Abstract
Growing literature indicates that emotional reactivity and regulation are strongly linked to genetic modulation of serotonergic neurotransmission. However, until now, most studies have focused on the relationship between genotypic markers, in particular the serotonin transporter-linked polymorphic region (5-HTTLPR), and neural structures using MRI. The current study aimed to bridge the gap between the relevant MRI literature on the effects of the 5-HTTLPR genotype and the research tradition focusing on transient lateralized changes of electrocortical activity in the prefrontal cortex using electroencephalography (EEG). Lateral shifts of EEG alpha asymmetry in response to an aversive film consisting of scenes of real injury and death were assessed in healthy participants (n = 165). To evaluate the specificity of the 5-HTTLPR effect, participants were also tested for the COMT Val158Met polymorphism which is linked to dopamine inactivation. While viewing the film, individuals homozygous for the 5-HTTLPR short allele displayed a clear lateral shift of dorsolateral frontal activity to the right, which was virtually absent in participants carrying the long allele. The heightened electrocortical response to the aversive stimulation and its direction indicates a greater propensity of s/s homozygotes to experience withdrawal oriented affect in response to negative emotion cues in the environment. Moreover, together with previous research the findings support the notion of a link between the serotonergic system and self-regulation related to avoidance motivation, and a link between the dopaminergic system and self-regulation related to approach motivation.
Keywords: EEG asymmetry, genetic polymorphisms, emotional reactivity, withdrawal motivation
Emotional reactivity and regulation is strongly linked to serotonergic neuromodulation. A key regulator of serotonin (5-HT) function is the serotonin transporter (5-HTT), which facilitates the reuptake of 5-HT from the synaptic cleft. One common polymorphic variant of the serotonin transporter linked promoter region (5-HTTLPR), located on chromosome 17q11.1-q12, results in two prevalent alleles: the short allele (s-allele) and the long allele (l-allele). The s-allele is associated with lower 5-HTT expression and function compared with the l-allele (Caspi et al., 2003; Hu et al., 2005). A growing body of literature has associated the s-allele with increased negative emotionality (Beevers et al., 2011; Carver, Johnson, & Joormann, 2008; Munafo, Brown, & Hariri, 2008) including anxiety-related personality traits (for meta-analysis see Minelli, Bonvincini, Scasselllati, Sartori, & Gennarelli, 2011) as well as an attention bias for negative emotional information (for meta-analysis see Pergamin-Hight, Bakemans-Kranenburg, van Izendoom, & Bar-Haim, 2012). Furthermore, considerable attention has focused on the 5-HTTLPR as a putative moderator of the stress-depression relationship (for a recent meta-analysis see Karg, Burmeister, Shedden, & Sen, 2011).
Besides the numerous association studies between 5-HTTLPR and common psychological disorders like affective disorders, posttraumatic stress disorder, and personality disorders (e.g.,Blom et al., 2011; Hankin et al., 2011; Wang et al., 2011; Xie, Kranzler, Farrer, & Gelernter, 2012), the strategy to elucidate relationships between genotypic markers and neural structures or processes has gained great scientific interest in this context. Previous functional MRI studies revealed heightened amygdala activation in response to negative emotional stimuli in s-allele carriers compared to l-allele carriers (for a review see Scharinger, Rabl, Sitte, & Pezawas, 2010; for meta-analysis see Murphy et al., 2012), which may lead to enhanced susceptibility for affective disorders (Hariri et al., 2002). However, until now no study has related the 5-HTTLPR genotype to transient lateralized electrocortical responses in the prefrontal cortex in response to aversive emotional information with traumatic content. Therefore, the purpose of the present study was to bridge the gap between the relevant research on the effects of the 5-HTTLPR genotype that has largely focused on the amygdala using MRI and the research tradition focusing on transient lateralized changes of electrocortical activity in the prefrontal cortex using electroencephalography (EEG).
The amygdala and the dorsolateral prefrontal cortex are functionally interlinked. According to a model proposed by Johnstone and colleagues, the left ventrolateral prefrontal cortex modulates the activity of the amygdala in the context of affective processing via a relay station in the ventromedial prefrontal cortex (Johnstone, van Reekum, Urry, Kalin, & Davidson, 2007). This relay station is not only linked to the amygdala, but is also directly linked to the dorsolateral prefrontal cortex (Barbas, 1995; Ongur & Price, 2000). Moreover, there is evidence of an emotion-related information-processing path between the amygdala and the dorsolateral prefrontal cortex via the orbitofrontal cortex that may be involved in the generation of emotional states (Phillips, Drevets, Rauch, & Lane, 2003; Phillips, Ladouceur, & Drevets, 2008).
Transient changes of dorsolateral prefrontal EEG alpha asymmetry are considered to represent an objective correlate of the relative activation of two motivational systems: an approach system, associated with the left prefrontal cortex, and a withdrawal system, associated with the right prefrontal cortex (Davidson, 1998; Harmon-Jones, Gable, & Peterson, 2010). The approach-withdrawal model is conceptually closely related to the dichotomy of self-regulatory systems in motivational theories such as the Reinforcement Sensitivity Theory (Demaree, Everhart, Youngstrom, & Harrison, 2005). In the revised version of Gray’s (1994) theory, the Fight-Flight-Freeze System (FFFS) is posited to be a motivational system responsible for mediating reactions to aversive stimuli. Its activation is associated with avoidance and escape behaviors and negative hedonic experience. The Behavioral Approach system (or Behavioral Activation System, BAS) is assumed to be sensitive to signals of reward and to mediate reactions to appetitive stimuli. Activation of the BAS is associated with approach behaviors and mostly positive emotions (Gray & McNaughton, 2000). A lateral shift toward relatively greater activity in the right prefrontal cortex (indexed by attenuated EEG alpha band activity, which is inversely related to cortical activity) is posited to indicate a withdrawal-oriented and more negative affective state. A lateral shift to the left is thought to indicate a more approach oriented affective state (Davidson, 1998; Harmon-Jones et al., 2010). Consequently, environmental stimuli that encourage withdrawal responses and, therefore, activate the withdrawal system of FFFS should result in increased right versus left prefrontal activation.
Empirical studies have confirmed these associations between the response of the motivational systems and transient changes of prefrontal EEG asymmetry. These associations have been demonstrated in studies directly manipulating reward and punishment contingencies or using conditioned aversive stimuli to produce withdrawal or approach oriented motivational states (Flo et al., 2011; Shankman, Klein, Tenke, & Bruder, 2007; Shankman, Sarapas, & Klein, 2011; Sobotka, Davidson, & Senulis, 1992). Additionally, studies have used various methods such as emotional films, voluntary facial expressions, social-evaluative stress, or anger provocation to induce withdrawal-oriented emotional states such as disgust, fear or sadness, and approach-oriented emotional states such as joy or anger (e.g., Coan & Allen, 2004; Coan, Allen, & Harmon-Jones, 2001; Davidson, Ekman, Saron, Senulis, & Friesen, 1990; Davidson, Marshall, Tomarken, & Henriques, 2000; Ekman & Davidson, 1993; Harmon-Jones & Sigelman, 2001; Peterson, Gravens, & Harmon-Jones, 2011; Verona, Sadeh, & Curtin, 2009). The most common site of these effects is the dorsolateral prefrontal cortex in the region of the EEG electrode positions F3 and F4. Importantly, in this general context, the relative difference between the hemispheres is more important than the absolute level of independent left or right hemisphere activity per se. Consequently, relationships often have not been observed if only the absolute activity at individual sites was examined and data of the left and the right hemisphere were not related to each other, for instance, using appropriate laterality coefficients (e.g., Blackhart & Kline, 2005; Cole, Zapp, Nelson, & Perez-Edgar, 2012; Harmon-Jones, 2006; Papousek & Schulter, 2004; Papousek, Schulter, & Lang, 2009; Shankman et al., 2011).
