Abstract
Wnt morphogens released by neural precursor cells were recently reported to control blood–brain barrier (BBB) formation during development. Indeed, in mouse brain endothelial cells, activation of the Wnt/β-catenin signaling pathway, also known as the canonical Wnt pathway, was shown to stabilize endothelial tight junctions (TJs) through transcriptional regulation of the expression of TJ proteins. Because Wnt proteins activate several distinct β-catenin-dependent and independent signaling pathways, this study was designed to assess whether the noncanonical Wnt/Par/aPKC planar cell polarity (PCP) pathway might also control TJ integrity in brain endothelial cells. First we established, in the hCMEC/D3 human brain endothelial cell line, that the Par/aPKC PCP complex colocalizes with TJs and controls apicobasal polarization. Second, using an siRNA approach, we showed that the Par/aPKC PCP complex regulates TJ stability and reassembling after osmotic shock. Finally, we provided evidence that Wnt5a signals in hCMEC/D3 cells through activation of the Par/aPKC PCP complex, independently of the Wnt canonical β-catenin-dependent pathway and significantly contributes to TJ integrity and endothelial apicobasal polarity. In conclusion, this study suggests that the Wnt/Par/aPKC PCP pathway, in addition to the Wnt/β-catenin canonical pathway, is a key regulator of the BBB.
Keywords: blood–brain barrier, brain endothelial cells, planar cell polarity, tight junction, Wnt
Introduction
The blood–brain barrier (BBB) is a physiologic interface between the central nervous system and the blood. It strictly limits the passive diffusion of polar substances from the blood to the brain, providing brain parenchyma with nutrients and efflux from the brain of toxic metabolites and xenobiotics by active mechanisms and controlling the migration of circulating immune cells.1 The unique phenotype of BBB endothelial cells is characterized by the expression of tight junctions (TJ) and adherens junctions, two distinct, although functionally related, junctional complexes. Tight junctions are essential for BBB properties not only by restricting the passive diffusion of ions or macromolecules,2 but also by preventing the lateral diffusion of lipids and integral membrane proteins, thus controlling the brain endothelial apicobasal polarity.3
Tight junction complexes are composed of transmembrane proteins, notably claudin (cldn) −3, −52 and occludin,4 which are crucial to maintain TJ integrity together with several immunoglobulin superfamily members, such as junctional adhesion molecules.5 In addition, cytosolic proteins, such as the ZO family (ZO−1, −2, −3) directly bind the intracellular domains of these transmembrane proteins and act as molecular links to the cytoskeleton and signal transducers. We recently identified Gαi2 as a new cldn-5 partner that regulates TJ integrity in the hCMEC/D3 human brain endothelial cell line, an extensively used in vitro model of the human BBB,6 initially produced and characterized by us.7, 8
Development and maintenance of the BBB needs a dynamic dialog between endothelial and perivascular cells of the so-called neurovascular unit: indeed, neural precursor cells were shown to secrete factors that have a central role in BBB formation and integrity.9 Among these factors, Wnt morphogens appear as key regulators of the BBB phenotype. The Wnt family includes 19 secreted glycoproteins, which primarily signal through seven-pass transmembrane receptors, the Frizzled (Fzd) family in various cell types, including epithelial and endothelial cells.10, 11 Wnts activate several signaling pathways known as the β-catenin-dependent canonical pathway and alternative noncanonical pathways. The canonical pathway, leading to β-catenin cytoplasmic accumulation downstream of Fzd activation, followed by its nuclear translocation and transcription of multiple target genes, was identified, by us and others, as a regulator of brain angiogenesis and BBB phenotype establishment in vivo and in vitro.12, 13, 14 We recently confirmed that this pathway controls the expression of several TJ proteins in hCMEC/D3 cells.15 The noncanonical Wnt pathways are alternative β-catenin-independent cascades: a Wnt/calcium pathway and a Wnt/planar cell polarity (PCP) pathway have been documented in insect and mammalian epithelial morphogenesis.16, 17 The latter involves three interacting proteins, PAR-3, PAR-6, and the atypical protein kinase C-ζ.18 In epithelial cells, these proteins, associated with junctional adhesion molecules, are involved in the formation of TJ complexes and in apicobasal polarity,19 whereas their role in endothelial cells is linked to arterial polarization and lumen formation.20 Only recently, their expression was confirmed in brain endothelial cells.21
The present study aimed to assess whether the Wnt/Par/aPKC PCP pathway, in parallel to the Wnt canonical pathway, might directly control TJ integrity and apicobasal polarity in brain endothelial cells. Here we show that the Par/aPKC PCP complex controls hCMEC/D3 cell polarity; in addition, PAR-3 downregulation by specific small interfering RNAs (siRNAs) increases endothelial permeability and delays TJ recovery after hyperosmotic treatment. Finally, we demonstrate that Wnt5a, through a selective activation of the Wnt/Par/aPKC PCP pathway, significantly contributes to TJ integrity, strongly suggesting that the Wnt/Par/aPKC PCP pathway is a key regulator of the BBB.
Materials and Methods
Cell Culture Conditions
The immortalized human brain microvessel endothelial cell line (hCMEC/D3) was cultured at density of 50,000 cells per cm2 on Transwell inserts (0.4 μm pore size; Corning, Lowell, MA, USA) coated with 150 μg/mL rat tail collagen type I (R&D Systems, Minneapolis, MN, USA). The culture medium contained EBM-2 basal medium (Lonza, Walkersville, MD, USA) supplemented with 5% fetal bovine serum ‘Gold' (PAA Laboratories GmbH, Pasching, Austria), 10 mmol/L HEPES, 1% penicillin–streptomycin, 1% chemically defined lipid concentrate (Life Technologies SAS, Saint Aubin, France), 1.4 μmol/L hydrocortisone, 5 μg/mL ascorbic acid and 1 ng/ml basic fibroblast growth factor (Sigma-Aldrich, St Louis, MO, USA). Cells were grown for 6 days at 37°C in a in a 5% CO2 incubator and the medium was changed 3 days after seeding. Culture medium was supplemented with lithium chloride (Merck Millipore SAS, Molsheim, France) as indicated.
Small Interfering RNA Experiments
Small Interfering RNA transfections were performed using various Stealth RNAi duplexes against PAR-3 (#HSS183488, #HSS183489), aPKC-ζ (#HSS183348, #HSS183325), cldn-5 (#HSS186370), and nontargeting siRNA as control (Life Technologies SAS). Cells were plated onto culture inserts (Corning, Lowell, MA, USA) or culture E-plates wells for xCELLigence technology in culture medium without antibiotics. One hour after cells seeding (day 0), the transfection mix containing Lipofectamine RNAiMAX (Life Technologies SAS), 50 nmol/L of siRNAs and Opti-MEM Reduced Serum Medium (Life Technologies SAS) was added to the culture medium according to the manufacturer's instructions. Cells were incubated for 6 days at 37°C in a 5% CO2 incubator and culture medium was changed at day 3 with fresh siRNA-supplemented transfection medium. The efficacy of PAR-3, aPKC-ζ, and cldn-5 knockdown was assessed by immunoblotting (see below).
