Abstract
The lower limit of autoregulation of cerebral blood flow (CBF) can be modulated with both angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARB). The influence of bradykinin antagonism on ARB-induced changes was the subject of this study. CBF was measured in Sprague–Dawley rats with laser Doppler technique. The blood pressure was lowered by controlled bleeding. Six groups of rats were studied: a control group and five groups given drugs intravenously: an ACE inhibitor (enalaprilat), an ARB (candesartan), a bradykinin-2 receptor antagonist (Hoe 140), a combination of enalaprilat and Hoe 140, and a combination of candesartan and Hoe 140. In the control group, the lower limit of CBF autoregulation was 54±9 mm Hg (mean±s.d.), with enalaprilat it was 46±6, with candesartan 39±8, with Hoe 140 53±6, with enalaprilat/Hoe 140 52±6, and with candesartan/Hoe 140 50±7. Both enalaprilat and candesartan lowered the lower limit of autoregulation of CBF significantly. The bradykinin antagonist abolished not only the effect of the ACE inhibitor but surprisingly also the effect of the ARB on the lower limit of CBF autoregulation, the latter suggesting an effect on intravascular bradykinin.
Keywords: autoregulation, bradykinin, cerebral blood flow, renin-angiotensin system
Introduction
The renin-angiotensin system (RAS) exerts a tone in the resistance vessels of the brain. Inhibition of this system with either an angiotensin-converting enzyme inhibitor (ACE inhibitor) or an angiotensin receptor blocker (ARB) shifts the lower and upper limits of autoregulation of cerebral blood flow (CBF) toward lower blood pressure levels. This has been shown with the ACE inhibitors captopril and ceranopril1, 2 and the ARBs candesartan and valsartan3, 4, 5 that block the angiotensin II subtype 1 (AT1) receptor. By contrast, the ARB losartan in one study shifted the upper limit of CBF autoregulation toward higher blood pressure.6 The angiotensin II subtype 2 (AT2) receptor blocker PD123319 does not influence the lower limit of autoregulation of CBF.7 Angiotensin-converting enzyme is identical to kininase II and inactivates bradykinin and other kinins.8 The ACE inhibitors thus cause a rise in circulating bradykinin and a fall in angiotensin II levels whereas ARBs have no effect on circulating bradykinin and cause an increase in angiotensin II levels.9, 10 Bradykinin is a potent vasodilator and acts by releasing prostacyclin, NO, and endothelial-derived hyperpolarizing factor.11
Hoe 140 is a selective blocker of the subtype 2 bradykinin (B2) receptor as has been shown in both in vitro and in vivo experiments.12, 13 Takada et al14 have shown that blocking the B2 receptor with Hoe 140 abolishes the effect of captopril on the lower limit of autoregulation of CBF. It is thus possible that the effect of captopril on autoregulation of CBF is caused by accumulation of bradykinin. Bradykinin and the B2 receptor may also participate in the effect of ARBs on the AT1 receptor. Thus, in rats with nitric oxide synthetase inhibitor-induced hypertension, the effects of ARBs on endothelial vasodilator function were found to be mediated by bradykinin and the B2 receptor.15 Angiotensin II can via the AT2 receptor induce vasodilatation and that effect is dependent on bradykinin and the B2 receptor.16 Whether bradykinin antagonism modulates the effect of ARBs on the lower limit of CBF autoregulation has not been shown and is the subject of the present study.
Materials and Methods
Studies of CBF autoregulation were carried out in anesthetized male Sprague–Dawley (SPRD) rats obtained from Charles River, Germany.
Animal Experimentation Ethics
The experiments were approved by the Animal Experiments Inspectorate under the Danish Ministry of Food, Agriculture, and Fisheries (license number: 2002/561-527and 2007/561-1320). The animals were at all times handled by trained personnel in accordance with the Guidelines for the Care and Use of Laboratory Animals in a facility fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Surgical Procedures
Male SPRD rats were studied under general anesthesia with Isoflurane (Baxter, Deerfield, IL, USA) and N2O. The Isoflurane dose was 5% during induction, 2.5% during the surgical procedures, and 1.7% during measurements. A tube was surgically placed in the trachea for mechanical ventilation. Polyethylene catheters were inserted in both femoral arteries and both femoral veins. One arterial catheter was used for monitoring blood pressure and the other for arterial blood sampling to monitor paCO2. One of the venous catheters was used to infuse study medication. After the insertion of the catheters, the animal was placed in a stereotactic apparatus. With a small dental drill, a craniotomy was done and care was taken not to damage the dura that was kept intact. A laser Doppler probe was placed onto the dura away from the larger vessels, to measure CBF.