Moreover, it has been suggested that interindividual differences in lateral shifts of prefrontal EEG alpha asymmetry can be used to assess the proneness or capability for approach- versus withdrawal-tendencies dominated responses during emotionally salient events (e.g., Coan, Allen, & McKnight, 2006). In line with this proposition, stronger lateral shifts to the left or to the right to relevant stimuli were observed in individuals characterized by a predisposition or capability for more pronounced approach- or withdrawal-oriented responses respectively: for instance, individuals with greater social anxiety, better emotion perception skills, greater liking ratings of objects, and relevant neurophysiological traits (Cole et al., 2012; Davidson et al., 2000; Gable & Harmon-Jones, 2008; Harmon-Jones & Gable, 2009; Papousek, Freudenthaler, & Schulter, 2011; Papousek, Reiser, Weber, Freudenthaler, & Schulter, 2012). Taken together, the evidence indicates that interindividual differences in prefrontal EEG alpha asymmetry responses reflect an individual’s sensitivity to environmental triggers of withdrawal or approach oriented affect (see also Cole et al., 2012).
Research has suggested that the 5-HTTLPR short allele is specifically associated with the strength of avoidance-oriented motivation but not of the BAS. This was shown using a self-report questionnaire assessing the strength of Gray’s motivational systems as a trait characteristic (Whisman, Richardson, & Smolen, 2011). In line with this, the 5-HTTLPR short allele was specifically associated with heightened amygdala responses to sad but not happy faces, suggesting that the 5-HTTLPR genotype impacts the processing of negative environmental cues but not emotionally salient stimuli in general (Dannlowski et al., 2010). In contrast, there is evidence that the strength of the BAS is associated with another genetic polymorphism: the Val158Met single nucleotide polymorphism (rs4680) of the gene coding for the catechol-O-methlytransferase (COMT) enzyme that inactivates extraneuronal dopamine. Homozygotes for the high-activity Val allele, who are expected to have lower prefrontal dopamine levels but at the same time higher striatal dopamine levels, showed higher trait BAS scores than carriers of the low-activity Met allele (Wacker, Mueller, Hennig, & Stemmler, 2012). In an EEG study in which participants were exposed to an approach motivational context, individuals homozygous for the Val allele showed greater relative activation of the left over the right dorsolateral prefrontal cortex than carriers of the Met allele (Wacker, Mueller, Pizzagalli, Hennig, & Stemmler, 2013). To our knowledge, the emerging evidence for an effect of the dopamine-related COMT Val158Met polymorphism on approach-related electrocortical responses is not complemented by studies addressing a potential relationship between the serotonin-related 5-HTTLPR polymorphism and electrocortical responses indicating the sensitivity to environmentally triggered affect in withdrawal-related contexts. There has been some sparse research on potential effects of the 5-HTTLPR genotype on prefrontal EEG measures in resting conditions, but this does not allow clear conclusions (Bismark et al., 2010; Lee et al., 2011).
Healthy participants were confronted with an aversive film consisting of scenes of real injury and death, to activate withdrawal motivation. The size of the response to the aversive film in terms of lateral shifts of EEG alpha asymmetry was assessed as an objective indicator of an individuals’ proneness for withdrawal motivation dominated affect. No regulation instructions were given, in order to study characteristic and predominantly automatic responses (e.g., Jackson et al., 2003). According to theoretical approaches, approach and avoidance temperaments are relatively reflexive, that is, they are involuntary tendencies to approach incentives and avoid threats. The automatic response tendencies may be moderated by effortful (deliberate) control, which is not thought to be specific to the motivational systems (Carver et al., 2008). Studying automatic response tendencies, therefore, fits in with the theoretical framework of the present study and the examination of lateralized EEG responses in the prefrontal cortex. To evaluate the specificity of the 5-HTTLPR effect, the participants were also tested for the COMT Val158Met polymorphism. Participants homozygous for the 5-HTTLPR short allele were expected to show a more pronounced electrocortical response indicating withdrawal motivation dominated affect compared with participants carrying the long allele.
Method
Participants
The sample comprised 165 right-handed female Caucasian university students aged between 18 to 59 years (M = 22.5, SD = 4.8). They were drawn from a larger screening sample on the basis of their 5-HTTLPR genotype. Buccal swabs were taken to genotype all participants for the 5-HTTLPR and COMT Met158Val polymorphism. No other polymorphisms were examined. Table 1 shows the distribution of genotypes. Individuals who reported having a neuropsychiatric disease or using psychoactive medication and individuals who reported traumatic experiences related to car crashes, surgery or death of a close person within the past 12 months were excluded from the study (n = 6). In addition, only participants who had at least 30 s of artifact free EEG data in each of the experimental conditions were included in the final sample (n = 165). A female only sample was chosen, because previous research showed sex differences moderating the association between 5-HTTLPR and emotion-related phenotypes (Cannon et al., 2013; Mizuno et al., 2006) and the neural mechanisms underlying emotional processes (Whittle, Yücel, Yap, & Allen, 2011). Handedness was assessed by a standardized hand performance test (Hand Dominance Test; Papousek & Schulter, 1999; Steingrüber & Lienert, 1971). Participants were requested to refrain from alcohol for 12 hours and from coffee and other stimulating beverages for 2 hours prior to their lab appointment, and to come to the session well rested. The study was performed in accordance with the American Psychological Association’s Ethics Code and the 1964 Declaration of Helsinki and was approved by the local ethics committee. Participants gave their written consent to participate in the study.
Table 1. Distribution of Genotypes.
| COMT | |||||
|---|---|---|---|---|---|
| Genotype | Val/Val | Val/Met | Met/Met | Row total | |
| 5-HTTLPR | s/s | 11 | 23 | 16 | 50 |
| s/l | 12 | 25 | 20 | 57 | |
| l/l | 12 | 26 | 20 | 58 | |
| Col. total | 35 | 74 | 56 | 165 | |
Genotyping
DNA Extraction
DNA was extracted from individual saliva swabs using a modified Chelex (Biorad, Hercules, CA) method (Berger et al., 2013).
Genotyping of 5-HTTLPR
Polymerase chain reaction (PCR) followed the protocol published by Hu et al. (2006) with modifications. The amplification cocktails had a volume of 10 μl containing 5 μl TaqMan Universal PCR Master Mix (Life Technologies, Paisley, United Kingdom), 2.5 μg nonacetylated bovine serum albumin (Sigma-Aldrich, St. Louis, MO), 4% (vol/vol) dimethyl sulfoxide, 100 nM primer HTTLPR_F: FAM-GCAACCTCCCAGCAACTCCCTGTA, 100 nM primer HTTLPR_R: GAGGTGCAGGGGGATGCTGGAA, and 2 μl sample DNA. The thermal cycling conditions were set according to Hu et al. (2006). Genotypes were determined by capillary electrophoretic separation of the PCR products on an ABI Prism 3100 Genetic Analyzer (Life Technologies).
