Abstract
Network activation triggers a significant energy metabolism increase in both neurons and astrocytes. Questions of the primary neuronal energy substrate (e.g., glucose vs. lactate) as well as the relative contributions of glycolysis and oxidative phosphorylation and their cellular origin (neurons vs. astrocytes) are still a matter of debates. Using simultaneous measurements of electrophysiological and metabolic parameters during synaptic stimulation in hippocampal slices from mature mice, we show that neurons and astrocytes use both glycolysis and oxidative phosphorylation to meet their energy demands. Supplementation or replacement of glucose in artificial cerebrospinal fluid (ACSF) with pyruvate or lactate strongly modifies parameters related to network activity-triggered energy metabolism. These effects are not induced by changes in ATP content, pHi, [Ca2+]i or accumulation of reactive oxygen species. Our results suggest that during network activation, a significant fraction of NAD(P)H response (its overshoot phase) corresponds to glycolysis and the changes in cytosolic NAD(P)H and mitochondrial FAD are coupled. Our data do not support the hypothesis of a preferential utilization of astrocyte-released lactate by neurons during network activation in slices—instead, we show that during such activity glucose is an effective energy substrate for both neurons and astrocytes.
Keywords: astrocytes, energy metabolism, glycolysis, lactate, network activity, neurons
Introduction
High cellular energy demands during network activation are met by upregulation of cytosolic glycolysis and mitochondrial oxidative phosphorylation. Mitochondrial metabolism provides most of the ATP but glycolysis is also enhanced and may contribute to the energy production.1, 2, 3, 4 For elucidation of the cellular basis of neuroenergetics, measurements of metabolic signals including the oxygen utilization and NAD(P)H/FAD autofluorescence provide valuable information for connecting energy metabolism with neuronal activity. NADH (reduced form) is fluorescent when excited with UV light whereas NAD+ is not, leading to a decrease in observed fluorescence as a result of NADH oxidation. In contrast, FAD (oxidized form) is fluorescent, so the oxidation of FADH2 to FAD causes an increase in fluorescence. The fluorescence of NADH cannot be separated from that of NADPH and their emission is measured in concert (NAD(P)H). NAD(P)H fluorescence represents a ‘mixed' signal since this cofactor can be produced by both glycolysis and mitochondria, whereas FAD fluorescence is entirely mitochondrial.5, 6 Measurements of these parameters in combination with electrophysiological recordings have been used in many studies to monitor the energy status during neuronal activity in brain tissues.
Typically, NAD(P)H transients induced by synaptic stimulation have a characteristic biphasic waveform: the initial short dip is followed by a long-lasting overshoot. While there exists a common consensus that the initial decrease in fluorescence is due to mitochondrial function, the origin of the overshoot is as yet unclear. A radical solution to this problem has been proposed by Kaschiske et al.7 and suggests that during evoked synaptic activity, the NAD(P)H ‘dip' represents neuronal oxidative phosphorylation while the overshoot is a result of astrocytic glycolysis. In contrast, other studies suggested that both phases of NAD(P)H signaling reflect neuronal mitochondrial processes without any significant contribution of glycolysis.6, 8 The FAD transients, which seem to be symmetrically inverted in respect to those of NAD(P)H, were taken as an argument in favor of the mitochondrial origin of both signals.8 Major mitochondrial contribution to the energy supply was also suggested recently by Hall et al.9 Finally, it has been reported that the overshoot is strongly sensitive to oxygen deficiency and it is the balance between oxygen demand and supply that ultimately affects the shape of NAD(P)H fluorescence response.10 Indeed, in vivo NAD(P)H measurements displayed smaller overshoot values (compared with the oxidation phase) than those seen in slices,11, 12 a phenomenon that may be associated with a more adequate oxygen supply present in vivo. Thus, the unequivocal interpretation of NAD(P)H transients has yet to be reached.
Another important issue concerns the preferred energy substrate for supporting cellular activity. Glucose is the main energy substrate supplied to the adult brain under normal conditions, although the exact supply pathways and cell types that consume glucose during network activation are not fully elucidated. Brain cells are capable of utilizing varying energy substrates in addition to glucose.13 For instance, the ‘lactate shuttle' hypothesis postulates that lactate, as the end product of astrocytic glycolysis triggered by glutamate uptake, is the main fuel for neurons during their activity.14 Glucose is thus the essential substrate for energy generation throughout network activity, but its metabolism is segregated into two cell types—glycolysis in astrocytes and oxidation in neurons. The relevance of such a scenario, however, has been questioned (for review, refs. 15, 16, 17) and views on the major fuel source for activated neurons and the corresponding neuron–astrocyte interactions are controversial.
Therefore, the roles of glycolysis and oxidative phosphorylation in energy metabolism of neurons and astrocytes during network activation are still debatable. This is an issue of principal importance for furthering our understanding of cellular specificities of energy metabolism and their coupling with neuronal–astrocytic interactions. We addressed these questions in the present study, utilizing simultaneous electrophysiological and metabolic recordings in hippocampal slices from mature mice.
Materials and methods
Tissue Slice Preparation
Brain slices were prepared from P19-P44 Swiss mice of both sexes. However, from 64 mice used in the experiments, only 5 mice were younger 30 days, and therefore we mention the animals as ‘mature' in the text. All animal protocols conformed the INSERM guidelines on the use of laboratory animals and are approved by the Ethics Committee for Animal Experimentation of Marseille (#30-03102012). A mouse anesthetized with isoflurane was decapitated, the brain was rapidly removed from the skull and placed in the ice-cold artificial cerebrospinal fluid (ACSF). The ACSF solution consisted of (in mmol/L): NaCl 124, KCl 3.50, NaH2PO4 1.25, NaHCO3 25, CaCl2 2.00, MgCl2 1.30, and dextrose 10, pH 7.4. ACSF was aerated with 95% O2/5% CO2 gas mixture. Sagittal slices (350 μm) were cut using a tissue slicer (Leica VT 1200s, Leica Microsystem, Wetzlar, Germany). During cutting, slices were submerged in an ice-cold (<6°C) solution consisting of (in mmol/L): K-gluconate 140, HEPES 10, Na-gluconate 15, EGTA 0.2, NaCl 4, pH adjusted to 7.2 with KOH. Slices were immediately transferred to a multisection, dual-side perfusion holding chamber with constantly circulating ACSF and allowed to recover for 2 hours at room temperature (22°C to 24°C). Slices were then transferred to a recording chamber continuously superfused (15 mL/min) with ACSF (33°C to 34°C) containing either 5 mmol/L glucose or 10 mmol/L pyruvate (unless otherwise noted) with access to both slice sides. No more than two slices from each brain were used in the experiment.
Synaptic Stimulation and Field Potential Recordings
Shaffer collateral/commissural pathway was stimulated using the DS2A isolated stimulator (Digitimer Ltd, Hertfordshire, UK) with a bipolar metal electrode situated in the stratum radiatum of CA1 hippocampal region. To reduce the stimulated area in some experiments, we used a bipolar glass electrode (30 μm tip diameter) pulled from a borosilicate theta-tube (TG150-4; Warner Instruments, Hamden, CT, USA). Stimulus current was adjusted using single pulses (40 to 170 μA, 200 μs, 0.15 Hz) to induce a local field potential (LFP) of nearly 50% of maximal amplitude. Local field potentials were recorded using glass microelectrodes filled with ASCF, placed in stratum pyramidale and connected to the ISO DAM-8A amplifier (WPI, Sarasota, FL, USA). Synaptic stimulation consisting of a 10-second stimulus train (200 μs pulses at 10 Hz) was used to trigger autofluorescence responses of NAD(P)H and FAD. Local field potentials were quantified by calculating their integrals as previously described.18 The second stimulation approach utilized a glutamate (10 mmol/L) application consisting of 1- or 5-second trains (10 Hz) of short (10 ms) puffs from the glass pipette (∼30 μm diameter) connected to the Toohey Spritzer pressure system (Toohey Co., Fairfield, NJ, USA). The pressure and pipette tip position were adjusted to produce no tissue motion during glutamate application.
