), obtained from rats, used as model to study the effect of ASL on transfection efficiency. For the formulation work, LAH4-L1 was used as a model for the class to investigate the feasibility of formulating the peptide/DNA system into dry powders for inhalation. Both SD and SFD were employed and the physicochemical characteristics, aerodynamic performances as well as the in vitro biological activities, in particular the ability to mediate delivery via a twin-state impinger, of these powder formulations were examined and compared.
2. MATERIALS AND METHODS
2.1 Materials
LAH4-L1 peptide (> 70% purity) was purchased from ChinaPeptide (Shanghai, China) and used as provided. For other peptides, their detailed synthetic procedures and characterisation are delineated in another manuscript (Abbate et al., unpublished). The sequences of LAH or LADap peptides used in this study are shown in Table 1. Plasmid DNA (gWIZ™ Luciferase) was purchased from Aldevron (Fargo, ND, USA). Mannitol (Pearlitol 160C) was purchased from Roquette (Lestrem, France). Dulbecco’s modified eagle medium (DMEM), OptiMEM-1 reduced serum medium, antibiotic-animycotic liquid, fetal bovine serum (FBS) and Lipofectamine™ 2000 were purchased from Invitrogen (CA, USA). The luciferase assay system was purchased from Promega (Madison, WI, USA). GelRed™ nucleic acid stain was purchased from Biotium (Hayward, CA, USA). All other reagents and solvents were purchased from Sigma (Poole, UK) and were of analytical grade or better.
Table 1. Sequences of LAH or LADap peptides used in this study.
pH responsive components are highlighted in bold. X = Dap, X1 = N-methyl Dap
| Peptide | Sequence |
|---|---|
| LAH4-L1 | KKALLAHALHLLALLALHLAHALKKA-NH2 |
| LAH6-X1L-W | KHKLLHLLHLLALLWLHLLHLLKHK-NH2 |
| LAH6-X1-L | KHKLLHLLHLLALLALHLLHLLKHK-NH2 |
| LADap4-L1 | KKALLAXALXLLALLALXLAXALKKA- NH2 |
| LADap(Me)4-L1 | KKALLAX1ALX1LLALLALX1LAX1ALKKA- NH2 |
| LADap6-L1 | KKXLLAXLLXLLALLALXLLXALKXK- NH2 |
| LADap(Me)6-L1 | KKX1LLAX1LLX1LLALLALX1LLX1ALKX1K-NH2 |
2.2 Cell Culture
A549 (human lung adenocarcinoma epithelial cells) were obtained from ATCC (Manassas, VA, USA). The cells were maintained at 5% CO2, 37°C in DMEM supplemented with 10% FBS, 100 units/ml penicillin, 100 μg/mL streptomycin and 0.25 μg/mL amphotericin B. The cells were subcultured twice weekly.
2.3 Preparation of peptide/DNA complexes
Peptide/DNA complexes were prepared at 10:1 ratio (w/w) for all the transfection experiments. Peptide solution and DNA solution were prepared separately in ultrapure water. Equal volumes of peptide solution and DNA solution were mixed to give peptide/DNA complexes with 0.02 mg/mL DNA concentration. The mixture was allowed to incubate for 30 min at room temperature before further processing.
2.4 Bronchoalveolar lavage fluid (BALF) collection
Bronchoalveolar lavage fluid (BALF) was collected by cannulating the trachea of rats (Sprague-Dawley) and washing the airway with 2 mL of PBS. The collected BALF was centrifuged and the supernatant was collected and stored on ice before use. All animal works were approved by the committee on the use of live animals in teaching and research (CULATR), the laboratory animal unit, The University of Hong Kong.
2.5 In vitro transfection in culture plate
A549 cells were transfected with peptide/DNA complexes at 1 μg DNA per well or dry powder formulations equivalent to 1 μg DNA per well in 24-well plates in OptiMEM-I reduced serum medium or 50% BALF (v/v). After 4 h of incubation at 37°C, the cells were washed with PBS. Fresh DMEM supplemented with 10% FBS were added to the cells. At 48 h post-transfection, the luciferase expression was detected using the luciferase assay system according to the manufacturer’s protocol. The cells were also transfected with Lipofectamine™ 2000 under the same condition.
2.6 Powders deposition and in vitro transfection in twin stage impinger
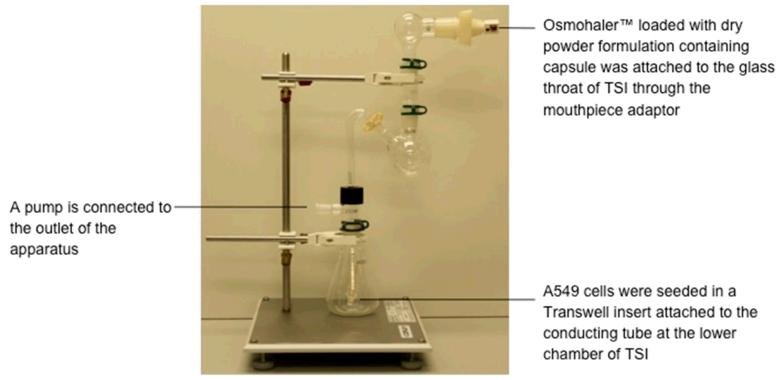
A glass twin stage impinger (TSI) (Copley Scientific, Nottingham, UK) was used to evaluate the aerosolisation and deposition, as well as the transfection efficiency of the powder formulations at the same time. The glass TSI was assembled as described in the British Pharmacopeia with minor modification (Fig. 1). A549 cells were seeded on Transwell inserts (6.5 mm diameter, 0.4 μm pore size, Costar, High Wycombe, UK) at a density of 30,000 cells/cm2 and were cultured for three days. Transwell insert (empty or seeded with cells) was fitted to the TSI conducting tube in the lower impingement chamber. Test powders were loaded into size 3 empty capsules and placed into a dry powder inhaler (Osmohaler™, Plastiape, Osnago, Italy) located in the mouthpiece adapter of the glass throat of TSI. A vacuum pump (DA-60D, ULVAC Kiko, Japan) was attached to the TSI which captured all the emitted particles from the inhaler. The powders were aerosolised at a flow rate of 60 L/min with the pump for 5 s, according to the British Pharmacopoeia procedure [33]. To evaluate powder deposition, the empty Transwell insert was removed and weighted after aerosolization. The amount of powder reached the Transwell was quantified by the weight difference of Transwell before and after powders deposition. It was compared to the initial amount of powders loaded into the empty capsules and the % (w/w) of deposition was calculated. For the in vitro transfection experiment, the cell containing Transwell insert was removed from the TSI and transferred to a well of a 24-well plate containing 1 mL of OptiMEM-1 reduced serum medium. 200 μL OptiMEM-1 reduced serum medium was gently added into the Transwell insert to dissolve all the powder adhering to its inner surface. After 4 h of incubation at 37°C, medium was changed as described before and the luciferase assay was carried out at 48 h post-transfection.
Fig. 1. The glass twin-stage impinger (TSI) setup for transfection experiment.
