Abstract
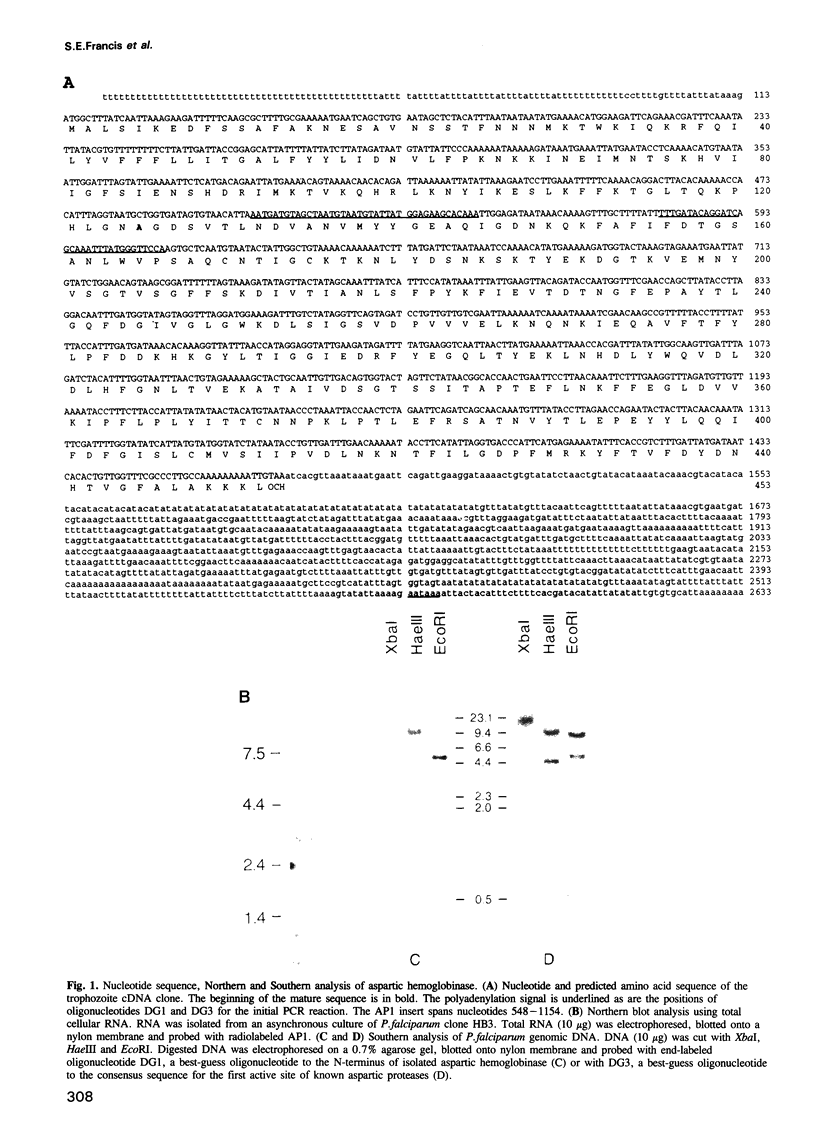
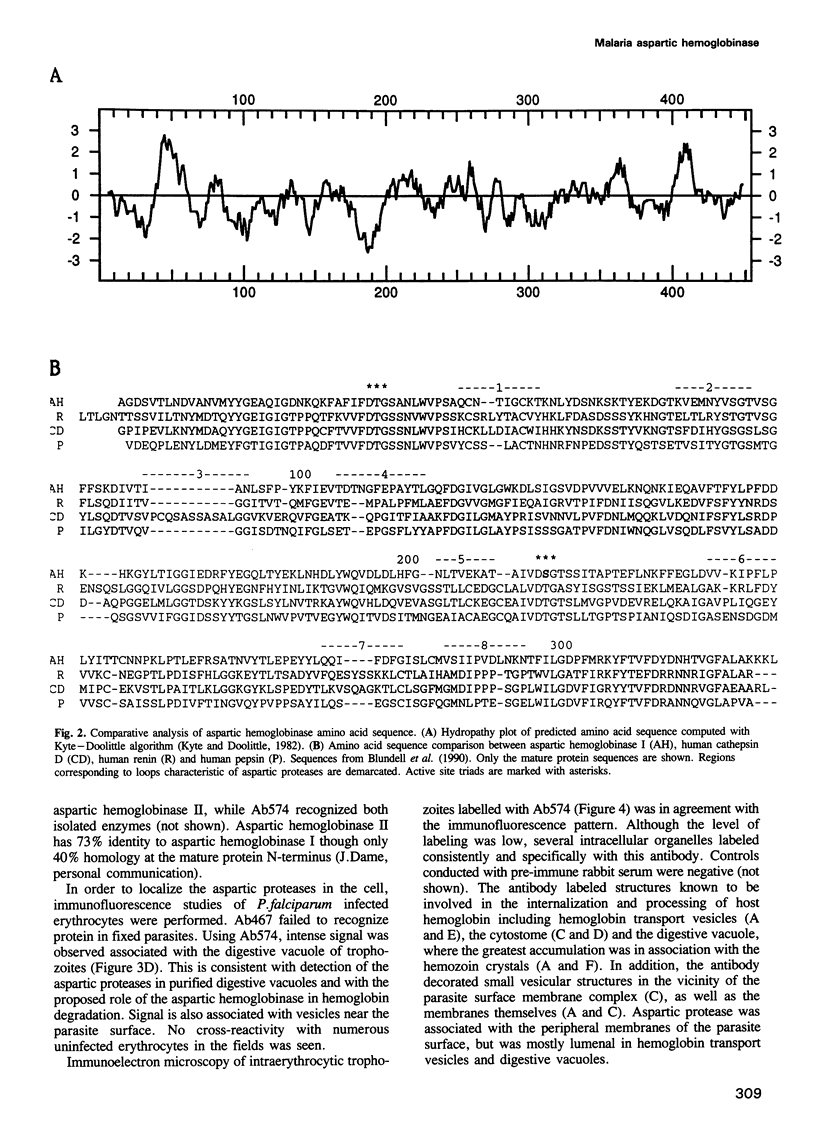
Intraerythrocytic malaria parasites rapidly degrade virtually all of the host cell hemoglobin. We have cloned the gene for an aspartic hemoglobinase that initiates the hemoglobin degradation pathway in Plasmodium falciparum. It encodes a protein with 35% homology to human renin and cathepsin D, but has an unusually long pro-piece that includes a putative membrane spanning anchor. Immunolocalization studies place the enzyme in the digestive vacuole and throughout the hemoglobin ingestion pathway, suggesting an unusual protein targeting route. A peptidomimetic inhibitor selectively blocks the aspartic hemoglobinase, prevents hemoglobin degradation and kills the organism. We conclude that Plasmodium hemoglobin catabolism is a prime target for antimalarial chemotherapy and have identified a lead compound towards this goal.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashorn P., McQuade T. J., Thaisrivongs S., Tomasselli A. G., Tarpley W. G., Moss B. An inhibitor of the protease blocks maturation of human and simian immunodeficiency viruses and spread of infection. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7472–7476. doi: 10.1073/pnas.87.19.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blundell T. L., Jenkins J. A., Sewell B. T., Pearl L. H., Cooper J. B., Tickle I. J., Veerapandian B., Wood S. P. X-ray analyses of aspartic proteinases. The three-dimensional structure at 2.1 A resolution of endothiapepsin. J Mol Biol. 1990 Feb 20;211(4):919–941. doi: 10.1016/0022-2836(90)90084-Y. [DOI] [PubMed] [Google Scholar]
- Braun M., Waheed A., von Figura K. Lysosomal acid phosphatase is transported to lysosomes via the cell surface. EMBO J. 1989 Dec 1;8(12):3633–3640. doi: 10.1002/j.1460-2075.1989.tb08537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowman A. F., Karcz S., Galatis D., Culvenor J. G. A P-glycoprotein homologue of Plasmodium falciparum is localized on the digestive vacuole. J Cell Biol. 1991 Jun;113(5):1033–1042. doi: 10.1083/jcb.113.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desjardins R. E., Canfield C. J., Haynes J. D., Chulay J. D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob Agents Chemother. 1979 Dec;16(6):710–718. doi: 10.1128/aac.16.6.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divo A. A., Geary T. G., Davis N. L., Jensen J. B. Nutritional requirements of Plasmodium falciparum in culture. I. Exogenously supplied dialyzable components necessary for continuous growth. J Protozool. 1985 Feb;32(1):59–64. doi: 10.1111/j.1550-7408.1985.tb03013.x. [DOI] [PubMed] [Google Scholar]