NAD(P)H and FAD Fluorescence Imaging
NADPH and NADH have similar optical properties; therefore, it is expected that NADPH may contribute to the total autofluorescence signal. Changes in NAD(P)H fluorescence in hippocampal slices were monitored using a 290–370 nm excitation filter and a 420 nm long-pass filter for the emission (Omega Optical, Brattleboro, VT, USA). In the case of FAD imaging, 500 to 570 nm emission was excited with 400 to 490 nm light. The light source was the Intensiligh C-HGFI illuminator (Nikon Instruments Europe B.V., Amsterdam, Netherland) equipped with a mercury arc lamp. Slices were epi-illuminated and imaged through a Nikon upright microscope (FN1, Eclipse; Nikon Instruments Europe B.V.) with × 4/0.10 Nikon Plan objective. Images were acquired using a linear, cooled 12-bit CCD camera (Sensicam; PCO AG, Kelheim, Germany) with a 640 × 480 digital spatial resolution. Because of a low level of fluorescence emission, NAD(P)H or FAD images were acquired every 600 to 800 ms as 8 × 8 binned images (effective spatial resolution of 80 × 60 pixels). The exposure time was adjusted to obtain a baseline fluorescence intensity between 2,000 and 3,000 optical intensity levels. Fluorescence intensity changes in stratum radiatum near sites of LFP and O2 recordings were measured in three to five regions of interest (ROIs) using the ImageJ software (NIH, USA). Data were expressed as the percentage changes in fluorescence over a baseline ((ΔF/F) × 100). In analysis, peak amplitudes of different response phases were used. High variability in NADH/FAD transients measured in different experiments in control (glucose-ACSF) complicated statistical analysis of the effects when data were pooled together. To overcome this problem and satisfy the normality condition test (Shapiro-Francia normality test), we scaled the data in each experiment using the oxidative dip (peak) and overshoot (undershoot) amplitudes of NADH/FAD transients recorded in control (glucose-ACSF) as scaling factors. Signal analysis was performed using the IgorPro software (WaveMetrics, Inc., Portland, OR, USA).
Oxygen Measurements
A Clark-style oxygen microelectrode (tip diameter 10 μm; Unisense Ltd, Denmark) was used to measure slice tissue pO2. The electrode was connected to a picoammeter (PA2000, Unisense Ltd). A two-point calibration was performed by inserting the electrode in normal saline solution (at 33°C) equilibrated with either 95% O2 or ambient air. Calibrations were repeated after each experiment to determine the pO2 values. Electrode tip penetration depth was controlled via the LinLab software (Scientifica Ltd, Uckfield, UK). In analysis, oxygen consumption was estimated as an integral of pO2 transient below the baseline.
Intracellular pH and Ca2+ Imaging
pH and Ca2+ were measured in two separate experiment series using ratiometric imaging approach. For Ca2+ measurements, brain slices were loaded with Fura-2 AM (Molecular Probes, Life Technologies, St Aubin, France), and with SNARF-1 (Invitrogen, Life Technologies) for pH evaluation (see details in Supplementary Methods).
ATP Measurements
Brain slices from one animal were divided into two groups. One group was incubated for 40 minutes at 32°C in 5 mmol/L glucose-ACSF whereas another one in 10 mmol/L pyruvate-ACSF. ATP quantity was determined in each group using the Stay Brite ATP Bioluminiscence assay kit (BioVision Inc., Milpitas, CA, USA). ATP content in slices was normalized to total protein content determined using Bio-Rad protein assay (see Supplementary Methods).
Pharmacology
Drugs used were purchased from Sigma (Sigma-Aldrich Chimie S.a.r.l., Lyon, France) (L-lactate sodium salt, glutamate sodium salt, pyruvate sodium salt, and TEMPOL) and Tocris (Tocris Bioscience, Bristol, UK) (NBQX, AP-5, gabazine, E4CPG, and TFB-TBOA).
Statistical Analysis
Group measures were expressed as means±s.e.m. Statistical significance was assessed using Student's paired t-test or ANOVA if data distribution was confirmed with the Shapiro-Francia normality test. Otherwise, the Wilcoxon's signed paired test was applied. The level of significance was set at P<0.05.
Results
In several studies on slices, it has been shown that the waveshape of NAD(P)H response to synaptic stimulation depends strongly on the availability of oxygen: an insufficient pO2 results in a decrease in oxidation phase and an increase in overshoot.10, 18, 19, 20 In brain slices, pO2 decreases with depth and cells located at different depths may contribute differentially to the acquired NAD(P)H signal, hindering its adequate interpretation. Therefore, we first had to confirm that the NAD(P)H recordings were not ‘contaminated' by such artifactual conditions and that the NAD(P)H transient waveshapes are similar throughout the slice depth. We used a high superfusion rate (15 mL/min) together with solution access to the both sides of the slice to ensure adequate slice oxygenation18, 21, 22 and tested NAD(P)H signaling using a local Shaffer collaterals stimulation by a double-barrel glass electrode while simultaneously measuring pO2 at the same depth (Figure 1A). Note that the variability in electrodes' location during intrusion into the slice underlies the difference in response values in Figure 1A. For instance, a smaller NAD(P)H response at 300 μm compared with that at 175 μm is due more to the difference in stimulation electrode location at these depths rather than to the oxygen insufficiency. To evaluate the NAD(P)H waveshape, we used a ratio of the oxidation dip amplitude to the overshoot peak amplitude (oxidation/overshoot ratio) as this ratio best characterizes the relative contribution of oxidative phase and overshoot to a signal, and depends neither on a signal size nor on a change in basal fluorescence. The results showed that the oxidation/overshoot ratio of NAD(P)H transients was independent of the depth (Figure 1B), indicating that our experimental conditions were adequate for the metabolic measurements.
Figure 1.
NAD(P)H transient waveshape is similar throughout the depth of the slice. (A) Example of recording electrodes' configuration and regions of interest for fluorescence measurements in a slice (photo on the left); original NAD(P)H transients and the corresponding oxygen transients measured at different depths (distance from the upper slice surface) of the same slice. Signals were induced by a 10- Hz, 10-second stimulation of Schaffer collaterals. Note that the variable location of the stimulation electrode due to its moving into the slice explains the difference in response values. (B) The dependence of NAD(P)H oxidation/overshoot amplitudes ratio on the depth in slice. Linear regression (straight line) shows no correlation between these two parameters (R2=0.013).
Contribution of Neurons and Astrocytes to the Energy Production During Evoked Activity
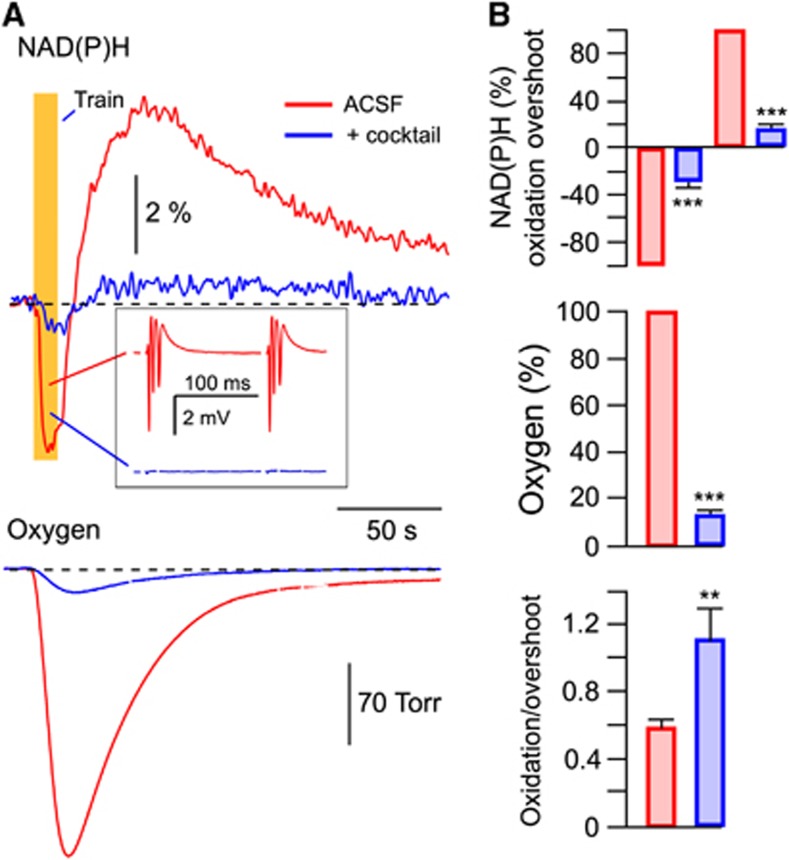
Stimulation-triggered presynaptic neurotransmitter release from Shaffer collateral terminals activates energy metabolism in both CA1 neurons and astrocytes. In neurons, energy is mainly spent on recovering the transmembrane potential and ion gradients perturbed by the various ion channel-mediated currents, whereas in astrocytes energy is mostly spent on the glutamate and potassium uptake.2, 3, 9 To pharmacologically separate the postsynaptic neuronal fraction of the observed response, we used a mixture (cocktail) of postsynaptic receptor blockers consisting of NBQX (antagonist of AMPA receptors, 10 μmol/L), AP-5 (antagonist of NMDA receptors, 40 μmol/L), gabazine (antagonist of GABAA receptors, 10 μmol/L), and E4CPG (nonselective antagonist of metabotropic glutamate receptors, 500 μmol/L). Application of this cocktail completely abolished LFPs (see inset in Figure 2A, blue trace) and resulted in a strong decrease in both NAD(P)H (oxidation phase by 71.6±5.5% (from −2.51±0.26% to −0.62±0.06% amplitude), overshoot by 83.7±3% (from 4.38±0.46% to 0.68±0.11% amplitude); n=10) and pO2 (by 86.7±1.9%) transients (Figures 2A and 2B). The remaining responses could be attributed to presynaptic energy metabolism and/or activation of astrocytes by the increased extracellular concentrations of glutamate and K+. To distinguish between these potential components we applied TFB-TBOA, an antagonist of glial glutamate transporters.23 In most experiments, neither NAD(P)H nor O2 responses could be detected after the TFB-TBOA (10 μmol/L) application. In some experiments, however, small NAD(P)H and O2 transients could still be identified (see an example in Supplementary Figure 1). These results suggest that the main metabolic response remaining after the cocktail application is a result of astrocytic activity. Note also that the contribution of the ‘after-cocktail' component to the total ‘before-cocktail' response is likely underestimated since the neuronal activity during stimulation in ‘cocktail-free' conditions is strongly potentiated due to short-term synaptic plasticity (see inset in Figure 2A) that should result in a larger subsequent glutamate and K+ release and, as a consequence, an enhanced astrocytic metabolism. It is clear, however, that during periods of synaptic activation neurons are the major consumers of oxygen and the major contributors to NAD(P)H overshoot. Interestingly, the oxidation/overshoot ratio became significantly larger after the cocktail application (Figure 2B). This may indicate a smaller glycolytic contribution to the NAD(P)H transients in astrocytes compared with neurons (see below).
