Abstract
Previous studies have shown that intraparenchymal transplantation of neural stem cells (NSCs) ameliorates neurologic deficits in animals with intracerebral hemorrhage (ICH). However, massive grafted cell death after transplantation, possibly caused by a hostile host brain environment, lessens the effectiveness of this approach. We focused on the effect of oxidative stress against grafted NSCs and hypothesized that conferring antioxidant properties to transplanted NSCs may overcome their death and enhance neuroprotection after ICH. Copper/zinc-superoxide dismutase (SOD1) is a specific antioxidant enzyme that counteracts superoxide anions. We investigated whether genetic manipulation to overexpress SOD1 enhances survival of grafted NSCs and accelerates amelioration of ICH. Neural stem cells that overexpress SOD1 were administered intracerebrally 3 days after ICH in a mouse model. Histologic and behavioral tests were examined after ICH. Copper/zinc-superoxide dismutase overexpression protected the grafted NSCs via a decrease in production of reactive oxygen species. This resulted in an increase in paracrine factors released by the NSCs, and an increase in surviving neurons in the striatum and a reduction in striatal atrophy. In addition, SOD1 overexpression showed progressive improvement in behavioral recovery. Our results suggest that enhanced antioxidative activity in NSCs improves efficacy of stem cell therapy for ICH.
Keywords: cell transplantation therapy, copper/zinc-superoxide dismutase, intracerebral hemorrhage, neural stem cell, reactive oxygen species
Introduction
Intracerebral hemorrhage (ICH) accounts for ∼10% to 15% of all strokes that occur in the world each year, and hypertension is the main cause of spontaneous ICH. The prognosis of ICH is poor, with an overall 30-day mortality rate of ∼40%.1 Most survivors experience persistent, severe neurologic deficits.2 Medical therapy for ICH, such as mechanical removal of the hematoma, pharmacological prevention of edema formation, and reduction in intracranial pressure, shows only limited effectiveness.3 It is important to explore alternative approaches.
Intracerebral hemorrhage results in both primary and secondary injury. The primary injury results from disruption of adjacent tissue and mass effect.4 Secondary injury occurs with the development of edema, free radical formation, inflammation, and direct cellular toxicity caused by the hematoma and subsequent degradation byproducts.5 Neuroprotective strategies might prevent additional cell loss in the perihematomal area caused by secondary injury, thereby improving outcome after ICH.
Previous studies have shown that intraparenchymal transplantation of stem cells ameliorates neurologic deficits in animals with ICH.6, 7, 8 Stem cell transplantation might not only result in integration of the transplanted cells into the host brain, but might also contribute to neuroprotection. The optimal time window for transplantation after ICH is yet to be determined. Neuroprotective mechanisms might be most effective if the stem cells are transplanted early after the insult. However, the host's hostile environment during the acute stage after ICH impairs survival of the grafted cells.9, 10, 11, 12 Furthermore, there is little information regarding the mechanism involved in the death of grafted cells. Increased free radicals and reactive oxygen species (ROS) production have important roles in ICH damage. Hemoglobin (Hb) and its breakdown products, such as iron, are the major sources of ROS and mediate the pathologic process after ICH.13, 14 Therefore, ROS production after ICH might be involved in the accelerated death of the grafted stem cells, resulting in diminished effectiveness of stem cell therapy.
Copper/zinc-superoxide dismutase (SOD1) is a dimeric cytosolic enzyme that detoxifies superoxide anions to H2O2. We have shown that SOD1 overexpression reduces oxidative stress induced by Hb, resulting in the reduction of blood–brain barrier disruption and the decrease in apoptotic cell death in the rat brain.14 We have also reported that transplantation of neural stem cells (NSCs) that overexpress SOD1 provides extended survival of these grafted cells and facilitates functional recovery in ischemic stroke animals.15
The purpose of this study was to determine whether ROS cause grafted cell death and whether SOD1 overexpression in NSCs can increase their survival after early transplantation in mice with ICH and can enhance functional recovery. We also sought to investigate whether NSCs that overexpress SOD1 enhance neuroprotection in vitro after they are treated with Hb.
Materials and Methods
Animals
All animals were treated in accordance with Stanford University guidelines and the animal protocols were approved by Stanford University's Administrative Panel on Laboratory Animal Care. Homozygous green fluorescent protein (GFP) transgenic (Tg) mice (C57BL/6-Tg [UBC-GFP] 30Scha/J; The Jackson Laboratory, Bar Harbor, ME, USA) were bred with heterozygous SOD1 Tg mice (C57BL/6 background, backcrossed with C57BL/6 for more than 10 generations) to generate heterozygous GFP Tg mice (wild-type (WT) mice) and heterozygous SOD1/GFP double Tg mice (Tg mice).16 These animals were used for isolation of NSCs. We also used C57BL/6 mice (The Jackson Laboratory) for ICH models and for preparation of Hb.
Isolation and Culture of Neural Stem Cells
Neural stem cells were isolated from the subventricular zones of postnatal day 1 WT mice and Tg mice as described.15 In brief, bilateral subventricular zones were dissected and mechanically dissociated. The cells were collected and suspended in Neurobasal-A medium (Invitrogen, Carlsbad, CA, USA) containing B-27 supplement (Invitrogen), L-glutamine (Invitrogen), 20 ng/mL mouse fibroblast growth factor basic (PeproTech, Rocky Hill, NJ, USA), and 10 ng/mL mouse epidermal growth factor (PeproTech). Cells were grown as adherent monolayers. The medium was changed every 2 days and cells were passaged weekly. Cells that had been passaged 5 to 10 times were used for the experiments.
Preparation of Hemoglobin for Treatment in Neural Stem Cells
In prior cell culture studies, Hb was shown to be an oxidative cytotoxin.17, 18 Hemoglobin was prepared as described.13 The mice were killed with isoflurane and blood was drawn by cardiac puncture. After centrifugation of the blood at 1,250 g for 5 minutes at 4°C, the supernatant was removed and the pellet was washed, resuspended in sterile saline, and lysed by two freeze–thaw cycles. The sample was then centrifuged again and most of the supernatant was removed. The Hb concentration of this sample was determined with an Hb assay kit (BioChain, Newark, CA, USA). Hemoglobin was then diluted with sterile saline, aliquoted, and stored at −80°C until used.
Cell Viability Assay
Cell viability was assessed with a cell proliferation reagent using a WST-1 assay kit (Roche Diagnostics, Indianapolis, IN, USA).19 The NSCs were cultured on 96-well plates and were exposed to different doses of Hb for 24 hours. The NSCs were then washed with phosphate-buffered saline (PBS) and incubated with 90 μL of new medium with 10 μL WST-1 assay reagent for 1.5 to 2 hours. The absorbance of the samples was measured at 450 nm using a microplate reader. We used four samples for each condition and repeated this experiment six times for each condition (n=6).