Groups of Animals and Medication
Six groups, each of 10 to 12 of male SPRD rats, weight ∼350 g (Table 1), were studied. (1) a control group. A placebo was not given. The other groups were given drugs intravenously as follows: (2) the ACE inhibitor enalaprilat, 2 mg/kg (Merck Frosst Canada Inc, Kirkland, QC, Canada), (3) the ARB candesartan, 0.2 mg/kg (AstraZeneca, Sodertalje, Sweden), (4) the bradykinin B2 receptor antagonist Hoe 140, 4 nmol/kg and 2 nmol/kg every 15 minutes hereafter (Sigma-Aldrich, Munich, Germany), (5) a combination of Hoe 140 in the same doses as group 4 with the addition of enalaprilat in the same doses as group 2, given 10 minutes after the first Hoe 140 injection and (6) a combination of Hoe 140 in the same doses as group 4 with the addition of candesartan in the same doses as group 3 given 10 minutes after the first Hoe 140 injection. The Hoe 140 doses given in our study were doses that in other experiments have averted bradykinin-induced hypotension.14 Average injectate volume in each group ranged from 0.19 mL in the candesartan group to 1.00 mL in the enalaprilat plus HOE140 group.
Table 1. Physiologic variables.
| Control (n=10) | Enalaprilat (n=10) | Candesartan (n=12) | Hoe 140 (n=11) | Enalaprilat & Hoe 140 (n=10) | Candesartan & Hoe 140 (n=10) | |
|---|---|---|---|---|---|---|
| Mean±s.d. | Mean±s.d. | Mean±s.d. | Mean±s.d. | Mean±s.d. | Mean±s.d. | |
| Weight (g) | 336.8±34.7 | 322.9±27.0 | 377.4±48.6 | 368.6±17.3 | 376.1±51.1 | 379.8±30.2 |
| Start paCO2 (kPa) | 5.7±0,3 | 5.8±0,5 | 5.3±0,5 | 5.5±0,3 | 5.6±0,4 | 5.8±0.3 |
| End paCO2 (kPa) | 5.8±0.4 | 5.8±0.7 | 5.3±0.4 | 5.8±0.6 | 6.0±0,7 | 5,4±0,9 |
The groups are similar regarding basic physiologic variables.
Measurements and Data Analysis
To prevent an initial fall in the blood pressure below the lower limit of the autoregulation of CBF, norepinephrine 2 to 8 μg/minute was infused intravenously keeping MAP between 85 to 100 mm Hg in all groups of animals. Infusate volume was less than 0.4 mL. After injection of study medication and a period of 20 minutes with stable blood pressure and CBF, the experiment started with slowly reducing and finally discontinuing the norepinephrine infusion followed by controlled stepwise bleeding to lower the blood pressure. Approximately 2.5 to 4.0 mL of blood was removed over 20 to 25 minutes. Arterial blood samples were collected to measure PaCO2 at the start, midway, and at the end of the experiment. Before craniotomy, 0.25 mL of blood was drawn for control of hemoglobin, electrolytes, and creatinine. At the start of the experiment, 0.4 mL was drawn for PaCO2 control. The respirator was adjusted as necessary to maintain stable paCO2. Data were collected continuously on a PC running Perisoft 2.5 from Perimed AB, Jarfalla, Sweden.
The laser Doppler flowmetry is a validated method to estimate the lower limit of CBF autoregulation.17 The method can continuously monitor local cortical cerebral perfusion by multiple measurements, which give relative values of CBF with baseline registered as 100%.