Figure 2.
Neuronal and astrocytic metabolic signaling induced by synaptic stimulation. (A) NAD(P)H and oxygen transients induced by a 10-Hz, 10-second stimulation of Schaffer collaterals. Red, signals recorded in artificial cerebrospinal fluid (ACSF); blue, signals recorded after the addition of a cocktail of blockers consisting of NBQX (10 μmol/L), AP-5 (40 μmol/L), gabazine (10 μmol/L), and E4CPG (500 μmol/L). The inset (A) shows local field potentials (LFPs) within the stimulation train. Note multiple population spikes induced by short-term synaptic plasticity. (B) Summary of all similar experiments. The NAD(P)H oxidation dip and overshoot amplitudes were used in analysis. Oxygen consumption was estimated as an integral of pO2 transient below the baseline. Responses are normalized to the amplitudes in ‘cocktail-free' ACSF. **P<0.01, ***P<0.001.
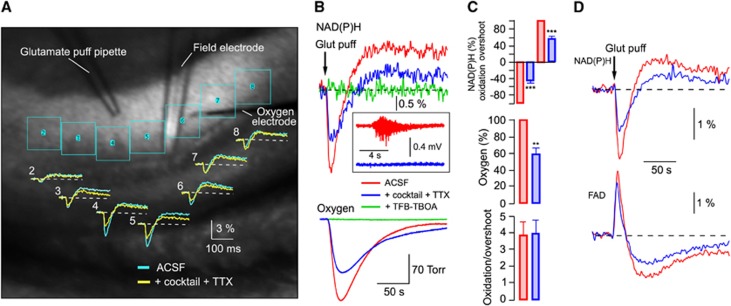
To reveal in detail the postsynaptic neuronal and astrocytic metabolic activities ‘uncontaminated' by presynaptic components, we utilized a different type of stimulation—a 10-Hz train of short (10 ms) puffs of glutamate (10 mmol/L) directly onto the recorded region. Glutamate application can serve as metabolic stimulation due to induced neuronal electrical activity via postsynaptic AMPA and NMDA receptors as well as astrocytic activity mostly via glutamate transporters. The neuronal activity can be prevented at large by the application of glutamate receptor blockers cocktail+TTX. Figure 3A shows an example of such an experiment. The local application of 1-second glutamate puff train in stratum radiatum induced NAD(P)H transients whose magnitude decreased with a horizontal distance from the pipette, likely being proportional to the glutamate concentration within each ROI (see ROIs in Figure 3A). In the vicinity of application pipette, the glutamate concentration was large enough to induce neuronal firing (Figure 3B, inset) via activation of AMPA receptors. The same was not the case in regions more distant from the pipette, where glutamate concentration decreased below AMPA receptor activation threshold (EC50>500 μmol/L). The metabolic changes observed at distal points can therefore be attributed to glutamate uptake-induced astrocytic activation effective at lower neurotransmitter concentrations (Km of 2 to 90 μmol/L). We thus performed our recordings in the proximal region of neuronal firing where the contribution of neurons to energy consumption would be significant. After the application of cocktail+TTX that blocked neuronal activation thus revealing the astrocytic fraction, NAD(P)H responses became smaller but still possessed a pronounced oxidation phase followed by an overshoot. Such NAD(P)H transient waveshape was observed in all ROIs and therefore was independent of glutamate concentration. Note that the expected difference of ‘before cocktail' and ‘after cocktail' responses is more prominent closer to the application pipette. Figures 3B–3D show the results of similar experiments in more detail. In control, the glutamate puff train induced neuronal firing that was completely abolished by the application of cocktail+TTX (Figure 3B, inset). It also resulted in a decrease in both NAD(P)H (oxidation phase from −2.48±0.23% to −1.15±0.18% amplitude; overshoot from 0.91±0.14% to 0.42±0.08% amplitude; n=12) and O2 (by ∼40%) transients (Figure 3C) although to a much smaller extent than during electrical stimulation as in Figure 2A. The astrocytic origin of responses was verified by the application of TFB-TBOA, which completely abolished them (n=3). Interestingly, at both types of stimulation (synaptic and glutamate puffs), the astrocytic NAD(P)H transients had a prominent oxidation phase followed by an overshoot of smaller amplitude and were also associated with a significant oxygen consumption that was especially pronounced during glutamate application. This indicates that astrocytes efficiently employ not only glycolysis but also oxidative phosphorylation for covering their energy requirements induced by glutamate uptake.
Figure 3.
Neuronal and astrocytic metabolic signaling induced by the glutamate puff train stimulation. (A) Electrodes' configuration and regions of interest (ROIs) for fluorescence measurements in a slice. Original NAD(P)H transients in each ROI in response to a 10-ms, 10-Hz, 1-second glutamate (10 mmol/L) puff train stimulation. In blue, signals recorded in standard artificial cerebrospinal fluid (ACSF), in yellow—after addition a cocktail of blockers consisting of NBQX (10 μmol/L), AP-5 (40 μmol/L), gabazine (10 μmol/L), E4CPG (500 μmol/L), and TTX (1 μmol/L). Reference ROI (#1) is not shown. (B) NAD(P)H and oxygen transients induced by a 10-ms, 10-Hz, 5-second glutamate (10 mmol/L) puff stimulation in ACSF (red), ACSF+cocktail+TTX (blue) and ACSF+cocktail+TTX+TFB-TBOA (green). The inset shows field recordings during the stimulation train. (C) Summary of similar experiments. Responses are normalized to the amplitudes in ‘cocktail-free' ACSF. (D) Averaged transients of NAD(P)H and FAD responses (n=4) to the glutamate puffs train in ACSF (red) and ACSF+cocktail+TTX (blue). **P<0.01, ***P<0.001.
To further confirm this conclusion, we recorded the FAD autofluorescence originating from mitochondria.5, 6 In six experiments, we recorded both NAD(P)H and FAD transients induced by the 5-second, 10-Hz glutamate puff trains. Figure 3D shows these signals' combined average. Prominent FAD fluorescence remaining after the cocktail+TTX application confirms the intense mitochondrial activity in astrocytes. Note that with both types of stimulation (electrical-synaptic and glutamate puffs) a significant fraction of the overshoot phase disappeared after the cocktail administration, indicating that it corresponds to neuronal activity. Thus, the metabolic processes associated with the oxidation and overshoot phases of NAD(P)H transients are active in both neurons and astrocytes.