2.7 Spray drying and spray freeze drying
To prepare spray dried powders, a solution (Solution A) containing LAH4-L1/DNA complexes was prepared as described in Section 2.3. Another solution (Solution B) containing 20 mg/mL of mannitol in ultrapure water was made separately. Equal volumes of Solutions A and B were mixed together by gentle stirring just prior to spray drying. Thus the final concentrations of mannitol, peptide, and DNA were 10, 0.1, and 0.01 mg/mL, respectively. This solution was spray dried using a Büchi B-191 Mini Spray Dryer with a high performance cyclone (Büchi Labortechnik, Flawil, Switzerland) under the following conditions: inlet temperature of 50°C, outlet temperature of 28°C, aspiration at 100% (60 m3/h), compressed air atomisation flow rate at 750 NL/h, and liquid feed rate at 2 mL/min. The SD powder was collected and stored in a desiccator with silica gel at 4°C until further analysis. To prepare spray freeze dried powders, a solution containing mannitol/LAH4-L1/DNA was prepared as described in section 2.3. The solution was transferred into a syringe and was fed to an ultrasonic atomizer nozzle (130K50ST, Sonaer®, Farmingdale, NY, USA) operating at 130 kHz powered by digital ultrasonic generator for atomizers (Sonaer®, NY, USA) at a rate of 0.55 mL/min via an injection pump (Model 975, Harvard Apparatus, MA, USA). The sprayed liquid droplets were collected in liquid nitrogen to allow instant freezing. The liquid nitrogen containing the frozen droplets was subjected to two phases of freeze drying process. The samples first underwent freeze-drying under vacuum at −70°C for 5 days, followed by further drying at ambient temperature for 3 days. The SFD powders were collected and stored in desiccator with silica gel at 4°C until further analysis.
2.8 Extraction of DNA from powders and determination of DNA recovery
DNA was extracted from the SD or SFD powders by phenol/chloroform extraction method. In brief, the SD or SFD powders were dissolved in distilled water. SDS solution was added to the samples to allow the dissociation of DNA from the peptides. Equal volume of phenol/chloroform/isoamyl alcohol (25/24/1) was added. The samples were centrifuged and the upper aqueous phase was collected. Ice-cold 95% ethanol and sodium acetate solution were added to the aqueous layer. The mixtures were incubated at −80°C. The samples were then centrifuged. The supernatants were removed and the samples were washed with 70% ethanol. The pellet was air-dry at room temperature. The sample was reconstituted in sterile DEPC treated water and the extracted DNA was quantified by measuring the UV absorbance at 260 nm and 280 nm. The DNA recovery was determined as the difference in theoretical amount of DNA in dry powder formulations and the measured amount DNA after extraction. The efficiency of DNA extraction was not taken in account when calculating the percentage recovery.
2.9 Study of integrity of DNA by gel retardation assay and particle size analysis
The integrity of DNA was evaluated by agarose gel electrophoresis. The extracted DNA samples were loaded into a 1% agarose gel containing GelRed™ nucleic acids stains. Gel electrophoresis was run in TAE buffer at 100 V for 60 min and the gel was visualized under the UV illumination.
2.10 Particle size of dry powder and molecular complexes
Volume size distributions of the SD and SFD particles were determined in triplicate by laser diffraction on a Mastersizer 2000 laser diffractometer (Malvern Instruments, Worcestershire, UK). The powders were dispersed by a Scirocco 2000 dry powder feeder (Malvern Instruments, Worcestershire, UK) from 0.7 to 4.0 bar through the diffractometer. The particle refractive index and absorption were 1.544 and 0.1, respectively, which are the parameters for the major constituent, mannitol. The dispersant refractive index was 1.000 for air. The size data were expressed as D10, D50, D90, which are equivalent spherical volume diameters at 10%, 50% and 90% cumulative volume, respectively. The size distribution of LAH4-L1/DNA complexes after the reconstitution of SD and SFD powders was measured in triplicate by Photon Correlation Spectroscopy (Delsa™Nano C, Beckman Coulter, CA, USA). Dry powders were reconstituted in filtered ultrapure water to give a final volume of 50 μL containing 5 μg DNA. The size of LAH4-L1/DNA complexes, at the same DNA concentration as the dry powder formulations, was also measured as control.
2.11 Morphology study by SEM
SD and SFD powders were sprinkled onto carbon sticky tape mounted on SEM stubs and sputter coated with approximately 11 nm palladium/gold coating. The surface morphology of the powders was observed by field emission scanning electron microscopy (SEM) at 2.0 kV (Hitachi S-4800 FEG, Hitachi, Tokyo, Japan).
2.12 In vitro aerosol performance
The in vitro aerosol performance of SD and SFD particles was tested by dispersing the powders into a Next Generation Impactor (NGI) at 100 L/min of air flow for 2.4 s, according to the British Pharmacopoeia procedure [33]. Size 3 hydroxypropyl methylcellulose capsules (Capsugel, West Ryde, NSW, Australia) were used for powder loading. Each capsule contained 2.0 ± 1.0 mg of powder and two capsules were used per run. The runs were performed in triplicate. The impactor stages were coated with silicon grease (Slipicone; DC Products, Waverley, VIC, Australia) prior to testing to minimise particle bounce. After dispersion, the powder deposits on each inhaler and NGI part were exhaustively washed with 4 mL of deionised water. The solutions were assayed by high performance liquid chromatography (HPLC). The HPLC system comprised of a Shimadzu CBM-20A controller, LC-20AT pump, SIL-20A HT autosampler, RID-10A refractive index detector and LCsolution Version 1.24 SP1 control and analysis software (Shimadzu Corporation, Kyoto, Japan). Samples (100 μL) were injected into a Resolve C-18 column (150 mm × 3.9 mm, 5 μm; Waters, Milford, MA) with deionised water as the mobile phase pumped at a flow rate of 1 mL/min. The standard solutions were made with the SD and SFD powders. Since refractive index detection is species non-specific, using the powders as their own standards took into account the presence of all ingredients in the samples. The limit of detection of this method was 4 μg/mL of powder. The sum of powder mass assayed on all inhaler and NGI parts was the recovered dose. The emitted fraction (EF) was the fraction of powder that exited the inhaler with respect to the recovered dose. The fine particle fraction (FPF) was defined as the mass fraction of particles < 5.0 μm with respect to the recovered dose. At 100 L/min, the upper aerodynamic cutoff diameters of Stages 2 and 3 are 6.12 and 3.42 μm, respectively. The cumulative mass fraction < 5.0 μm (i.e. the FPF) was obtained by interpolation between the two cumulative mass fractions corresponding to the cutoff diameters for Stages 2 and 3 at the respective flow rate.
2.13 Statistical Analysis
Statistical testing was performed by Prism software (version 5.0d) and the data were analysed by one-way ANOVA followed by Bonferroni’s post test.