Assessment of Cell Death In Vitro
Cell death was assessed by LIVE/DEAD Viability/Cytotoxicity assay kit (Life Technologies, Grand Island, NY, USA).20 The NSCs were cultured on 8-well chamber slides (ThermoFisher Scientific, Waltham, MA, USA) and were exposed to 20 μmol/L Hb for 12 hours. The NSCs were then washed with PBS and incubated with 4 μmol/L ethidium homodimer-1 and 2 μmol/L calcein AM for 15 minutes. Calcein is retained very well within live cells, producing an intense uniform green fluorescence. Ethidium homodimer-1 enters cells with damaged membranes and produces a bright red fluorescence in dead cells.
Western Blot Analysis In Vitro
To investigate whether Hb induces changes in SOD1 expression in NSCs, Western blotting was performed. We also investigated whether overexpression of SOD1 involves the phosphorylated serine threonine kinase, pAkt, in NSCs under normal conditions and after exposure to Hb. After exposure to 20 μmol/L Hb for 4, 8, and 24 hours, 50 μmol/L Hb for 4 hours, and 100 μmol/L H2O2 for 4 hours, the NSCs were washed and treated with cell lysis buffer (Cell Signaling Technology, Beverly, MA, USA) and used as whole-cell lysate samples. Protein concentrations were examined by comparison with a known concentration of bovine serum albumin using a kit (ThermoFisher Scientific). Equal amounts of the samples (20 μg) were loaded per lane and analyzed by SDS-PAGE on a 10% NuPAGE Bis-Tris gel (Invitrogen) and then immunoblotted. The primary antibodies were a 1:3,000 dilution of rabbit polyclonal anti-SOD1 (molecular weight: ∼19 kDa) (Enzo Life Sciences, Farmingdale, NY, USA), a 1:500 dilution of rabbit polyclonal anti-pAkt (Ser473) (molecular weight: ∼60 kDa) (Cell Signaling Technology), a 1:2,000 dilution of rabbit polyclonal anti-Akt (molecular weight: ∼60 kDa) (Cell Signaling Technology), and a 1:100,000 dilution of mouse monoclonal anti-β-actin (molecular weight: ∼42 kDa) (Sigma-Aldrich, St Louis, MO, USA). After incubation with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG) (Cell Signaling Technology) or anti-rabbit IgG (Cell Signaling Technology), the antigen was detected by SuperSignal West Pico substrates (ThermoFisher Scientific). Images were captured with a GS-700 imaging densitometer (Bio-Rad, Hercules, CA, USA) and the results were quantified using MultiAnalyst software (Bio-Rad).
Immunofluorescent Staining
For immunocytochemistry, the NSCs were cultured on 8-well chamber slides, then washed with PBS, and fixed with 4% paraformaldehyde in PBS. For immunohistochemistry, the animals were anesthetized and perfused with PBS followed by 4% paraformaldehyde in PBS. The brains were postfixed overnight in the same fixative at 4°C. For cryosectioning, fixed tissue was cryoprotected in 10% sucrose in PBS overnight at 4°C, then in 20% sucrose in PBS overnight at 4°C, and embedded in Tissue-Tek O.C.T. compound (Sakura Finetek, Torrance, CA, USA). Cryostat sections (20 μm) were cut and affixed to glass slides. Cells or tissue sections were subsequently incubated overnight at 4°C in an appropriate mixture of primary antibodies. The following antibodies were used: rabbit anti-GFP (1:200, Invitrogen), goat anti-GFP (1:200, LifeSpan BioSciences, Seattle, WA, USA), mouse anti-Nestin (1:100, BD Biosciences Pharmingen, San Jose, CA, USA), mouse anti-mitogen-activated protein 2 (MAP2) (1:200, Sigma-Aldrich), rabbit anti-glial fibrillary acidic protein (GFAP) (1:200, Millipore, Billerica, MA, USA), mouse anti-NG2 (1:200, Abcam, Cambridge, MA, USA), and rabbit anti-Ki-67 (1:200, Abcam). After three washes, cells or tissue sections were incubated for 1 hour with a 1:500 dilution of the following secondary antibodies: Alexa Fluor 488-conjugated donkey anti-rabbit IgG, Alexa Fluor 594-conjugated donkey anti-rabbit IgG, Alexa Fluor 488-conjugated donkey anti-goat, and Alexa Fluor 594-conjugated donkey anti-mouse IgG (all from Invitrogen). Slides were covered with VECTASHIELD mounting medium with 4′,6 diamidino-2-phenylindole. The samples were examined by confocal or fluorescence microscopy (Axioplan 2; Carl Zeiss, Thornwood, NY, USA).
Oxidative Damage
Production of superoxide anions in vitro was shown by oxidized hydroethidine as described.21 The cell culture was incubated with 5 μmol/L hydroethidine solution (Invitrogen) for 15 minutes, followed by fixation with 4% paraformaldehyde in PBS. Slides were covered with VECTASHIELD mounting medium with 4′,6 diamidino-2-phenylindole and observed with a fluorescence microscope. The oxidized hydroethidine fluorescence was examined at an excitation of 510 nm and emission of 580 nm and quantified with ImageJ software (version 1.42q; NIH, Bethesda, MD, USA). For an in vivo study, we observed the carbonyl proteins as indicators of oxidative protein damage, with the use of a commercial kit (Millipore).14 The mice were killed 2 days after NSC transplantation and tissue sections were prepared. Wild-type NSCs were also transplanted into the striatum without ICH as a control. The sections were incubated with 2,4-dinitrophenylhydrazone, and 2,4-dinitrophenyl-derivatized carbonyl proteins were detected by immunostaining with an anti-2,4-dinitrophenyl biotinylated antibody (1:200) and streptavidin-Cy3 (1:500). Fluorescence intensity was examined at an excitation of 510 nm and emission of 580 nm and quantified with ImageJ software.
Intracerebral Hemorrhage Model with Autologous Blood Infusion
We used an experimental ICH procedure described previously,22, 23 with some modifications. Male C57BL/6 mice (14 to 15 weeks old, 25 to 30 g) were anesthetized with 2.0% isoflurane in 30% oxygen and 70% nitrous oxide and placed in a stereotactic frame. The rectal temperature was controlled at 37.0±0.5°C with a homeothermic blanket. A midline scalp incision was made and a hole was drilled in the right side of the skull (0.0 mm anterior and 2.5 mm lateral of the bregma) in preparation for the infusion. The mouse tail tip was cut off and some drops of blood were allowed to fall on parafilm. The blood (20 μL) was collected without the use of an anticoagulant and used for injection. A 30-gauge needle attached to a 50-μL Hamilton microsyringe was inserted 3.5 mm ventral from the surface of the skull, with adjustment of the stereotaxic arm to a point 5° medially relative to the vertical axis. After waiting 2 minutes, the needle was withdrawn 0.5 mm, and left for another 5 minutes. Ten microliters of blood was injected over 10 minutes using a microsyringe pump. The remaining 10 μL of blood was injected using the same procedure. After infusion, the needle was left in place for 25 minutes and then slowly removed (Supplementary Figure 1A). Animal mortality was rare during surgery (<5%) and was related mostly to respiratory disorder. No animals died after surgery. All underwent behavioral testing 1 day after ICH and those with low behavioral scores were excluded from the experiment (cylinder test<0.6, corner turn test<0.8; 40% were excluded).