Permeability Assays
HCMEC/D3 cells were seeded onto culture inserts for 6 days and permeability assays with 50 μmol/L Lucifer Yellow (LY) were performed as previously described.6 Results are expressed as permeability coefficients (Pe) in 10−3 cm per minute or as the percentage of control permeability (corresponding to either untreated cells or control-conditioned medium or control siRNA-treated cells, as indicated).
Immunofluorescence Microscopy
HCMEC/D3 cells were cultured for 6 days on insert filters, washed with phosphate-buffered saline (PBS) and fixed with 2% paraformaldehyde for 10 minutes at room temperature; then cells were permeabilized with 0.1% Triton X-100 in PBS for 5 minutes at room temperature, blocked in 3% bovine serum albumin in PBS (blocking buffer) for 30 minutes, and stained with the indicated primary antibodies (diluted in the blocking buffer overnight at 4°C), directed against the following proteins: ZO-1 (monoclonal or polyclonal antibodies, Life Technologies SAS), VE-cadherin (Clone BV6; Merck Millipore SAS), Podocalyxin (Santa Cruz Biotechnology, Heidelberg, Germany), PAR-3 (Merck Millipore SAS), GLUT-1 (Epitomics, Burlingame, USA). After three washes with PBS, cells were incubated for 1 hour with Alexa488- or Alexa568-conjugated secondary antibodies (Life Technologies SAS). Cells were then washed three times with PBS and preparations were mounted in Glycergel medium (Dako, Carpinteria, CA, USA) supplemented with 0.1% 4′,6-diamidino-2-phenylindole. Cells were observed with a Zeiss Axio Observer Z1 microscope using a × 40 Oil Objective (Olympus, Rungis, France). For apicobasal polarity ratio assessment, cell membranes were stained before cell fixation with 5 μmol/L, chloromethylbenzamido (Cell tracker CM-diI, Life Technologies SAS) in PBS for 5 minutes at 37°C, then left for another 5 minutes at 4°C. After three washes in PBS, cells were fixed and permeabilized as described above. Staining of P-gp, Podocalyxin or GLUT-1 proteins was performed. Image stacks were acquired with 0.3 nm z-step ( × 100 oil objective; Olympus) using Yokogawa CSU X1 Spinning Disk (Yokogawa, Tokyo, Japan) coupled with a DMI6000B Leica microscope (Leica microsystems Gmbh, Wetzlar, Germany). Acquisitions were made with MetaMorph 7 software.
Image Analysis
Analyses were performed using homemade ImageJ routine (http://www.imagej.nih.gov/ij/). For apicobasal polarity ratio assessment, the quantification of fluorescence intensity was estimated at the apical and basal plasma membranes, respectively above and below the nucleus, as illustrated in Figure 2B, taking advantage of the larger thickness of the cells at the level of the nucleus. Using nuclear signal z-projection as binary mask for each cell, fluorescence intensities of CM-d-labeled membranes and immunolabeled-targeted proteins were automatically measured for every slice of z-stack acquisition.
To establish the apicobasal ratio, CM-Dil labeling was used to define the apical and basal membrane boundaries. Then total fluorescence intensity of targeted protein was evaluated in those both domains and the apicobasal ratio was calculated.
Immunoblotting Assays
HCMEC/D3 cells were washed with PBS, lysed for 10 minutes with ice-cold Laemmli lysis buffer and scraped. Proteins of the whole-cell lysate were heated at 95°C for 5 minutes and processed for immunoblotting as previously described.6
xCELLigence Assays
The xCELLigence system (ACEA, San Diego, USA) is a cell-based label-free instrument that measure in real time electrical impedance across gold microelectrodes integrated on the bottom of culture E-plates. HCMEC/D3 cells were seeded in standard culture medium without antibiotics at a density of 50,000 cells per cm2 onto 96-well E-Plates coated with 150 μg/mL rat tail collagen type I. One hour after cell adhesion, the siRNA transfection mix was added (see protocol of siRNAs above) and cells were cultured for 7 days. As indicated, at 72 hours of culture, the confluent monolayer of hCMEC/D3 cells was treated with 1 mol/L mannitol (osmotic shock) in serum-free culture medium for 30 minutes. Then, mannitol solution was removed and standard culture medium was added for a recovery period up to 4 days. Impedance measurement was displayed in real time as a cell index (CI, an arbitrary unit) by the xCELLigence RTCA software (ACEA, San Diego, CA, USA). Results are presented as mean values of CI±s.d. against time in each condition.
Quantitative Real Time Polymerase Chain Reaction
Axin2 (a β-catenin target gene) expression profile was performed by qRT-PCR. Total RNA was isolated using the RNeasy mini kit (Qiagen Gmbh, Hilden, Germany). RNA purity and concentration were determined with a NanoDrop 2000 spectrophotometer (Thermo Scientific Gmbh, Ulm, Germany). Conversion to cDNA was performed by reverse transcription. One microgram of total RNA was added in a mixture, which consisted of 500 μmol/L of each dNTP, 10 mmol/L DTT, 1.5 μmol/L random hexanucleotide primers, 20 U RNase out, and 100 U SuperScript II reverse transcriptase (Life Technologies, SAS). Hexamers were annealed at 25°C for 10 minutes, products were extended at 42°C for 30 minutes, and the reaction was terminated by heating to 99°C for 5 minutes before being quick chilled to 4°C and stored at −80°C.
The cDNA template (diluted 1:20) was then mixed with forward, reverse primers (0.5 μmol/L) and LightCycler 480 SYBR Green I Master (Roche, Basel, Switzerland) according to the manufacturer's instructions. Quantitative real time polymerase chain reaction was performed on a Roche Applied Science LightCycler 480 system (Roche,). Raw data were analyzed with LightCycler 480 software (Roche,) using the ΔΔCt method (relative quantification). Primer pairs:
Axin2: forward (5′-CGCCAACGACAGTGAGAT-3′); reverse (5′-TGCCCACACGATAAGGAG-3′)
β-Actin: forward (5′-CTGGCCCGGACCTGACAGA-3′); reverse (5′-GCCGCAGTGGCCATCTCTT-3′)
RT2 Profiler Polymerase Chain Reaction Array
Total RNA was isolated using the RNeasy mini kit (Qiagen). RNA purity and concentration were determined with a NanoDrop 2000 spectrophotometer (Thermo Scientific). One microgram of total RNA was converted to cDNA using RT2 First Strand Kit (Qiagen). cDNA was mixed with RT2 SYBR Green Master mix and dispensed into the RT2 Profiler Array (Human WNT Signaling Pathway PAHS-043 F-2) (Qiagen). Quantitative real time polymerase chain reaction was performed on a Roche Applied Science LightCycler 480 system (Roche). Raw data were analyzed with LightCycler 480 software (Roche) using the ΔΔCt method (relative quantification).
Statistical Analysis
Data are presented as means±s.d. Statistical analysis was performed using Student's t-tests with differences between means considered significant when P<0.05.