3. RESULTS
3.1 DNA transfection mediated by pH responsive peptides in the presence of BALF
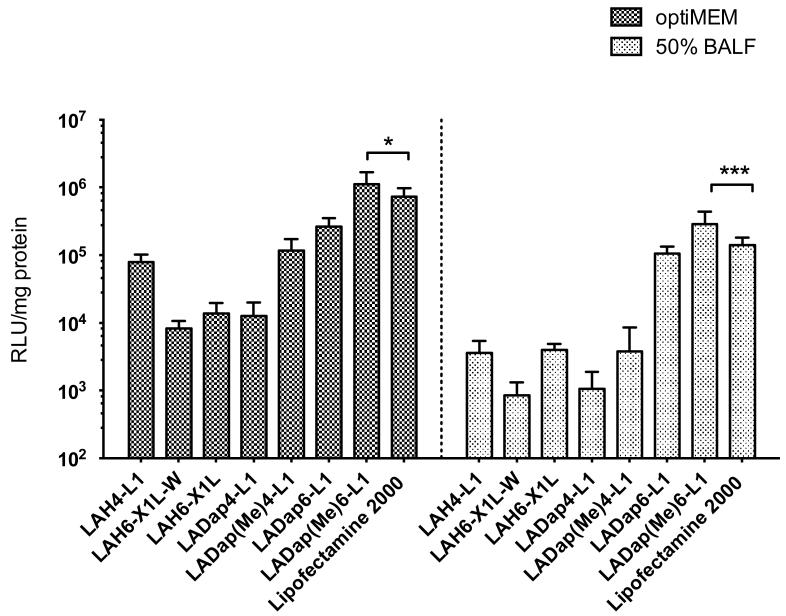
The DNA transfection efficiency of pH responsive peptides was assessed in A549 cells in OptiMEM-1 reduced serum medium or in the presence of 50% BALF (Fig. 2). In general, LADap peptides performed substantially better than the LAH peptides. Within the LAH series, LAH4-L1 had the highest transfection efficiency (7.9 × 104 RLU/mg protein) in OptiMEM-1 reduced serum medium. Among all the peptides tested, LADap(Me)6-L1 had the highest transfection efficiency (1.1 × 106 RLU/mg protein) and performed significantly better than Lipofectamine™ 2000 (p < 0.05). In the presence of 50% BALF, there was a significant reduction of transfection efficiency with all the peptides as well as with Lipofectamine™ 2000. The biggest reduction in transfection was observed with LAH4-L1 (35-fold reduction), and the lowest reduction of transfection was observed with LADap6-L1 (2-fold reduction). However, LADap(Me)6-L1 retained the highest transfection efficiency (2.8 × 105 RLU/mg protein) and performed significantly better than Lipofectamine™ 2000 (p < 0.001) with both LADap(Me)6-L1 and Lipofectamine™ 2000 suffering from an approximate 4-fold reduction in transfection efficiency after the addition of 50% BALF. For reasons of cost and availability, LAH4-L1 was used as a representative of the pH responsive peptide class for the formulation studies.
Fig. 2. DNA transfection of A549 cells mediated by pH responsive peptides in OptiMEM-1 or 50% BALF.
Each well of a 24-well plate contained 1 μg of luciferase plasmid DNA. Lipofectamine™ 2000 was used as control. Transfection efficiency and protein level were evaluated at 48 h post-transfection. Transfection efficiency expressed as relative light unit (RLU)/mg protein. Bars shown as mean ± standard deviation (n = 6). Significance difference was determined using one way ANOVA analysis followed by Bonferroni’s post test. *p < 0.05, ***p < 0.001.
3.2 Recovery and integrity of DNA in SD and SFD powders
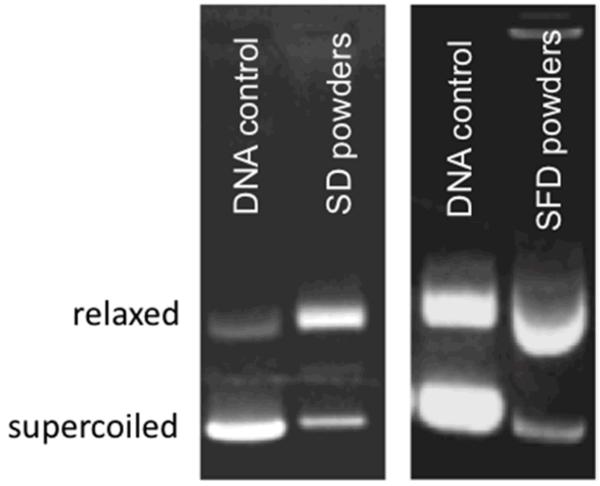
After the extraction and purification of DNA from the dry powder formulations, the amount of DNA was quantified by UV absorbance and the % recovery was calculated. The DNA recovery for SD and SFD powders were 76.1 % and 82.7% respectively (Table 2). The A260/280 ratio was close to 1.8 in both samples, indicating little protein contamination of the extracted DNA samples. To ensure that the integrity of DNA was successfully preserved during the drying processes, gel electrophoresis of DNA was performed after extraction from the powders and release from LAH4-L1 peptides. Compared with the control, the DNA extracted from the powders remained intact since low molecular weight DNA fragments were not detected on the gel (Fig. 3). However, it was noticed that the DNA extracted from SD or SFD powders adopted the more relaxed structure instead of the original supercoiled structure, indicating that both drying processes had some effect on the conformation of DNA.
Table 2. The percentage recovery and UV absorbance of DNA after extraction from spray dried (SD) and spray freeze dried (SFD) formulations.
DNA was extracted and quantified after spray drying and spray freeze drying process. The amount of DNA in the dry powder formulation after extraction was compared to the initial theoretical DNA amount and the recovery in % (w/w) was calculated.
| Samples | DNA recovery | A260/280 |
|---|---|---|
| SD powders | 76.1% | 1.82 |
| SFD powders | 82.7% | 1.74 |
Fig. 3. Gel retardation assay of plasmid DNA extracted from spray dried (SD) and spray freeze dried (SFD) formulations on 1% agarose gel.
DNA was stained with GelRed™ stain. The conformation of the extracted DNA was compared with the control DNA.
3.3 Particle size distribution and morphology
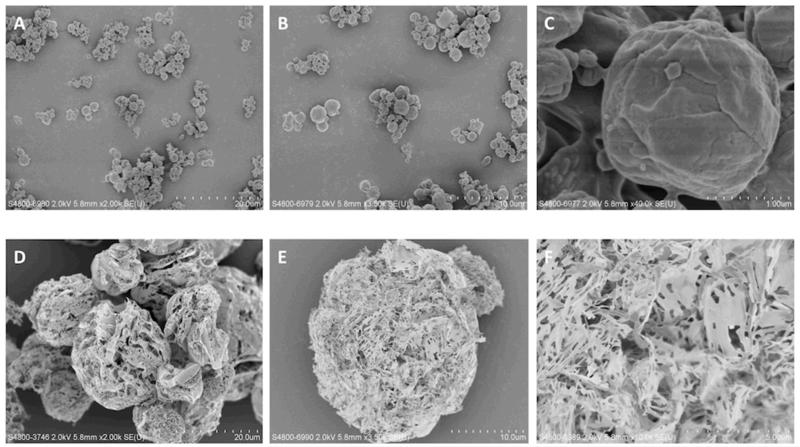
Particle size distributions of SD and SFD powders at various dispersion pressures are plotted in Fig. 4. The D90 of SD particles reduced with increasing dispersion pressure, while the D10 and D50 remained relatively stable. No agglomerates > 10 μm were present in this powder either. This indicates that inter-particulate cohesion for this powder was low. On the other hand, SFD particles were larger and their sizes decreased markedly with dispersion pressure (Fig. 4). The particle size reduction might be due to deagglomeration and/or fragmentation of the primary particles. The difference in the SD and SFD particle sizes is clearly shown in the SEM images (Fig. 5). Both types of particle were spherical overall. However, the SD particles were small with a slightly wrinkled surface, while the SFD particles were large and porous. The particle and agglomerate sizes observed under the SEM for both samples were corroborated by their respective laser diffraction data. SD and SFD powders were reconstituted in distilled water and the particle size distribution of the LAH4-L1/DNA complexes was measured (Table 3). The complexes sizes were compared with the fresh LAH4-L1/DNA complexes at the same DNA concentration as the reconstituted samples. The sizes of DNA complexes of the reconstituted SD and SFD samples were 212.4 ± 3.3 nm and 163.2 ± 12.6 nm respectively. Both of formulated complexes were larger than the fresh DNA complexes which were around 133 ± 6.5 nm. The polydispersity indexes of the samples were similar, all within the range of 0.20 to 0.30.
