Abstract
Acute-phase proteins (APPs) are key effectors of the immune response and are routinely used as biomarkers in cerebrovascular diseases, but their role during brain inflammation remains largely unknown. Elevated circulating levels of the acute-phase protein pentraxin-3 (PTX3) are associated with worse outcome in stroke patients. Here we show that PTX3 is expressed in neurons and glia in response to cerebral ischemia, and that the proinflammatory cytokine interleukin-1 (IL-1) is a key driver of PTX3 expression in the brain after experimental stroke. Gene deletion of PTX3 had no significant effects on acute ischemic brain injury. In contrast, the absence of PTX3 strongly compromised blood–brain barrier integrity and resolution of brain edema during recovery after ischemic injury. Compromised resolution of brain edema in PTX3-deficient mice was associated with impaired glial scar formation and alterations in scar-associated extracellular matrix production. Our results suggest that PTX3 expression induced by proinflammatory signals after ischemic brain injury is a critical effector of edema resolution and glial scar formation. This highlights the potential role for inflammatory molecules in brain recovery after injury and identifies APPs, in particular PTX3, as important targets in ischemic stroke and possibly other brain inflammatory disorders.
Keywords: brain inflammation, cerebral ischemia, edema, glial scar, IL-1, pentraxin-3
Introduction
Inflammation is critically implicated in the pathogenesis of ischemic and hemorrhagic stroke, and is generally associated with poor clinical outcome.1, 2, 3, 4 A new mediator of inflammation is the acute-phase protein pentraxin-3 (PTX3), which has an emerging role in cardiovascular and cerebrovascular disorders. PTX3 has an important role in innate immunity,5 vascular inflammation,6 and extracellular matrix functionality7, 8, 9 in the periphery. Importantly, elevated PTX3 plasma levels are recognized as an independent predictor of mortality at 3 months after acute myocardial infarction,10 and is associated with the incidence of heart disease11, 12, 13 and hypoxic respiratory failure.14 Furthermore, PTX3 has been recently identified as a novel and independent prognostic marker in ischemic stroke.15 Despite the strong clinical association between plasma PTX3 levels and vascular disease, no studies have addressed whether PTX3 is expressed in the brain after stroke and whether PTX3 contributes to stroke pathology.
Inflammation in the brain after stroke is critically regulated by the cytokine interleukin-1 (IL-1); IL-1 expression increases early after ischemic injury contributing to neurotoxicity,16 and early inhibition of IL-1 actions by the naturally occurring IL-1 receptor antagonist (IL-1Ra) markedly reduces brain damage induced by experimental ischemia.17 We and others have shown that PTX3 expression can be induced by treatment with IL-118, 19 but whether this occurs during cerebral ischemia is unknown. While detrimental actions mediated by IL-1 in the early phase of the central inflammatory response are well characterized, the role of IL-1 during the later stages of inflammation in recovery and brain repair is completely unknown. Recent research suggests, however, that inflammation could have some beneficial effects when repair mechanisms are initiated,20 although mechanisms by which inflammation mediates repair are not well understood.
We demonstrate here that IL-1 induces PTX3 expression in the brain after cerebral ischemia and that in addition to its well recognized role in the periphery, PTX3 has unique actions in the brain mediating the formation of the glial scar and resolution of brain edema. Thus, we identify a link between proinflammatory factors and brain repair after cerebral ischemia and show that PTX3 is a key mediator of this process.
Materials and Methods
Animals
C57BL/6 wild-type (WT) mice were supplied by Harlan Olac (UK), IL-1α/β double knockout (KO) and IL-1β KO mice were provided by Prof Yoichiro Iwakura (University of Tokyo, Japan), and PTX3 KO mice were provided by Dr Cecilia Garlanda (Humanitas Clinical and Research Center, Rozzano, Italy). All mice were on C57BL/6 background and were bred in-house. Age (12 to 20-week-old) and weight-matched male littermates were used for all experiments. Pentraxin-3 KO mice were bred as heterozygous and litter genotyping was performed as described previously.21 (A sample of genotyping is included in Supplementary Figure 1). Levels of plasma PTX3 measured in a representative group of WT and PTX3 KO mice confirmed that PTX3 was not expressed in PTX3 KO mice (see Supplementary Figure 2).
Animals were maintained at 21±1°C, 55±10% humidity, in a 12 hour light–dark cycle with free access to food and water. All animal procedures were performed under the Home office (UK) project license number (40/3076), and were carried out in accordance with STAIR and ARRIVE guidelines, the European Council directives (86/609/EEC), and the Animal Scientific Procedures Act (UK) 1986.