To calculate the lower limit of autoregulation of CBF, a computer program was used. In brief, this program determines the lower limit of autoregulation of CBF by ranking the measurements by blood pressure values from 1 to N. The lower limit is then calculated as the breaking point between a slope regression line, which represents the measured levels below the lower limit and a plateau regression line, which in turn represents the measurements above the lower limit. The lines are found by including more and more measurements in the slope until a best fit is found, defined as the least sum of squares of the deviations from the different sets of lines. The method of calculation represents a modification of the method described in detail in a previously published paper.18 In the earlier paper, a horizontal line was used to fit the plateau, but here we allowed this line to have a slope. This is a DOS program that was run on a MacBook Pro running OS X 10.5.8, emulating DOS using DOSBox v.0.72. Statistical analysis was done on a MacBook Pro running OS X 10.5.8 using Prism 5.0b. Data were analyzed with one-way analysis of variance followed by Dunnett's multiple comparison test where the five treatment groups were compared with the control group.
Results
There was no significant difference between the six groups with regard to the weight of the animals and the level of paCO2 at the beginning and at the end of the study (Table 1).
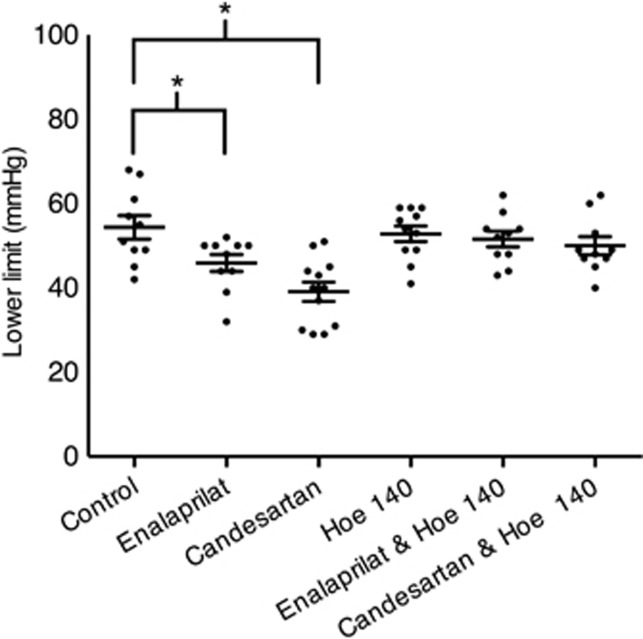
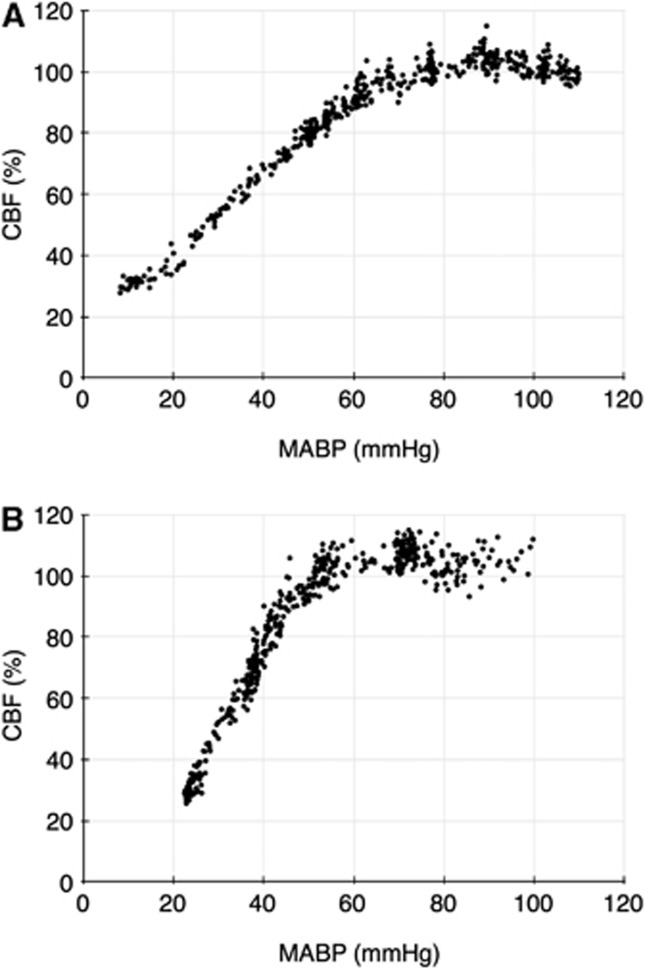
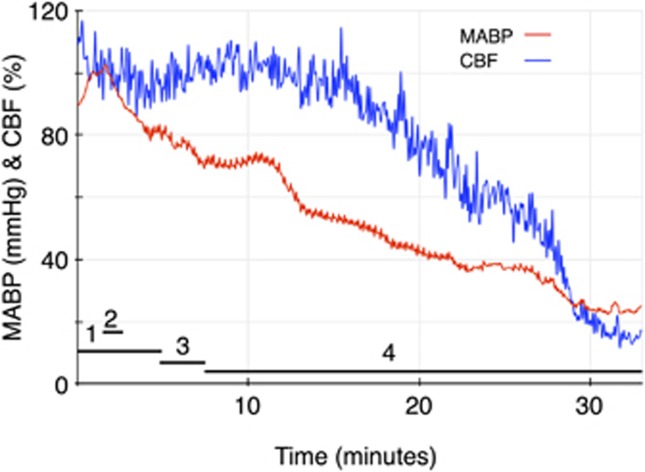
The mean and s.d. for the lower limit of CBF autoregulation in the six groups of animals are shown in Table 2, and all lower limit values are shown in Figure 1. Both enalaprilat and candesartan caused a shift in the lower limit of autoregulation toward lower blood pressure levels that was statistically significant when compared with the control group. The B2 receptor antagonist Hoe 140 by itself did not influence autoregulation. The addition of the B2 receptor antagonist to either enalaprilat or candesartan abolished the effect of both antihypertensive drugs on the lower limit of CBF autoregulation. Examples of individual autoregulation curves are shown in Figure 2. Figure 3 shows the time course of the candesartan experiment in Figure 2B.
Table 2. Results.