Fig. 4. Particle size profiles of spray dried (SD) and spray freeze dried (SFD) formulations determined by laser diffraction.
Data presented as mean ± standard deviation (n = 3).
Fig. 5. SEM images of spray dried (SD) and spray freeze dried (SFD) formulations.
(A) SD powders at × 2,000 magnification; (B) SD powders at × 3,500 magnification; (C) SD powders at × 40,000 magnification; (D) SFD powders at × 2,000 magnification; (E) SFD powders at × 3,500 magnification; (F) SFD powders at × 10,000 magnification.
Table 3. Particle size analysis of LAH4-L1/DNA complexes.
The hydrodynamic diameter and polydispersity index of DNA complexes were measured by photon correlation spectroscopy (PCS) after the spray dried (SD) or spray freeze dried (SFD) powders were reconstituted in distilled water. Fresh LAH-L1/DNA complexes were also measured for comparison. Data presented as mean ± standard deviation (n = 3).
| Samples | Hydrodynamic diameter (nm) |
Polydispersity Index |
|---|---|---|
| LAH4-L1/DNA complexes | 133.1 ± 6.5 | 0.283 ± 0.02 |
| DNA complexes of reconstituted SD powders | 212.4 ± 3.3 | 0.200 ± 0.04 |
| DNA complexes of reconstituted SFD powders | 163.2 ± 12.6 | 0.241 ± 0.01 |
3.4 Aerodynamic performance
The NGI dispersion data are plotted in Fig. 6. Although the EF of the SFD powder was higher, the FPFs of both powders were approximately 50%. This was because the SFD powder showed high deposition on Stage 1 (cutoff diameter > 6.12 μm), which was too large to be considered as fine particles suitable for inhalation. The percentage of powders deposited in lower chamber of TSI was shown in Table 4. 4.4 ± 1.3 % of SD powders reached the lower chamber. SFD formulation had 17.8 ± 2.8 % powders deposited in the lower chamber, which was significantly better than SD powders.
Fig. 6. NGI dispersion data of spray dried (SD) (white bars) and spray freeze dried (SFD) (grey bars) formulations.
Data presented as mean ± standard deviation (n = 3). S1–S7 denotes impactor stages 1–7, followed by the corresponding upper aerodynamic cutoff diameter in parentheses. MOC is the micro-orifice collector in the NGI.
Table 4. The deposition of spray dried (SD) and spray freeze dried (SFD) formulations in the lower chamber of twin stage impinger (TSI).
SD or SFD powders were loaded into size 3 empty capsules, aerosolized by Osmohaler™ and deposited in the Transwell in TSI. The amount of powder reached the Transwell was compared to the initial amount of powders loaded into the capsules, and the % (w/w) of deposition was calculated. Data presented as mean ± standard deviation (n = 3)
| Sample | Powder deposition in lower chamber of TSI |
|---|---|
| SD powders | 4.4 ± 1.3 % |
| SFD powders | 17.8 ± 2.8 % |
3.5 In vitro transfection activity of SD and SFD powders
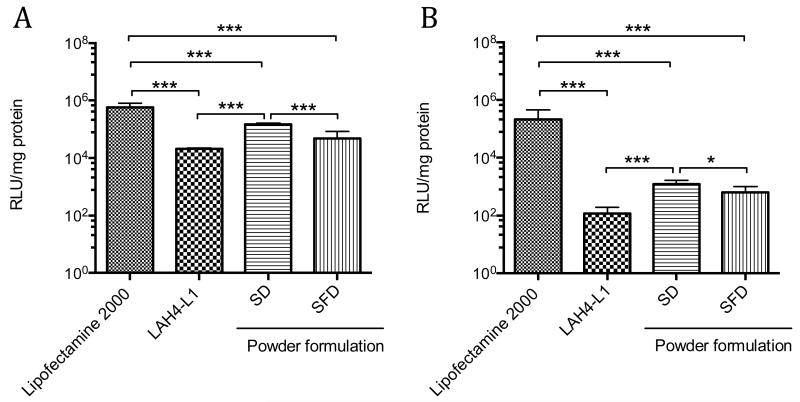
The transfection efficiency of the SD and SFD powders was determined in A549 cells. The powders were reconstituted either in OptiMEM-1 medium or 50% BALF (Fig. 7) before added to the cells. The transfection efficiency of both dry powder formulations after reconstitution in OptiMEM-1 was not inferior to the freshly prepared LAH4-L1/DNA complexes at equivalent amount of DNA per well, indicating that the SD or SFD process did not deteriorate the biological activities of the DNA. The luciferase gene expression of SD formulation was indeed significantly enhanced by approximately 7.0-fold compared with the freshly prepared LAH4-L1/DNA complexes (p < 0.001). In addition, the luciferase gene expression of SD formulation was also significantly higher than that of SFD formulation (p < 0.001). However, no significant difference was observed between SFD formulation and the fresh LAH4-L1/DNA complexes. When SD and SFD powders were reconstituted in 50% BALF, the transfection efficiency was significantly reduced for both formulations in comparison to the reconstitution in OptiMEM-1. The transfection efficiency of SD powders in 50% BALF was significantly higher than the fresh LAH4-L1/DNA complexes (p < 0.001) as well as the SFD powders (p < 0.05), but no significant difference was observed between the SFD formulation and the fresh LAH4-L1/DNA complexes.
Fig. 7. In vitro transfection of powder formulations on A549 cells.
SD or SFD powders were reconstituted in (A) OptiMEM-1 or (B) 50% BALF before added to the cells. Each well of 24-well plate contained sample equivalent to 1 μg of DNA. Freshly prepared DNA complexes with Lipofectamine™ 2000 and LAH4-L1 were used as control. Transfection efficiency and protein level were evaluated at 48 h post-transfection. Transfection efficiency expressed as relative light unit (RLU)/mg protein. Bars shown as mean ± SD (n = 6-8). Significance difference was determined using one way ANOVA analysis followed by Bonferroni’s post test. *p < 0.05, ***p < 0.001
To compare the toxicity of the samples to the A549 cells, the protein levels of the transfected cells were examined (Fig. S1). It was apparent that the cells transfected with Lipofectamine™ 2000 had significantly lower cellular protein level than all of the LAH4-L1 peptide formulations, despite the same initial seeding density and culture condition of all samples, suggesting that the Lipofectamine™ 2000 was substantially more cytotoxic than the LAH4-L1 peptide.
The effect of mannitol on transfection efficiency was investigated (Fig. S2). The presence of mannitol improved the transfection efficiency in a dose dependent manner. The luciferase gene expression level was enhanced by 1.3-fold, 1.6-fold and 2.1-fold at mannitol to DNA ratios (w/w) of 1000/1, 3000/1 and 5000/1 respectively. A significant difference was observed at ratios 3000/1 and 5000/1 only.