The Link between NAD(P)H Overshoot and Glycolysis
The cell type-specific role of cytosolic glycolysis and mitochondrial oxidative phosphorylation involved in energy generation is a question of primary importance for the conceptual understanding of energy metabolism and neuronal–astrocytic interactions. Since NAD(P)H is produced by both glycolysis and citric acid cycle, the analysis of NAD(P)H autofluorescence may provide important information on energy metabolism components. Indeed, glucose metabolism by glycolysis provides the end product pyruvate, which further serves as a primary substrate for mitochondrial metabolism. Therefore, to distinguish the glycolytic and mitochondrial contributions to the NAD(P)H fluorescence, we compared metabolic signaling recorded in standard ACSF with that recorded after complete replacement of ACSF glucose with pyruvate. Such a substitution was expected to arrest glycolysis while maintaining the mitochondrial function. Importantly, the substitution of energy substrates did not result in any change in ATP content in slices (nmoles/mg of total protein: 9.38±1.05 in 5 mmol/L glucose-ACSF and 9.47±1.28 in 10 mmol/L pyruvate-ACSF; n=4, P>0.4).
In each experiment, similar stimulation intensities (single-pulse Shaffer collateral stimulation) in both substrate-containing solutions activated a similar number of nerve fibers, as manifested by the unaffected size of presynaptic volley (e.g., see Supplementary Figure 2A). However, in all such single-pulse experiments (n=45), the exchange of glucose-ACSF for pyruvate-ACSF was associated with a decrease in resulting LFP integral (by 31±9%, Supplementary Figure 2A) as was also reported previously.24 Since adenosine release during glycolysis inhibition has been suggested in previous reports, we tested the effect of DPCPX, an antagonist of type 1 adenosine receptors expressed in synaptic terminals.25 DPCPX (100 nmol/L) did not cause any changes in such action of pyruvate (Supplementary Figure 2B). Interestingly, with the use of the train (10 Hz, 10 seconds) stimulation, despite the decrease in the first LFP, the following and total train LFP integral did not differ significantly between glucose and pyruvate conditions (Supplementary Figure 2C). Since the recorded LFP integrals are proportional to neural activity, this suggests that the overall neural activity during the train is similar in glucose vs. pyruvate and the energy demands therefore can be assumed to be similar for both substrates.
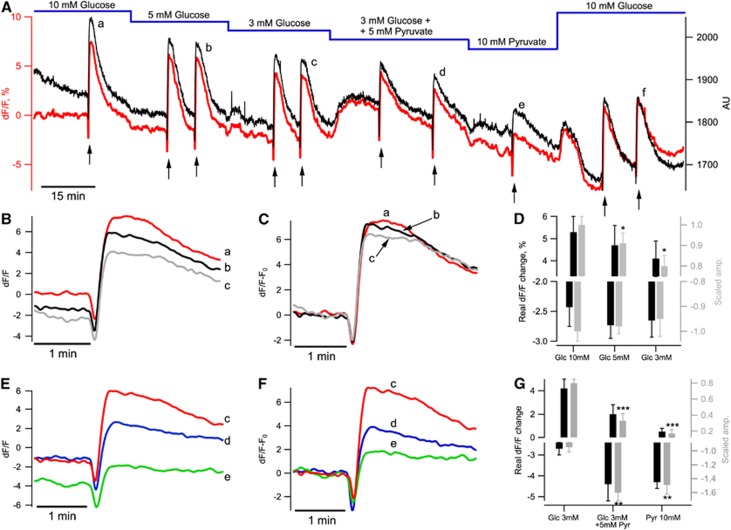
The glucose-for-pyruvate exchange could modify the basal cellular redox state and shift NAD(P)H/FAD basal fluorescences (F) to new steady-state levels. This should be taken into account when comparing transient fluorescent responses with stimulation (ΔF/F). Therefore, in four experiments, NADH was monitored continuously during the entire experiment duration (130 minutes on average; Figure 4A). At the beginning of recordings, the basal NAD(P)H emission intensity was 2,149±200 arbitrary units and declined slowly to 2,046±142 arbitrary units in 15 minutes (Figure 4A, black trace). During this time, Shaffer collaterals were stimulated with single pulses every 10 seconds without ACSF type exchange. The decrease in NAD(P)H fluorescence could be explained by both a change in parameters of experimental equipment (e.g., mercury lamp intensity stabilization) and a change in the brain slice itself. To evaluate the equipment-dependent component, we fitted the fluorescence during first 15 minutes with an exponential decay function. This function was then extrapolated to the entire experiment duration. The resulting curve was used as a baseline time course, F0(t), in the calculation of ΔF/F=(F(t)−F0(t))/F0(t). As shown in Figure 4A, this method allows to subtract the equipment-dependent decay while preserving NAD(P)H fluorescence intensity variations due to physiologic modifications (see red trace in Figure 4A).
Figure 4.
NADH fluorescence depends on the glucose and pyruvate content in artificial cerebrospinal fluid (ACSF). (A) Example of a long-lasting NAD(P)H fluorescence recording in a slice of P44 mouse. Black trace shows raw data from one region of interest (ROI) (right Y axis). The initial fragment of baseline recording (15-minute duration) was used to fit the baseline drift with an exponential decay function. This function was extrapolated to the entire experiment duration and applied as F0(t) to the calculation of ΔF(t)/F0(t) (red trace). Black arrows indicate stimulations of Shaffer collaterals (10 Hz, 10 seconds). (B, C) NAD(P)H responses to stimulations at different glucose concentrations in ACSF shown in panel A (a–c) are shown. (D) Summary of four similar experiments. NAD(P)H overshoot amplitude decreased with a decrease in glucose concentration (top graph, black bars) whereas NAD(P)H oxidation dip was insensitive to such change (lower graph, black bars). Gray bars show data scaled to the responses measured in 10 mmol/L glucose. (E, F) NAD(P)H responses to stimulations shown in panel A (c–e) are shown. (G) Summary of four similar experiments. Pyruvate modified both phases of NADH transients (top and lower graphs black bars). Gray bars show data scaled to the initial responses measured in 10 mmol/L glucose (see text for details). *P<0.05, **P<0.01, ***P<0.001.
After the initial control 15 minutes, we started Shaffer collateral pulse train stimulation (10 Hz, 10 seconds) and also exchanged ACSF energy substrates (Figure 4A, red). Train stimulations were applied 1 to 2 times in each condition. A decrease in glucose concentration from 10 to 5 mmol/L did not induce significant decreases in basal NAD(P)H fluorescence (−1.9±0.8%, n=4, P=0.07) but the baseline decline became significant in ACSF containing 3 mmol/L glucose (−2.6±1.0%, n=4, P=0.03). After supplementing 3 mmol/L glucose with 5 mmol/L pyruvate, NAD(P)H fluorescence increased transiently (to −0.16±0.80%, n=4, P=0.02) and then started to decline slowly. Complete glucose replacement with 10 mmol/L pyruvate decreased the basal fluorescence to −5.7±1.5% (n=4, P=0.02) whereas following the pyruvate washout by 10 mmol/L glucose-containing ACSF, NAD(P)H fluorescence started to increase after the initial transient. Therefore, compared with glucose-ACSF, pyruvate-ACSF results in a smaller amount of reduced cofactor (NAD(P)H) in cells.
We also compared NAD(P)H transients evoked by Shaffer collateral train stimulation (shown by arrows in Figure 4A) at different stages of the experiment. A decrease in ACSF glucose concentration had no effect on the oxidative dip (−2.4±0.3%, −2.7±0.2%, and −2.7±0.3% in 10, 5, and 3 mmol/L glucose, respectively; Figure 4D, black bars (corresponded to −1.00±0.04%, −0.98±0.03%, −0.95±0.07% of response in 10 mmol/L glucose, n>6; ANOVA, P=0.8; Figure 4D, gray bars)) but affected the overshoot amplitude (5.3±0.7%, 4.7±0.9%, and 4.2±0.8% in 10, 5, and 3 mmol/L glucose, respectively, Figure 4D, black bars (corresponded to 1.00±0.04%, 0.91±0.05%, 0.80±0.05% of response in 10 mmol/L glucose, n>6; ANOVA, P=0.025; Figure 4D, gray bars)) (see Figures 4B and 4C). Addition of 5 mmol/L pyruvate in the 3 mmol/L glucose-ACSF or complete glucose replacement with 10 mmol/L pyruvate strongly changed both phases of NAD(P)H transients: increased oxidative dip (−4.4±0.6% and −4.3±0.5 vs. −2.7±0.3% in 3 mmol/L glucose, respectively; Figure 4G; black bars) and decreased overshoot amplitude (2.0±0.8% and 0.5±0.3% vs. 4.2±0.8% in 3 mmol/L glucose, respectively; Figure 4G; black bars) (see Figures 4E and 4F). The pyruvate washout and return to the initial glucose concentration (10 mmol/L) recovered the waveshape of NAD(P)H transients. ANOVA test applied to the scaled data (see ‘Materials and Methods, NAD(P)H and FAD fluorescence imaging') verified statistical significance of the pyruvate-induced modifications of NAD(P)H transients (Figure 4G, gray bars, P=0.0012 for oxidative deep amplitudes and P<0.001 for overshoot amplitudes, n>5).