Intracerebral Transplantation
The mice were anesthetized 72 hours after ICH and the NSCs were transplanted using a 10-μL Hamilton syringe with a 31-gauge needle. The mice were given two 1.0-μL deposits of single-cell suspension in PBS (1 × 105 cells/μL) along the anterior–posterior axis into the striatum at these coordinates: (1) anterior–posterior (A–P), 0.5; medial–lateral (M–L), 2.5; dorsal–ventral (D–V), 3.0; (2) A–P, −0.75; M–L, 2.5; D–V, 3.0. These targets approximated the edge of the ICH. Deposits were delivered at 0.5 μL/minute, and the needle was left in place for 5 minutes post injection. The control group had PBS injected without NSCs. In the WT group, the mice had grafted WT NSCs, and in the Tg group, the mice had grafted Tg NSCs.
Detection of Paracrine Factors
Fresh brain was removed 5 and 14 days after ICH in each group (WT, Tg, PBS control) (Supplementary Figure 1B). Blocks of tissue were cut from 1 mm rostral to 2 mm caudal to the bregma. From these samples, the NSC-transplanted side of the striatum was dissected with the use of a microscope and used as samples that were treated with cell lysis buffer (Cell Signaling Technology) and used as whole-cell lysate samples. Commercial glial cell-derived neurotrophic factor (GDNF) (Promega, Madison, WI, USA) and vascular endothelial growth factor (VEGF) (R&D Systems, Minneapolis, MN, USA) ELISA kits were used to quantify the concentration of GDNF and VEGF in each sample.
Quantification of Survival of Transplanted Neural Stem Cells
To quantify survival of the grafted cells, the animals were killed 14 and 35 days after ICH and tissue sections were prepared. After immunostaining with GFP, as described above, the transplanted GFP-positive cells were counted using unbiased computational stereology (fractionator method, using STEREOINVESTIGATOR software (MicroBrightfield, Williston, VT, USA)), as described.18 All the GFP-positive cells were counted on 12 serial coronal sections per brain (0.25 mm apart).
Assessment of Neural Stem Cell Proliferation and Differentiation
After culturing in differentiation medium containing 1% fetal bovine serum for 5 days, the NSCs were stained with a proliferation marker (Ki-67) or lineage-specific marker (Nestin, MAP2, GFAP, NG2) to assess NSC proliferation and differentiation in vitro. The proportion of grafted cells was also determined by staining with Ki-67 or MAP2 and with GFAP and 4′,6 diamidino-2-phenylindole as described above.
Measurement of Striatum Atrophy
The brain sections were stained with cresyl violet. We estimated the striatum size as a percentage of the ipsilateral striatum. The area of both sides of the striatum was measured on nine serial coronal sections per brain (0.25 mm apart) and the size of the striatum was analyzed by an observer masked to the experimental cohort.
Quantification of Neuronal Survival
The brain sections were stained with NeuN (a neuronal marker) and 4′,6 diamidino-2-phenylindole as described above. Surviving neurons were analyzed in a masked manner by counting the number of cells or profiles in six defined regions of interest per striatum, measuring 62,500 μm2, both ipsilateral and contralateral to the ICH using unbiased computational stereology.24
Behavioral Analysis
A cylinder test and a corner turn test were used for behavioral analysis.22, 25 To obtain three groups with an equal post-ICH behavior score, the animals were ranked based on their post-day-1 results and assigned to one of the three groups. The animals were retested just before cell transplantation and 7, 14, 21, 28, and 35 days after ICH (Supplementary Figure 1C). Forelimb use during exploratory activity was analyzed in a transparent cylinder (12 cm in diameter). The behavior was scored according to the following criteria: independent use of the left or right forelimb for contacting the wall while the animals were standing on their hindlimbs to initiate a weight-shifting movement and simultaneous use of both the left and right forelimbs to contact the wall. Behavior was quantified by determining the number of times the ipsilateral (unimpaired) forelimb (I), contralateral forelimb (C), and both forelimbs (B) were used as a percentage of total number of limb usage. A single, overall limb-use asymmetry score was calculated as follows: forelimb-use asymmetry score (I/(I+C+B)) − (C/(I+C+B)). The second behavioral analysis involved a corner turn test. The mouse was allowed to proceed into a corner, the angle of which was 30°. To exit the corner, the animal could turn either to the left or right. When the mouse turned, its choice of direction was recorded. This was repeated 10 to 15 times and the percentage of right turns was calculated.
Statistical Analysis
Behavioral data were assessed using repeated measures of ANOVA followed by the Tukey test. For the paracrine factor experiment, measurement of striatum atrophy, and neuronal survival, comparisons among multiple groups were performed with one-way ANOVA, followed by the Tukey test. For other experiments, comparisons between two groups were achieved with Student's unpaired t-test. Data are expressed as the mean±s.d. Significance was accepted with P<0.05.
Results
Characterization of Neural Stem Cells
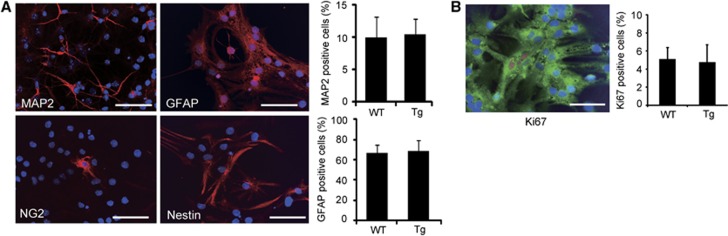
We isolated NSCs from fetal GFP Tg mice (WT NSCs) or SOD1/GFP double Tg mice (Tg NSCs). These NSCs, grown as adherent cultures, were self-renewing and multipotent (Figure 1A). The percentage of neurons (9.9±3.2% and 10.4±2.5%, n=4) and astrocytes (66.3±8.9% and 68.7±10.1%, n=4) differentiated from the WT and Tg NSCs was similar after culturing in differentiation medium containing 1% fetal bovine serum for 5 days (Figure 1A). Moreover, no difference was observed in the percentage of Ki-67-positive cells (n=4) (Figure 1B).
Figure 1.
Cell differentiation and proliferation in vitro. (A) Neural stem cells (NSCs) were stained with the neuronal marker mitogen-activated protein 2 (MAP2) (red), glial fibrillary acidic protein (GFAP) (red), the oligodendrocytic marker NG2 (red), and the immature cell marker Nestin (red), and 4′,6 diamidino-2-phenylindole (blue). The NSCs differentiated into neurons, astrocytes, and oligodendrocytes. Some cells were still stained with Nestin. Scale bars, 50 μm. (B) Fluorescent staining with the proliferation marker Ki-67 (red), green fluorescent protein (green), and 4′,6 diamidino-2-phenylindole (blue) after culturing in differentiation medium. Scale bar, 50 μm. WT, wild-type; Tg, transgenic.