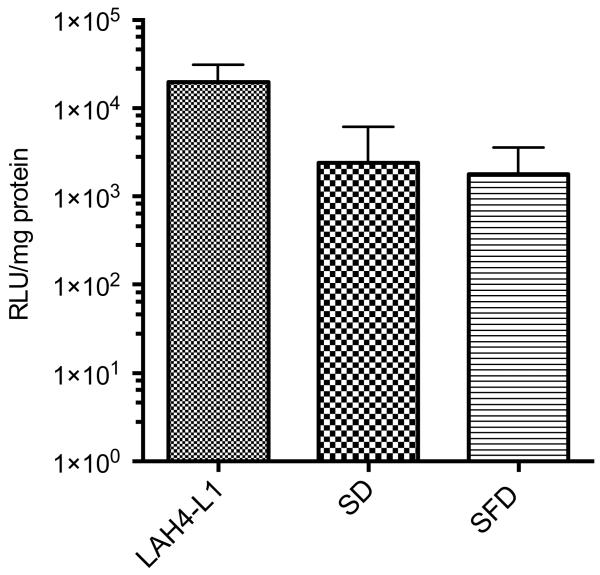
The transfection of SD or SFD powders after reconstitution did not take into account the dispersity and aerodynamic properties of the powders. To evaluate the gene delivery efficiency of the formulations closer to the physiological condition, the in vitro transfection study was performed in TSI (Fig. 8). Control LAH4-L1/DNA complexes were directly added to the Transwell insert without going through the TSI setup. The luciferase gene expression levels of both SD and SFD powder formulations were lower compared with the control, probably because only a proportion of powders reached the cells in the lower chamber. However, no significant difference was established between the control LAH4-L1/DNA complexes and either of the dry powder formulations.
Fig. 8. In vitro transfection of powder formulations on A549 cells.
Transfection was performed in twin stage impinger (TSI). A549 cells were seeded in Transwell at the lower chamber. SD or SFD powders were aerosolized by Osmohaler™ and deposited to the cells in TSI. LAH4-L1/DNA complexes which were added directly to the cells in Transwell insert were served as control. Transfection efficiency and protein level were evaluated at 48 h post-transfection. Transfection efficiency expressed as relative light unit (RLU)/mg protein. Bars shown as mean ± SD (n = 3). No significant difference was observed between groups using one way ANOVA analysis followed by Bonferroni’s post test.
4. DISCUSSION
In this work, we aimed to develop a peptide-based DNA delivery system for pulmonary delivery via inhalation. Previous studies have already demonstrated that pH responsive peptides are effective in mediating DNA transfection in a variety of mammalian cell lines [15]. Here we further investigate the suitability of the pH responsive peptides for delivering DNA to airway tissues. Since the ASL covering the airway epithelial cells represents an early barrier for DNA delivery following pulmonary administration, we examined the effect of ASL on DNA transfection of the peptides using BALF collected from rats as model. BALF is a representative sample of aqueous components continuously covering the alveolar epithelium [34]. Similar to ASL, the major components of BALF are phospholipids (90%), mainly phophatidylcholins, and proteins (10%) including variable serum proteins and surfactant proteins [20, 35]. Pulmonary surfactant layer poses one of the critical barriers for the transfection efficiency of non-viral gene delivery. Inhibition of luciferase plasmid delivery by BALF was observed with all the peptides tested as well as with Lipofectamine™ 2000. This could be attributed to the presence of negatively charged proteins and surfactants in BALF, which may alter the overall charges of DNA complexes, leading to the reduction of interaction between DNA complexes and surfaces, or causing premature dissociation of the non-covalent peptide/DNA complexes [36-38]. In general, peptides containing six pH responsive residues were found to be more effective in resisting the deleterious effect of BALF as has been observed previously when similarly assessing the affect of serum [39]. In particular, delivery mediated by LADap6-L1 and LADap(Me)6-L1 were the least affected by the presence of BALF and these peptides provided the highest overall transfection efficiency in A549 cells. LADap(Me)6-L1 continued to outperform Lipofectamine™ 2000 in the presence of BALF, and this, together with its low associated cytotoxicity (Abbate et al., unpublished data), makes it a very promising candidate for lung delivery.
The feasibility of formulating the peptide-based DNA system as dry powder for inhalation was explored. For reasons of cost and availability, LAH4-L1 was selected as a representative of the pH responsive peptide class and dry powders of LAH4-L1/DNA were prepared by both spray drying and spray freeze drying. Both formulations contained the LAH4-L1 peptide and mannitol at the same mannitol/LAH4-L1/DNA ratio of 1000/10 /1 (w/w/w). Mannitol has emerged as a promising carrier in inhalable dry powder formulation. It is less hygroscopic than the commonly used lactose and, more importantly, does not have a reducing sugar function which makes it more compatible with the peptide vector in our formulations [40]. One of the major considerations with the preparation of DNA powder formulations is to maintain the stability of DNA, especially the physical stability. Breakage of DNA strands could disrupt the gene sequences and affect their transcription and the subsequent translation of functional proteins. Among the various drying techniques employed by the pharmaceutical industry, spray drying and spray freeze drying are the most commonly used methods to produce dry powder aerosols of biological macromolecules [41, 42]. Spray drying is a one-step process that is easier for industrial scale-up, but the molecules being dried are inevitably exposed to harsh environment such as raised temperature and high shear stress, thereby increasing the risk of DNA damage. In contrast, spray freeze drying is more suitable for thermolabile biological molecules due to its milder conditions but it is less favoured on an industrial scale as this multi-step process is time consuming and expensive.
The DNA recovery of the SD and SFD formulations was evaluated and was found to be around 80% for both formulations. Although the recovery of DNA was good, the calculation was based on UV absorbance at 260 / 280 nm which did not reveal whether the plasmid DNA remained intact after the drying procedures. Gel electrophoresis provides information on the integrity of the plasmid DNA. The DNA extracted from both SD and SFD powders remained physically intact, suggesting that DNA of the formulations could withstand the physical stress during the two drying procedures. However, a change of DNA conformation was observed, with the plasmid DNA converted from the supercoiled structure to the relaxed structure. It has been previously reported that the level of supercoiling of plasmid DNA has no significant effect in the transfection efficiency of a non-viral vector [43].
One of the crucial factors that determine the success of the inhalable dry powder formulations is the particle size distribution which strongly affects the aerodynamic performance and lung deposition of the powders. Various techniques were employed here to characterize the particle size distribution and aerosol performance of SD and SFD powders. In general, the powders produced by both drying methods were spherical with the size and morphology of the two types of particles conforming to that expected from their respective particle production methods. Spray freeze drying produces large, porous particles [41, 44], which, due to their porous structures, are relatively fragile; thus fragmentation of the primary particles during the laser diffraction measurements may be possible. Despite their large geometric diameters, SFD particles possess low aerodynamic diameters because of their low densities. This was reflected in the similar FPFs of the two powders produced in this study even though their geometric diameters differed by about 10-fold. In addition, since large particles have better flowability, more SFD powder emptied from the capsule and inhaler than the SD powder, and achieved a higher EF and better deposition in the lower chamber of TSI. However, some large agglomerates were likely to be present in the emitted SFD dose because the deposition on Stage 1 was higher than that of the SD powder.
Both formulations investigated in this study contained 98.9% mannitol by mass. However, the surfaces of the resultant particles did not show the typical finger-like nano-scale crystals on SD or SFD pure mannitol particles [44, 45]. Rather, the SD particles produced in the current study appeared to have a wrinkled coating while the SFD particles were composed of porous, folded sheets. This may be due to surface enrichment by LAH4-L1/DNA complexes; when a solution droplet containing macromolecules (e.g. peptides and nucleic acids) and small molecular excipients (e.g. mannitol) is drying, the latter would diffuse more rapidly into the core of the particle [46]. Therefore, the dried particle surface would contain a higher proportion of the macromolecular materials that rendered the mannitol crystals indistinct. It should be noted that both particle production methods employed in this study subjected the peptide and DNA to thermal and mechanical stresses with the macromolecules being exposed to heating and freezing in SD and SFD, respectively. Moreover, the spraying and drying in both techniques exposed the peptide and DNA to potential mechanical shear and air-liquid surface denaturation. The biological activity of the LAH4-L1/DNA complexes was not affected by these treatments. Interestingly, the level of luciferase gene expression of SD formulation was significantly enhanced in comparison to the freshly prepared LAH4-L1/DNA complexes. The presence of mannitol, which was the sole extra excipient in the powder formulations, could partly explain the increase in transfection efficiency. Hyperosmotic mannitol solution is known to be able to open up tight junctions between adjacent cells, therefore increasing the accessibility of DNA complexes to the cell surface [47, 48] and the increase in transfection appeared in a mannitol dose dependent manner. However the mannitol in our powder formulations would have been present at a final concentration of 0.25% (w/v) and this may be too low to produce any prominent osmotic effect to the cells.