These results show that a low glucose concentration (3 mmol/L) in ACSF or supplementing/replacement glucose with pyruvate decreased both the overshoot of NAD(P)H transients and the basal NAD(P)H fluorescence. However, the maximal baseline fluorescence fluctuations did not exceed 200 arbitrary units in long-lasting (>2 hours) recordings, representing ∼10% of the NAD(P)H fluorescence at the beginning of experiments. Thus, the maximal error that might be introduced by ΔF/F evaluation could not exceed 10% of the obtained values.
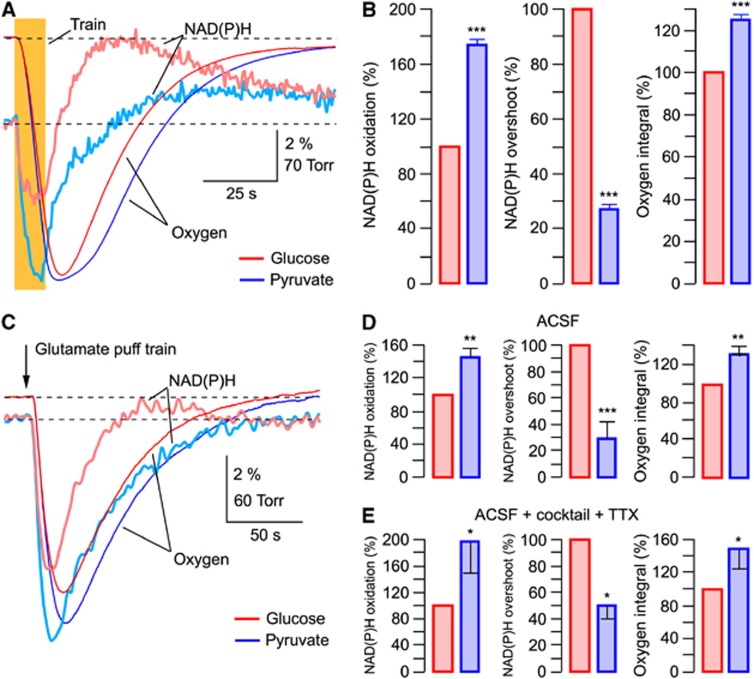
Figure 5A shows the original traces from an experiment in which recordings have been performed on the same slice and under similar conditions initially in glucose-ACSF followed by those in pyruvate-ACSF. Train stimulation of Shaffer collaterals resulted in radically different NAD(P)H responses in the presence of either glucose or pyruvate: in pyruvate, the oxidation phase became considerably larger (by 62±5% (from −3.31±0.11% to −5.33±0.2% amplitude)) while the overshoot strongly decreased (by 72±3% (from 4.13±0.41% to 1.15±0.15% amplitude)). Similar changes were observed in all 24 experiments (Figure 5B; P<0.001) and did not depend on the stimulation intensity (Supplementary Figure 3).
Figure 5.
Overshoot in NAD(P)H transients mainly reflects the glycolysis-generated NAD(P)H. (A) Replacement of glucose (5 mmol/L, red) with pyruvate (10 mmol/L, blue) in artificial cerebrospinal fluid (ACSF) results in robust changes in the NAD(P)H response to synaptic stimulation and in the increased oxygen consumption. (B) Summary of similar experiments. Responses are normalized to the amplitudes in glucose-ACSF. (C) Similar recordings as in (A) but with the glutamate (10 mmol/L) puff (10 ms) train stimulation (10 Hz, 1 second). (D) Summary of experiments as in panel D. (E) Summary of similar to (D) experiments but with cocktail+TTX added to ACSF to reveal the astrocytic fraction of responses. Cocktail of blockers consists of NBQX (10 μmol/L), AP-5 (40 μmol/L), gabazine (10 μmol/L), and E4CPG (500 μmol/L). *P<0.05, **P<0.01, ***P<0.001.
When NAD(P)H profiles are aligned with pO2 traces (Supplementary Figure 4) it is evident that in glucose-ACSF, NAD(P)H fluorescence decreases to its minimum and starts to recover while oxygen consumption (d(pO2)/dt) is at its maximal, i.e., before (∼10 seconds in Supplementary Figure 4) pO2 reaches its minimum. In contrast, in pyruvate-ACSF when glycolysis is downregulated but mitochondria still have their primary energy substrate, the NAD(P)H oxidation dip is much larger, the NAD(P)H recovery is much slower and the overshoot is relatively small. The time course of NAD(P)H recovery, as estimated by a single-exponential approximation, showed that in pyruvate-ACSF it was by 178±39% slower than in glucose-ACSF (characteristic time 21.8±6.99 seconds vs. 7.7±1.29 seconds, respectively; n=15; P<0.001). Therefore, a strongly biphasic autofluorescence response in glucose-ACSF is likely due to a significant contribution of glycolytic NAD(P)H generation to autofluorescence. This process also coincides with the mitochondrial NADH oxidation and recovery.
It is important to note that the oxygen consumption after synaptic activation was always significantly larger in pyruvate-ACSF (by 25±4%) pointing to a more active oxidative phosphorylation compared with glucose-ACSF. This suggests that in pyruvate-ACSF, mitochondrial metabolism is enhanced to compensate for a significant part of energy needs which are normally covered by the glycolysis-produced ATP and NADH in glucose-ACSF (see Discussion).
We also performed analogous experiments using the glutamate puff train stimulation (Figures 5C to 5E). After the exchange of glucose-ACSF for pyruvate-ACSF, NAD(P)H transients were modified in a similar way as with electrical stimulation (Figure 5C): the oxidation phase strongly increased (by 45±13%, n=5; from −2.6±0.65% to −3.8±1.05%) while the overshoot decreased (by 70±13% from 0.58±0.07% to 0.16±0.05%). The oxygen consumption was always larger in pyruvate than in glucose as well (by 30±9%). Figure 5D shows a summary of these experiments. The exchange of glucose-ACSF for pyruvate-ACSF induced similar effects on ‘astrocytic' responses revealed by supplementing cocktail+TTX to the perfusate: in pyruvate, NAD(P)H oxidation phase increased by 98±45%, n=4 (from −1.27±0.5% to −1.9±0.54%); NAD(P)H overshoot decreased by 49±10% (from 0.35±0.05% to 0.18±0.05%); and oxygen consumption increased by 47±24% (Figure 5E).
Altogether, these results indicate that a substantial part of NAD(P)H overshoot in both neurons and astrocytes is likely related to glycolysis. An alternative possibility is that the mitochondrial function strongly differs in glucose- and pyruvate-containing ACSF. We addressed this issue in detail in the following experiments.
Potential Caveats in Exchange of Glucose to Pyruvate
Extracellular pyruvate as well as lactate are transported into cells by the monocarboxylate transporters in conjunction with H+26 and can therefore potentially induce intracellular acidification. This in turn may modulate a number of cellular processes including mitochondrial function.27 We therefore measured the change in neuronal pHi after the replacement of 10 mmol/L glucose with 10 mmol/L pyruvate or lactate in ACSF (Figure 6D). In CA1 pyramidal neurons, during the glucose–pyruvate transition, the histogram peaks at ΔpHi=0.05 (n=115), while during the glucose–lactate transition the peak is at ΔpHi=0.01 (n=66). Interestingly, in the presence of both pyruvate and lactate, the initial acidification was followed by a gradual (10 to 20 minutes) recovery of pHi to the basal level (data not shown). The absence of significant changes of pHi in pyruvate- or lactate-ACSF might be the result of activation of compensatory mechanisms, which may be energy dependent and thus utilize a part of available oxygen. Gradual recovery of pHi in the presence of 20 mmol/L lactate has been also observed previously in cultured astrocytes and cultured or isolated neurons.28, 29 We conclude that addition of neither pyruvate nor lactate evokes a significant intracellular acidification that could affect the interpretation of our results.