| Control (n=10) | Enalaprilat (n=10) | Candesartan (n=12) | Hoe 140 (n=11) | Enalaprilat & Hoe 140 (n=10) | Candesartan & Hoe 140 (n=10) | |
|---|---|---|---|---|---|---|
| Mean±s.d. | Mean±s.d. | Mean±s.d. | Mean±s.d. | Mean±s.d. | Mean±s.d. | |
| Lower limit of CBF (mm Hg) | 54±9 | 46±6* | 39±8* | 53±6 | 52±6 | 50±7 |
Lower limit of autoregulation. *P<0.05 compared with the control group using Dunnett's post analysis after analysis of variance.
Figure 1.
Results. The figure shows a scatter plot of the results and the bars are s.e.m. There is a statistically significant difference between enalaprilat versus control and candesartan versus control (*P<0.05 analysis of variance with Dunnett's post analysis).
Figure 2.
(A) Cerebral blood flow autoregulation in a control rat. An autoregulation curve from a rat in the control group with blood pressure on the x-axis and CBF on the y-axis. The calculated lower limit of autoregulation of CBF is 67 mm Hg. (B) Cerebral blood flow autoregulation in a candesartan-treated rat. An autoregulatory curve from a rat in the candesartan group with blood pressure on the x-axis and CBF on the y-axis. The calculated lower limit of autoregulation of CBF is 52 mm Hg. MABP, mean arterial blood pressure.
Figure 3.
Time course of the candesartan experiment shown in Figure 2B. The experiment lasts 33 minutes. The horizontal bars denote the following: (1) stable norepinephrine infusion, (2) candesartan injection, (3) tapering of norepinephrine, (4) controlled hemorrhage. CBF, cerebral blood flow; MABP, mean arterial blood pressure.
Discussion
There are two new findings in the present study. The first and novel observation in the present study is that the effect of the ARB candesartan on the lower limit of CBF autoregulation was abolished by the B2 receptor antagonist Hoe 140, which by itself did not influence autoregulation. Secondly, we found that the ACE inhibitor enalaprilat caused a shift in the lower limit of autoregulation of CBF toward lower blood pressure levels similar to the effects of other studied ACE inhibitors1, 2 and ARBs.4, 5 The ACE inhibitors exert their effect on the CBF by dilating the larger resistance vessels.19 At low blood pressure, this contributes to autoregulation, which takes place further downstream. The ARBs would be expected also to preferentially dilate the larger cerebral resistance vessels.