Results
The Par/aPKC Planar Cell Polarity Complex is Expressed in hCMEC/D3 cells
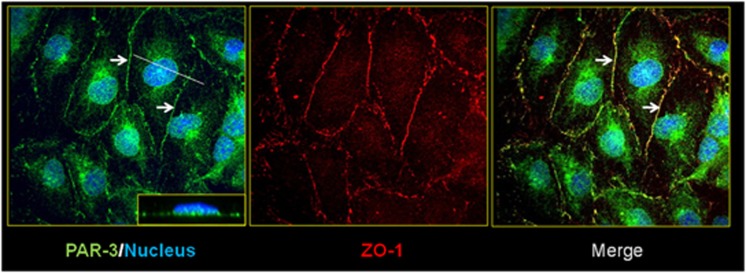
Given that the Par/aPKC PCP complex is localized at TJs in epithelial cells,19 we investigated its expression and localization in the human brain endothelial hCMEC/D3 cell line, a validated model of the human BBB.8 As illustrated in Figure 1, PAR-3 expression could be detected at cell–cell junctions, in addition to nuclear and perinuclear localizations (Figure1, left panel). PAR-3 expressed at cell–cell junctions colocalized with the TJ protein ZO-1 (Figure 1, center and right panels: arrows) suggesting that it might be located at TJs in brain endothelial cells, as previously reported in epithelial cells. In addition, we confirmed by western Blot analysis (data not shown) that hCMEC/D3 cells also express aPKC-ζ and PAR-6, the two other constituents of the Par/aPKC PCP complex, as recently reported.22
Figure 1.
Par/aPKC planar cell polarity complex is expressed in hCMEC/D3 cells and colocalized with ZO-1. HCMEC/D3 cells grown at confluence for 6 days were fixed and permeabilized. Double immunofluorescence labeling was performed with a ZO-1 polyclonal antibody and PAR-3 monoclonal antibody. Nuclei were labeled with 4′,6-diamidino-2-phenylindole. Inset in right panel x–z plan, which represent PAR-3 staining.
The Par/aPKC Planar Cell Polarity Complex Controls Polarization of hCMEC/D3 Cells
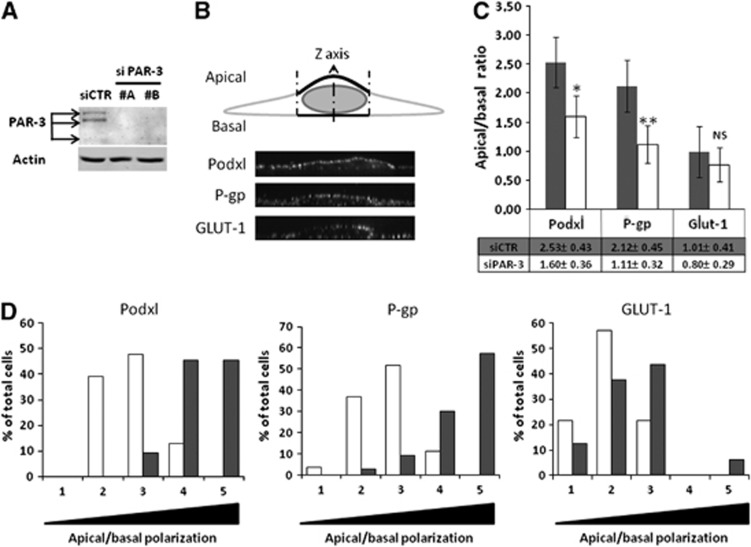
It has been well established that the Par/aPKC PCP complex is a regulator of cell polarization in the epithelia. Because brain endothelial cells are also polarized cells, the putative role of Par/aPKC PCP complex in controlling hCMEC/D3 polarization was investigated. Together with podocalyxin (Podxl), an apical marker of endothelial cells, the ABC-transporter P-gp, selectively expressed by brain endothelial cells, is highly enriched at their apical membrane; by contrast, the glucose transporter GLUT-1 is known to be expressed at apical and basal membranes of brain endothelial cells.23 By confocal immunofluorescence analysis of these proteins, we then compared their apical versus basal expression in hCMEC/D3 cells after siRNA-mediated PAR-3 knockdown or after treatment with scrambled siRNA as control.
Quantification of the apical versus basal expression of these three proteins was described in the M&M section and illustrated in Figure 2B. As expected, Podxl and P-gp were expressed in control cells at a significantly higher level in the apical membrane (apical/basal ratio: 2.53±0.43 and 2.12±0.45, respectively) (Figure 2C), whereas GLUT-1 expression was not significantly different in the two compartments (apical/basal ratio: 1.01±0.41) (Figure 2C). After confirming that two individual siRNAs substantially decreased PAR-3 expression in hCMEC/D3 cells (Figure 2A), we quantified the apical versus basal expression of Podxl, P-gp, and GLUT-1 in cells treated by PAR-3 siRNA (#A), the most efficient siRNA used. PAR-3 knockdown significantly decreased the polarization of Podxl and P-gp expression (apical/basal ratio: 1.60±0.36 and 1.11±0.32, respectively) (Figure 2C); as control, GLUT-1 localization was not affected (apical/basal ratio: 0.80±0.29) (Figure 2C). In Figure 2D, the same results are presented as the proportion of analyzed cells with increasing mean apical/basal ratios, from 0 to 0.5 (1) to 2.0 to 2.5 (5): the shift to the left of Podxl and P-gp expression in PAR-3 knockdown cells (Figure 2D: open bars) clearly illustrates that PAR-3 knockdown drastically reduced the spontaneous polarization of hCMEC/3 cells.
Figure 2.
The Par/aPKC planar cell polarity complex controls hCMEC/D3 cells polarization. (A) Immunoblot analysis of PAR-3 and actin expression. hCMEC/D3 cells were treated with control small interfering RNA (si CTR) or two distinct siRNAs against PAR-3 (#A, #B). Cells were grown at confluence and whole-cell lysates were generated. Proteins were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis gel followed by immunoblotting with anti-PAR-3 or anti-actin monoclonal antibodies. (B) Cell membranes were stained using CM-dil then cells were fixed and permeabilized. Immunostaining was performed using Podxl or P-gp monoclonal antibodies or a GLUT-1 polyclonal antibody. Nuclei were labeled with DAPI. Membranous immunofluorescence intensity was evaluated along cells Z-axis above and below the nucleus using ImageJ software and curve was generated using KaleidaGraph software. Apical or basal fluorescence intensity was calculated by integrating area under apical or basal peak defined by membrane labeling with CM-dil. (C) HCMEC/D3 cells treated with control siRNA (gray bars) or PAR-3 siRNA (#A) (white bars) were grown at confluence for 6 days on culture inserts. Mean apical/basal fluorescence intensity of 40 different cells from three independent experiments was calculated. Indicated P values were obtained using Student's t-test: *P<0.005; **P<0.001; NS, not significant. (D) Cell repartition in percent between apical/basal ratio range divided in five equal categories (gray: siCTR; white: siPAR-3). Category 1 includes cells with lower apical/basal ratio (0 to 0.5) to category 5, which includes cells with higher apical/basal ratio (2 to 2.5).