Another possibility is that the size distribution and structures of LAH4-L1/DNA complexes changed during the drying (and freezing in case of SFD) and reconstitution processes, as the cellular uptake is highly dependent on the particle size. Rejman et al. [49] used a range of latex beads of defined sizes to investigate the effect of particle size on cellular uptake pathway. It was found that as the particle size increased from 200 nm to 500 nm, the cellular uptake pathway shifted from clathrin-mediated endocytosis to caveolae-mediated endocytosis. The latter route was claimed to be a more efficient pathway for DNA delivery, possibly because the materials internalized through this route can avoid the degradative endosomes/lysosomes [50-52]. Our previous study showed that LAH4-L1/DNA complexes enter cells predominantly via clathrin-mediated endocytosis. On the contrary, LAH4-L1/siRNA complexes, which have larger particle size than DNA complexes, were found to enter cells mainly through caveolae-mediated endocytosis [15]. In this study, the particle size of DNA complexes of the reconstituted SD powders and SFD powders were larger than that of the freshly prepared complexes and it is possible that a higher proportion of DNA complexes of the reconstituted SD and SFD powders entered the cells through the more efficient caveolae-mediated endocytosis, thus achieving higher transfection efficiency. A similar phenomenon was observed by Mishra et al. [53] who found that the reconstitution of lyophilised micelle/DNA/PEI complexes significantly enhanced the transfection efficiencies up to 16 times in MCF-7 cells compared to the freshly prepared counterparts and this was related to higher cellular uptake.
In the presence of 50% BALF, both SD and SFD formulations of LAH4-L1/DNA complexes experienced significant reduction of luciferase protein expression. This was not surprising as the LAH4-L1 peptide was sensitive to the adverse effect of BALF. More importantly, substantial transfection of the two formulations was observed, and the performance of SD formulation in BALF was still superior to the freshly prepared LAH4-L1/DNA complexes and SFD formulation. The result confirmed the biological activities of the dry powder formulations.
The physicochemical characterisation and the transfection studies of the reconstituted dry powders demonstrate that both SD and SFD formulations are suitable for inhalation with good transfection efficiency. To further investigate the biological activities and the aerodynamic properties of the formulations at the same time, transfection experiment was also carried out in a TSI setting in which the powders were aerosolised and dispersed from an inhaler device. Such an experiment provides invaluable information that cannot be obtained in animal studies since there are considerable anatomical and physiological differences between the respiratory tracts of animals (such as the frequently used rodents) and humans. To evaluate the likely performance of inhaled drug powder formulations in vivo, intratracheal administration or Dry Powder Insufflator™ are typically employed [54]. These methods bypass the extrathoracic deposition and the aerosols are always well deposited into the lungs of animals. Such results could sometimes be misleading as the aerodynamic properties of the particles, which are critical in determining the success of the aerosol formulations, are not considered. In contrast, TSI (which is described in the British Pharmacopoeia) is a simple apparatus that is commonly used to evaluate the aerodynamic performance of inhaler devices and formulations. It can be easily adapted to study the biological activities of aerosol particles on airway epithelial cells [55, 56]. Transfection studies in a TSI setting takes into account the powder dispersion and deposition, as well as the biological activities of the powders.
TSI was adapted to accommodate A549 cells in the lower chamber where respirable powders of an aerodynamic diameter of < 6.4 μm are deposited [57]. After aerosolization a significant amount of powders was likely to be lost in the capsule shells, inhaler devices, adaptors and other parts of the TSI without reaching the cells, as demonstrated in the dispersion study with NGI. Transfection results showed that almost a 10-fold reduction in the level of gene expression compared with the control was observed with both dry powder formulations with control LAH4-L1/DNA complexes directly added to the Transwell without going through the TSI. In addition, a larger proportion of SFD powders successfully reached the cell layer on the Transwell of the TSI than SD powders. This explains why the transfection efficiency in TSI between SD and SFD powders did not have any significant difference even though the in vitro transfection efficiency of SD powders was superior to that of SFD powders. Nevertheless, the aerosol dispersions and the biological activities of the dry powder formulations were evident and, though reduced, transfection remained effective.
When comparing the overall performance of the SD and SFD powders, the former appeared to be superior in several aspects. From the aspect of industrial manufacture, spray drying is always more desirable as the process is simpler, cheaper and easier to scale up and here, the SFD powders appeared to be more fragile than the SD powders due to their highly porous structures which could be damaged relatively easily. This poses a challenge to maintain the long-term physical stability of SFD powders during manufacturing, transportation, handling and storage. The transfection efficiency of the SD powders was also significantly higher than SFD powders. In addition, the SD powders were of a narrower and better controlled particle size distribution.
5. CONCLUSIONS
Robust gene delivery is afforded by pH responsive peptides in the presence of BALF used to model the affect of airway surface liquid. Inhalable, pH responsive, peptide-based, plasmid DNA dry powder formulations can be successfully produced by spray drying and spray freeze drying using mannitol as carrier. Both formulations showed promising aerodynamic properties and biological activities but spray dried powders were physically more robust with better transfection efficiency and higher industrial potential.
Supplementary Material
6. ACKNOWLEDGEMENTS
This work was supported by Seed Funding Programme for Basic Research (No. 201111159114), The University of Hong Kong. The authors thanked Dr Judith Mak (LKS Faculty of Medicine, The University of Hong Kong) for her assistance with the BALF collection. The authors thank Dr Vincenzo Abbate and Dr Sukhi Bansal for the synthesis of LADap peptides which was supported by MRC NIRG G0801072/87482 (to AJM).
