Abstract
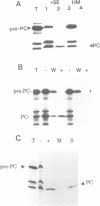
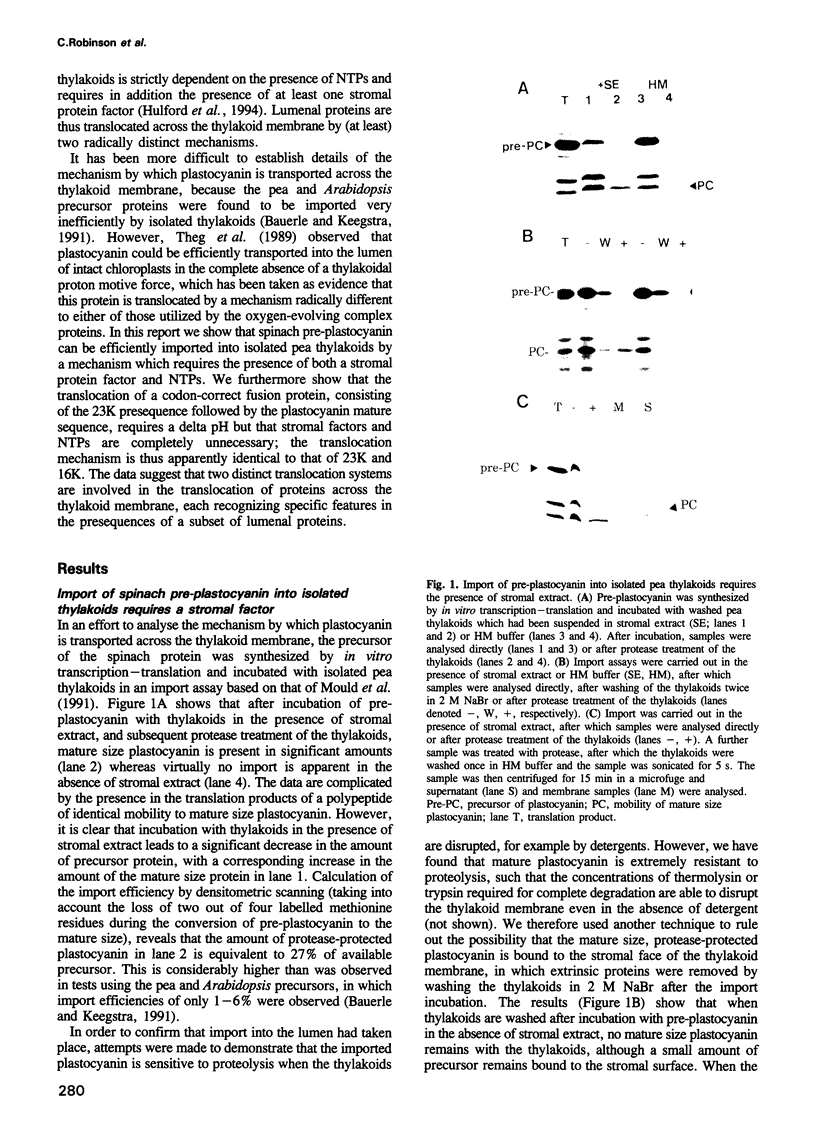
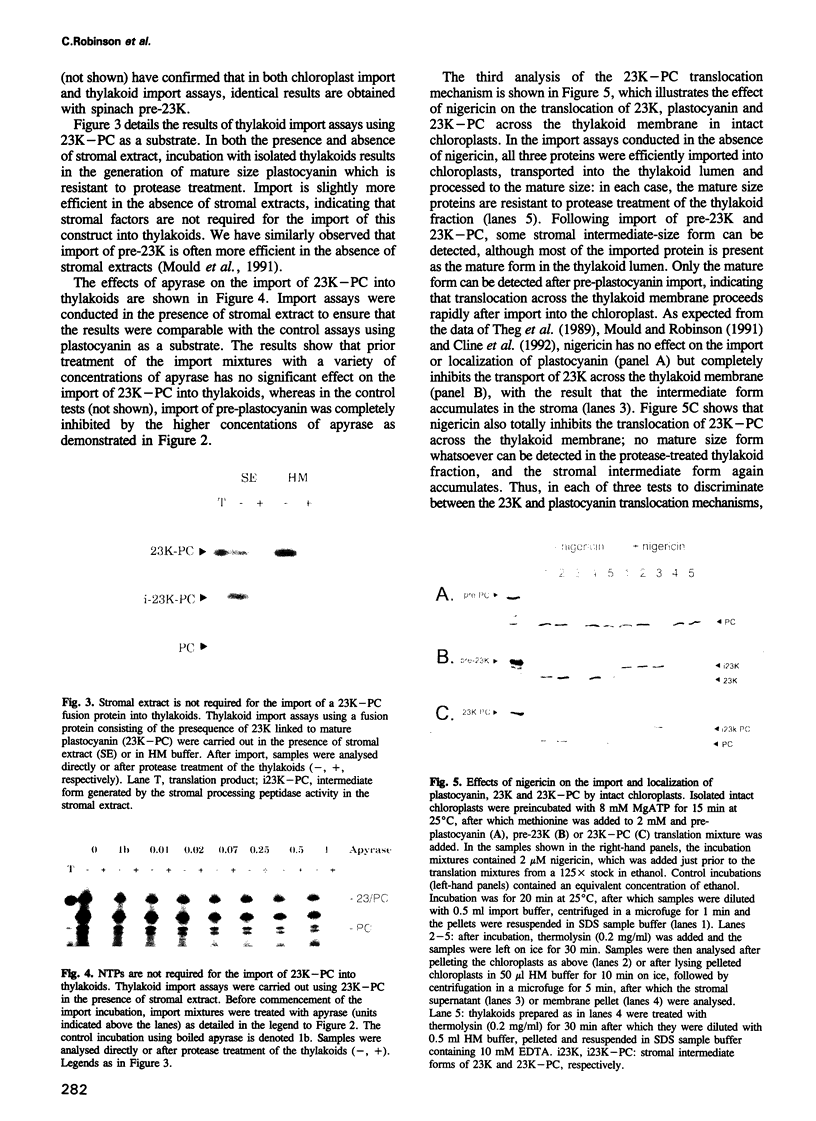
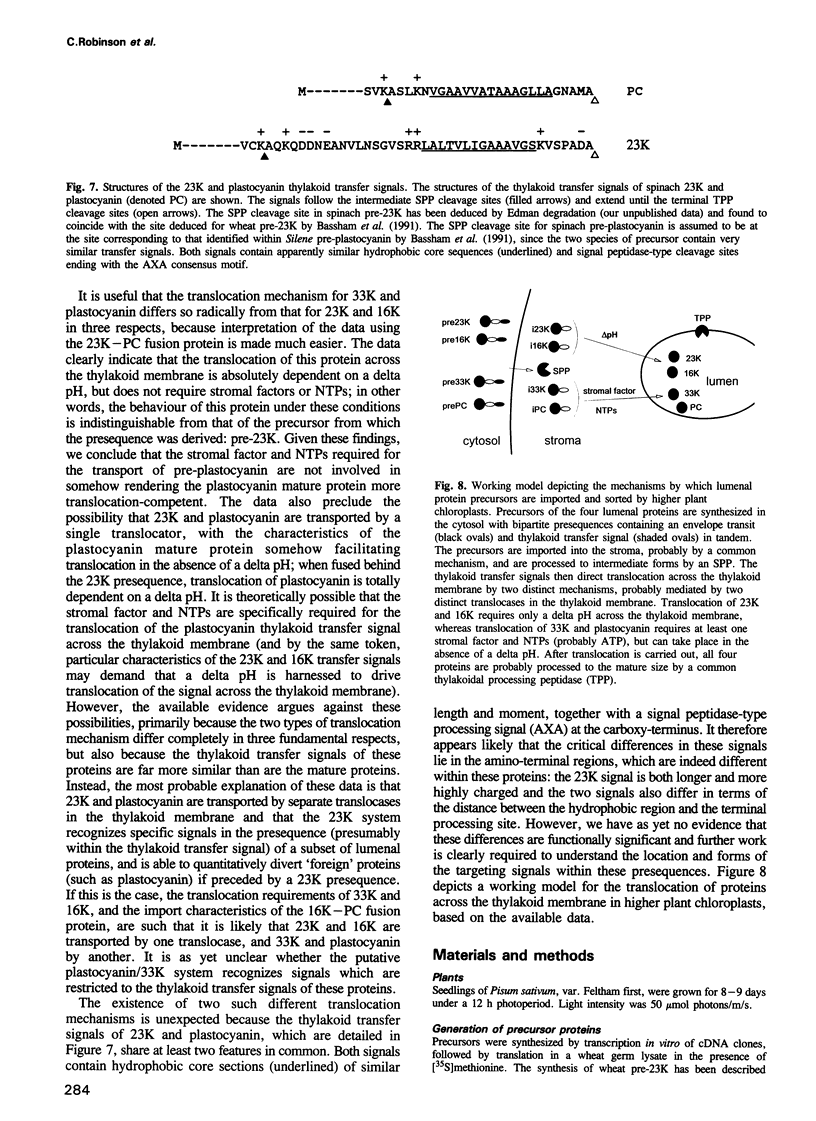
The translocation of plastocyanin across the thylakoid membrane in Pisum sativum has been studied in reconstitution assays and using chimeric constructs. The reconstitution assays demonstrate that plastocyanin translocation is absolutely dependent on the presence of a stromal factor(s) and nucleotide triphosphates (NTPs), whereas neither element is required for the translocation of the 23 or 16 kDa proteins of the oxygen-evolving complex. Previous studies had revealed that the transthylakoidal delta pH is essential for translocation of the 23 and 16 kDa proteins but unnecessary for plastocyanin translocation. The basis for these mechanistic differences has been tested by analysing the translocation of a chimeric construct consisting of the presequence of the 23 kDa protein linked to the mature plastocyanin sequence. This construct is efficiently imported into thylakoids in the absence of stromal extracts or NTPs and translocation across the thylakoid membrane within intact chloroplasts is totally inhibited by the uncoupler nigericin: the translocation requirements are thus identical to those of the pre-23 kDa protein and diametrically opposite to those of pre-plastocyanin. Transport across the thylakoid membrane of a second fusion protein, consisting of the presequence of the 16 kDa protein linked to mature plastocyanin, is also dependent on a delta pH. The data suggest that two distinct systems are involved in the translocation of proteins across the thylakoid membrane, with each system recognizing specific