Neural Stem Cell Death after Exposure to Hemoglobin
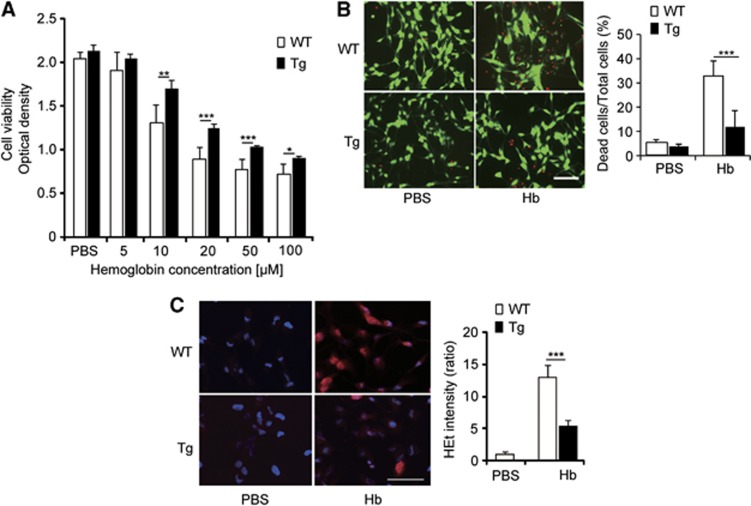
We investigated whether the levels of SOD1 expression could rescue NSCs from death after exposure to Hb. After 24 hours of exposure to Hb, cell viability decreased depending on the concentration of Hb, using the WST-1 assay (n=6) (Figure 2A). Cell viability was significantly higher in the Tg NSCs compared with the WT NSCs (n=6) (Figure 2A). This cytoprotective effect in the Tg NSCs was confirmed by a LIVE/DEAD assay, where there was a significant decrease in death in the Tg NSCs (11.9±7.0%) compared with the WT NSCs (32.9±5.9%) (n=5, P<0.001) after 12 hours of exposure to 20 μmol/L Hb (n=5, P<0.01) (Figure 2B). After 4 hours of exposure to 20 μmol/L Hb, the WT NSCs showed a significant increase in hydroethidine staining, which indicates production of superoxide anions (Figure 2C). This signal was significantly reduced in the Tg NSCs compared with the WT NSCs (n=5, P<0.001).
Figure 2.
Reduced death in copper/zinc-superoxide dismutase transgenic (Tg) neural stem cells after exposure to hemoglobin (Hb). (A) WST-1 assay after exposure to Hb. (B) Neural stem cell death analyzed by LIVE/DEAD assay. Live cells (green) and dead cells (red) after exposure to Hb. Scale bar, 100 μm. (C) Superoxide anion production analyzed by hydroethidine (HEt) (red) and 4′,6 diamidino-2-phenylindole (blue) after exposure to Hb. Scale bar, 50 μm. *P<0.05, **P<0.01, ***P<0.001. WT, wild-type; PBS, phosphate-buffered saline.
Copper/Zinc-Superoxide Dismutase and Phosphorylated Serine Threonine Kinase Expression in Neural Stem Cells after Exposure to Hemoglobin
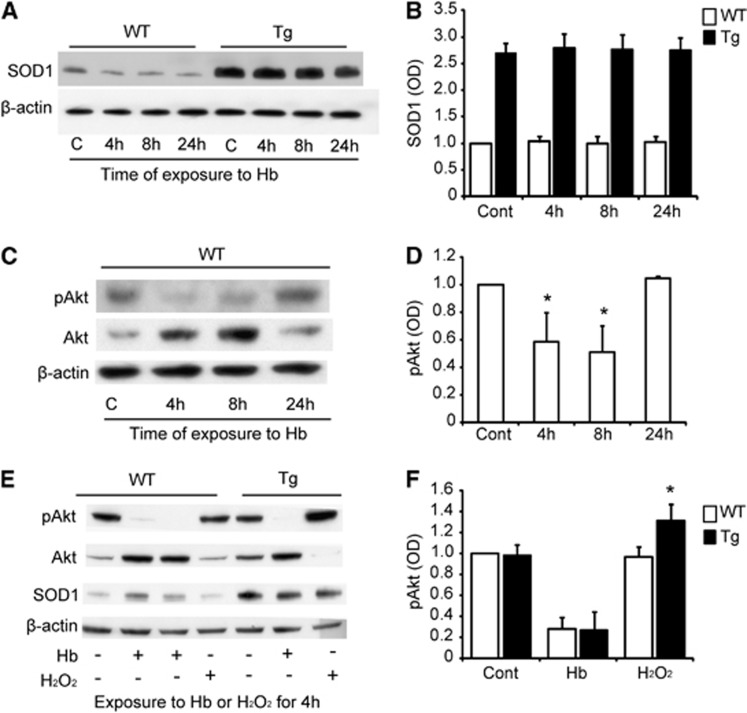
We next investigated whether SOD1 expression is changed by exposure to Hb. Western blot analysis revealed that, as expected, SOD1 expression was significantly higher in the untreated Tg NSCs (2.46±0.16-fold). Hemoglobin exposure did not change SOD1 expression in the WT and Tg NSCs (n=4) (Figures 3A and 3B). Phosphorylated serine threonine kinase levels were significantly reduced after exposure to Hb for 4 and 8 hours in the WT NSCs (n=4, P<0.05) (Figures 3C and 3D). There were no differences in pAkt expression between the WT and SOD1 Tg NSCs before exposure to Hb or after exposure to Hb for 4 hours (n=4). In contrast, pAkt was significantly higher in the Tg NSCs compared with the WT NSCs after exposure to H2O2 (n=4, P<0.05) (Figures 3E and 3F).
Figure 3.
Copper/zinc-superoxide dismutase (SOD1) and phosphorylated serine threonine kinase (pAkt) expression in neural stem cells (NSCs) in vitro. (A, B) Western blot analysis of SOD1 expression after exposure to 20 μmol/L hemoglobin (Hb). (C, D) Western blot analysis of pAkt expression after exposure to 20 μmol/L Hb in wild-type (WT) NSCs. (E, F) Western blot analysis of pAkt expression after exposure to 50 μmol/L Hb and 100 μmol/L H2O2 for 4 hours. *P<0.05. Tg, SOD1 transgenic; C and Cont, control; OD, optical density.
Protein Oxidation in Grafted Neural Stem Cells
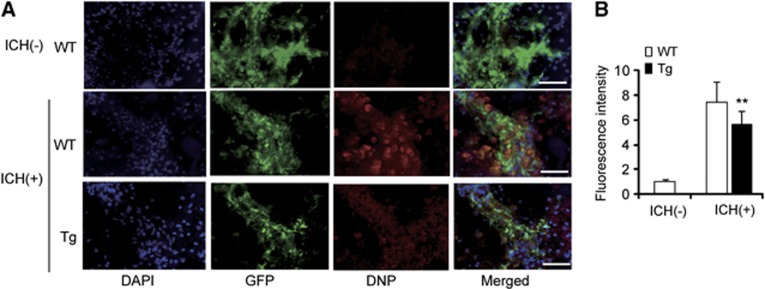
We transplanted WT and Tg NSCs into the perihematomal striatum 3 days after ICH. In the WT NSC transplantation, fluorescence intensity of 2,4-dinitrophenyl drastically increased 2 days afterwards compared with WT NSC transplantation in intact brains (Figures 4A and 4B). This increase was significantly diminished in the SOD1 Tg NSCs (n=5, P<0.01) (Figures 4A and 4B).