Bradykinin blockade has been shown in a study by Takada et al14 to abolish the effect of the ACE inhibitor captopril on the lower limit of autoregulation of CBF. We found a similar influence of bradykinin blockade on the effect of the ACE inhibitor enalaprilat on the lower limit. The definition of the lower limit of autoregulation of CBF in our study differs from that of Takada et al14 who estimated the lower limit of the autoregulation during controlled hypotension in rats as the blood pressure where CBF was 20% below baseline. In the present study, we defined the lower limit as the point where the plateau phase of CBF changes to a slope. This would seem to be the more accurate method, as shown by examples of autoregulation curves in Figure 2. The fact that we were able to reproduce the observations of Takada et al14 despite this difference in methodology, strengthen the biologic observation that bradykinin-2 receptor antagonism abolished the effect of the ACE inhibitors on the lower limit of autoregulation.
It was an unexpected finding that bradykinin blockade also abolished the effect of the ARB candesartan on the lower limit of CBF autoregulation. Angiotensin-converting enzyme degrades bradykinin to inactive substances and inhibiting this enzyme with ACE inhibitors causes an increase in blood bradykinin levels, whereas the ARBs do not cause such an increase. It is therefore conceptually plausible that bradykinin could have a role in the modulation of CBF autoregulation with ACE inhibitors, whereas it is less clear how the effect of ARBs should be similarly influenced. Several in vitro studies have, however, shown that a local kallikrein system exists in the vessel wall,20, 21, 22, 23 that angiotensin II via the AT2 receptor causes activation of this kinin system,24 and that the bradykinin B2 receptor has a role in vasodilatation induced by the AT2 receptor.16, 25 An in vivo study in rats performed by Gohlke et al26 showed that an increase in cGMP during infusion of angiotensin II was mediated via bradykinin and NO, as this effect could be abolished with either Hoe 140 or an NO synthase inhibitor. Another interesting study in kininogen-deficient rats showed a significant reduction in AT2-dependent vasodilatory response compared with wild type and that the same vasodilatory response was significantly suppressed in SPRD rats that were treated with the B2 antagonist FR173657.27 Finally, a study by Austinat et al28 showed that blocking the B1 receptor in an experimental ischemic stroke model in mice caused a reduction of infarct volume whereas the blocking of the B2 receptor did not have this effect. It is thus apparent that both the RAS and the kinin system exert a role in the vasculature of the central nervous system.
The ARBs may modulate CBF autoregulation through a local kinin system in the vessel wall. When Hoe 140 is given before either enalaprilat or candesartan, the endogenous bradykinin released from the vessel wall may be unable to bind to the B2 receptor and cause vasodilatation via prostacyclin, NO, and endothelial-derived hyperpolarizing factor. Our group has shown that the effect of captopril on autoregulation of CBF is independent of circulating renin, as this effect persists 48 hours after bilateral nephrectomy when renin is no longer present in the blood and the animal is kept alive by peritoneal dialysis.18 This suggests the importance of a local RAS in the vessels of the brain with compounds either taken up from the blood or synthesized locally. It seems possible that interplay of local RAS and local kallikrein system in the larger arteries of the brain have an important role in the modulation of autoregulation of CBF.
Isoflurane can, like all volatile anesthetics, influence both CBF and its autoregulation. It has, however, been shown that at low doses, the effect is minimal, especially in the cortex.29 In the present study, Isoflurane doses were low at the time of measurements and the same in all groups of animals.
In the present study, drugs were given acutely, so the effects found on CBF autoregulation are exclusively of a pharmacological nature. The use of controlled bleeding to lower blood pressure may cause activation of the RAS, with an amplification of the acute effect of ACEIs and ARBs on autoregulation. In earlier studies, this effect has been demonstrated to be of the same order of magnitude in hypertensive and normotensive rats.2, 4
If ACEIs or ARBs or other antihypertensives are given for prolonged periods, structural changes may develop in the cerebral arterial walls, leading to an additional shift of the lower limit of autoregulation toward even lower blood pressure.3, 30
Angiotensin-converting enzyme inhibitors and ARBs are widely used in antihypertensive therapy, and it has been shown in controlled trials that these drugs may have a beneficial effect beyond blood pressure lowering, in particular, protecting against stroke.31, 32, 33, 34 Interestingly, several studies in animals point to a cerebroprotective effect of ARBs not readily seen with ACE inhibitors.35, 36, 37 Thus, Fournier et al9 have suggested that ARBs, by way of increasing angiotensin II and influencing neuroprotective AT2 receptors in the brain, afford better stroke protection than ACE inhibitors. A meta-analysis of controlled clinical trials, although, found no difference between ACE inhibitors and ARBs with respect to stroke prevention, both groups of drugs being equally superior to placebo.38
From experimental studies such as the present, it may be inferred that part of the beneficial effect of the RAS blockers seen in the clinical trials may be due to a shift in the lower limit of autoregulation of CBF, improving the tolerance to lowering of blood pressure. Bradykinin may be involved in this via the B2 receptor.