- Dottavio-Martin D., Ravel J. M. Radiolabeling of proteins by reductive alkylation with [14C]formaldehyde and sodium cyanoborohydride. Anal Biochem. 1978 Jul 1;87(2):562–565. doi: 10.1016/0003-2697(78)90706-6. [DOI] [PubMed] [Google Scholar]
- Fujioka H., Aikawa M. Morphological changes of clefts in Plasmodium-infected erythrocytes under adverse conditions. Exp Parasitol. 1993 May;76(3):302–307. doi: 10.1006/expr.1993.1036. [DOI] [PubMed] [Google Scholar]
- Goldberg D. E. Hemoglobin degradation in Plasmodium-infected red blood cells. Semin Cell Biol. 1993 Oct;4(5):355–361. doi: 10.1006/scel.1993.1042. [DOI] [PubMed] [Google Scholar]
- Goldberg D. E., Slater A. F., Beavis R., Chait B., Cerami A., Henderson G. B. Hemoglobin degradation in the human malaria pathogen Plasmodium falciparum: a catabolic pathway initiated by a specific aspartic protease. J Exp Med. 1991 Apr 1;173(4):961–969. doi: 10.1084/jem.173.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg D. E., Slater A. F., Cerami A., Henderson G. B. Hemoglobin degradation in the malaria parasite Plasmodium falciparum: an ordered process in a unique organelle. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2931–2935. doi: 10.1073/pnas.87.8.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui G. S., Palmer K. L., Siddiqui W. A. Use of human plasma for continuous in vitro cultivation of Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1984;78(5):625–626. doi: 10.1016/0035-9203(84)90222-0. [DOI] [PubMed] [Google Scholar]
- Jacobs G. H., Oduola A. M., Kyle D. E., Milhous W. K., Martin S. K., Aikawa M. Ultrastructural study of the effects of chloroquine and verapamil on Plasmodium falciparum. Am J Trop Med Hyg. 1988 Jul;39(1):15–20. doi: 10.4269/ajtmh.1988.39.15. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krogstad D. J., Gluzman I. Y., Kyle D. E., Oduola A. M., Martin S. K., Milhous W. K., Schlesinger P. H. Efflux of chloroquine from Plasmodium falciparum: mechanism of chloroquine resistance. Science. 1987 Nov 27;238(4831):1283–1285. doi: 10.1126/science.3317830. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lambros C., Vanderberg J. P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979 Jun;65(3):418–420. [PubMed] [Google Scholar]
- Lingappa V. R. More than just a channel: provocative new features of protein traffic across the ER membrane. Cell. 1991 May 17;65(4):527–530. doi: 10.1016/0092-8674(91)90081-9. [DOI] [PubMed] [Google Scholar]
- Lipp J., Dobberstein B. Signal recognition particle-dependent membrane insertion of mouse invariant chain: a membrane-spanning protein with a cytoplasmically exposed amino terminus. J Cell Biol. 1986 Jun;102(6):2169–2175. doi: 10.1083/jcb.102.6.2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKay V. L., Welch S. K., Insley M. Y., Manney T. R., Holly J., Saari G. C., Parker M. L. The Saccharomyces cerevisiae BAR1 gene encodes an exported protein with homology to pepsin. Proc Natl Acad Sci U S A. 1988 Jan;85(1):55–59. doi: 10.1073/pnas.85.