From this set of experiments, we concluded that the Par/aPKC PCP complex efficiently controls the polarization of brain endothelial cells.
The Par/aPKC Planar Cell Polarity Complex Controls Tight Junction Integrity
Because the Par/aPKC PCP complex has been described during epithelial morphogenesis to be involved in TJ complex formation and stability, we intended to investigate its function in brain endothelial cells, using the hCMEC/D3 model.
First, we assessed the consequences of PAR-3 or aPKC-ζ knockdown on hCMEC/D3 permeability to Lucifer Yellow (LY), a 457-Da fluorescent marker of paracellular permeability. As presented in Figure 3, PAR-3 knockdown significantly increased hCMEC/D3 permeability to LY. Similarly, two individual aPKC-ζ siRNAs increased LY permeability, although to various extents (likely due to incomplete protein knockdown, as shown in Figure 3A), suggesting that the Par/aPKC PCP complex is involved in the control of TJ integrity.
Figure 3.
Par/aPKC planar cell polarity complex knockdown increases hCMEC/D3 permeabilty to Lucifer Yellow (LY). (A) Immunoblot analysis of atypical protein kinase C-ζ (aPKC-ζ) and actin expression. HCMEC/D3 cells were treated with control small interfering RNA (si CTR) or two distinct siRNAs against aPKC-ζ. Cells were grown at confluence and whole-cell lysates were generated. Proteins were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis gel followed by immunoblotting with anti-aPKC-ζ or anti-actin monoclonal antibodies. aPKC-ζ immunoblot quantification using ImageJ software, relative expression of aPKC-ζ normalized to actin level (lower panel). (B) HCMEC/D3 cells were treated with control siRNA or PAR-3 siRNA (#A) or two distinct aPKC-ζ siRNA (#1, #2) and grown at confluence for 6 days on culture inserts. Permeability assays to LY were performed and the permeability coefficient (Pe) was quantified. Results are indicated as permeability ratio, normalized relative to the Pe of cells treated with control siRNA. The basal permeability level was 1.42±0.16.10−3 cm/minute. Results are means±s.d. for three independent experiments. Indicated P values were obtained using Student's t-test. *P<0.02; **P<0.005.
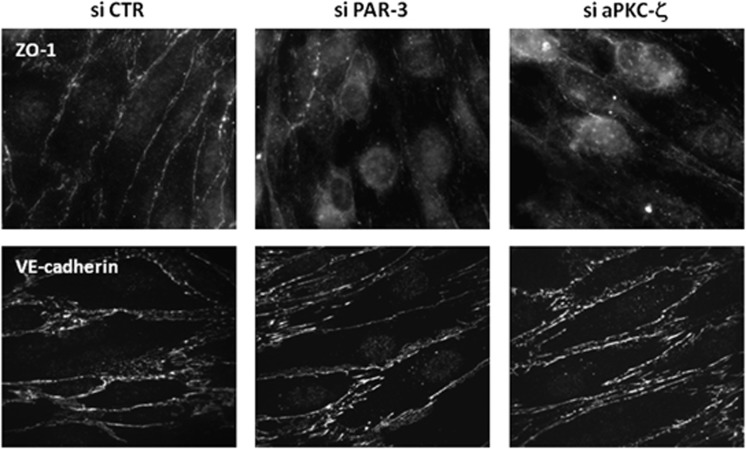
Second, immunofluorescence analysis of junction proteins showed that PAR-3 or aPKC-ζ knockdown decreased ZO-1 localization at cell–cell junctions, compared with the continuous staining observed in control cells (Figure 4, upper panel). By contrast, VE-cadherin expression/localization was not affected.
Figure 4.
Par/aPKC planar cell polarity complex knockdown destabilizes tight junction complexes. HCMEC/D3 cells were treated with control small interfering RNAs (si CTR), PAR-3 siRNA (A) or atypical protein kinase C-ζ (aPKC-ζ) siRNA (#2), and grown at confluence for 6 days on culture inserts. Cells were fixed and permeabilized. Immunofluorescence labeling was performed with a ZO-1 monoclonal antibody and VE-cadherin (VE-cad) polyclonal antibody.
Altogether, these observations indicate that, although adherens junction complex assembly appears independent of the Par/aPKC PCP-complex, TJ complexes do not properly form in brain endothelial cells in absence of PAR-3 or aPKC-ζ, thereby affecting TJ integrity.
The Par/aPKC Planar Cell Polarity Complex Controls Tight Junction Reassembling after Osmotic Shock
We previously established that knockdown of cldn-5, a key TJ protein for BBB integrity in vivo,6 strongly increased hCMEC/D3 permeability to LY and delayed TJ reassembling after osmotic shock by 1 mol/L mannitol.6 Along the same line, here we intended to assess the putative contribution of the Par/aPKC PCP complex to this process.
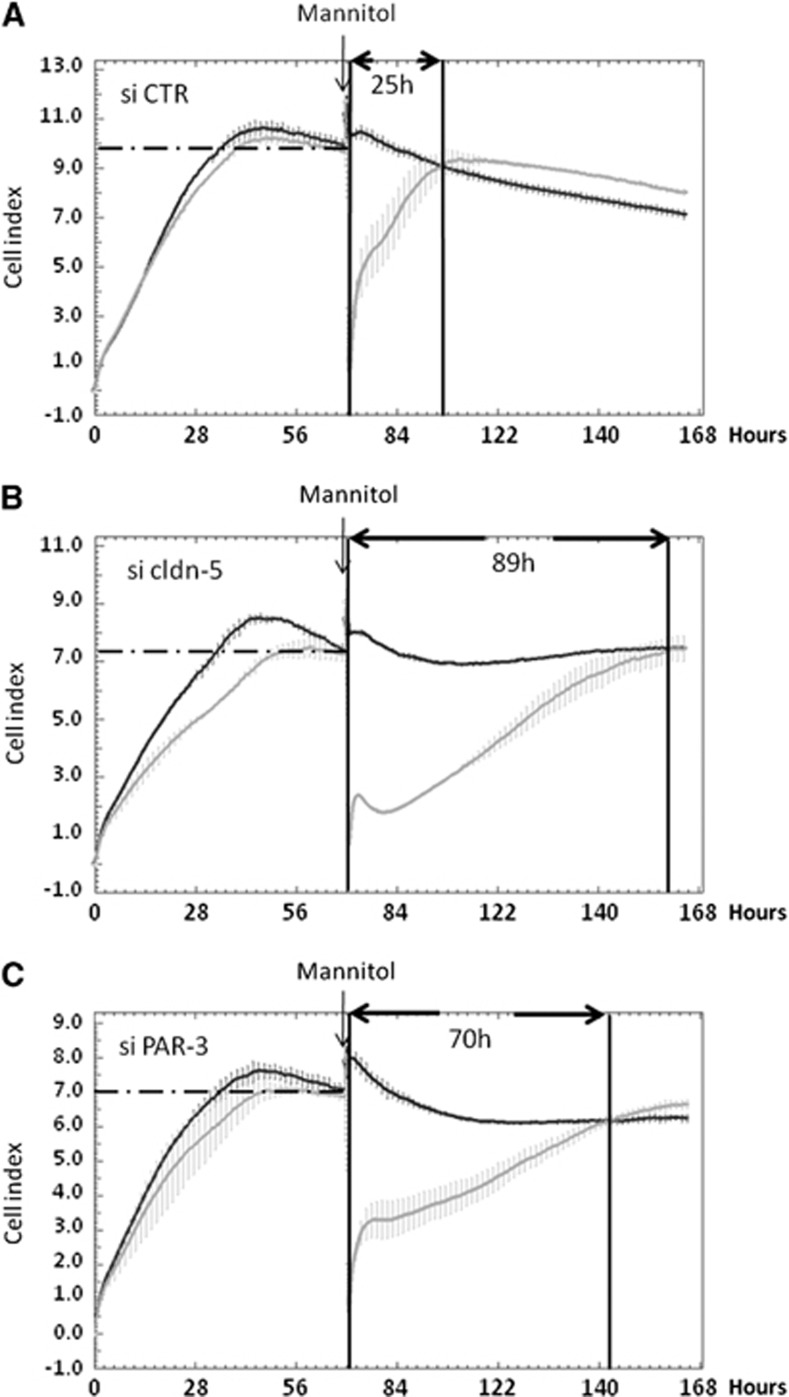
After a 72-hour period of proliferation, confluent control siRNA-treated hCMEC/D3 cells or PAR-3 or cldn-5 knockeddown cells were submitted to osmotic shock by 30-minute treatment with 1 mol/L mannitol; then monolayer impedance (assessed as CI) was monitored using the ACEA xCELLigence system over a time period of 96 hours. After cell proliferation, when cell confluence was reached, the CI of cldn-5 or PAR-3 knockeddown cells plateaued at similar levels (CI/cldn-5 knock-down=6.95±0.13; CI/PAR-3 knock-down=6.7±0.65), significantly lower than that of control siRNA-treated cells (CI=10.16±0.25) (Figure 5), which is in agreement with our observations that cldn-5 or PAR-3 deficiency did not prevent cells to reach confluence, but somehow destabilized TJs and increased LY permeability (Figure 4).6
Figure 5.