Conclusion
In the present study, candesartan and enalaprilat both acutely shifted the lower limit of autoregulation of CBF toward lower blood pressure, and this effect was dependent on bradykinin. The bradykinin antagonist Hoe 140 did not influence autoregulation on its own. An increase in bradykinin is seen in the systemic circulation during ACE inhibition, and may take place locally in the vessels during ARB treatment by way of an angiotensin effect on the AT2 receptors.
The authors declare no conflict of interest.
Footnotes
This work and related studies were supported by the Danish Heart Association, the Danish Society of Nephrology, the Research Council at Copenhagen University Hospital in Herlev, the A.P. Møller Foundation for the Advancement of Medical Science, the Ellen and Aage Fausbøll Health Foundation of 1975, the Edith and Frode Waagens Foundation, the Jørgen Wendelbo Foundation and the King Christian X Foundation. Professor J. David Spence, Robarts Research Institute, Western University, London, Canada, kindly helped to obtain enalaprilat for the study. Candesartan was kindly donated by AstraZeneca, Sweden.
References
- Barry DI, Jarden JO, Paulson OB, Graham DI, Strandgaard S. Cerebrovascular aspects of converting-enzyme inhibition i: effects of intravenous captopril in spontaneously hypertensive and normotensive rats. J Hypertens. 1984;2:589–597. doi: 10.1097/00004872-198412000-00003. [DOI] [PubMed] [Google Scholar]
- Torup M, Waldemar G, Paulson OB. Ceranapril and cerebral blood flow autoregulation. J Hypertens. 1993;11:399–405. doi: 10.1097/00004872-199304000-00010. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Ito T, Hoe K, Saavedra JM. Chronic peripheral administration of the angiotensin II at(1) receptor antagonist candesartan blocks brain at(1) receptors. Brain Res. 2000;871:29–38. doi: 10.1016/s0006-8993(00)02377-5. [DOI] [PubMed] [Google Scholar]
- Vraamark T, Waldemar G, Strandgaard S, Paulson OB. Angiotensin II receptor antagonist CV-11974 and cerebral blood flow autoregulation. J Hypertens. 1995;13:755–761. [PubMed] [Google Scholar]
- Takada J, Ibayashi S, Ooboshi H, Ago T, Ishikawa E, Kamouchi M, et al. Valsartan improves the lower limit of cerebral autoregulation in rats. Hypertens Res. 2006;29:621–626. doi: 10.1291/hypres.29.621. [DOI] [PubMed] [Google Scholar]
- Naveri L, Stromberg C, Saavedra JM. Angiotensin II AT2 receptor stimulation extends the upper limit of cerebral blood flow autoregulation: agonist effects of CGP 42112 and PD 123319. J Cereb Blood Flow Metab. 1994;14:38–44. doi: 10.1038/jcbfm.1994.6. [DOI] [PubMed] [Google Scholar]
- Estrup TM, Paulson OB, Strandgaard S. No effect of angiotensin II AT(2)-receptor antagonist PD 123319 on cerebral blood flow autoregulation. J Renin Angiotensin Aldosterone Syst. 2001;2:188–192. doi: 10.3317/jraas.2001.026. [DOI] [PubMed] [Google Scholar]
- Linz W, Wiemer G, Gohlke P, Unger T, Scholkens BA. Contribution of kinins to the cardiovascular actions of angiotensin-converting enzyme inhibitors. Pharmacol Rev. 1995;47:25–49. [PubMed] [Google Scholar]
- Fournier A, Messerli FH, Achard JM, Fernandez L. Cerebroprotection mediated by angiotensin II: a hypothesis supported by recent randomized clinical trials. J Am Coll Cardiol. 2004;43:1343–1347. doi: 10.1016/j.jacc.2003.10.060. [DOI] [PubMed] [Google Scholar]
- Werner C, Baumhakel M, Teo KK, Schmieder R, Mann J, Unger T, et al. Ras blockade with ARB and ACE inhibitors: current perspective on rationale and patient selection. Clin Res Cardiol. 2008;97:418–431. doi: 10.1007/s00392-008-0668-3. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM. Endothelium and control of vascular function. State of the art lecture. Hypertension. 1989;13:658–667. doi: 10.1161/01.hyp.13.6.658. [DOI] [PubMed] [Google Scholar]