Genotyping of COMT Val158Met (rs4680)
For the determination of the COMT Val158Met genotypes high resolution melting (HRM) curve profiles of short PCR amplicons were used. PCR and subsequent HRM analysis were conducted on a Rotor-Gene Q real-time PCR cycler (Qiagen, Hilden, Germany). The amplification cocktail had a total volume of 20 μl and consisted of 1x Type-it HRM PCR master mix (Qiagen), 250 nM primer COMT-F-44 and COMT-R+27 (both Oberacher et al., 2006), 5 μg nonacetylated bovine serum albumin, and 2 μl DNA extract. The thermal cycling conditions were set according to Oberacher et al. (2006). Following amplification, the PCR products were subjected to HRM analysis. For the automated COMT Val158Met genotype calling the Rotor-Gene Screen-Clust HRM software (v 1.10.1.2, Qiagen; Reja et al., 2010) was run in the supervised analysis mode. In initial Experiments 99.5% concordant genotype inferences were obtained for a set of 189 samples with known rs4680 genotype. Due to an additional sequence variant (C > T at chr22:19,951,246, GRCh37/hg19) in the amplicon, in the initial concordance study a single sample was attributed erroneously the A/A (Met/Met) instead of the actual G/A (Val/Met) genotype.
Stimulus Material
The aversive film (approx. 10 min in length) contained 11 clips that have been used in previous studies as an experimental analogue of psychological trauma. In stressful film studies, affect reliably deteriorates over film viewing (Holmes & Bourne, 2008). For instance, in the studies of Holmes, James, Coode-Bate, and Deeprose (2009) and Holmes, James, Kilford, and Deeprose (2010), which used the same film as the current study, negative mood was measured using a composite score comprising of sadness, hopelessness, and depression. These studies demonstrated a significant increase in negative affect from pre- to postfilm. Results extended with an adapted version of the film used in the current study (Deeprose, Zhang, Dejong, Dalgleish, & Holmes, 2012). Indeed, recent work combining data from 16 studies has shown that withdrawal-oriented negative affective states are reliably induced from the trauma film paradigm (Clark, Mackay, & Holmes, 2013). Effects of the film were generally found to be higher on scales representing depressed than fearful affect. The EEG was recorded during the last 5 minutes of the film during which there were five clips depicting several car accidents and a rampaging elephant injuring people at a circus. These clips included graphic scenes of severely injured, dying, and mourning people. The film was displayed on a 21” computer monitor viewed at 100 cm and was presented without sound, so that the stimulation was dominated by the visual information for all participants. The neutral visual display showed a green circle (diameter 75 mm) at the center of the screen.
EEG Recording and Quantification
The EEG was recorded from 19 channels according to the international 10–20 system, using a Brainvision BrainAmp Research Amplifier (Brain Products) and a stretchable electrode cap, and was rereferenced to a mathematically averaged ears reference (Hagemann, 2004). Impedance was kept below 5 kΩ for all electrodes. Horizontal and vertical EOG measures were obtained for identification of ocular artifacts. According to the research question, the dorsolateral frontal positions F3 and F4 were used for the analyses. All data were inspected visually, in order to eliminate intervals in which ocular or muscle artifacts occurred. Power spectra (epoch length 1 s, overlapping 50%, Hanning window) were averaged across all artifact-free intervals for an individual. (The mean numbers of artifact-free epochs were M = 150.0, SD = 46.7 for the initial recording at rest, and M = 121.1, SD = 43.7, M = 224.1, SD = 10.05, and M = 129.3, SD = 43.9 for the baseline, film, and postfilm recordings, respectively). Following a common approach in the field, power within the alpha frequency band (8 Hz–12 Hz) was used for the analyses. EEG laterality coefficients (LC) were computed as follows: LC = [(R − L)/(R + L)] × 100. Positive values indicate higher alpha activity in the right than in the left hemisphere (i.e., greater left hemisphere cortical activity). The calculation of LC has a long tradition in laterality research, because it separates the variance in asymmetry from the variance in general magnitude (e.g., Porac & Coren, 1981). In EEG studies, this asymmetry ratio is equivalent to another common metric (lnL − lnR), with which it is virtually perfectly correlated (Davidson, 1988; Papousek & Schulter, 2002). However, LC allows easier comparison of data from different studies, different frequency bands, and locations (Pivik et al., 1993).
Self-Report Measures
A composite mood score was calculated by summing participants’ ratings on three 10 cm horizontal visual analogue scales for feeling “sad.” “depressed,” and “hopeless” (Deeprose et al., 2012; Holmes et al., 2009, 2010). The responses were scored in millimeters from 0 (not at all) to 100 (extremely).
Depressive affect was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D; German version by Hautzinger & Bailer, 1993). It is comprised of 20 items referring to mood and attributions over the past week (internal consistency reliability in the present sample α = .87), designed for measuring subclinical depressive experiences in the general population (Wood, Taylor, & Joseph, 2010). In addition, the trait form of the Stait-Trait Anxiety Inventory was administered (STAI, German version by Laux, Glanzmann, Schaffner, & Spielberger, 1981; 20 items; α = .91).
Procedure
After completing the handedness test and the CES-D, participants were seated in an acoustically and electrically shielded examination chamber, electrodes were attached, and participants completed the mood rating scales using the computer mouse. The EEG was recorded in an initial two minutes rest period with closed eyes. Participants were then instructed that they would see a film to which they should direct their whole attention. They were asked to view the film as if they were really there, like a bystander at the scene of the events and to not close their eyes or look away. The film was immediately preceded and followed by recording periods in which the participants were exposed to the neutral visual display (2 min each; baseline and postfilm recording). Participants were instructed to watch the green circle. The experimenter was positioned outside the examination chamber, and participants were monitored using a camera. After the last recording period, the participants completed the rating scales again.
Statistical Analysis
The general impact of the aversive stimulation on dorsolateral frontal EEG asymmetry (LC) was assessed using a one-way repeated-measures analysis of variance, with recording period (baseline, aversive film, postfilm recording) as the independent variable. A paired t test comparing the composite mood scores obtained before and after the EEG recordings was used to test the film’s effect on subjective withdrawal-related affect.
To evaluate the specific hypothesis of the main research question, that is, the impact of the 5-HTTLPR genotype on the size of withdrawal-related EEG responses to the aversive stimulation, a planned comparison was performed. This comparison tested the specific a priori contrast s/s versus s/L, l/L in a 3 × 3 analysis of variance, with the 5-HTTLPR genotype (s/s, s/L, l/L) and the COMT genotype (Met/Met, Met/Val, Val/Val) as the between-subjects factors, and EEG reactivity (LC film minus LC baseline) as the dependent variable. Results of the omnibus F tests are also reported. The two genotypes were simultaneously entered in the analysis to test the effect of the 5-HTTLPR genotype independently of the COMT Val158Met genotype (using the regression approach to the analysis of variance, which also controls for unequal cell sizes).
An analysis analogous to that of the EEG changes was conducted using the composite mood score (rating at the end of the experiment minus rating at the beginning) as the dependent variable. In addition, potential effects of genotype on interindividual differences in dorsolateral prefrontal EEG asymmetry at rest (first recording period, closed eyes) were tested (analysis of variance with 5-HTTLPR and COMT as the independent variables). Two supplementary analyses tested whether the genotype groups differed in their trait depression or anxiety scores. Estimates of effect size are reported using partial eta-squared (ηp2). The sphericity assumption was not violated. A significance level of p < .05 was used for all analyses.
Results are reported in terms of cortical activation: a relative change of asymmetry “to the right” indicates an increase of right versus left sided cortical activity (i.e., a decrease of right vs. left sided EEG alpha values, because alpha power is inversely related to cortical activity).