PAR-3 knockdown delays tight junction complex reassembling after 1 mol/L mannitol treatment. HCMEC/D3 cells treated with control (A), claudin-5 (cldn-5) (B) or PAR-3 (C) small interfering RNAs were grown on a 96-well E-plate. Impedance measurement was monitored in real time by xCELLigence system and curves were generated using the RTCA Software. After 72 hours, cells were treated with 1 mol/L mannitol. After another 30 minutes, medium was changed to normal growth medium for a recovery period of up to 4 days. Black curve: untreated cells; light gray curve: 1 mol/L mannitol-treated cells. Results are mean cell index (CI) values±s.d. (n=3 wells) from one representative experiment out of three independent experiments.
Mannitol treatment, known to induce transient TJ destabilization at the BBB in vivo,24 induced a dramatic drop in CI values (CI=0.5) (Figure 5 light gray curve) in the three conditions. After medium change, the CI of control cells gradually increased to reach a similar value as the plateau before mannitol treatment after ∼25 hours (Figure 5A), likely reflecting a progressive and biphasic TJ reassembly during the recovery period: a rapid increase of the CI value within 2 hours, followed by a slower increase to the initial CI value. Cells silenced for cldn-5 or PAR-3 presented a clear delay in recovery, especially regarding the second phase: ∼89 hours and 70 hours, respectively (Figures 5B and 5C)
Altogether, these data illustrate that the absence of PAR-3, like the absence of cldn-5, strongly affects the integrity of cell–cell junctions in hCMEC/D3 cells by disturbing the process of junction reassembly during recovery after an osmotic shock.
Wnt5a Regulation of hCMEC/D3 Permeability is Mediated by the Par/aPKC Planar Cell Polarity Complex
It is well established that the Par/aPKC PCP complex can be activated in epithelial cells by some Wnt factors, including Wnt5a. This functional link is being known as the Wnt/PCP pathway. Accordingly, we investigated a putative role of Wnt5a in controlling TJ integrity in hCMEC/D3 cells through the Par/aPKC PCP complex.
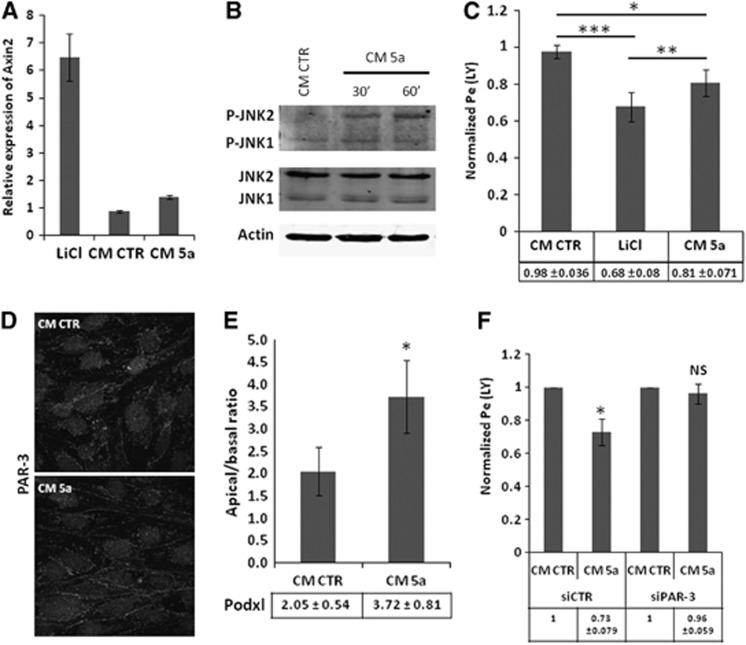
In a first step, we confirmed by qRT-PCR analysis that hCMEC/D3 cells express the major components of Wnt signaling pathways (Table 1), like Fzd −1 to −8 (except Fzd-3), Dishevelled proteins-1 and -2, together with Dishevelled-associated activator of morphogenesis 1, which is known to be triggered by Wnt5a.25 As a next step, hCMEC/D3 cells were treated during 6 days with Wnt5a (40% conditioned medium of Wnt5a producing LM (TK) cells (ATCC #CRL-2814), as previously described.26 As control, lithium chloride 10 mmol/L was used as an activator of the Wnt/β-catenin canonical pathway: indeed, we observed that treatment of hCMEC/D3 cells with 10 mmol/L lithium chloride strongly increased the transcription of Axin2 mRNA, a marker of activation of the Wnt/β-catenin pathway. By contrast, no effect was observed in response to Wnt5a-conditioned medium, confirming in this system that Wnt5a does not trigger the Wnt canonical pathway (Figure 6A).