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf P., Fusek M. Two crystal structures for cathepsin D: the lysosomal targeting signal and active site. EMBO J. 1993 Apr;12(4):1293–1302. doi: 10.1002/j.1460-2075.1993.tb05774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orjih A. U., Banyal H. S., Chevli R., Fitch C. D. Hemin lyses malaria parasites. Science. 1981 Nov 6;214(4521):667–669. doi: 10.1126/science.7027441. [DOI] [PubMed] [Google Scholar]
- Parks G. D., Lamb R. A. Topology of eukaryotic type II membrane proteins: importance of N-terminal positively charged residues flanking the hydrophobic domain. Cell. 1991 Feb 22;64(4):777–787. doi: 10.1016/0092-8674(91)90507-u. [DOI] [PubMed] [Google Scholar]
- Peters C., Braun M., Weber B., Wendland M., Schmidt B., Pohlmann R., Waheed A., von Figura K. Targeting of a lysosomal membrane protein: a tyrosine-containing endocytosis signal in the cytoplasmic tail of lysosomal acid phosphatase is necessary and sufficient for targeting to lysosomes. EMBO J. 1990 Nov;9(11):3497–3506. doi: 10.1002/j.1460-2075.1990.tb07558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouvelle B., Spiegel R., Hsiao L., Howard R. J., Morris R. L., Thomas A. P., Taraschi T. F. Direct access to serum macromolecules by intraerythrocytic malaria parasites. Nature. 1991 Sep 5;353(6339):73–75. doi: 10.1038/353073a0. [DOI] [PubMed] [Google Scholar]
- Rao J. K., Erickson J. W., Wlodawer A. Structural and evolutionary relationships between retroviral and eucaryotic aspartic proteinases. Biochemistry. 1991 May 14;30(19):4663–4671. doi: 10.1021/bi00233a005. [DOI] [PubMed] [Google Scholar]
- Robson K. J., Jennings M. W. The structure of the calmodulin gene of Plasmodium falciparum. Mol Biochem Parasitol. 1991 May;46(1):19–34. doi: 10.1016/0166-6851(91)90195-c. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. J., McKerrow J. H., Aikawa M., Nagasawa H., Leech J. H. A malarial cysteine proteinase is necessary for hemoglobin degradation by Plasmodium falciparum. J Clin Invest. 1988 Nov;82(5):1560–1566. doi: 10.1172/JCI113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P. J., Nelson R. G. Isolation and characterization of a cysteine proteinase gene of Plasmodium falciparum. Mol Biochem Parasitol. 1992 Mar;51(1):143–152. doi: 10.1016/0166-6851(92)90209-3. [DOI] [PubMed] [Google Scholar]