Results
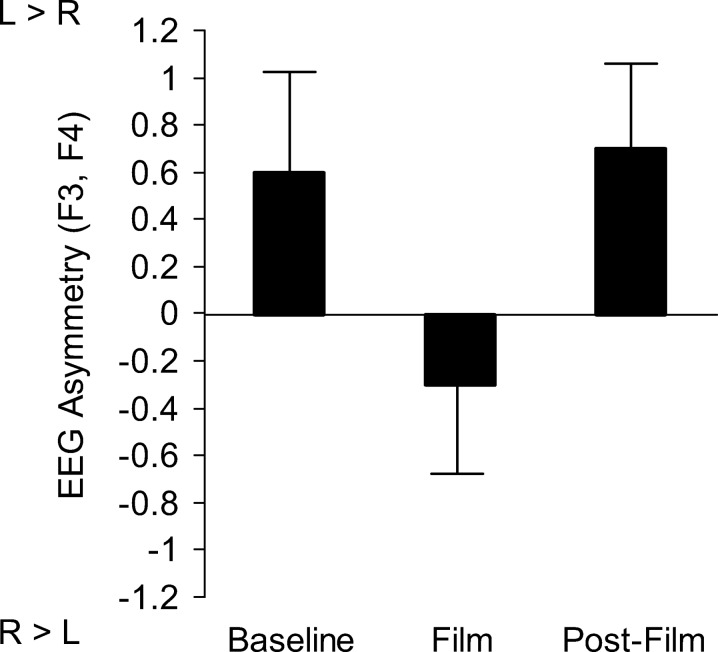
Watching the aversive film provoked the expected transient shift of dorsolateral prefrontal EEG asymmetry to the right (F2,328 = 3.4, p < .05, ηp2 = 0.021). Figure 1 shows the average changes across the baseline, aversive film, and postfilm recordings (baseline vs. film t164 = 2.0, p < .05; film vs. postfilm recording t164 = 2.5, p < .05). The composite mood rating indicated significant mood deterioration in terms of sadness, depression, and hopelessness following the film (t164 = 14.7, p < .001; M = 19.1, SD = 37.6 and M = 87.4, SD = 58.9).
Figure 1.
Average impact of watching the aversive film scenes on dorsolateral frontal EEG asymmetry. Note: Laterality coefficients (LC); L = left, R = right; L > R, R > L denote asymmetries in terms of activity (i.e., inverse of alpha). Whiskers indicate standard errors.
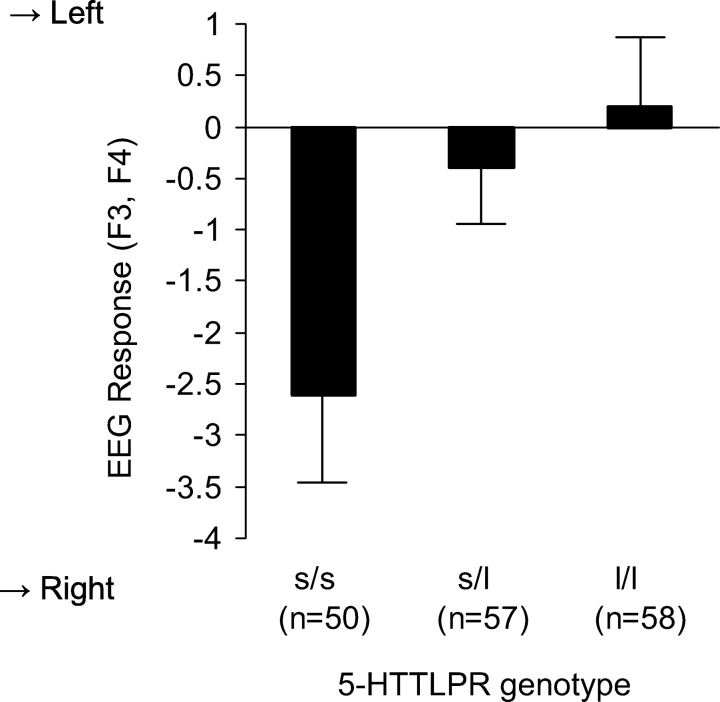
The main analysis tested the effect of the 5-HTTLPR genotype on individual differences in the EEG responses to the stimulation. The hypothesis-driven a priori contrast (t156 = 2.44, p < .05) indicated that compared with the other 5-HTTLPR genotype groups, participants homozygous for the short allele (s/s) showed the most pronounced EEG response to the film, that is, a clear lateral shift of dorsolateral frontal asymmetry to the right from baseline to viewing the aversive film. The other two groups did not display a comparable response (see Figure 2). The F test for the main effect of 5-HTTLPR in the omnibus analysis of variance was also significant (F2,156 = 3.1, p < .05, ηp2 = 0.039). There was no significant main effect of COMT (F2,156 = 1.0, ns.) or 5-HTTLPR by COMT interaction (F2,156 = 1.4, ns.).1 Baseline asymmetry scores of the three HTTLPR genotype groups were s/s: M = 1.78 (SD = 7.64), s/L: M = −0.07 (SD = 5.46), l/L: M = 0.05 (SD = 6.10; F2,162 = 1.4, ns.).
Figure 2.
Effect of 5-HTTLPR genotype on the size of the lateralized EEG response indicating withdrawal motivation dominated affect. Note: Reactivity to stimulation with aversive emotional information (difference scores LC film minus LC baseline). “→ Right” denotes a relative increase of right versus left sided activity in the dorsolateral frontal cortex (i.e., inverse of alpha); higher negative values indicate a more pronounced withdrawal motivation related response. Whiskers indicate standard errors.
In the analysis using the participants’ mood changes from the beginning to the end of the experiment as the dependent variable, none of the effects were significant (t156 = 0.3, ns.; 5-HTTLPR F2,156 = 0.1, ns.; COMT F2,156 = 1.3, ns.; 5-HTTLPR by COMT F2,156 = 0.7, ns.). No relationship between genotype and dorsolateral prefrontal resting asymmetry in the first baseline recording was observed (5-HTTLPR F2,156 = 1.1, ns.; COMT F2,156 = 0.9, ns.; 5-HTTLPR by COMT F4,156 = 1.1, ns.).
The genotype groups did not differ in their trait depression scores (5-HTTLPR F2,164 = 0.6, ns.; COMT F2,164 = 1.8, ns.; 5-HTTLPR by COMT F4,164 = 0.5, ns.), nor in their trait anxiety scores (5-HTTLPR F2,164 = 0.1, ns.; COMT F2,164 = 0.2, ns.; 5-HTTLPR by COMT F4,164 = 0.5, ns.)
Discussion
The results showed that individuals homozygous for the 5-HTTLPR s-allele displayed a clear lateral shift of dorsolateral frontal activity to the right while viewing a film containing traumatic content, which was virtually absent in participants carrying the long allele. According to the relevant literature, the heightened electrocortical response to the aversive stimulation and its direction indicates a greater propensity of s/s homozygotes to experience withdrawal-oriented affect in response to negative emotion cues in the environment (Coan et al., 2006; Cole et al., 2012; Harmon-Jones et al., 2010). Although observed in the context of a different research tradition, this novel finding is in line with the association of the 5-HTTLPR s-allele to heightened amygdala responsiveness to negative emotional stimulation (e.g., Domschke et al., 2012; Munafo et al., 2008; von dem Hagen, Passamonti, Nutland, Sambrook, & Calder, 2011), with which activity changes in the dorsolateral prefrontal cortex are interlinked (Johnstone et al., 2007; Phillips et al., 2003, 2008; see introduction). Lateralized prefrontal changes as a function of the 5-HTTLPR genotype have not been evident in functional MRI (fMRI) studies, because fMRI studies are typically not specifically concerned with differences between the activity in the two hemispheres, but only focus on whether an active area meets a particular statistical threshold in one hemisphere as well as the other (Davidson, 1998; Herrington et al., 2005). In the context of individual differences in responses to emotional cues, the relative difference in activation between the hemispheres seems to be more important than the absolute level of independent left or right hemisphere activation. That is, no effect of increased left-hemisphere activation on approach and avoidance motivation may be expected if the right hemisphere is also more activated (Davidson, Chapman, Chapman, & Henriques, 1990; Harmon-Jones, 2006; Heller, Nitschke, Etienne, & Miller, 1997). The easier implementation of appropriate methods to relate left and right hemisphere data to one another may be one reason why EEG has had a predominance in research on lateralized brain responses to date (see, e.g., Seghier, 2008). However, directly comparing left to right activation in the dorsolateral frontal cortex by calculating an asymmetry score according to the convention in the EEG literature, the link between increased right versus left activation during avoidance versus approach actions has also be demonstrated using fMRI methods (Berkman & Lieberman, 2010).