Table 1. Expression of genes involved in Wnt signaling pathways in hCMEC/D3 cells.
| Name | Symbol | Expression level |
|---|---|---|
| Frizzled family receptor 1 | Fzd-1 | ++ |
| Frizzled family receptor 2 | Fzd-2 | ++ |
| Frizzled family receptor 3 | Fzd-3 | ND |
| Frizzled family receptor 4 | Fzd-4 | +++ |
| Frizzled family receptor 5 | Fzd-5 | ++ |
| Frizzled family receptor 6 | Fzd-6 | +++ |
| Frizzled family receptor 7 | Fzd-7 | + |
| Frizzled family receptor 8 | Fzd-8 | ++ |
| Dishevelled, dsh homolog 1 (Drosophila) | DVL-1 | ++ |
| Dishevelled, dsh homolog 2 (Drosophila) | DVL-2 | ++ |
| Dishevelled-associated activator of morphogenesis 1 | DAAM1 | +++ |
| Adenomatous polyposis coli | APC | ++ |
| Glycogen synthase kinase 3 beta | GSK3-b | +++ |
| Axin 1 | AXIN1 | ++ |
| Low-density lipoprotein receptor-related protein 5 | LRP-5 | ++ |
| Low-density lipoprotein receptor-related protein 6 | LRP-6 | ++ |
(+++: 20<Ct<25; ++: 25<Ct<30; +: 30<Ct<35; ND, not detectable Ct>35).
Figure 6.
Wnt5a-triggered control of hCMEC/D3 permeability is mediated by Par/aPKC planar cell polarity complex. (A) HCMEC/D3 cells were incubated with EBM-2 medium or with EBM-2 containing 10 mmol/L lithium chloride (LiCl), 40% of control (CM CTR) or Wnt5a (CM 5a)-conditioned medium and grown at confluence for 6 days on culture inserts. Total RNA extracts were obtained using RNA kit extraction. Reverse transcription was performed and Axin2 messenger RNA expression was evaluated by quantitative real time polymerase chain reaction using LightCycler 480 device. Results are visualized as relative expression compared with EBM-2 medium. (B) Immunoblot analysis of JNK phosphorylation and actin expression. HCMEC/D3 cells grown at confluence for 6 days were treated for 30 minutes or 1 hour with EBM-2 completed with 40% of CM CTR or CM 5a. Whole-cell lysates were generated. Proteins were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis gel followed by immunoblotting with anti-Phospho-JNK polyclonal antibody, anti-JNK monoclonal antibody or anti-actin monoclonal antibody. (C) HCMEC/D3 cells were incubated with EBM-2 medium or with EBM-2 containing 10 mmol/L LiCl, 40% of CM CTR or CM 5a-conditioned medium and grown at confluence for 6 days on culture inserts. Permeability assays to Lucifer Yellow (LY) were performed and the permeability coefficient was quantified. Results are expressed as permeability ratio, normalized relative to the permeability coefficient for EBM-2 cultured cells. The basal permeability level was 1.8±0.1.10−3 cm/minute. Results are means±s.d. for seven independent experiments. Indicated P values were obtained using Student's t-test. *P<0.01; **P<0.003, ***P<0.002. (D) HCMEC/D3 cells were treated with EBM-2 complement with 40% of CM CTR or CM 5a and grown at confluence for 6 days on culture inserts. Then cells were fixed and permeabilized. Immunofluorescence labeling was performed with a PAR-3 polyclonal antibody. (E) HCMEC/D3 cells were treated with EBM-2 completed with 40% of CM CTR or CM 5a and grown at confluence for 6 days on culture inserts. Mean apical/basal fluorescence intensity of 40 different cells from at least three independent experiments is shown. Indicated P value was obtained using Student's t-test: *P<0.0001. (F) HCMEC/D3 cells treated with control siRNA or PAR-3 siRNA (#A) were grown at confluence for 6 days in EBM-2 medium completed with 40% of CM CTR or CM 5a on culture inserts. Permeability assays to LY were performed and the permeability coefficient was quantified. Results are expressed as permeability ratio, normalized relative to the permeability coefficient for CM CTR. The basal permeability level was 1.65±0.2.10−3 cm/minute. Results are means±s.d. for five independent experiments. Indicated P values were obtained using Student's t-test. *P<0.02; NS, not significant.
Because C-JUN-N-terminal kinase (JNK) has been reported as a downstream effector of the Wnt/PCP pathway, the putative phosphorylation of JNK, reflecting its activation, was assessed in hCMEC/D3 cells in response to Wnt5a. As illustrated in Figure 6B, Wnt5a-conditioned medium increased JNK-2 phosphorylation in 30 to 60 minutes, strongly suggesting that the Par/aPKC PCP complex is activated by Wnt5a. After 6 days of incubation with Wnt5a-conditioned medium, LY permeability of hCMEC/D3 cells was moderately but significantly decreased as compared with cells treated with control-conditioned medium (0.81±0.07 as compared with 1 for normalized permeability of control cells) (Figure 6C); as control, lithium chloride also decreased LY permeability (0.68±0.08), as previously reported.15
In order to establish whether this effect of Wnt5a might be mediated by the Par/aPKC PCP complex, we first monitored PAR-3 expression and localization in hCMEC/D3 cells after Wnt5a stimulation. As illustrated in Figure 6D, PAR-3 localization to cell–cell contacts was stronger after Wnt5a treatment, in line with its putative activation-induced translocation to the plasma membrane. Furthermore, we observed by confocal fluorescence microscopy that the apical/basal ratio of Podxl expression was significantly increased after Wnt5a treatment: apical/basal ratio: 3.72±0.81 versus 2.05±0.54 in control condition (Figure 6E).
Finally, PAR-3-deficient or control hCMEC/D3 cells were treated with Wnt5a-conditioned medium, or control-conditioned medium. Whereas Wnt5a-conditioned medium significantly decreased LY permeability of control hCMEC/D3 cells (as reported above), no effect was observed in PAR-3-deficient cells (Figure 6F); as control, Wnt5a increased JNK phosphorylation in PAR-3-deficient as well as control cells, confirming that the proximal step of the Wnt/PCP pathway was activated by Wnt5a in both cell types (data not shown).
Altogether, these results indicate that Wnt5a stabilizes brain endothelial TJs through activation of the Par/aPKC PCP complex, independently of the Wnt canonical pathway.
Discussion
It is now well established that the Wnt/β-catenin pathway (or: ‘canonical' pathway) controls the development and integrity of the BBB; this key observation has also been confirmed in vitro in brain endothelial cells and multiple target genes have been identified.12, 13, 15 We demonstrate in the present study that the noncanonical Wnt signaling pathway, coupled to activation of the Par/aPKC PCP complex, also contributes to the control of the apicobasal polarization of human brain endothelial cells and to the regulation of Tj integrity.