signals within the presequences of a subset of lumenal protein precursors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bassham D. C., Bartling D., Mould R. M., Dunbar B., Weisbeek P., Herrmann R. G., Robinson C. Transport of proteins into chloroplasts. Delineation of envelope "transit" and thylakoid "transfer" signals within the pre-sequences of three imported thylakoid lumen proteins. J Biol Chem. 1991 Dec 15;266(35):23606–23610. [PubMed] [Google Scholar]
- Bauerle C., Keegstra K. Full-length plastocyanin precursor is translocated across isolated thylakoid membranes. J Biol Chem. 1991 Mar 25;266(9):5876–5883. [PubMed] [Google Scholar]
- Clausmeyer S., Klösgen R. B., Herrmann R. G. Protein import into chloroplasts. The hydrophilic lumenal proteins exhibit unexpected import and sorting specificities in spite of structurally conserved transit peptides. J Biol Chem. 1993 Jul 5;268(19):13869–13876. [PubMed] [Google Scholar]
- Cline K., Ettinger W. F., Theg S. M. Protein-specific energy requirements for protein transport across or into thylakoid membranes. Two lumenal proteins are transported in the absence of ATP. J Biol Chem. 1992 Feb 5;267(4):2688–2696. [PubMed] [Google Scholar]
- Hageman J., Baecke C., Ebskamp M., Pilon R., Smeekens S., Weisbeek P. Protein Import into and Sorting inside the Chloroplast Are Independent Processes. Plant Cell. 1990 May;2(5):479–494. doi: 10.1105/tpc.2.5.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpin C., Elderfield P. D., James H. E., Zimmermann R., Dunbar B., Robinson C. The reaction specificities of the thylakoidal processing peptidase and Escherichia coli leader peptidase are identical. EMBO J. 1989 Dec 1;8(12):3917–3921. doi: 10.1002/j.1460-2075.1989.tb08572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G., Merchant S. Maturation of thylakoid lumen proteins proceeds post-translationally through an intermediate in vivo. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1862–1866. doi: 10.1073/pnas.90.5.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James H. E., Bartling D., Musgrove J. E., Kirwin P. M., Herrmann R. G., Robinson C. Transport of proteins into chloroplasts. Import and maturation of precursors to the 33-, 23-, and 16-kDa proteins of the photosynthetic oxygen-evolving complex. J Biol Chem. 1989 Nov 25;264(33):19573–19576. [PubMed] [Google Scholar]
- Klösgen R. B., Brock I. W., Herrmann R. G., Robinson C. Proton gradient-driven import of the 16 kDa oxygen-evolving complex protein as the full precursor protein by isolated thylakoids. Plant Mol Biol. 1992 Mar;18(5):1031–1034. doi: 10.1007/BF00019226. [DOI] [PubMed] [Google Scholar]
- Ko K., Cashmore A. R. Targeting of proteins to the thylakoid lumen by the bipartite transit peptide of the 33 kd oxygen-evolving protein. EMBO J. 1989 Nov;8(11):3187–3194. doi: 10.1002/j.1460-2075.1989.tb08477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows J. W., Robinson C. The full precursor of the 33 kDa oxygen-evolving complex protein of wheat is exported by Escherichia coli and processed to the mature size. Plant Mol Biol. 1991 Dec;17(6):1241–1243. doi: 10.1007/BF00028739. [DOI] [PubMed] [Google Scholar]
- Mould R. M., Robinson C. A proton gradient is required for the transport of two lumenal oxygen-evolving proteins across the thylakoid membrane. J Biol Chem. 1991 Jul 5;266(19):12189–12193. [PubMed] [Google Scholar]