Figure 6.
Coupling between cytosolic and mitochondrial redox states. (A) NAD(P)H and FAD transients in response to synaptic stimulation in 5 mmol/L glucose-artificial cerebrospinal fluid (ACSF) (red) and 10 mmol/L pyruvate-ACSF (blue). Black bar indicates stimulation. (B) Summary of similar experiments. Responses are normalized to the amplitudes in glucose-ACSF. (C) Averaged (n=6) transients recorded consequently in 5 mmol/L glucose-ACSF (red), 10 mmol/L pyruvate-ACSF (blue), and 10 mmol/L lactate-ACSF (green). In each experiment, signals were normalized to the overshoot (undershoot) amplitude in glucose-ACSF. (D) Histograms of changes (maximal values) in neuronal pHi after replacement of glucose in ACSF with pyruvate or lactate. ***P<0.001.
One more factor that could affect metabolic parameters after the exchange of energy substrates might be an increase in intracellular Ca2+ concentration.30 However, measurements of [Ca2+]i in CA1 pyramidal cells induced by stimulation of Shaffer collaterals (10 Hz, 10 seconds) revealed no significant difference between values recorded in glucose-ACSF and pyruvate-ACSF (Supplementary Figure 5A).
In addition, glutathione is the most abundant mammalian antioxidant. It is likely that in the absence of glucose, the cytosolic glutathione-based antioxidant system is inhibited and that may lead to the accumulation of reactive oxygen species. Therefore, we tested effects of an antioxidant (TEMPOL) on NADH/FAD transients. Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-1-oxyl) is a member of family of nitroxide compounds that has been studied extensively in animal models of increased reactive oxygen species and is one of the most effective antioxidants.31 In three experiments, addition of TEMPOL (2 mmol/L) to glucose- and pyruvate-ACSF did not induce significant changes in pyruvate effects on NADH/FAD autofluorescence (Supplementary Figure 5B).
The results from these experiments make unlikely the assumption that the changes in pHi, Ca2+i, and reactive oxygen species during energy substrate substitution in ACSF are the possible reasons for the observed modifications in NADH/FAD autofluorescence.
Glycolysis Affects the Mitochondrial Redox State
To further evaluate the influence of ACSF glucose-to-pyruvate transition on mitochondrial metabolism, we measured FAD autofluorescence that has been frequently used as a selective marker of mitochondrial redox state.5, 6 As was the case with NAD(P)H (Figure 4A), the FAD baseline fluorescence decreased when pyruvate was either supplemented to or completely replaced glucose in ACSF (−13.3±4.2%, n=3; Supplementary Figures 6A and B, left).
As has been observed previously,8, 19 FAD transients were inverted in respect to NAD(P)H responses and possessed a prominent undershoot, although in our experiments NAD(P)H and FAD signals clearly did not mirror each other and displayed significantly different dynamics (Figure 6A). Surprisingly, an exchange of glucose in ACSF for pyruvate resulted in a strong modification of not only NAD(P)H but also FAD transients with an almost complete disappearance of the latter's undershoot phase. Similar results were obtained in 14 experiments (Figure 6B): in pyruvate, FAD peak did not change (P>0.3) while FAD undershoot decreased by 72±5% (from −6.29±0.7% to −1.77±0.31%). Thus, in spite of the same primary energy substrate (pyruvate) for the mitochondrial functioning, mitochondrial FAD fluorescence differs drastically in glucose- and pyruvate-ACSF. This type of strong redox coupling between cytosol and mitochondria has not been observed previously and therefore requires a hypothesis of its origin. One previously reported process that could fit our observations was a glycerol-3-phosphate shuttle32 which transfers the reducing equivalents from cytosolic NADH to mitochondrial FAD leading to the FAD–FADH2 transition (see Discussion). In light of this shuttle activity, the decrease in FADH2 (smaller FAD undershoot) in pyruvate-ACSF could be explained by the lack of glycolytic NADH production leading to the downregulation of glycerol-3-phosphate shuttle activity. To reinforce the proposition of operational NADH-FAD shuttle, we switched to lactate as a primary energy substrate (lactate-ACSF), using a rationale that the transition from lactate to pyruvate by lactate dehydrogenase is associated with generation of cytosolic NADH in neurons and astrocytes; compared with pyruvate's effects, this should lead to an increase in both NADH overshoot and FAD undershoot. Indeed, lactate substitution resulted in such effects in our experiments: the NAD(P)H overshoot/FAD undershoot were larger than those in pyruvate but still smaller than those seen in glucose-ACSF. Figure 6C shows the NAD(P)H and FAD response average from six experiments (in each experiment, signals were normalized to the overshoot (undershoot) amplitude in glucose-ACSF). Fluorescent transients obtained in lactate-ACSF are thus situated (in respect to both the amplitudes and dynamics) in between those of glucose- and pyruvate-ACSF.
Clearly, direct verification of the glycerol-3-phosphate shuttle involvement in glycolysis–mitochondrial interactions could be provided by a shuttle blockade. Unfortunately, the pharmacological tools for specific inhibition of the glycerol-3-phosphate shuttle are not yet available (McKenna et al32; but see Kao et al33). Therefore, detailed investigation of glycerol-3-phosphate shuttle activity in brain cells is a matter for future studies.
Discussion
Metabolic Processes in Neurons and Astrocytes
Network activation stimulates energy metabolism in both neurons and astrocytes. The relative contribution of different cell types to the energy utilization is of interest since the energy use of astrocytes has often been ignored. To separate the neuronal and astrocytic fractions of the metabolic process, we utilized a pharmacological dissection by applying a cocktail of postsynaptic receptor antagonists, blocking neuronal activation. In the case of synaptic stimulation such a blockade was not complete as the remaining responses still contained a presynaptic neuronal fraction. An alternative stimulation by direct application of glutamate provided more evident results since here the presynaptic neuronal fraction could be eliminated by TTX. Separation of neuronal and astrocytic responses reveals their qualitative similarity: NAD(P)H transients in both cell types possess pronounced dip and overshoot (Figures 2 and 3). During synaptic stimulation, however, the relative contribution of oxidation phase (dip) was larger in astrocytes compared with neurons (see Figure 2B). Importantly, the observed NADH dip and overshoot do not directly reflect the actual amount of NADH utilized and generated and therefore cannot be used for quantitative estimation of energy production.9 These signals may indicate, however, the relative contribution of oxidative phosphorylation and glycolysis (see below) to the overall process. In contrast, oxygen consumption is a direct correlate of oxidative phosphorylation and mitochondrial ATP generation. When induced by synaptic stimulation, astrocytic activity constitutes a notable fraction of total oxygen utilization (at least ∼15% Figure 2B), suggesting that glial energy demands during synaptic stimulation are significant and should not be ignored when estimating the energy consumption by different network components.
Contribution of Glycolysis to the NAD(P)H Overshoot
The origin of NAD(P)H overshoot is still the matter of debates. Two opposing points of view associate the overshoot either with glycolytic activity7, 20, 21 or primarily with mitochondrial one.8 The contradictory results of these studies may be explained in part by the experimental confines characteristic for slice preparations. Experimental observations that in vivo the overshoot is much less pronounced than that in most in vitro studies11, 12 imply a possible dissociation between the oxygen supply and the mitochondrial processing due to oxygenation limitations in slices.10, 34 Indeed, the overshoot value was correlated with the O2 tension in slice tissue, increasing with lower O2 levels.20 Unfortunately, the importance of a parameter as vital for energy metabolism as oxygen tension was ignored in some studies, rendering the conclusions reached therein questionable. In addition, in some other reports the definition of ‘physiologic oxygen levels' was automatically applied to slice preparations while slices, compared with the in vivo tissue, possess strongly modified characteristics related to energy metabolism, including their oxygen consumption.18, 35 In our experiments, we used a high rate of slice superfusion18, 21 that provided sufficient slice oxygenation manifested by a similar waveshape of NAD(P)H transients throughout the slice depth (Figure 1). Note, however, that even at such conditions the rate of oxygen consumption was lower than in vivo as we showed previously.18
To uncover the purely mitochondrial fraction of NAD(P)H signaling during synaptic stimulation, we exchanged ACSF glucose to pyruvate. This resulted in a significant decline in both NAD(P)H and FAD basal fluorescence (Figure 4A; Supplementary Figure 6A). Therefore, the mitochondrial reduction (FADH2) increased in contrast with a decreased amount of NAD(P)H.