Figure 4.
Reduced oxidative stress in copper/zinc-superoxide dismutase transgenic (Tg) neural stem cells (NSCs) in vivo. (A) Fluorescent staining with 4′,6 diamidino-2-phenylindole (DAPI) (blue), green fluorescent protein (GFP) (green), and 2,4-dinitrophenylhydrazone and 2,4-dinitrophenyl (DNP) (red) in brain sections 2 days after transplantation. (B) DNP signals increased in the wild-type (WT) NSCs, but this increase was significantly reduced in the Tg NSCs (n=5). Scale bars, 50 μm. **P<0.01. ICH, intracerebral hemorrhage.
Survival of Grafted Neural Stem Cells
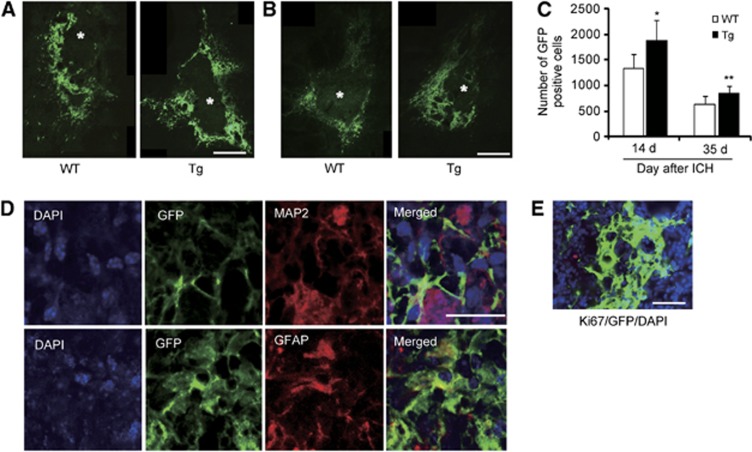
Green fluorescent protein staining revealed that grafted cells surrounded the ICH lesion in the WT and Tg NSC groups 14 days (Figure 5A) and 35 days (Figure 5B) after ICH. The number of surviving grafted cells was significantly higher in the Tg NSC group compared with the WT NSC group both 14 and 35 days after ICH (n=7, 14 days: P<0.05; 35 days: P<0.01) (Figure 5C). There were no signs of tumor formation caused by the transplanted NSCs 35 days after ICH in either group.
Figure 5.
Increased cell survival in vivo. Fluorescent staining with green fluorescent protein (GFP) (green) revealed that the grafted cells surrounded the intracerebral hemorrhage (ICH) lesion 14 days (A) and 35 days (B) afterwards. The images were taken at a × 40 objective, and were put together to make an entire image of the grafted cells and ICH using the STEREOINVESTIGATOR system (MicroBrightfield, Williston, VT, USA). * represents ICH. Scale bars, 200 μm. (C) The number of surviving grafted cells significantly increased in the copper/zinc-superoxide dismutase transgenic (Tg) neural stem cell (NSC) group compared with the wild-type (WT) NSC group 14 and 35 days after ICH (n=7). (D) Fluorescent staining with GFP (green), 4′,6 diamidino-2-phenylindole (DAPI) (blue) and mitogen-activated protein 2 (MAP2) (red), or glial fibrillary acidic protein (GFAP) (red) revealed that the grafted NSCs differentiated into neurons (MAP2+) and astrocytes (GFAP+) 32 days after transplantation. (E) Fluorescent staining with GFP (green), DAPI (blue), and the proliferation marker Ki-67 (red) revealed that no Ki-67-positive grafted cells were observed 32 days after transplantation. Scale bar, 20 μm. *P<0.05, **P<0.01.
Cell Differentiation after Transplantation
Fluorescent staining with GFP, MAP2, and GFAP revealed that the grafted NSCs differentiated into neurons (MAP2) and astrocytes (GFAP) 35 days after ICH (Figure 5D). The percentage of neurons (3.8±3.6% versus 5.3±4.2%, n=4) and astrocytes (88.2±5.3% versus 86.9±7.6%, n=4) differentiated from the grafted cells was similar between the WT and Tg NSCs. There were no cells positive for Ki-67 (cell proliferation marker) 35 days after ICH (Figure 5E).
Paracrine Factor Expression in the Striatum
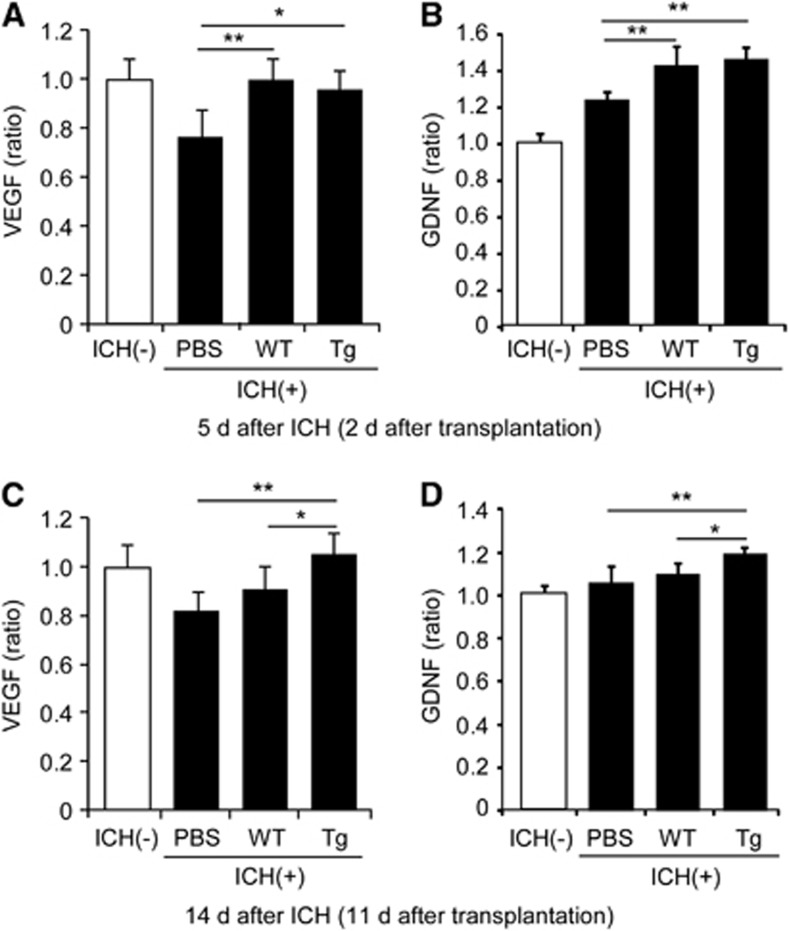
We measured VEGF and GDNF levels in the striatum using ELISA. These paracrine factors significantly increased in both the WT and Tg NSC groups compared with the PBS control group 2 days after transplantation (n=5) (Figures 6A and 6B), and significantly increased in the Tg NSC group compared with both the WT NSC and PBS control groups 11 days after transplantation (n=5) (Figures 6C and 6D).