- Mould R. M., Shackleton J. B., Robinson C. Transport of proteins into chloroplasts. Requirements for the efficient import of two lumenal oxygen-evolving complex proteins into isolated thylakoids. J Biol Chem. 1991 Sep 15;266(26):17286–17289. [PubMed] [Google Scholar]
- Musgrove J. E., Elderfield P. D., Robinson C. Endopeptidases in the stroma and thylakoids of pea chloroplasts. Plant Physiol. 1989 Aug;90(4):1616–1621. doi: 10.1104/pp.90.4.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rother C., Jansen T., Tyagi A., Tittgen J., Herrmann R. G. Plastocyanin is encoded by an uninterrupted nuclear gene in spinach. Curr Genet. 1986;11(3):171–176. doi: 10.1007/BF00420603. [DOI] [PubMed] [Google Scholar]
- Seidler A., Michel H. Expression in Escherichia coli of the psbO gene encoding the 33 kd protein of the oxygen-evolving complex from spinach. EMBO J. 1990 Jun;9(6):1743–1748. doi: 10.1002/j.1460-2075.1990.tb08298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S., Bauerle C., Hageman J., Keegstra K., Weisbeek P. The role of the transit peptide in the routing of precursors toward different chloroplast compartments. Cell. 1986 Aug 1;46(3):365–375. doi: 10.1016/0092-8674(86)90657-4. [DOI] [PubMed] [Google Scholar]
- Theg S. M., Bauerle C., Olsen L. J., Selman B. R., Keegstra K. Internal ATP is the only energy requirement for the translocation of precursor proteins across chloroplastic membranes. J Biol Chem. 1989 Apr 25;264(12):6730–6736. [PubMed] [Google Scholar]
- von Heijne G., Steppuhn J., Herrmann R. G. Domain structure of mitochondrial and chloroplast targeting peptides. Eur J Biochem. 1989 Apr 1;180(3):535–545. doi: 10.1111/j.1432-1033.1989.tb14679.x. [DOI] [PubMed] [Google Scholar]