- Hock FJ, Wirth K, Albus U, Linz W, Gerhards HJ, Wiemer G, et al. Hoe 140 a new potent and long acting bradykinin-antagonist: in vitro studies. Br J Pharmacol. 1991;102:769–773. doi: 10.1111/j.1476-5381.1991.tb12248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth K, Hock FJ, Albus U, Linz W, Alpermann HG, Anagnostopoulos H, et al. Hoe 140 a new potent and long acting bradykinin-antagonist: in vivo studies. Br J Pharmacol. 1991;102:774–777. doi: 10.1111/j.1476-5381.1991.tb12249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada J, Ibayashi S, Nagao T, Ooboshi H, Kitazono T, Fujishima M. Bradykinin mediates the acute effect of an angiotensin-converting enzyme inhibitor on cerebral autoregulation in rats. Stroke. 2001;32:1216–1219. doi: 10.1161/01.str.32.5.1216. [DOI] [PubMed] [Google Scholar]
- De Gennaro Colonna V, Fioretti S, Rigamonti A, Bonomo S, Manfredi B, Muller EE, et al. Angiotensin II type 1 receptor antagonism improves endothelial vasodilator function in l-name-induced hypertensive rats by a kinin-dependent mechanism. J Hypertens. 2006;24:95–102. doi: 10.1097/01.hjh.0000194116.89356.66. [DOI] [PubMed] [Google Scholar]
- Bergaya S, Hilgers RH, Meneton P, Dong Y, Bloch-Faure M, Inagami T, et al. Flow-dependent dilation mediated by endogenous kinins requires angiotensin AT2 receptors. Circ Res. 2004;94:1623–1629. doi: 10.1161/01.RES.0000131497.73744.1a. [DOI] [PubMed] [Google Scholar]
- Tonnesen J, Pryds A, Larsen EH, Paulson OB, Hauerberg J, Knudsen GM. Laser Doppler flowmetry is valid for measurement of cerebral blood flow autoregulation lower limit in rats. Exp Physiol. 2005;90:349–355. doi: 10.1113/expphysiol.2004.029512. [DOI] [PubMed] [Google Scholar]
- Pedersen TF, Paulson OB, Nielsen AH, Strandgaard S. Effect of nephrectomy and captopril on autoregulation of cerebral blood flow in rats. Am J Physiol Heart Circ Physiol. 2003;285:H1097–H1104. doi: 10.1152/ajpheart.00098.2003. [DOI] [PubMed] [Google Scholar]
- Postiglione A, Bobkiewicz T, Vinholdt-Pedersen E, Lassen NA, Paulson OB, Barry DI. Cerebrovascular effects of angiotensin converting enzyme inhibition involve large artery dilatation in rats. Stroke. 1991;22:1363–1368. doi: 10.1161/01.str.22.11.1363. [DOI] [PubMed] [Google Scholar]
- Schmaier AH, Kuo A, Lundberg D, Murray S, Cines DB. The expression of high molecular weight kininogen on human umbilical vein endothelial cells. J Biol Chem. 1988;263:16327–16333. [PubMed] [Google Scholar]
- Oza NB, Schwartz JH, Goud HD, Levinsky NG. Rat aortic smooth muscle cells in culture express kallikrein, kininogen, and bradykininase activity. J Clin Invest. 1990;85:597–600. doi: 10.1172/JCI114479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mombouli JV, Vanhoutte PM. Kinins mediate kallikrein-induced endothelium-dependent relaxations in isolated canine coronary arteries. Biochem Biophys Res Commun. 1992;185:693–697. doi: 10.1016/0006-291x(92)91681-f. [DOI] [PubMed] [Google Scholar]
- Wolf WC, Harley RA, Sluce D, Chao L, Chao J. Localization and expression of tissue kallikrein and kallistatin in human blood vessels. J Histochem Cytochem. 1999;47:221–228. doi: 10.1177/002215549904700210. [DOI] [PubMed] [Google Scholar]
- Okamoto H, Yayama K, Shibata H, Nagaoka M, Takano M. Kininogen expression by rat vascular smooth muscle cells: stimulation by lipopolysaccharide and angiotensin II. Biochim Biophys Acta. 1998;1404:329–337. doi: 10.1016/s0167-4889(98)00074-3. [DOI] [PubMed] [Google Scholar]