Figure 6.
Induction of paracrine factors in the striatum in vivo. ELISA of the striatum revealed significant elevations of vascular endothelial growth factor (VEGF) and glial cell-derived neurotrophic factor (GDNF) in both the copper/zinc-superoxide dismutase transgenic (Tg) neural stem cell (NSC) group and the wild-type (WT) NSC group compared with the phosphate-buffered saline (PBS) group 2 days after transplantation (A, B). Significant elevations in VEGF and GDNF in the Tg NSCs compared with the WT NSCs 11 days after transplantation (C, D). *P<0.05, **P<0.01. ICH, intracerebral hemorrhage.
Neural Stem Cells Promote Perilesional Tissue Protection
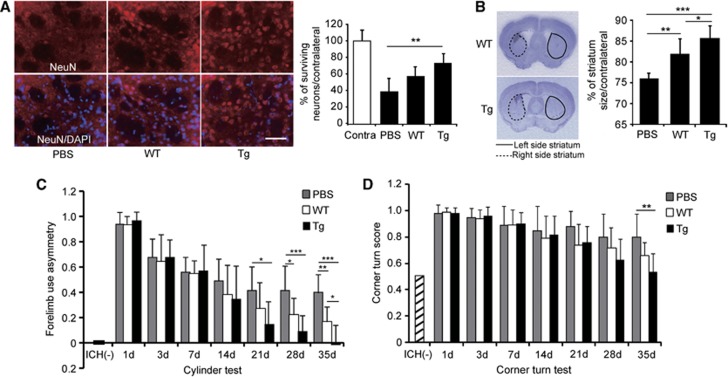
To assess whether SOD1 overexpression in NSC transplantation influences protection of peri-ICH tissue, histochemical studies were performed. NeuN staining revealed that neuronal survival was significantly increased in the Tg NSC group 35 days after ICH compared with the PBS control group (n=6, P<0.01), although there was no significance between the WT NSC group and the PBS control group (n=6) (Figure 7A). Furthermore, NSC transplantation prevented striatal atrophy, and the Tg NSCs significantly attenuated this atrophy compared with the WT NSCs (n=7, P<0.05) (Figure 7B).
Figure 7.
Copper/zinc-superoxide dismutase transgenic (Tg) neural stem cells (NSCs) promote perilesional tissue protection and enhance functional recovery. (A) Enhanced neuronal survival with transplantation of Tg NSCs. Fluorescent staining of NeuN (a neuronal marker) (red) and 4′,6 diamidino-2-phenylindole (DAPI) (blue) and quantification of NeuN-positive cells in the striatum. Scale bar, 50 μm. (B) Striatal atrophy 35 days after intracerebral hemorrhage (ICH). The Tg NSCs significantly reduced atrophy compared with the wild-type (WT) NSC and phosphate-buffered saline (PBS) groups 35 days after ICH. Behavioral performance using the cylinder test (C) and the corner turn test (D). The Tg NSC group showed significant functional improvement compared with the WT NSC and PBS groups. *P<0.05, **P<0.01, ***P<0.001. Contra, contralateral striatum.
Functional Recovery
We analyzed behavioral performance using the cylinder test and corner turn test. Mice that were transplanted with Tg NSCs showed significant functional improvement from day 21 compared with the PBS control group and at day 35 compared with the WT NSC group according to the cylinder test (n=9) (Figure 7C). The corner turn test revealed that although the Tg NSC group showed a statistical significance at day 35 compared with the PBS control group (n=9, P<0.01), there was no significant behavioral improvement at any time point between these two groups (n=9) (Figure 7D).
Discussion
Reactive oxygen species mediate oxidative brain damage after ICH. Cell culture studies have shown that Hb-induced neurotoxicity is mediated by iron released from Hb as free radical scavengers, and that an iron chelator blocked Hb-induced neurotoxicity.26, 27 Injection of autologous lysed erythrocytes, Hb, and iron into the brain produces ROS, causing brain damage in animal models.14, 28, 29 In this study, we demonstrated that (i) Hb led to NSC death via ROS production, which may also mediate grafted cell death in ICH; (ii) overexpression of SOD1 in NSCs reduced ROS and increased survival of these cells after exposure to Hb and after transplantation in the ICH brain; and (iii) transplantation of SOD1 Tg NSCs enhanced neuroprotection and facilitated behavioral recovery in mice with ICH.
Although the vulnerability and response of NSCs to Hb cytotoxicity have not yet been examined, several studies have shown a rapid and massive loss of NSCs under oxidative stress.30, 31 We recently showed that ROS production resulted in a reduction in grafted cells and diminishment of cell transplantation therapy for ischemic stroke.15, 19, 21 Similarly, in the present study, we showed that ROS induced by Hb accelerated NSC death. Hydroethidine staining was enhanced in WT NSCs that were treated with Hb, whereas it was reduced in SOD1 Tg NSCs exposed to Hb. This demonstrates that ROS have an important role and are affected by exposure to Hb. Furthermore, we showed that ROS accelerated death of grafted NSCs after ICH, where carbonyl protein increased in WT NSCs after transplantation whereas it was reduced in SOD1 Tg NSCs. These findings suggest that ROS not only cause host tissue damage but also hinder survival of grafted NSCs in ICH.
Research on ROS has focused on their effect on toxicity in cells, but recent studies suggest that low levels of ROS contribute to stem cell proliferation, self-renewal, and neurogenesis under physiologic conditions.32, 33 However, in our study, SOD1 Tg NSCs demonstrated similar proliferation and differentiation capacities compared with WT NSCs in vitro and similar differentiation in vivo. This discrepancy might be because the basal expression of antioxidant or pro-oxidant enzymes in Tg NSCs is altered in association with SOD1 expression and compensates for the regulation of ROS levels. Further study is needed to clarify this important issue.
Serine threonine kinase is a key molecule in cell survival not only in ischemic stroke but also in hemorrhagic stroke.34, 35 However, the role of Akt signaling in Hb cytotoxicity in NSCs has not been reported. Overexpression of Akt1 enhanced survival of grafted human NSCs transplanted into mouse brains with ICH induced by striatal collagenase injection, as demonstrated by Lee et al.10 Reactive oxygen species contributed to enhanced Akt activity in NSCs,32, 33 and an increase in Akt activity was induced by intracellular H2O2 converted from superoxide by SOD1.36 Although pAkt expression increased in the SOD1 Tg NSCs compared with the WT NSCs after exposure to H2O2, there was no difference in pAkt expression between SOD1 Tg NSCs and WT NSCs after exposure to Hb in this study. An earlier report by our group showed that cortical neurons that overexpress SOD1 had a tolerance against ischemic insults via upregulation of Akt signaling. The increase in pAkt was observed in the penumbral region of the cortex, whereas it was decreased in the ischemic core after transient focal cerebral ischemia.37 These findings suggest that relatively mild insults upregulate Akt phosphorylation, while severe damage downregulates it. Thus, Hb stimulation might be too strong and detrimental for Akt activation in our study.