- Rosenthal P. J., Wollish W. S., Palmer J. T., Rasnick D. Antimalarial effects of peptide inhibitors of a Plasmodium falciparum cysteine proteinase. J Clin Invest. 1991 Nov;88(5):1467–1472. doi: 10.1172/JCI115456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D. G., Xu S., Chakraborty P. Intracellular trafficking and the parasitophorous vacuole of Leishmania mexicana-infected macrophages. J Cell Sci. 1992 Dec;103(Pt 4):1193–1210. doi: 10.1242/jcs.103.4.1193. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul A., Battistutta D. Analysis of the sequences flanking the translational start sites of Plasmodium falciparum. Mol Biochem Parasitol. 1990 Aug;42(1):55–62. doi: 10.1016/0166-6851(90)90112-y. [DOI] [PubMed] [Google Scholar]
- Sherman D. R., Kloek A. P., Krishnan B. R., Guinn B., Goldberg D. E. Ascaris hemoglobin gene: plant-like structure reflects the ancestral globin gene. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11696–11700. doi: 10.1073/pnas.89.24.11696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater A. F., Cerami A. Inhibition by chloroquine of a novel haem polymerase enzyme activity in malaria trophozoites. Nature. 1992 Jan 9;355(6356):167–169. doi: 10.1038/355167a0. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Spigelman M. K., Zappulla R. A., Goldberg J. D., Goldsmith S. J., Marotta D., Malis L. I., Holland J. F. Effect of intracarotid etoposide on opening the blood-brain barrier. Cancer Drug Deliv. 1984 Summer;1(3):207–211. doi: 10.1089/cdd.1984.1.207. [DOI] [PubMed] [Google Scholar]
- Tang J., Wong R. N. Evolution in the structure and function of aspartic proteases. J Cell Biochem. 1987 Jan;33(1):53–63. doi: 10.1002/jcb.240330106. [DOI] [PubMed] [Google Scholar]
- Trager W., Jensen J. B. Human malaria parasites in continuous culture. Science. 1976 Aug 20;193(4254):673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- Vander Jagt D. L., Hunsaker L. A., Campos N. M. Comparison of proteases from chloroquine-sensitive and chloroquine-resistant strains of Plasmodium falciparum. Biochem Pharmacol. 1987 Oct 1;36(19):3285–3291. doi: 10.1016/0006-2952(87)90646-0. [DOI] [PubMed] [Google Scholar]
- Weber J. L. Molecular biology of malaria parasites. Exp Parasitol. 1988 Aug;66(2):143–170. doi: 10.1016/0014-4894(88)90087-2. [DOI] [PubMed] [Google Scholar]
- Wellems T. E. Molecular genetics of drug resistance in Plasmodium falciparum malaria. Parasitol Today. 1991 May;7(5):110–112. doi: 10.1016/0169-4758(91)90168-n. [DOI] [PubMed] [Google Scholar]
- van Noort J. M., van der Drift A. C. The selectivity of cathepsin D suggests an involvement of the enzyme in the generation of T-cell epitopes. J Biol Chem. 1989 Aug 25;264(24):14159–14164. [PubMed] [Google Scholar]
- vander Jagt D. L., Hunsaker L. A., Campos N. M., Scaletti J. V. Localization and characterization of hemoglobin-degrading aspartic proteinases from the malarial parasite Plasmodium falciparum. Biochim Biophys Acta. 1992 Aug 21;1122(3):256–264. doi: 10.1016/0167-4838(92)90401-x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]