This result cannot be explained if the mitochondrial citric acid cycle is assumed to be the major source for autofluorescence—strongly suggesting that the absence of glycolytic NAD(P)H generation underlies our observations.
Considerable overshoot reduction during stimulation in the presence of pyruvate has been reported in both early21 and recent studies8, 18, 20 where supplementing glucose in ACSF with pyruvate was used for revealing the glycolytic NAD(P)H production. The effects of pyruvate became more prominent after a complete replacement of glucose in ACSF that resulted in: (1) a robust increase in the oxidation phase; (2) considerable slowing down of NAD(P)H recovery to baseline; (3) a strong overshoot decrease; (4) a significant enhancement (by ∼25%) of oxygen consumption. The effects (1) and (4) indicate the augmentation of mitochondrial functioning at similar energy demands and therefore suggest that in glucose-ACSF a part of energy requirements during network activity is covered by the glycolysis-generated ATP. Indeed, cytosolic glycolysis produces two ATP and two NADH. If reducing equivalents from these cytosolic NADH are transferred to mitochondrial NAD (or FAD) by the malate–aspartate (and, presumably, glycerol-phosphate shuttle) this produces two mitochondrial NADH (or FADH2) resulting in five (or three) ATP after oxidation in the respiratory chain. Therefore, glycolysis may potentially provide 5 to 7 ATPs that represents 17% to 22% of the total ATP yield (30 to 32 ATPs) per one glucose molecule. This is consistent with the observed rise in oxygen consumption after glucose exchange for pyruvate. A much faster NAD(P)H recovery and larger overshoot in glucose-ACSF indicate a significant contribution of glycolysis-produced NAD(P)H to the total autofluorescence response.
We observed similar effects of pyruvate using the alternative stimulation protocols, i.e., the glutamate puff trains (Figure 5C). Compared with glucose-ACSF, glutamate puff trains in pyruvate-ACSF induced the increase in NAD(P)H oxidation phase, enhancement of oxygen consumption and a decrease in NAD(P)H overshoot. Interestingly, pyruvate affected both the neuronal and astrocytic metabolic parameters in the same manner (Figure 5E).
Altogether, our results suggest that the NAD(P)H overshoot originates mostly from the glycolytic processes in both neurons and astrocytes that proceed in parallel with mitochondrial metabolism and provide a part of energy for covering cellular demands during network activation.
Astrocyte-Released Lactate Is Unlikely a Preferred Neuronal Fuel in Slices
The ‘lactate shuttle' hypothesis suggests lactate as a preferred energy fuel for neurons during network activation.14 In such a scenario in slices, neurons should cover their energy needs mainly by using lactate supplied by astrocytes. However, our observations of a robust exogenous pyruvate and lactate action on cell metabolic parameters contradict such a hypothesis. It is possible to suggest that the effects of lactate and pyruvate in our experiments result from the endogenous lactate washout from a slice tissue due to a high rate of perfusion (15 mL/min). This explanation is not valid due to at least three following reasons: (1) since our slices survived well for many hours in glucose-ACSF and showed electrical activity similar to that in pyruvate- or lactate-ACSF, that would mean that glucose was utilized efficiently by cells in the absence of lactate; (2) in studies that used the interface chambers with a very slow ACSF superfusion rate (1 to 2 mL/min) and high level of oxygenation,20, 36 the NAD(P)H waveform in standard ACSF is very similar to that we observed in our experiments in glucose-ACSF; (3) we have shown previously (Ivanov and Zilberter18; see also Figure 4 and Supplementary Figure 7) that in our conditions, not only a complete exchange by but also supplementation of glucose in ACSF with pyruvate or lactate results in a significant reduction in NAD(P)H overshoot.
Therefore, the observation that in slices, exogenous lactate strongly modifies the process of neuronal energy metabolism contradicts the assumption of a preferential neuronal utilization of astrocyte-released lactate. This does not imply, however, that glucose utilization only by itself is optimal for the neuronal functioning. We have shown previously that in both neonatal and adult slices, supplementing glucose in ACSF with pyruvate or lactate improves synaptic function.18, 37 In vivo, the interstitial fluid in neocortex and hippocampus contains ∼1 to 2 mmol/L of basal glucose and similar concentrations of lactate (for review, Zilberter et al38), and therefore cells are potentially able to utilize a combination of both substrates. It is also possible that in vivo, under specific physiologic conditions, the neuronal metabolism may be shifted toward lactate utilization.2
Redox Coupling Between Cytosol and Mitochondria: A Possible Involvement of the Glycerol-Phosphate Shuttle
During glucose utilization for energy generation, NADH produced by glycolysis in cytosol must be reoxidized to NAD+. Since NADH generated by glycolysis cannot penetrate the mitochondrial membrane but should be regenerated to NAD+, shuttling of reducing equivalents from the cytoplasm into mitochondria is necessary for both glycolytic pyruvate formation and lactate transformation to pyruvate. Two main intracellular redox shuttle systems, the glycerol-3-phosphate shuttle and malate–aspartate shuttle, have been described in the brain.32 The malate–aspartate shuttle is thought to be the major redox shuttle for regeneration of cytosolic NAD+.32 It transfers the reducing equivalents from cytosolic NADH to mitochondrial NAD+, forming NADH. Opposite to the malate–aspartate shuttle, the glycerol-3-phosphate shuttle transfers the reducing equivalents from cytosolic NADH to mitochondrial FAD leading to the FAD–FADH2 transition. The malate–aspartate is considered as the most important shuttle in brain while the functioning of glycerol-phosphate shuttle has been poorly investigated—although several studies on cultured astrocytes and cerebellar granule cells provided evidence of the shuttle activity in these cells.32 It has been reported recently that all components of the glycerol-3-phosphate shuttle are expressed in both neurons and astrocytes and suggested that the shuttle should be operational in both cell types.39 Moreover, it was reported that Ca2+ entry into mitochondria during cell activation inhibited the malate–aspartate shuttle and suggested that the decrease in reducing equivalents' transfer may be compensated by the glycerol-phosphate shuttle activity.40 Our experiments reveal a strong coupling between the glycolysis-generated NADH and mitochondrial FAD that may be relevant to the activity of glycerol-phosphate shuttle.
In conclusion, our results show that during periods of network activity in slices from mature mice, both neurons and astrocytes are significant consumers of energy produced in parallel by glycolysis and mitochondrial oxidative phosphorylation, and that glucose is an efficient fuel for these processes.
Acknowledgments
The authors thank Dr P Bregestovski for valuable discussion.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This study was supported by The French National Research Agency ‘METANEX' grant (ANR-2010-BLAN-1443-01) and the Alzheimer's Association research grant NESAD-12-242486.