Many studies have shown that the majority of exogenous, transplanted stem cells exert cytoprotection or stimulate endogenous repair mechanisms in the ICH brain.5, 6, 8, 38 The present study shows that transplantation of SOD1 Tg NSCs in ICH brains reduced their atrophy and death. Moreover, an improvement in the outcome of behavioral tests was observed in these animals, indicating that stem cells facilitate neuroprotection after being grafted to ICH brains. The present study also demonstrates that overexpression of SOD1 enhanced survival of grafted cells and increased paracrine factors in the ICH brain, resulting in enhanced neuroprotection. Levels of VEGF and GDNF in the striatum were significantly increased in both the WT and Tg NSCs compared with the controls 2 days after cell transplantation. There were no significant differences in expression of these paracrine factors between the WT and Tg NSCs at this time point, but higher levels of paracrine factors were detected in the Tg NSCs compared with the WT NSCs 11 days after transplantation. These differences might be caused by a decrease in grafted cells in the WT mice. The number of surviving grafted cells decreased more quickly in the WT mice compared with the Tg mice after transplantation. These results are consistent with previous studies. HB1.F3 gene-modified human NSCs that overexpress VEGF were found to produce a higher level of human VEGF at the transplantation site, and to provide neuroprotection, angiogenesis, and functional recovery in mouse ICH.9 Furthermore, HB1.F3 gene-modified human NSCs that overexpress GDNF, which can produce a fourfold higher amount of GDNF compared with parental F3 cells in vitro, reduced apoptotic cells around the ICH lesion and induced behavioral improvement after transplantation in mouse ICH.11 These studies show that direct secretion of trophic factors by the grafted cells enhance neuroprotection and neurorepair. In contrast, cell transplantation therapy after stroke can enhance endogenous secretion of trophic factors from host tissue.39
Genetic modification of stem cells generates a number of concerns, including tumor formation after transplantation.40 In this study, tumor formation was not observed and grafted cells positive for a proliferation marker were not detected 35 days after ICH. Tumor formation was also not observed 3 months after transplantation of SOD1 Tg NSCs in ischemic mouse brains in our recent report.15
There are reports of NSCs transplanted at different times and by different routes (intraparenchymal, intraventricular, intravenous, and intraarterial).5, 8, 38 In some reports, NSC transplantation was performed at later time points after ICH compared with this study, and they also describe improved behavioral recovery.6, 12 However, NSCs were transplanted at the acute stage of ICH in our study for examination of enhanced neuroprotection. As the environment of the host tissue at the acute stage of ICH is worse than at later stages due to edema, acute inflammation, and oxidative stress,12 we looked at the survivability of modified NSCs. We demonstrated that SOD1 overexpression overcomes this hostile host environment. Andres et al.5 has reported that notwithstanding the differences in the procedures, including the route, NSCs migrate into the perihematomal region and survive after transplantation in ICH animals, but clinically, surgical evacuation is done either with open craniotomy or stereotactic and endoscopic techniques to prevent expansion, to decrease mass effects, and to block the release of neuropathic products from hematomas at the acute stage of ICH.3 Therefore, intraparenchymal transplantation was used in this present study, because it is possible to graft cells during surgery.
In conclusion, we have shown an important role for SOD1 overexpression in NSC survival after transplantation in the ICH brain. Our findings indicate that conferring antioxidant properties on NSCs is a potential approach for enhancing the effectiveness of cell transplantation therapy in ICH.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported by grants RO1 NS025372 and RO1 NS038653 from the National Institutes of Health, and by the James R Doty Endowment.
Supplementary Material
References
- Godoy DA, Piñero G, Di Napoli M. Predicting mortality in spontaneous intracerebral hemorrhage. Can modification to original score improve the prediction. Stroke. 2006;37:1038–1044. doi: 10.1161/01.STR.0000206441.79646.49. [DOI] [PubMed] [Google Scholar]
- Dennis MS. Outcome after brain haemorrhage. Cerebrovasc Dis. 2003;16 (Suppl 1:9–13. doi: 10.1159/000069935. [DOI] [PubMed] [Google Scholar]
- Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009;373:1632–1644. doi: 10.1016/S0140-6736(09)60371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hoff JT. Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol. 2006;5:53–63. doi: 10.1016/S1474-4422(05)70283-0. [DOI] [PubMed] [Google Scholar]
- Andres RH, Guzman R, Ducray AD, Mordasini P, Gera A, Barth A, et al. Cell replacement therapy for intracerebral hemorrhage. Neurosurg Focus. 2008;24:E16. doi: 10.3171/FOC/2008/24/3-4/E15. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH, Park IH, et al. Brain transplantation of immortalized human neural stem cells promotes functional recovery in mouse intracerebral hemorrhage stroke model. Stem Cells. 2007;25:1204–1212. doi: 10.1634/stemcells.2006-0409. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lim IJ, Lee MC, Kim SU. Human neural stem cells genetically modified to overexpress brain-derived neurotrophic factor promote functional recovery and neuroprotection in a mouse stroke model. J Neurosci Res. 2010;88:3282–3294. doi: 10.1002/jnr.22474. [DOI] [PubMed] [Google Scholar]
- Wang Z, Cui C, Li Q, Zhou S, Fu J, Wang X, et al. Intracerebral transplantation of foetal neural stem cells improves brain dysfunction induced by intracerebral haemorrhage stroke in mice. J Cell Mol Med. 2011;15:2624–2633. doi: 10.1111/j.1582-4934.2011.01259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim KS, Park IH, Kim SU. Human neural stem cells over-expressing VEGF provide neuroprotection, angiogenesis and functional recovery in mouse stroke model. PLoS ONE. 2007;2:e156. doi: 10.1371/journal.pone.0000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Kim MK, Kim HJ, Kim SU. Human neural stem cells genetically modified to overexpress Akt1 provide neuroprotection and functional improvement in mouse stroke model. PLoS ONE. 2009;4:e5586. doi: 10.1371/journal.pone.0005586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Park IH, Kim HJ, Kim SU. Human neural stem cells overexpressing glial cell line-derived neurotrophic factor in experimental cerebral hemorrhage. Gene Ther. 2009;16:1066–1076. doi: 10.1038/gt.2009.51. [DOI] [PubMed] [Google Scholar]