- Tsutsumi Y, Matsubara H, Masaki H, Kurihara H, Murasawa S, Takai S, et al. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J Clin Invest. 1999;104:925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gohlke P, Pees C, Unger T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31:349–355. doi: 10.1161/01.hyp.31.1.349. [DOI] [PubMed] [Google Scholar]
- Katada J, Majima M. AT(2) receptor-dependent vasodilation is mediated by activation of vascular kinin generation under flow conditions. Br J Pharmacol. 2002;136:484–491. doi: 10.1038/sj.bjp.0704731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austinat M, Braeuninger S, Pesquero JB, Brede M, Bader M, Stoll G, et al. Blockade of bradykinin receptor b1 but not bradykinin receptor b2 provides protection from cerebral infarction and brain edema. Stroke. 2009;40:285–293. doi: 10.1161/STROKEAHA.108.526673. [DOI] [PubMed] [Google Scholar]
- Hoffman WE, Edelman G, Kochs E, Werner C, Segil L, Albrecht RF. Cerebral autoregulation in awake versus isoflurane-anesthetized rats. Anesth Analg. 1991;73:753–757. doi: 10.1213/00000539-199112000-00013. [DOI] [PubMed] [Google Scholar]
- Vorstrup S, Barry DI, Jarden JO, Svendsen UG, Braendstrup O, Graham DI, et al. Chronic antihypertensive treatment in the rat reverses hypertension-induced changes in cerebral blood flow autoregulation. Stroke. 1984;15:312–318. doi: 10.1161/01.str.15.2.312. [DOI] [PubMed] [Google Scholar]
- Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The heart outcomes prevention evaluation study investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, et al. Cardiovascular morbidity and mortality in the losartan intervention for endpoint reduction in hypertension study (life): a randomised trial against atenolol. Lancet. 2002;359:995–1003. doi: 10.1016/S0140-6736(02)08089-3. [DOI] [PubMed] [Google Scholar]
- Schrader J, Luders S, Kulschewski A, Hammersen F, Plate K, Berger J, et al. Morbidity and mortality after stroke, eprosartan compared with nitrendipine for secondary prevention: principal results of a prospective randomized controlled study (moses) Stroke. 2005;36:1218–1226. doi: 10.1161/01.STR.0000166048.35740.a9. [DOI] [PubMed] [Google Scholar]
- Sigurdsson ST, Strandgaard S. Blood pressure lowering in acute ischaemic stroke: an update on the role of angiotensin receptor blockers. J Hypertens. 2007;25:743–745. doi: 10.1097/HJH.0b013e3280be5af4. [DOI] [PubMed] [Google Scholar]
- Fagan SC, Kozak A, Hill WD, Pollock DM, Xu L, Johnson MH, et al. Hypertension after experimental cerebral ischemia: candesartan provides neurovascular protection. J Hypertens. 2006;24:535–539. doi: 10.1097/01.hjh.0000209990.41304.43. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Kawamata T, Shibata N, Okada Y, Kobayashi M, Hori T. Angiotensin II type 1 receptor blocker telmisartan reduces cerebral infarct volume and periinfarct cytosolic phospholipase A2 level in experimental stroke. J Neurotrauma. 2009;26:2355–2364. doi: 10.1089/neu.2009.0965. [DOI] [PubMed] [Google Scholar]
- Mecca AP, O'Connor TE, Katovich MJ, Sumners C. Candesartan pretreatment is cerebroprotective in a rat model of endothelin-1-induced middle cerebral artery occlusion. Exp Physiol. 2009;94:937–946. doi: 10.1113/expphysiol.2009.047936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu GC, Cheng JW, Zhu KM, Ma XJ, Shen FM, Su DF. A systematic review of angiotensin receptor blockers in preventing stroke. Stroke. 2009;40:3876–3878. doi: 10.1161/STROKEAHA.109.559989. [DOI] [PubMed] [Google Scholar]