It is of note that differences between the 5-HTTLPR genotype groups were only observed in the transient electrocortical responses to the triggers of withdrawal oriented affect (i.e., in the changes within individuals, provoked by the stimulation from the aversive scenes). The genotype groups did not differ in their EEG alpha asymmetry during the first stimulation-free baseline recording. Related to this, it was found that tryptophan depletion generally does not affect mood per se, but may exaggerate mood change in response to a stressor (Carver et al., 2008). However, Bismark et al. (2010) observed a small effect of the 5-HTTLPR s-allele on prefrontal EEG alpha asymmetry in resting conditions, which was qualified by an interaction with lifetime major depression disorder. It should be noted in this context that the application of the electrodes in the EEG laboratory can be an aversive situation for some people, accompanied by respective changes in prefrontal EEG asymmetry (Blackhart, Kline, Donohue, LaRowe, & Joiner, 2002). Another study exploring the potential effect of the 5-HTTLPR genotype on the EEG in resting conditions found no differences between s/s homozygotes and carriers of the l-allele in the absolute EEG power in various frequency bands and electrode positions including the alpha band power at prefrontal sites (Lee et al., 2011). The relative left to right hemisphere EEG activity was not examined in the study of Lee et al. Analogous to the present study, associations between the COMT Val158Met polymorphism and dorsolateral prefrontal EEG alpha asymmetry were only detectable in a situation in which approach motivation was highly salient, indicating that associations between genetic polymorphisms and brain indicators of individual traits may only become apparent in situational contexts relevant to the traits of interest (Wacker et al., 2013). Both the present findings and the findings of Wacker, Mueller, Pizzagalli, Hennig, and Stemmler (2013) support the notion that transient changes in prefrontal EEG alpha asymmetry are produced by the interaction between the emotional or motivational demands of a specific situation and the capability of the individual’s nervous system to produce (appropriate) and inhibit (inappropriate or unfavorable) responses in that context (Coan et al., 2006; Papousek et al., 2011, 2012).
Moreover, the pattern of findings of the present study and of Wacker et al. (2013) are consistent with the proposed links between the serotonergic system and self-regulation related to avoidance motivation, and between the dopaminergic system and self-regulation related to approach motivation. In addition to correlations of the 5-HTTLPR s-allele with self-report measures of the strength of avoidance-oriented motivation (but not the BAS; Whisman et al., 2011) and of the COMT Val-allele with self-report measures of the strength of the BAS (but not avoidance motivation; Wacker et al., 2012), there is also evidence from animal studies suggesting that avoidance-motivated behavior is largely controlled by serotonergic pathways, whereas approach-motivated behavior is mediated by mostly dopaminergic pathways (Demaree et al., 2005). The present study and the study of Wacker et al. (2013) add to this evidence by demonstrating corresponding links with electrocortical measures indicating the sensitivity to withdrawal or approach-oriented affect.
In contrast to the electrocortical measure of withdrawal motivation dominated responses during viewing the aversive film, the 5-HTTLPR polymorphism had no effect on a subjective measure of mood. Several explanations may account for this finding. The self-report measure obtained at the end of the experiment may provide a less precise measure than the EEG measure that was obtained directly during the stimulation. Further, the EEG response may be dominated by the expression of motivational direction (Harmon-Jones et al., 2010), whereas the mood ratings may have been associated more with the experience of negative affective valence than with withdrawal motivation. The 5-HTTLPR genotype may be more closely related to withdrawal motivation than to current mood per se. The self-report rating may also have captured situational or identity-related beliefs about what one ought to have felt, and to a lesser extent the actual emotional and motivational states during viewing the film, with the latter showing generally more clear relationships to biological variables (Steiner & Coan, 2011). This effect might have been amplified by introducing some temporal distance between the film and the ratings through the study design.
A limitation of the study may be that, following the tradition of MRI research that has almost exclusively been concerned with negative stimuli, an appetitive condition was not included, preventing direct comparison. However, it seems highly implausible that the s/s genotype might also be related to greater responsiveness to appetitive stimuli. In fact, it was directly demonstrated in a MRI study that the 5-HTTLPR impacts the amygdala responsiveness to negative environmental cues specifically, but not to emotionally salient stimuli in general (Dannlowski et al., 2010). A further limitation may be that the present study did not directly verify the validity of the EEG measure. However, the study is based on a well-founded theoretical background according to which transient changes of prefrontal EEG asymmetry to the left and to the right indicate activation of the approach and the withdrawal motivational system, respectively. Therefore, the study was not designed to reconfirm this link, which has been demonstrated in a large number of empirical studies (e.g., Coan & Allen, 2004; Coan et al., 2001; Davidson, Ekman, et al., 1990; Davidson et al., 2000; Ekman & Davidson, 1993; Flo et al., 2011; Harmon-Jones & Sigelman, 2001; Papousek et al., 2012; Peterson et al., 2011; Sobotka et al., 1992; Shankman et al., 2007, 2011; Verona et al., 2009).
In conclusion, the present findings provide a novel addition to the literature documenting associations between the serotonin related 5-HTTLPR genotype and a heightened sensitivity to emotional cues, which to date had primarily been based on amygdala responses in MRI studies. Linking this evidence with the research tradition focusing on transient lateralized changes of activity in the prefrontal cortex using electroencephalography, the current study demonstrated that the 5-HTTLPR short allele is associated with responses in the dorsolateral prefrontal cortex indicating a heightened sensitivity to environmentally triggered withdrawal-oriented affect.
Footnotes
Exploratory analyses showed that EEG responsiveness at other electrode positions than those for which the theoretical and empirical context of the study had suggested an effect did not vary as a function of genotypes.