REFERENCES
- [1].Sinn PL, Anthony RM, McCray PB., Jr. Genetic therapies for cystic fibrosis lung disease. Hum. Mol. Genet. 2011;20:R79–86. doi: 10.1093/hmg/ddr104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Zhang L, Button B, Gabriel SE, Burkett S, Yan Y, Skiadopoulos MH, Dang YL, Vogel LN, McKay T, Mengos A, Boucher RC, Collins PL, Pickles RJ. CFTR delivery to 25% of surface epithelial cells restores normal rates of mucus transport to human cystic fibrosis airway epithelium. PLoS Biol. 2009;7:e1000155. doi: 10.1371/journal.pbio.1000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Johnson LG, Olsen JC, Sarkadi B, Moore KL, Swanstrom R, Boucher RC. Efficiency of gene transfer for restoration of normal airway epithelial function in cystic fibrosis. Nat. Genet. 1992;2:21–25. doi: 10.1038/ng0992-21. [DOI] [PubMed] [Google Scholar]
- [4].Moon C, Oh Y, Roth JA. Current status of gene therapy for lung cancer and head and neck cancer. Clin. Cancer Res. 2003;9:5055–5067. [PubMed] [Google Scholar]
- [5].Vachani A, Moon E, Wakeam E, Albelda SM. Gene therapy for mesothelioma and lung cancer. Am. J. Respir. Cell Mol. Biol. 2010;42:385–393. doi: 10.1165/rcmb.2010-0026RT. [DOI] [PubMed] [Google Scholar]
- [6].Sumimoto H, Yamagata S, Shimizu A, Miyoshi H, Mizuguchi H, Hayakawa T, Miyagishi M, Taira K, Kawakami Y. Gene therapy for human small-cell lung carcinoma by inactivation of Skp-2 with virally mediated RNA interference. Gene Ther. 2005;12:95–100. doi: 10.1038/sj.gt.3302391. [DOI] [PubMed] [Google Scholar]
- [7].Al-Jamal R, Wallace WA, Harrison DJ. Gene therapy for chronic obstructive pulmonary disease: twilight or triumph? Expert Opin. Biol. Ther. 2005;5:333–346. doi: 10.1517/14712598.5.3.333. [DOI] [PubMed] [Google Scholar]
- [8].Huang HY, Chiang BL. siRNA as a therapy for asthma. Curr. Opin. Mol. Ther. 2009;11:652–663. [PubMed] [Google Scholar]
- [9].Factor P. Gene therapy for asthma. Mol. Ther. 2003;7:148–152. doi: 10.1016/s1525-0016(03)00003-0. [DOI] [PubMed] [Google Scholar]
- [10].Parry-Billings M, Ferrari N, Seguin R. Oligonucleotides: New therapeutic approaches for asthma and chronic obstructive pulmonary disease. Curr. Opin. Investig. Drugs. 2010;11:1276–1285. [PubMed] [Google Scholar]
- [11].Dave UP, Jenkins NA, Copeland NG. Gene therapy insertional mutagenesis insights. Science. 2004;303:333–333. doi: 10.1126/science.1091667. [DOI] [PubMed] [Google Scholar]
- [12].Kohn DB, Sadelain M, Glorioso JC. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat. Rev. Cancer. 2003;3:477–488. doi: 10.1038/nrc1122. [DOI] [PubMed] [Google Scholar]
- [13].Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11:S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- [14].Khalil IA, Kogure K, Akita H, Harashima H. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol. Rev. 2006;58:32–45. doi: 10.1124/pr.58.1.8. [DOI] [PubMed] [Google Scholar]
- [15].Lam JK, Liang W, Lan Y, Chaudhuri P, Chow MY, Witt K, Kudsiova L, Mason AJ. Effective endogenous gene silencing mediated by pH responsive peptides proceeds via multiple pathways. J. Control. Release. 2012;158:293–303. doi: 10.1016/j.jconrel.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Lan Y, Langlet-Bertin B, Abbate V, Vermeer LS, Kong X, Sullivan KE, Leborgne C, Scherman D, Hider RC, Drake AF, Bansal SS, Kichler A, Mason AJ. Incorporation of 2,3-diaminopropionic acid into linear cationic amphipathic peptides produces pH-sensitive vectors. Chembiochem. 2010;11:1266–1272. doi: 10.1002/cbic.201000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Langlet-Bertin B, Leborgne C, Scherman D, Bechinger B, Mason AJ, Kichler A. Design and Evaluation of Histidine-Rich Amphipathic Peptides for siRNA Delivery. Pharm. Res. 2010;27:1426–1436. doi: 10.1007/s11095-010-0138-2. [DOI] [PubMed] [Google Scholar]
- [18].Gerard X, Perrault I, Hanein S, Silva E, Bigot K, Defoort-Delhemmes S, Rio M, Munnich A, Scherman D, Kaplan J, Kichler A, Rozet J-M. AON-mediated Exon Skipping Restores Ciliation in Fibroblasts Harboring the Common Leber Congenital Amaurosis CEP290 Mutation. Mol. Ther. Nucleic Acids. 2012;1:e29. doi: 10.1038/mtna.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Zhang TT, Kang TH, Ma B, Xu Y, Hung CF, Wu TC. LAH4 enhances CD8+ T cell immunity of protein/peptide-based vaccines. Vaccine. 2012;30:784–793. doi: 10.1016/j.vaccine.2011.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sanders N, Rudolph C, Braeckmans K, De Smedt SC, Demeester J. Extracellular barriers in respiratory gene therapy. Adv. Drug Deliv. Rev. 2009;61:115–127. doi: 10.1016/j.addr.2008.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Goerke J. Pulmonary surfactant: functions and molecular composition. Biochim. Biophys. Acta. 1998;1408:79–89. doi: 10.1016/s0925-4439(98)00060-x. [DOI] [PubMed] [Google Scholar]
- [22].Lam JK, Liang W, Chan HK. Pulmonary delivery of therapeutic siRNA. Adv. Drug Deliv. Rev. 2012;64:1–15. doi: 10.1016/j.addr.2011.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Mastrandrea LD, Quattrin T. Clinical evaluation of inhaled insulin. Adv. Drug Deliv. Rev. 2006;58:1061–1075. doi: 10.1016/j.addr.2006.07.019. [DOI] [PubMed] [Google Scholar]
- [24].Codrons V, Vanderbist F, Verbeeck RK, Arras M, Lison D, Preat V, Vanbever R. Systemic delivery of parathyroid hormone (1-34) using inhalation dry powders in rats. J. Pharm. Sci. 2003;92:938–950. doi: 10.1002/jps.10346. [DOI] [PubMed] [Google Scholar]
- [25].Bai SH, Gupta V, Ahsan F. Inhalable Lactose-Based Dry Powder Formulations of Low Molecular Weight Heparin. J. Aerosol Med. Pulm. Drug Deliv. 2010;23:97–104. doi: 10.1089/jamp.2009.0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Rawat A, Majumder QH, Ahsan F. Inhalable large porous microspheres of low molecular weight heparin: In vitro and in vivo evaluation. J. Control. Release. 2008;128:224–232. doi: 10.1016/j.jconrel.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Jensen DMK, Cun D, Maltesen MJ, Frokjaer S, Nielsen H.M.r., Foged C. Spray drying of siRNA-containing PLGA nanoparticles intended for inhalation. J. Control. Release. 2010;142:138–145. doi: 10.1016/j.jconrel.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mohri K, Okuda T, Mori A, Danjo K, Okamoto H. Optimized pulmonary gene transfection in mice by spray-freeze dried powder inhalation. J. Control. Release. 2010;144:221–226. doi: 10.1016/j.jconrel.2010.02.018. [DOI] [PubMed] [Google Scholar]
- [29].Seville PC, Kellaway IW, Birchall JC. Preparation of dry powder dispersions for nonviral gene delivery by freeze-drying and spray-drying. J. Gene Med. 2002;4:428–437. doi: 10.1002/jgm.282. [DOI] [PubMed] [Google Scholar]