Although the role of the Par/aPKC PCP complex in epithelial polarity has been well established both in Drosophila and mammals,19, 27 evidence of its putative expression and function in vascular endothelial cells remained so far largely elusive or indirect. In particular, it was recently reported that the recruitment of this complex by bacterial pathogens adhering at the apical surface of hCMEC/D3 brain endothelial cells induced the formation of ectopic intercellular junctional domains at the site of bacteria host cell interaction and a subsequent destabilization of endothelial cell–cell junctions assessed by increased LY permeability.22 In addition, the apicobasal polarity in endothelial cells was shown to be controlled by VE-cadherin and associated proteins, via activation of the Par/aPKC PCP complex.28 We confirmed here, by confocal microscopy, that PAR-3 is expressed by hCMEC/D3 cells and partly localized at cell–cell junctions, together with ZO-1, a prototype TJ-associated protein; a proportion of PAR-3 was also detected in the cytosol and nucleus, in line with previous reports in different cell types.29, 30 We established here, by quantitative confocal microscopy analysis of Podxl and P-gp expression in individual cells, that hCMEC/D3 cells are polarized on Transwell inserts (Figure 2), as previously reported by electron microscopy monitoring of P-gp expression.23 We further demonstrated that siRNA-mediated PAR-3 knockdown largely prevented the apicobasal-polarized expression of these proteins. This result constitutes, to our knowledge, the first direct demonstration of the regulation by the Par/aPKC PCP complex of the apicobasal polarization of brain endothelial cells.
In addition, by knocking down the expression of PAR-3 or aPKC-ζ, we observed a significant increase in hCMEC/D3 permeability to the paracellular diffusion marker LY (Figure 3), together with a partial loss of ZO-1 expression at cell–cell contacts (Figure 4) and a delayed TJ reassembling after hyperosmotic shock (Figure 5). We recently reported similar results after siRNA-mediated deletion of cldn-5 expression in hCMEC/D3 cells,6 strongly suggesting that the Par/aPKC PCP complex, like the TJ protein cldn-5, is actively involved in maintaining TJ integrity in brain endothelial cells. Compared with the very transient opening of the BBB in vivo after an hyperosmotic shock (30 to 60 minutes), full recovery of the impedance of hCMEC/D3 monolayers (expressed here as CI) after mannitol treatment took much longer (∼24 hours in control condition). However, it must be noticed that BBB recovery assessed in patients by permeability to small molecules, rather than by Evans blue diffusion in preclinical models, may take 6 to 8 hours as reported by Siegal et al.31 We may then hypothesize that the rapid in vivo recovery (1 hour) might correspond to the early recovery phase (1 to 2 hours) observed in vitro and that full recovery, likely corresponding to a complete reorganization of junctional complexes, would take several hours to proceed as observed in the present study (Figure 5).
Our data are in agreement with the previous report that, although PAR-3 does not interact directly with cldns, it is recruited to TJ complexes through binding to JAM proteins.32 In epithelial cells, two alternative polarization complexes, the Crumbs and Scribble complexes, are known to be involved in TJ formation and apicobasal polarization and to functionally interact with the Par/aPKC PCP complex.27 Inasmuch as a recent report indicated that endothelial cells also express Scribble,33 it is tempting to speculate that this complex might also contribute to the apicobasal polarity of brain endothelial cells and BBB integrity.
Activation of the noncanonical Par/aPKC PCP complex has been extensively described as a signaling pathway downstream of the interaction between Wnt morphogens and their membrane receptors, the Fzd family and associated coreceptors.34 Because Wnts are now considered as key regulators of BBB formation and integrity, we intended here to explore the putative role of the Wnt/Par/aPKC PCP pathway in TJ integrity in hCMEC/D3 cells. The Wnt factor Wnt5a was previously described to selectively activate this pathway in various cell types,25, 35 to control multiple cellular functions (cell migration, proliferation, differentiation) and to promote proliferation or survival, particularly in endothelial cells.36 These pleiotropic effects are mediated through Wnt5a binding to several Fzds, including Fzd-2, Fzd-4, Fzd-5, and Fzd-7.37 In line with these observations, here we report in hCMEC/D3 cells, which express all these Fzds (Table 1), that Wnt5a induced JNK phosphorylation, a hallmark of activation of the Wnt/Par/aPKC PCP pathway,38 without activating the canonical Wnt/β-catenin pathway (Figure 6). In addition, we observed that Wnt5a increased the apicobasal polarization of hCMEC/D3 cells and reduced their permeability to LY, whereas this effect was abolished after siRNA-mediated PAR-3 knockdown. We previously reported that hCMEC/D3 monolayers display low transendothelial electrical resistance in standard culture conditions;7 however, preliminary results suggest that Wnt5a may increase these transendothelial electrical resistance values by ∼50% (not shown). Altogether, these results point to a key contribution of the Wnt/Par/aPKC PCP pathway to the maintenance of the specialized phenotype of brain endothelial cells in vitro and suggest that it may also be involved in the control of BBB integrity in situ. Recent reports showing that the Wnt noncanonical pathway activated by Wnt5a, as well as the Par/aPKC PCP complex contribute in vivo to retinal vessel maturation are in line with our proposition.39, 40
In conclusion, we propose that Wnt factors control BBB integrity through activation of multiple signaling pathways, including the canonical Wnt/β-catenin pathway12, 13, 15 and the Wnt/Par/aPKC PCP pathway (this study). Further investigations will aim at assessing whether additional pathways, such as the Wnt/calcium pathway, may also contribute to BBB integrity.
The authors declare no conflict of interest.
Footnotes
This work was supported by the European FP7 collaborative grants: the European Stroke Network (ESN, EU FP7, agreement No. 202213) and JUSTBRAIN (Health-F2-2009-241861).