References
- Barbas H. (1995). Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neuroscience and Biobehavioral Review, 19, 499–510. doi: 10.1016/0149-7634(94)00053-4 [DOI] [PubMed] [Google Scholar]
- Beevers C. G., Marti C. N., Lee H. J., Stote D. L., Ferrell R. E., Hariri A. R., & Telch M. J. (2011). Associations between serotonin transporter gene promotor region (5-HTTLPR) polymorphism and gaze bias for emotional information. Journal of Abnormal Psychology, 120, 187–197. doi: 10.1037/a0022125 [DOI] [PubMed] [Google Scholar]
- Berger B., Parson R., Clausen J., Berger C., Nachbaur D., & Parson W. (2013). Chimerism in DNA of buccal swabs from recipients after allogeneic hematopoietic stem cell transplantations: Implications for forensic DNA testing. International Journal of Legal Medicine, 127, 49–54. doi: 10.1007/s00414-012-0687-5 [DOI] [PubMed] [Google Scholar]
- Berkman E. T., & Lieberman M. D. (2010). Approaching the bad and avoiding the good: Lateral prefrontal cortical asymmetry distinguishes between action and valence. Journal of Cognitive Neuroscience, 22, 1970–1979. doi: 10.1162/jocn.2009.21317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bismark A. W., Moreno F. A., Stewart J. L., Towers D. N., Coan J. A., Oas J., et al. Allen J. J. B. (2010). Polymorphisms of the HTR1a allele are linked to frontal brain electrical asymmetry. Biological Psychology, 83, 153–158. doi: 10.1016/j.biopsycho.2009.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhart G. C., & Kline J. P. (2005). Individual differences in anterior EEG asymmetry between high and low defensive individuals during a rumination/distraction task. Personality and Individual Differences, 39, 427–437. doi: 10.1016/j.paid.2005.01.027 [DOI] [Google Scholar]
- Blackhart G. C., Kline J. P., Donohue K. F., LaRowe S. D., & Joiner T. E. (2002). Affective responses to EEG preparation and their link to resting anterior EEG asymmetry. Personality and Individual Differences, 32, 167–174. doi: 10.1016/S0191-8869(01)00015-0 [DOI] [Google Scholar]
- Blom R. M., Samuels J. F., Riddle M. A., Joseph Bienvenu O., Grados M. A., Reti I. M., et al. Nestadt G. (2011). Association between a serotonin transporter promoter polymorphism (5-HTTLPR) and personality disorder traits in a community sample. Journal of Psychiatric Research, 45, 1153–1159. doi: 10.1016/j.jpsychires.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon D. M., Klaver J. M., Klug S. A., Carlson P. J., Luckenbaugh D. A., Ichise M., & Drevets W. C. (2013). Gender-specific abnormalities in the serotonin transporter system in panic disorder. The International Journal of Neuropsychopharmacology, 16, 733–743. doi: 10.1017/S1461145712000776 [DOI] [PubMed] [Google Scholar]
- Carver C. S., Johnson S. L., & Joormann J. (2008). Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: What depression has in common with impulsive aggression. Psychological Bulletin, 134, 912–943. doi: 10.1037/a0013740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A., Sugden K., Moffitt T. E., Taylor A., Craig I. W., Harrington H., McClay J., et al. Poulton R. (2003). Influence of life stress on depression: Moderation by a polymorphism in the 5-HTT gene. Science, 301, 386–389. doi: 10.1126/science.1083968 [DOI] [PubMed] [Google Scholar]
- Clark I. A., Mackay C. E., & Holmes E. A. (2013). Why doesn’t everyone get flashbacks after trauma? Low emotional response to traumatic footage predicts an absence of flashbacks. Manuscript in preparation [DOI] [PMC free article] [PubMed]
- Coan J. A., & Allen J. J. B. (2004). Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology, 67, 7–50. doi: 10.1016/j.biopsycho.2004.03.002 [DOI] [PubMed] [Google Scholar]
- Coan J. A., Allen J. J. B., & Harmon-Jones E. (2001). Voluntary facial expression and hemispheric asymmetry over the frontal cortex. Psychophysiology, 38, 912–925. doi: 10.1111/1469-8986.3860912 [DOI] [PubMed] [Google Scholar]
- Coan J. A., Allen J. J. B., & McKnight P. E. (2006). A capability model of individual differences in frontal EEG asymmetry. Biological Psychology, 72, 198–207. doi: 10.1016/j.biopsycho.2005.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C., Zapp D. J., Nelson K., & Perez-Edgar K. (2012). Speech presentation cues moderate frontal EEG asymmetry in socially withdrawn young adults. Brain and Cognition, 78, 156–162. doi: 10.1016/j.bandc.2011.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U., Konrad C., Kugel H., Zwitserlood P., Domschke K., Schöning S., et al. Suslow T. (2010). Emotion specific modulation of automatic amygdala responses by 5-HTTLPR genotype. NeuroImage, 53, 893–898. doi: 10.1016/j.neuroimage.2009.11.073 [DOI] [PubMed] [Google Scholar]
- Davidson R. J. (1988). EEG measures of cerebral asymmetry: Conceptual and methodolocial issues. International Journal of Neuroscience, 39, 71–89. doi: 10.3109/00207458808985694 [DOI] [PubMed] [Google Scholar]
- Davidson R. J. (1998). Affective style and affective disorders: Perspectives from affective neuroscience. Cognition and Emotion, 12, 307–330. doi: 10.1080/026999398379628 [DOI] [Google Scholar]
- Davidson R. J., Chapman J. P., Chapman L. J., & Henriques J. B. (1990). Asymmetrical brain electrical activity discriminates between psychometrically-matched verbal and spatial cognitive tasks. Psychophysiology, 27, 528–543. doi: 10.1111/j.1469-8986.1990.tb01970.x [DOI] [PubMed] [Google Scholar]
- Davidson R. J., Ekman P., Saron C., Senulis J., & Friesen W. V. (1990). Approach/withdrawal and cerebral asymmetry: Emotional expression and brain physiology. Journal of Personality and Social Psychology, 58, 330–341. doi: 10.1037/0022-3514.58.2.330 [DOI] [PubMed] [Google Scholar]
- Davidson R. J., Marshall J. R., Tomarken A. J., & Henriques J. B. (2000). While a phobic waits: Regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biological Psychiatry, 47, 85–95. doi: 10.1016/S0006-3223(99)00222-X [DOI] [PubMed] [Google Scholar]
- Deeprose C., Zhang S., Dejong H., Dalgleish T., & Holmes E. A. (2012). Imagery in the aftermath of viewing a traumatic film: Using cognitive tasks to modulate the development of involuntary memory. Journal of Behavior Therapy and Experimental Psychiatry, 43, 758–764. doi: 10.1016/j.jbtep.2011.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaree H. A., Everhart D. E., Youngstrom E. A., & Harrison D. W. (2005). Brain lateralization of emotional processing: Historical roots and a future incorporating “dominance”. Behavioral and Cognitive Neuroscience Reviews, 4, 3–20. doi: 10.1177/1534582305276837 [DOI] [PubMed] [Google Scholar]
- Domschke K., Baune B. T., Havlik L., Stuhrmann A., Suslow T., Kugel H., et al. Dannlowski U. (2012). Catechol-O-methyltransferase gene variation: Impact on amygdala response to aversive stimuli. NeuroImage, 60, 2222–2229. doi: 10.1016/j.neuroimage.2012.02.039 [DOI] [PubMed] [Google Scholar]
- Ekman P., & Davidson R. J. (1993). Voluntary smiling changes regional brain activity. Psychological Science, 4, 342–345. doi: 10.1111/j.1467-9280.1993.tb00576.x [DOI] [Google Scholar]
- Flo E., Steine I., Blagstad T., Gronli J., Pallesen S., & Portas C. M. (2011). Transient changes in frontal alpha asymmetry as a measure of emotional and physical distress during sleep. Brain Research, 1367, 234–249. doi: 10.1016/j.brainres.2010.09.090 [DOI] [PubMed] [Google Scholar]