- [30].Kuo JH, Hwang R. Preparation of DNA dry powder for non-viral gene delivery by sprayfreeze drying: effect of protective agents (polyethyleneimine and sugars) on the stability of DNA. J. Pharm. Pharmacol. 2004;56:27–33. doi: 10.1211/0022357022494. [DOI] [PubMed] [Google Scholar]
- [31].Li HY, Neill H, Innocent R, Seville P, Williamson I, Birchall JC. Enhanced dispersibility and deposition of spray-dried powders for pulmonary gene therapy. J. Drug Target. 2003;11:425–432. doi: 10.1080/10611860410001659786. [DOI] [PubMed] [Google Scholar]
- [32].Jensen DK, Jensen LB, Koocheki S, Bengtson L, Cun D, Nielsen HM, Foged C. Design of an inhalable dry powder formulation of DOTAP-modified PLGA nanoparticles loaded with siRNA. J. Control. Release. 2012;157:141–148. doi: 10.1016/j.jconrel.2011.08.011. [DOI] [PubMed] [Google Scholar]
- [33].Commission BP, Council GM, Commission GBM. British pharmacopoeia. Her Majesty’s Stationery Office; 2001. [Google Scholar]
- [34].Rosenecker J, Naundorf S, Gersting S, Hauck R, Gessner A, Nicklaus P, Müller R, Rudolph C. Interaction of bronchoalveolar lavage fluid with polyplexes and lipoplexes: analysing the role of proteins and glycoproteins. J. Gene Med. 2003;5:49–60. doi: 10.1002/jgm.291. [DOI] [PubMed] [Google Scholar]
- [35].Stark B, Andreae F, Mosgoeller W, Edetsberger M, Gaubitzer E, Koehler G, Prassl R. Liposomal vasoactive intestinal peptide for lung application: protection from proteolytic degradation. Eur. J. Pharm. Biopharm. 2008;70:153–164. doi: 10.1016/j.ejpb.2008.04.015. [DOI] [PubMed] [Google Scholar]
- [36].Roesnecker J, Naundorf S, Gersting SW, Hauck RW, Gessner A, Nicklaus P, Muller RH, Rudolph C. Interaction of bronchoalveolar lavage fluid with polyplexes and lipoplexes: analysing the role of proteins and glycoproteins. J. Gene Med. 2003;5:49–60. doi: 10.1002/jgm.291. [DOI] [PubMed] [Google Scholar]
- [37].Duncan JE, Whitsett JA, Horowitz AD. Pulmonary surfactant inhibits cationic liposome-mediated gene delivery to respiratory epithelial cells in vitro. Hum. Gene Ther. 1997;8:431–438. doi: 10.1089/hum.1997.8.4-431. [DOI] [PubMed] [Google Scholar]
- [38].Ernst N, Ulrichskotter S, Schmalix WA, Radler J, Galneder R, Mayer E, Gersting S, Plank C, Reinhardt D, Rosenecker J. Interaction of liposomal and polycationic transfection complexes with pulmonary surfactant. J. Gene Med. 1999;1:331–340. doi: 10.1002/(SICI)1521-2254(199909/10)1:5<331::AID-JGM60>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- [39].Mason AJ, Leborgne C, Moulay G, Martinez A, Danos O, Bechinger B, Kichler A. Optimising histidine rich peptides for efficient DNA delivery in the presence of serum. J. Control. Release. 2007;118:95–104. doi: 10.1016/j.jconrel.2006.12.004. [DOI] [PubMed] [Google Scholar]
- [40].Saint-Lorant G, Leterme P, Gayot A, Flament MP. Influence of carrier on the performance of dry powder inhalers. Int. J. Pharm. 2007;334:85–91. doi: 10.1016/j.ijpharm.2006.10.028. [DOI] [PubMed] [Google Scholar]
- [41].Maa YF, Nguyen PA, Sweeney T, Shire SJ, Hsu CC. Protein inhalation powders: spray drying vs spray freeze drying. Pharm. Res. 1999;16:249–254. doi: 10.1023/a:1018828425184. [DOI] [PubMed] [Google Scholar]
- [42].Pilcer G, Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. Int. J. Pharm. 2010;392:1–19. doi: 10.1016/j.ijpharm.2010.03.017. [DOI] [PubMed] [Google Scholar]
- [43].Bergan D, Galbraith T, Sloane DL. Gene transfer in vitro and in vivo by cationic lipids is not significantly affected by levels of supercoiling of a reporter plasmid. Pharm. Res. 2000;17:967–973. doi: 10.1023/a:1007531405796. [DOI] [PubMed] [Google Scholar]
- [44].D’Addio SM, Chan JG, Kwok PC, Prud’homme RK, Chan HK. Constant size, variable density aerosol particles by ultrasonic spray freeze drying. Int. J. Pharm. 427:185–191. doi: 10.1016/j.ijpharm.2012.01.048. [DOI] [PubMed] [Google Scholar]
- [45].Adi H, Kwok PC, Crapper J, Young PM, Traini D, Chan HK. Does electrostatic charge affect powder aerosolisation? J. Pharm. Sci. 99:2455–2461. doi: 10.1002/jps.21996. [DOI] [PubMed] [Google Scholar]
- [46].Kwok PC, Chan HK. Pulmonary delivery of proteins and peptides. In: Van de Walle CF, editor. Peptide and Protein Delivery. Elsevier; Amsterdam: 2011. pp. 23–46. [Google Scholar]
- [47].Duchler M, Pengg M, Brunner S, Muller M, Brem G, Wagner E. Transfection of epithelial cells is enhanced by combined treatment with mannitol and polyethyleneglycol. J. Gene Med. 2001;3:115–124. doi: 10.1002/jgm.171. [DOI] [PubMed] [Google Scholar]
- [48].Nilsson H, Dragomir A, Ahlander A, Johannesson M, Roomans GM. Effects of hyperosmotic stress on cultured airway epithelial cells. Cell Tissue Res. 2007;330:257–269. doi: 10.1007/s00441-007-0482-7. [DOI] [PubMed] [Google Scholar]
- [49].Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin-and caveolae-mediated endocytosis. Biochem. J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Bathori G, Cervenak L, Karadi I. Caveolae - An alternative endocytotic pathway for targeted drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 2004;21:67–95. doi: 10.1615/critrevtherdrugcarriersyst.v21.i2.10. [DOI] [PubMed] [Google Scholar]
- [51].van der Aa M, Huth US, Hafele SY, Schubert R, Oosting RS, Mastrobattista E, Hennink WE, Peschka-Suss R, Koning GA, Crommelin DJA. Cellular uptake of cationic polymer-DNA complexes via caveolae plays a pivotal role in gene transfection in COS-7 cells. Pharm. Res. 2007;24:1590–1598. doi: 10.1007/s11095-007-9287-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol. Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- [53].Mishra D, Kang HC, Bae YH. Reconstitutable charged polymeric (PLGA)(2)-b-PEI micelles for gene therapeutics delivery. Biomaterials. 2011;32:3845–3854. doi: 10.1016/j.biomaterials.2011.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Sakagami M. In vivo, in vitro and ex vivo models to assess pulmonary absorption and disposition of inhaled therapeutics for systemic delivery. Adv. Drug Deliv. Rev. 2006;58:1030–1060. doi: 10.1016/j.addr.2006.07.012. [DOI] [PubMed] [Google Scholar]
- [55].Grainger CI, Greenwell LL, Martin GP, Forbes B. The permeability of large molecular weight solutes following particle delivery to air-interfaced cells that model the respiratory mucosa. Eur. J. Pharm. Biopharm. 2009;71:318–324. doi: 10.1016/j.ejpb.2008.09.006. [DOI] [PubMed] [Google Scholar]
- [56].Ong HX, Traini D, Bebawy M, Young PM. Epithelial profiling of antibiotic controlled release respiratory formulations. Pharm. Res. 28:2327–2338. doi: 10.1007/s11095-011-0462-1. [DOI] [PubMed] [Google Scholar]
- [57].Grainger CI, Greenwell LL, Lockley DJ, Martin GP, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm. Res. 2006;23:1482–1490. doi: 10.1007/s11095-006-0255-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.