Supplementary Material
References
- Schousboe A, Sickmann HM, Bak LK, Schousboe I, Jajo FS, Faek SA, et al. Neuron-glia interactions in glutamatergic neurotransmission: roles of oxidative and glycolytic adenosine triphosphate as energy source. J Neurosci Res. 2011;89:1926–1934. doi: 10.1002/jnr.22746. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Fueling and imaging brain activation. ASN Neuro. 2012;4:267–361. doi: 10.1042/AN20120021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L. Astrocytic energy metabolism and glutamate formation—relevance for 13C-NMR spectroscopy and importance of cytosolic/mitochondrial trafficking. Magn Reson Imaging. 2011;29:1319–1329. doi: 10.1016/j.mri.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Harris JJ, Jolivet R, Attwell D. Synaptic energy use and supply. Neuron. 2012;75:762–777. doi: 10.1016/j.neuron.2012.08.019. [DOI] [PubMed] [Google Scholar]
- Scholz R, Thurman RG, Williamson JR, Chance B, Bucher T. Flavin and pyridine nucleotide oxidation-reduction changes in perfused rat liver. I. Anoxia and subcellular localization of fluorescent flavoproteins. J Biol Chem. 1969;244:2317–2324. [PubMed] [Google Scholar]
- Shuttleworth CW. Use of NAD(P)H and flavoprotein autofluorescence transients to probe neuron and astrocyte responses to synaptic activation. Neurochem Int. 2010;56:379–386. doi: 10.1016/j.neuint.2009.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasischke KA, Vishwasrao HD, Fisher PJ, Zipfel WR, Webb WW. Neural activity triggers neuronal oxidative metabolism followed by astrocytic glycolysis. Science. 2004;305:99–103. doi: 10.1126/science.1096485. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Connor JA, Shuttleworth CW. NAD(P)H fluorescence transients after synaptic activity in brain slices: predominant role of mitochondrial function. J Cereb Blood Flow Metab. 2006;26:1389–1406. doi: 10.1038/sj.jcbfm.9600292. [DOI] [PubMed] [Google Scholar]
- Hall CN, Klein-Flugge MC, Howarth C, Attwell D. Oxidative phosphorylation, not glycolysis, powers presynaptic and postsynaptic mechanisms underlying brain information processing. J Neurosci. 2012;32:8940–8951. doi: 10.1523/JNEUROSCI.0026-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galeffi F, Somjen GG, Foster KA, Turner DA. Simultaneous monitoring of tissue PO(2) and NADH fluorescence during synaptic stimulation and spreading depression reveals a transient dissociation between oxygen utilization and mitochondrial redox state in rat hippocampal slices. J Cereb Blood Flow Metab. 2011;31:626–639. doi: 10.1038/jcbfm.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaManna JC, Light AI, Peretsman SJ, Rosenthal M. Oxygen insufficiency during hypoxic hypoxia in rat brain cortex. Brain Res. 1984;293:313–318. doi: 10.1016/0006-8993(84)91238-1. [DOI] [PubMed] [Google Scholar]
- Mayevsky A, Chance B. Oxidation-reduction states of NADH in vivo: from animals to clinical use. Mitochondrion. 2007;7:330–339. doi: 10.1016/j.mito.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Amaral AI. Effects of hypoglycaemia on neuronal metabolism in the adult brain: role of alternative substrates to glucose. J Inherit Metab Dis. 2012;36:621–634. doi: 10.1007/s10545-012-9553-3. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Magistretti PJ. Sweet sixteen for ANLS. J Cereb Blood Flow Metab. 2011;32:1152–1166. doi: 10.1038/jcbfm.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokoloff L. The physiological and biochemical bases of functional brain imaging. Cogn Neurodyn. 2008;2:1–5. doi: 10.1007/s11571-007-9033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Dienel GA. Brain lactate metabolism: the discoveries and the controversies. J Cereb Blood Flow Metab. 2012;32:1107–1138. doi: 10.1038/jcbfm.2011.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Zilberter Y. Critical state of energy metabolism in brain slices: the principal role of oxygen delivery and energy substrates in shaping neuronal activity. Front Neuroenergetics. 2011;3:9. doi: 10.3389/fnene.2011.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huchzermeyer C, Albus K, Gabriel HJ, Otahal J, Taubenberger N, Heinemann U, et al. Gamma oscillations and spontaneous network activity in the hippocampus are highly sensitive to decreases in pO2 and concomitant changes in mitochondrial redox state. J Neurosci. 2008;28:1153–1162. doi: 10.1523/JNEUROSCI.4105-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster KA, Beaver CJ, Turner DA. Interaction between tissue oxygen tension and NADH imaging during synaptic stimulation and hypoxia in rat hippocampal slices. Neuroscience. 2005;132:645–657. doi: 10.1016/j.neuroscience.2005.01.040. [DOI] [PubMed] [Google Scholar]
- Lipton P. Effects of membrane depolarization on nicotinamide nucleotide fluorescence in brain slices. Biochem J. 1973;136:999–1009. doi: 10.1042/bj1360999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajos N, Ellender TJ, Zemankovics R, Mann EO, Exley R, Cragg SJ, et al. Maintaining network activity in submerged hippocampal slices: importance of oxygen supply. Eur J Neurosci. 2009;29:319–327. doi: 10.1111/j.1460-9568.2008.06577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada S, Iino M, Takayasu Y, Shimamoto K, Ozawa S. Effects of a novel glutamate transporter blocker, (2S, 3S)-3-[3-[4-(trifluoromethyl)benzoylamino]benzyloxy]aspartate (TFB-TBOA), on activities of hippocampal neurons. Neuropharmacology. 2005;48:479–491. doi: 10.1016/j.neuropharm.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Cox DW, Bachelard HS. Partial attenuation of dentate granule cell evoked activity by the alternative substrates, lactate and pyruvate: evidence for a postsynaptic action. Exp Brain Res. 1988;69:368–372. doi: 10.1007/BF00247581. [DOI] [PubMed] [Google Scholar]
- Zhao YT, Tekkok S, Krnjevic K. 2-Deoxy-D-glucose-induced changes in membrane potential, input resistance, and excitatory postsynaptic potentials of CA1 hippocampal neurons. Can J Physiol Pharmacol. 1997;75:368–374. [PubMed] [Google Scholar]
- Halestrap AP. The SLC16 gene family—structure, role and regulation in health and disease. Mol Aspects Med. 2013;34:337–349. doi: 10.1016/j.mam.2012.05.003. [DOI] [PubMed] [Google Scholar]
- Obara M, Szeliga M, Albrecht J. Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem Int. 2008;52:905–919. doi: 10.1016/j.neuint.2007.10.015. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Goldman SA. Carrier-mediated transport of lactic acid in cultured neurons and astrocytes. Am J Physiol. 1993;265:R282–R289. doi: 10.1152/ajpregu.1993.265.2.R282. [DOI] [PubMed] [Google Scholar]
- Svichar N, Chesler M. Surface carbonic anhydrase activity on astrocytes and neurons facilitates lactate transport. Glia. 2003;41:415–419. doi: 10.1002/glia.10187. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Duchen MR. Mitochondria: the hub of cellular Ca2+ signaling. Physiology (Bethesda) 2008;23:84–94. doi: 10.1152/physiol.00046.2007. [DOI] [PubMed] [Google Scholar]
- Wilcox CS. Effects of tempol and redox-cycling nitroxides in models of oxidative stress. Pharmacol Ther. 2010;126:119–145. doi: 10.1016/j.pharmthera.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MC, Waagepetersen HS, Schousboe A, Sonnewald U. Neuronal and astrocytic shuttle mechanisms for cytosolic-mitochondrial transfer of reducing equivalents: current evidence and pharmacological tools. Biochem Pharmacol. 2006;71:399–407. doi: 10.1016/j.bcp.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Kao CC, Wu BT, Tsuei YW, Shih LJ, Kuo YL, Kao YH. Green tea catechins: inhibitors of glycerol-3-phosphate dehydrogenase. Planta Med. 2010;76:694–696. doi: 10.1055/s-0029-1240623. [DOI] [PubMed] [Google Scholar]
- Turner DA, Foster KA, Galeffi F, Somjen GG. Differences in O2 availability resolve the apparent discrepancies in metabolic intrinsic optical signals in vivo and in vitro. Trends Neurosci. 2007;30:390–398. doi: 10.1016/j.tins.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Nedden S, Hawley S, Pentland N, Hardie DG, Doney AS, Frenguelli BG. Intracellular ATP influences synaptic plasticity in area CA1 of rat hippocampus via metabolism to adenosine and activity-dependent activation of adenosine A1 receptors. J Neurosci. 2011;31:6221–6234. doi: 10.1523/JNEUROSCI.4039-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadgrove MP, Beaver CJ, Turner DA. Effects of relative hypoglycemia on LTP and NADH imaging in rat hippocampal slices. Brain Res. 2007;1165:30–39. doi: 10.1016/j.brainres.2007.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov A, Mukhtarov M, Bregestovski P, Zilberter Y. Lactate effectively covers energy demands during neuronal network activity in neonatal hippocampal slices. Front Neuroenergetics. 2011;3:1–14. doi: 10.3389/fnene.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y, Zilberter T, Bregestovski P. Neuronal activity in vitro and the in vivo reality: the role of energy homeostasis. Trends Pharmacol Sci. 2010;31:394–401. doi: 10.1016/j.tips.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Sonnewald U, Waagepetersen HS, Schousboe A, He W, Lin JH, et al. The transcriptome and metabolic gene signature of protoplasmic astrocytes in the adult murine cortex. J Neurosci. 2007;27:12255–12266. doi: 10.1523/JNEUROSCI.3404-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras L, Satrustegui J. Calcium signaling in brain mitochondria: interplay of malate aspartate NADH shuttle and calcium uniporter/mitochondrial dehydrogenase pathways. J Biol Chem. 2009;284:7091–7099. doi: 10.1074/jbc.M808066200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.