- Gable P., & Harmon-Jones E. (2008). Relative left frontal activation to appetitive stimuli: Considering the role of individual differences. Psychophysiology, 45, 275–278. doi: 10.1111/j.1469-8986.2007.00627.x [DOI] [PubMed] [Google Scholar]
- Gray J. A. (1994). Framework on taxonomy of psychiatric disorder In VanGoozen H. M., Van De Poll N. E., & Sergeant J. A. (Eds.), Emotions: Essays on emotion theory (pp. 29–59). Hillsdale, NJ: Erlbaum [Google Scholar]
- Gray J. A., & McNaughton N. (2000). The neuropsychology of anxiety. Oxford, UK: Oxford University Press [Google Scholar]
- Hagemann D. (2004). Individual differences in anterior EEG asymmetry: Methodological problems and solutions. Biological Psychology, 67, 157–182. doi: 10.1016/j.biopsycho.2004.03.006 [DOI] [PubMed] [Google Scholar]
- Hankin B. L., Barrocas A. L., Jenness J., Oppenheimer C. W., Badanes L. S., Abela J., et al. Smolen A. (2011). Association between 5-HTTLPR and borderline personality disorder traits among youth. Frontiers in Child and Neurodevelopmental Psychiatry, 2, 6. doi: 10.3389/fpsyt.2011.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri A. R., Mattay V. S., Tessitore A., Kolachana B., Fera F., Goldman D., et al. Weinberger D. R. (2002). Serotonin transporter genetic variation and the response of the human amygdala. Science, 297, 400–403. doi: 10.1126/science.1071829 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E. (2006). Unilateral right-hand contractions cause contralateral alpha power suppression and approach motivational affective experience. Psychophysiology, 43, 598–603. doi: 10.1111/j.1469-8986.2006.00465.x [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., & Gable P. A. (2009). Neural activity underlying the effect of approach-motivated positive affect on narrowed attention. Psychological Science, 20, 406–409. doi: 10.1111/j.1467-9280.2009.02302.x [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., Gable P. A., & Peterson C. K. (2010). The role of asymmetric frontal cortical activity in emotion-related phenomena: A review and update. Biological Psychology, 84, 451–462. doi: 10.1016/j.biopsycho.2009.08.010 [DOI] [PubMed] [Google Scholar]
- Harmon-Jones E., & Sigelman J. (2001). State anger and prefrontal brain activity: Evidence that insult-related relative left-prefrontal activation is associated with experienced anger and aggression. Journal of Personality and Social Psychology, 80, 797–803. doi: 10.1037/0022-3514.80.5.797 [DOI] [PubMed] [Google Scholar]
- Hautzinger M., & Bailer M. (1993). Allgemeine depressions skala [General depression scale]. Weinheim, Germany: Beltz [Google Scholar]
- Heller W., Nitschke J. B., Etienne M. A., & Miller G. A. (1997). Patterns of regional brain activity differentiate types of anxiety. Journal of Abnormal Psychology, 106, 376–385. doi: 10.1037/0021-843X.106.3.376 [DOI] [PubMed] [Google Scholar]
- Herrington J. D., Mohanty A., Koven N. S., Fisher J. E., Stewart J. L., Banich M. T., et al. Heller W. (2005). Emotion-modulated performance and activity in left dorsolateral prefrontal cortex. Emotion, 5, 200–207. doi: 10.1037/1528-3542.5.2.200 [DOI] [PubMed] [Google Scholar]
- Holmes E. A., & Bourne C. (2008). Inducing and modulating intrusive emotional memories: A review of the trauma film paradigm. Acta Psychologica, 127, 553–566. doi: 10.1016/j.actpsy.2007.11.002 [DOI] [PubMed] [Google Scholar]
- Holmes E. A., James E. L., Coode-Bate T., & Deeprose C. (2009). Can playing the computer game “Tetris” reduce the build-up of flashbacks for trauma? A proposal from cognitive science. PLoS One, 4, . doi: 10.1371/journal.pone.0004153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes E. A., James E. L., Kilford E. J., & Deeprose C. (2010). Key steps in developing a cognitive vaccine against traumatic flashbacks: Visuospatial Tetris versus verbal Pub Quiz. PLoS ONE, 5, . doi: 10.1371/journal.pone.0013706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X. Z., Lipsky R. H., Zhu G., Akhtar L. A., Taubman J., Greenberg B. D., et al. Goldman D. (2006). Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. American Journal of Human Genetics, 78, 815–826. doi: 10.1086/503850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Oroszi G., Chun J., Smith T. L., Goldman D., & Schuckit M. A. (2005). An expanded evaluation of the relationship of four alleles to the level of response to alcohol and the alcoholism risk. Alcoholism: Clinical and Experimental Research, 29, 8–16. doi: 10.1097/01.ALC.0000150008.68473.62 [DOI] [PubMed] [Google Scholar]
- Jackson D. C., Mueller C. J., Dolski I., Dalton K. M., Nitschke J. B., Urry H. L., et al. Davidson R. J. (2003). Now you feel it, now you don’t: Frontal brain electrical asymmetry and individual differences in emotion regulation. Psychological Science, 14, 612–617. doi: 10.1046/j.0956-7976.2003.psci_1473.x [DOI] [PubMed] [Google Scholar]
- Johnstone T., van Reekum C. M., Urry H. L., Kalin N. H., & Davidson R. J. (2007). Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience, 27, 8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg K., Burmeister M., Shedden K., & Sen S. (2011). The serotonin transporter promoter variant (5-HTTLPR), stress, and depression metaanalysis revisited: Evidence of genetic moderation. Archives of General Psychiatry, 68, 444–454. doi: 10.1001/archgenpsychiatry.2010.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux L., Glanzmann P., Schaffner P., & Spielberger C. D. (1981). Das State-Trait-Angstinventar [The State-Trait Anxiety Inventory]. Weinheim, Germany: Beltz [Google Scholar]
- Lee T.-W., Yu Y. W. Y., Hong C.-J., Tsai S.-J., Wu H.-C., & Chen T.-J. (2011). The influence of serotonin transporter polymorphisms on cortical activity: A resting EEG study. BMC Neuroscience, 12, 33. doi: 10.1186/1471-2202-12-33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minelli A., Bonvicini C., Scassellati C., Sartori R., & Gennarelli M. (2011). The influence of psychiatric screening in healthy populations selection: A new study and meta-analysis of functional 5-HTTLPR and rs25531 polymorphisms and anxiety-related personality traits. BMC Psychiatry, 11, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T., Aoki M., Shimada Y., Inoue M., Nakaya K., Takahashi T., et al. Fukudo S. (2006). Gender difference in association between polymorphism of serotonin transporter gene regulatory region and anxiety. Journal of Psychosomatic Research, 60, 91–97. doi: 10.1016/j.jpsychores.2005.06.068 [DOI] [PubMed] [Google Scholar]
- Munafo M. R., Brown S. M., & Hariri A. R. (2008). Serotonin transporter (5-HTTLPR) genotype and amygdala activation: A meta-analysis. Biological Psychiatry, 63, 852–857. doi: 10.1016/j.biopsych.2007.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy S. E., Norbury R., Godlewska B. R., Cowen P. J., Mannie Z. M., Harmer C. J., & Munafò M. R. (2012). The effect of the serotonin transporter polymorphism (5-HTTLPR) on amygdala function: A meta-analysis. Molecular Psychiatry, 18, 512–520. doi: 10.1038/mp.2012.19 [DOI] [PubMed] [Google Scholar]
- Oberacher H., Pitterl F., Niederstätter H., Weiss E. M., Stadelmann E., Marksteiner J., & Parson W. (2006). Direct molecular haplotyping of multiple polymorphisms within exon 4 of the human catechol-O-methyltransferase gene by liquid chromtaography-electrospray ionization time-of-flight mass spectrometry. Analytical and Bioanalytical Chemistry, 386, 83–91. doi: 10.1007/s00216-006-0589-9 [DOI] [PubMed] [Google Scholar]