- Li F, Liu Y, Zhu S, Wang X, Yang H, Liu C, et al. Therapeutic time window and effect of intracarotid neural stem cells transplantation for intracerebral hemorrhage. Neuroreport. 2007;18:1019–1023. doi: 10.1097/WNR.0b013e328165d170. [DOI] [PubMed] [Google Scholar]
- Qu Y, Chen J, Benvenisti-Zarom L, Ma X, Regan RF. Effect of targeted deletion of the heme oxygenase-2 gene on hemoglobin toxicity in the striatum. J Cereb Blood Flow Metab. 2005;25:1466–1475. doi: 10.1038/sj.jcbfm.9600143. [DOI] [PubMed] [Google Scholar]
- Katsu M, Niizuma K, Yoshioka H, Okami N, Sakata H, Chan PH. Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood–brain barrier dysfunction in vivo. J Cereb Blood Flow Metab. 2010;30:1939–1950. doi: 10.1038/jcbfm.2010.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, Niizuma K, Wakai T, Narasimhan P, Maier CM, Chan PH. Neural stem cells genetically modified to overexpress Cu/Zn-superoxide dismutase enhance amelioration of ischemic stroke in mice. Stroke. 2012;43:2423–2429. doi: 10.1161/STROKEAHA.112.656900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang T-T, Carlson EJ, Raineri I, Gillespie AM, Kozy H, Epstein CJ. The use of transgenic and mutant mice to study oxygen free radical metabolism. Ann NY Acad Sci. 1999;893:95–112. doi: 10.1111/j.1749-6632.1999.tb07820.x. [DOI] [PubMed] [Google Scholar]
- Rogers B, Yakopson V, Teng Z-P, Guo Y, Regan RF. Heme oxygenase-2 knockout neurons are less vulnerable to hemoglobin toxicity. Free Radic Biol Med. 2003;35:872–881. doi: 10.1016/s0891-5849(03)00431-3. [DOI] [PubMed] [Google Scholar]
- Chen-Roetling J, Li Z, Chen M, Awe OO, Regan RF. Heme oxygenase activity and hemoglobin neurotoxicity are attenuated by inhibitors of the MEK/ERK pathway. Neuropharmacology. 2009;56:922–928. doi: 10.1016/j.neuropharm.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, et al. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci. 2012;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narasimhan P, Liu J, Song YS, Massengale JL, Chan PH. VEGF stimulates the ERK 1/2 signaling pathway and apoptosis in cerebral endothelial cells after ischemic conditions. Stroke. 2009;40:1467–1473. doi: 10.1161/STROKEAHA.108.534644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, Narasimhan P, Niizuma K, Maier CM, Wakai T, Chan PH. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain. 2012;135:3298–3310. doi: 10.1093/brain/aws259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rynkowski MA, Kim GH, Komotar RJ, Otten ML, Ducruet AF, Zacharia BE, et al. A mouse model of intracerebral hemorrhage using autologous blood infusion. Nat Protoc. 2008;3:122–128. doi: 10.1038/nprot.2007.513. [DOI] [PubMed] [Google Scholar]
- Sansing LH, Kasner SE, McCullough L, Agarwal P, Welsh FA, Kariko K.Autologous blood injection to model spontaneous intracerebral hemorrhage in mice J Vis Exp 201154pii2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reitmeir R, Kilic E, Kilic Ü, Bacigaluppi M, ElAli A, Salani G, et al. Post-acute delivery of erythropoietin induces stroke recovery by promoting perilesional tissue remodelling and contralesional pyramidal tract plasticity. Brain. 2011;134:84–99. doi: 10.1093/brain/awq344. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Xi G, Hua Y, Schallert T, Hoff JT, Keep RF. Intracerebral hemorrhage in mice: model characterization and application for genetically modified mice. J Cereb Blood Flow Metab. 2004;24:487–494. doi: 10.1097/00004647-200405000-00002. [DOI] [PubMed] [Google Scholar]
- Wang X, Mori T, Sumii T, Lo EH. Hemoglobin-induced cytotoxicity in rat cerebral cortical neurons. Caspase activation and oxidative stress. Stroke. 2002;33:1882–1888. doi: 10.1161/01.str.0000020121.41527.5d. [DOI] [PubMed] [Google Scholar]
- Regan RF, Rogers B. Delayed treatment of hemoglobin neurotoxicity. J Neurotrauma. 2003;20:111–120. doi: 10.1089/08977150360517236. [DOI] [PubMed] [Google Scholar]
- Wu J, Hua Y, Keep RF, Schallert T, Hoff JT, Xi G. Oxidative brain injury from extravasated erythrocytes after intracerebral hemorrhage. Brain Res. 2002;953:45–52. doi: 10.1016/s0006-8993(02)03268-7. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Keep RF, Hua Y, Schallert T, Hoff JT, Xi G. Deferoxamine-induced attenuation of brain edema and neurological deficits in a rat model of intracerebral hemorrhage. J Neurosurg. 2004;100:672–678. doi: 10.3171/jns.2004.100.4.0672. [DOI] [PubMed] [Google Scholar]
- Lin HJ, Wang X, Shaffer KM, Sasaki CY, Ma W. Characterization of H2O2-induced acute apoptosis in cultured neural stem/progenitor cells. FEBS Lett. 2004;570:102–106. doi: 10.1016/j.febslet.2004.06.019. [DOI] [PubMed] [Google Scholar]
- Theus MH, Wei L, Cui L, Francis K, Hu X, Keogh C, et al. In vitro hypoxic preconditioning of embryonic stem cells as a strategy of promoting cell survival and functional benefits after transplantation into the ischemic rat brain. Exp Neurol. 2008;210:656–670. doi: 10.1016/j.expneurol.2007.12.020. [DOI] [PubMed] [Google Scholar]
- Pervaiz S, Taneja R, Ghaffari S. Oxidative stress regulation of stem and progenitor cells. Antioxid Redox Signal. 2009;11:2777–2789. doi: 10.1089/ars.2009.2804. [DOI] [PubMed] [Google Scholar]
- Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, et al. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell. 2011;8:59–71. doi: 10.1016/j.stem.2010.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A, Tang Y, Ran R, Ardizzone TL, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2006;26:230–252. doi: 10.1038/sj.jcbfm.9600183. [DOI] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Yu F, Chan PH. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3β survival signaling. J Cereb Blood Flow Metab. 2007;27:975–982. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai C-Y, Finley JC, Ali SS, Patel HH, Howell SB. Copper influx transporter 1 is required for FGF, PDGF and EGF-induced MAPK signaling. Biochem Pharmacol. 2012;84:1007–1013. doi: 10.1016/j.bcp.2012.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noshita N, Sugawara T, Lewén A, Hayashi T, Chan PH. Copper-zinc superoxide dismutase affects Akt activation after transient focal cerebral ischemia in mice. Stroke. 2003;34:1513–1518. doi: 10.1161/01.STR.0000072986.46924.F4. [DOI] [PubMed] [Google Scholar]
- Lee S-T, Chu K, Jung K-H, Kim S-J, Kim D-H, Kang K-M, et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain. 2008;131:616–629. doi: 10.1093/brain/awm306. [DOI] [PubMed] [Google Scholar]
- Chen J, Zhang ZG, Li Y, Wang L, Xu YX, Gautam SC, et al. Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res. 2003;92:692–699. doi: 10.1161/01.RES.0000063425.51108.8D. [DOI] [PubMed] [Google Scholar]
- Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.