References
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13–25. doi: 10.1016/j.nbd.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- Dragsten PR, Blumenthal R, Handler JS. Membrane asymmetry in epithelia: is the tight junction a barrier to diffusion in the plasma membrane. Nature. 1981;294:718–722. doi: 10.1038/294718a0. [DOI] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Padura I, Lostaglio S, Schneemann M, Williams L, Romano M, Fruscella P, et al. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luissint AC, Federici C, Guillonneau F, Chretien F, Camoin L, Glacial F, et al. Guanine nucleotide-binding protein Galphai2: a new partner of claudin-5 that regulates tight junction integrity in human brain endothelial cells. J Cereb Blood Flow Metab. 2012;32:860–873. doi: 10.1038/jcbfm.2011.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, et al. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Weksler B, Romero IA, Couraud PO. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS. 2013;10:10–25. doi: 10.1186/2045-8118-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luissint AC, Artus C, Glacial F, Ganeshamoorthy K, Couraud PO. Tight junctions at the blood brain barrier: physiological architecture and disease-associated dysregulation. Fluids Barriers CNS. 2012;9:23–34. doi: 10.1186/2045-8118-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald BT, Tamai K, He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejana E. The role of wnt signaling in physiological and pathological angiogenesis. Circ Res. 2010;107:943–952. doi: 10.1161/CIRCRESAHA.110.223750. [DOI] [PubMed] [Google Scholar]
- Daneman R, Agalliu D, Zhou L, Kuhnert F, Kuo CJ, Barres BA. Wnt/beta-catenin signaling is required for CNS, but not non-CNS, angiogenesis. Proc Natl Acad Sci USA. 2009;106:641–646. doi: 10.1073/pnas.0805165106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebner S, Corada M, Bangsow T, Babbage J, Taddei A, Czupalla CJ, et al. Wnt/beta-catenin signaling controls development of the blood-brain barrier. J Cell Biol. 2008;183:409–417. doi: 10.1083/jcb.200806024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattelino A, Liebner S, Gallini R, Zanetti A, Balconi G, Corsi A, et al. The conditional inactivation of the beta-catenin gene in endothelial cells causes a defective vascular pattern and increased vascular fragility. J Cell Biol. 2003;162:1111–1122. doi: 10.1083/jcb.200212157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paolinelli R, Corada M, Ferrarini L, Devraj K, Artus C, Czupalla CJ, et al. Wnt activation of immortalized brain endothelial cells as a tool for generating a standardized model of the blood brain barrier in vitro. PLoS ONE. 2013;8:e70233. doi: 10.1371/journal.pone.0070233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeman MT, Axelrod JD, Moon RT. A second canon. Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev Cell. 2003;5:367–377. doi: 10.1016/s1534-5807(03)00266-1. [DOI] [PubMed] [Google Scholar]
- Klein TJ, Mlodzik M. Planar cell polarization: an emerging model points in the right direction. Annu Rev Cell Dev Biol. 2005;21:155–176. doi: 10.1146/annurev.cellbio.21.012704.132806. [DOI] [PubMed] [Google Scholar]
- Schlessinger K, McManus EJ, Hall A. Cdc42 and noncanonical Wnt signal transduction pathways cooperate to promote cell polarity. J Cell Biol. 2007;178:355–361. doi: 10.1083/jcb.200701083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells. 2002;7:1161–1171. doi: 10.1046/j.1365-2443.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Luque A, Turlo KA, Hofmann JJ, Yee KM, Becker MS, et al. Beta1 integrin establishes endothelial cell polarity and arteriolar lumen formation via a Par3-dependent mechanism. Dev Cell. 2010;18:39–51. doi: 10.1016/j.devcel.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daneman R, Zhou L, Agalliu D, Cahoy JD, Kaushal A, Barres BA. The mouse blood-brain barrier transcriptome: a new resource for understanding the development and function of brain endothelial cells. PLoS ONE. 2010;5:e13741. doi: 10.1371/journal.pone.0013741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coureuil M, Mikaty G, Miller F, Lecuyer H, Bernard C, Bourdoulous S, et al. Meningococcal type IV pili recruit the polarity complex to cross the brain endothelium. Science. 2009;325:83–87. doi: 10.1126/science.1173196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LM, Reddy PS, Lopez-Ramirez MA, Davies HA, Male DK, Loughlin AJ, et al. Polarized P-glycoprotein expression by the immortalised human brain endothelial cell line, hCMEC/D3, restricts apical-to-basolateral permeability to rhodamine 123. Brain Res. 2009;1292:14–24. doi: 10.1016/j.brainres.2009.07.039. [DOI] [PubMed] [Google Scholar]
- Rapoport SI. Osmotic opening of the blood-brain barrier: principles, mechanism, and therapeutic applications. Cell Mol Neurobiol. 2000;20:217–230. doi: 10.1023/a:1007049806660. [DOI] [PubMed] [Google Scholar]
- Ju R, Cirone P, Lin S, Griesbach H, Slusarski DC, Crews CM. Activation of the planar cell polarity formin DAAM1 leads to inhibition of endothelial cell proliferation, migration, and angiogenesis. Proc Natl Acad Sci USA. 2010;107:6906–6911. doi: 10.1073/pnas.1001075107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, ten Berge D, Brown J, Ahn S, Hu LA, Miller WE, et al. Dishevelled 2 recruits beta-arrestin 2 to mediate Wnt5A-stimulated endocytosis of Frizzled 4. Science. 2003;301:1391–1394. doi: 10.1126/science.1082808. [DOI] [PubMed] [Google Scholar]
- Morais-de-Sa E, Mirouse V, St, Johnston D. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell. 2010;141:509–523. doi: 10.1016/j.cell.2010.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Orsenigo F, Rudini N, Maddaluno L, Boulday G, Chapon F, et al. CCM1 regulates vascular-lumen organization by inducing endothelial polarity. J Cell Sci. 2010;123:1073–1080. doi: 10.1242/jcs.059329. [DOI] [PubMed] [Google Scholar]
- Gao L, Joberty G, Macara IG. Assembly of epithelial tight junctions is negatively regulated by Par6. Curr Biol. 2002;12:221–225. doi: 10.1016/s0960-9822(01)00663-7. [DOI] [PubMed] [Google Scholar]
- Fang L, Wang Y, Du D, Yang G, Tak Kwok T, Kai Kong S, et al. Cell polarity protein Par3 complexes with DNA-PK via Ku70 and regulates DNA double-strand break repair. Cell Res. 2007;17:100–116. doi: 10.1038/sj.cr.7310145. [DOI] [PubMed] [Google Scholar]
- Siegal T, Rubinstein R, Bokstein F, Schwartz A, Lossos A, Shalom E, et al. In vivo assessment of the window of barrier opening after osmotic blood-brain barrier disruption in humans. J Neurosurg. 2000;92:599–605. doi: 10.3171/jns.2000.92.4.0599. [DOI] [PubMed] [Google Scholar]
- Itoh M, Sasaki H, Furuse M, Ozaki H, Kita T, Tsukita S. Junctional adhesion molecule (JAM) binds to PAR-3: a possible mechanism for the recruitment of PAR-3 to tight junctions. J Cell Biol. 2001;154:491–497. doi: 10.1083/jcb.200103047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaelis UR, Chavakis E, Kruse C, Jungblut B, Kaluza D, Wandzioch K, et al. The polarity protein Scrib is essential for directed endothelial cell migration. Circ Res. 2013;112:924–934. doi: 10.1161/CIRCRESAHA.112.300592. [DOI] [PubMed] [Google Scholar]
- Gao C, Chen YG. Dishevelled: the hub of Wnt signaling. Cell Signal. 2010;22:717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- Qian D, Jones C, Rzadzinska A, Mark S, Zhang X, Steel KP, et al. Wnt5a functions in planar cell polarity regulation in mice. Dev Biol. 2007;306:121–133. doi: 10.1016/j.ydbio.2007.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CW, Yeh JC, Fan TP, Smith SK, Charnock-Jones DS. Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochem Biophys Res Commun. 2008;365:285–290. doi: 10.1016/j.bbrc.2007.10.166. [DOI] [PubMed] [Google Scholar]
- Kikuchi A, Yamamoto H, Sato A, Matsumoto S. Wnt5a: its signalling, functions and implication in diseases. Acta Physiol (Oxf) 2012;204:17–33. doi: 10.1111/j.1748-1716.2011.02294.x. [DOI] [PubMed] [Google Scholar]
- Wang Y. Wnt/Planar cell polarity signaling: a new paradigm for cancer therapy. Mol Cancer Ther. 2009;8:2103–2109. doi: 10.1158/1535-7163.MCT-09-0282. [DOI] [PubMed] [Google Scholar]
- Stefater JA, 3rd, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, et al. Regulation of angiogenesis by a non-canonical Wnt-Flt1 pathway in myeloid cells. Nature. 2011;474:511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HC, et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat Cell Biol. 2013;15:249–260. doi: 10.1038/ncb2679. [DOI] [PMC free article] [PubMed] [Google Scholar]