- Ongur D., & Price J. L. (2000). The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cerebral Cortex, 10, 206–219. doi: 10.1093/cercor/10.3.206 [DOI] [PubMed] [Google Scholar]
- Papousek I., Freudenthaler H. H., & Schulter G. (2011). Typical performance measures of emotion regulation and emotion perception and frontal EEG asymmetry in an emotional contagion paradigm. Personality and Individual Differences, 51, 1018–1022. doi: 10.1016/j.paid.2011.08.013 [DOI] [Google Scholar]
- Papousek I., Reiser E. M., Weber B., Freudenthaler H. H., & Schulter G. (2012). Frontal brain asymmetry and affective flexibility in an emotional contagion paradigm. Psychophysiology, 49, 489–498. doi: 10.1111/j.1469-8986.2011.01324.x [DOI] [PubMed] [Google Scholar]
- Papousek I., & Schulter G. (1999). Quantitative assessment of five behavioural laterality measures: Distribution of scores and intercorrelations among right-handers. Laterality, 4, 345–362 [DOI] [PubMed] [Google Scholar]
- Papousek I., & Schulter G. (2002). Covariations of EEG asymmetries and emotional states indicate that activity at frontopolar locations is particularly affected by state factors. Psychophysiology, 39, 350–360. doi: 10.1017/S0048577201393083 [DOI] [PubMed] [Google Scholar]
- Papousek I., & Schulter G. (2004). Manipulation of frontal brain asymmetry by cognitive tasks. Brain and Cognition, 54, 43–51. doi: 10.1016/S0278-2626(03)00258-6 [DOI] [PubMed] [Google Scholar]
- Papousek I., Schulter G., & Lang B. (2009). Effects of emotionally contagious films on changes in hemisphere specific cognitive performance. Emotion, 9, 510–519. doi: 10.1037/a0016299 [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L., Bakermans-Kranenburg M. J., van Ijzendoorn M. H., & Bar-Haim Y. (2012). Variations in the promoter region of the serotonin transporter gene and biased attention for emotional information: A meta-analysis. Biological Psychiatry, 71, 373–379. doi: 10.1016/j.biopsych.2011.10.030 [DOI] [PubMed] [Google Scholar]
- Peterson C. K., Gravens L. C., & Harmon-Jones E. (2011). Asymmetric frontal cortical activity and negative affective responses to ostracism. Social Cognitive and Affective Neuroscience, 6, 277–285. doi: 10.1093/scan/nsq027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. L., Drevets W. C., Rauch S. L., & Lane R. (2003). Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biological Psychiatry, 54, 504–514. doi: 10.1016/S0006-3223(03)00168-9 [DOI] [PubMed] [Google Scholar]
- Phillips M. L., Ladouceur C. D., & Drevets W. C. (2008). A neural model of voluntary and automatic regulation: Implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Molecular Psychiatry, 13, 833–857. doi: 10.1038/mp.2008.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivik R. T., Broughton R. J., Coppola R., Davidson R. J., Fox N., & Nuwer M. R. (1993). Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology, 30, 547–558. doi: 10.1111/j.1469-8986.1993.tb02081.x [DOI] [PubMed] [Google Scholar]
- Porac C., & Coren S. (1981). Lateral preferences and human behavior. New York, NY: Springer. doi: 10.1007/978-1-4613-8139-6 [DOI] [Google Scholar]
- Reja V., Kwok A., Stone G., Yang L., Missel A., Menzel C., & Bassam B. (2010). ScreenClust: Advanced statistical software for supervised and unsupervised resolution melting (HRM) analysis. Methods, 50, S10–S14. doi: 10.1016/j.ymeth.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Scharinger C., Rabl U., Sitte H. H., & Pezawas L. (2010). Imaging genetics of mood disorders. NeuroImage, 53, 810–821. doi: 10.1016/j.neuroimage.2010.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seghier M. L. (2008). Laterality index in functional MRI: Methodological issues. Magnetic Resonance Imaging, 26, 594–601. doi: 10.1016/j.mri.2007.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankman S. A., Klein D. N., Tenke C. E., & Bruder G. E. (2007). Reward sensitivity in depression: A biobehavioral study. Journal of Abnormal Psychology, 116, 95–104. doi: 10.1037/0021-843X.116.1.95 [DOI] [PubMed] [Google Scholar]
- Shankman S. A., Sarapas C., & Klein D. N. (2011). The effect of pre- vs. post-reward attainment on EEG asymmetry in melancholic depression. International Journal of Psychophysiology, 79, 287–295. doi: 10.1016/j.ijpsycho.2010.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobotka S. S., Davidson R. J., & Senulis J. A. (1992). Anterior brain electrical asymmetries in response to reward and punishment. Electroencephalography and Clinical Neurophysiology, 83, 236–247. doi: 10.1016/0013-4694(92)90117-Z [DOI] [PubMed] [Google Scholar]
- Steiner A. R. W., & Coan J. A. (2011). Prefrontal asymmetry predicts affect, but not beliefs about affect. Biological Psychology, 88, 65–71. doi: 10.1016/j.biopsycho.2011.06.010 [DOI] [PubMed] [Google Scholar]
- Steingrüber H., & Lienert G. (1971). Hand-dominanz-test. Göttingen, Germany: Hogrefe [Google Scholar]
- Verona E., Sadeh N., & Curtin J. J. (2009). Stress-induced asymmetric frontal brain activity and aggression risk. Journal of Abnormal Psychology, 118, 131–145. doi: 10.1037/a0014376 [DOI] [PubMed] [Google Scholar]
- von dem Hagen E. A. H., Passamonti L., Nutland S., Sambrook J., & Calder A. J. (2011). The serotonin transporter gene polymorphism and the effect of baseline on amygdala response to emotional faces. Neuropsychologia, 49, 674–680. doi: 10.1016/j.neuropsychologia.2010.12.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacker J., Mueller E. M., Hennig J., & Stemmler G. (2012). How to consistently link extraversion and intelligence to the Catechol-O-Methyltransferase (COMT) Gene: On defining and measuring psychological phenotypes in neurogenetic research. Journal of Personality and Social Psychology, 102, 427–444. doi: 10.1037/a0026544 [DOI] [PubMed] [Google Scholar]
- Wacker J., Mueller E. M., Pizzagalli D. A., Hennig J., & Stemmler G. (2013). Dopamine-d2-receptor blockade reverses the association between trait approach motivation and frontal asymmetry in an approach motivational context. Psychological Science, 24, 489–497. doi: 10.1177/0956797612458935 [DOI] [PubMed] [Google Scholar]
- Wang Z., Baker D. G., Harrer J., Hamner M., Price M., & Amstadter A. (2011). The relationship between combat-related posttraumatic stress disorder and the 5-HTTLPR/rs25531 polymorphism. Depression and Anxiety, 28, 1067–1073. doi: 10.1002/da.20872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whisman M. A., Richardson E. D., & Smolen A. (2011). Behavioral inhibition and triallelic genotyping of the serotonin transporter (5-HTTLPR) polymorphism. Journal of Research in Personality, 45, 706–709. doi: 10.1016/j.jrp.2011.08.009 [DOI] [Google Scholar]
- Whittle S., Yücel M., Yap M. B., & Allen N. B. (2011). Sex differences in the neural correlates of emotion: Evidence from neuroimaging. Biological Psychology, 87, 319–333. doi: 10.1016/j.biopsycho.2011.05.003 [DOI] [PubMed] [Google Scholar]
- Wood A. M., Taylor P. J., & Joseph S. (2010). Does the CES-D measure a continuum from depression to happiness? Comparing substantive and artifactual models. Psychiatry Research, 177, 120–123. doi: 10.1016/j.psychres.2010.02.003 [DOI] [PubMed] [Google Scholar]
- Xie P., Kranzler H. R., Farrer L., & Gelernter J. (2012). Serotonin transporter 5-HTTLPR genotype moderates the effects of childhood posttraumatic stress disorder risk: A replication study. American Journal of Medical Genetics, 159B, 644–652. doi: 10.1002/ajmg.b.32068 [DOI] [PMC free article] [PubMed] [Google Scholar]