Cerebral Ischemia Induced by Transient Middle Cerebral Artery Occlusion
Transient middle cerebral artery occlusion (MCAo) was performed using the intraluminal filament method as described previously.22 To investigate PTX3 expression in the brain and periphery after MCAo, the occlusion time was 45 minutes, as this length of occlusion in our hands ensures the development of a severe striatal and cortical infarct. To study the role of PTX3 in brain injury and repair over time (48 hours and 6 days post MCAo), 30-minute occlusion was used, as this duration of MCAo increases long-term animal survival and is suitable to study either neuroprotection or increased brain injury in this animal model. Monitoring of cerebral blood flow within the middle cerebral artery territory was performed during the entire surgical procedure by using a laser Doppler (Moor Instruments, Devon, UK). Doppler signal showed no significant difference between WT and PTX3 KO mice before MCAo, after occlusion (77±7% and 76±5% drop in WT and PTX3 KO mice, respectively) and during reperfusion. Mice that did not show at least a 70% drop or a Doppler signal that did not recover fully within 5 minutes after reperfusion were excluded from the study (n=1). We also confirmed that absence of PTX3 is not associated with altered vascular density after 48-hour reperfusion (assessed by PECAM-1 and ICAM-1 quantification; see Supplementary Figure 3). After surgery, mice were subjected to reperfusion and allowed to recover for 24 hours, 48 hours, or 6 days. Mice were injected subcutaneously with 0.5 mL of sterile saline daily for rehydration and continuously monitored for neurologic symptoms. To assess cell proliferation, mice euthanized at 48 hours and 6 days reperfusion were intraperitoneally injected twice daily with bromodeoxyuridine (BrdU) (50 mg/kg, Sigma-Aldrich, Dorset, UK), dissolved in sterile phosphate-buffered saline.
IL-1β Administration
Mice were randomized and were injected intraperitoneally with 4 μg/kg of recombinant human IL-1β (National Institute for Biologic Standards and Control, NIBSC, Potters Bar, UK) dissolved in sterile phosphate-buffered saline containing 0.1% low-endotoxin bovine serum albumin. Four hours or 24 hours after injection, animals were transcardially perfused with 0.9% saline to remove blood contamination.
Tissue Processing
For immunohistochemistry, mice were perfused transcardially with 0.9% saline followed by 4% paraformaldehyde. Brains were removed, postfixed overnight in 20% sucrose/paraformaldehyde, and sectioned on a sledge microtome (Bright, Cambridgeshire, UK). Typically, 20 parallel series of 20 μm (for mice undergoing 24-hour or 48-hour reperfusion) or 25 μm (for mice undergoing 6 days reperfusion) coronal brain sections were cut and stored in antifreeze solution at −20°C. Cardiac blood was collected from the right ventricle before perfusion. Plasma was separated from blood cells by centrifugation at 1,700 g 4°C for 10 minute. For enzyme-linked immunosorbent assay measurements, organs were collected and tissues were homogenized as previously described.23 Protein concentrations were measured with bicinchoninic acid assay (Pierce, Thermo-Fisher Scientific, Hemel Hempstead, UK).
Enzyme-linked immunosorbent assay
Pentraxin-3 levels were measured by enzyme-linked immunosorbent assay (R&D Systems, Abingdon, UK). Absorbance was read at 450 nm and corrected at 570 nm with a plate reader (Synergy HT, BioTek, UK). The limit of detection for PTX3 was 1.35 ng/mL in plasma samples and 110 pg/mL in other samples.
Measurement of Infarct Size, Brain Edema and Blood–Brain Barrier Breakdown
Infarct size was measured using Nissl staining as described previously24 and IgG infiltration in the brain was used to assess blood–brain barrier (BBB) breakdown as reported earlier.25 Eight defined coronal levels according to Bregma were measured, and integrated to obtain the infarct and BBB damage volume. Edema was calculated as the percentage of ipsilateral hemisphere volume increased compared with the contralateral hemisphere. Infarct and BBB damage volumes were corrected for edema to avoid possible masking effects of edema as described previously.25
Immunofluorescence
Free floating brain sections were incubated in 2% normal donkey serum (Jackson laboratories, Bar Harbor, ME, USA) in primary diluent (0.3% Triton X-100 in phosphate-buffered saline) for 1 hour. Suitable combinations of primary antibodies were added and left overnight. The primary antibodies used were rat anti-PECAM-1 (1:250, BD Biosciences, Oxford, UK), goat anti-ICAM-1 (1:250, R&D Systems), goat anti-MAP-2 (1:500, Santa Cruz Biotechnology, Dallas, TX, USA), rat anti-CD45 (1:250, Serotec, Kidlington, UK), rat anti-myelin basic protein (1:1000, Abcam, Cambridge, UK), goat anti-IL-1β (1:100, R&D Systems), chicken anti-PGP9.5 (1:500, Santa Cruz Biotechnology), chicken anti-glial fibrillary acidic protein (GFAP) (1:1000, Abcam), goat anti-laminin-α 4 (1:100, Santa Cruz Biotechnology), rabbit anti-PTX3 (1:500, Abcam). A second PTX3 antibody (rabbit anti-PTX3, 1:500, kindly donated by Prof A Mantovani was used to ensure specificity of PTX3 staining. Staining was abolished by 5-minute preincubation with 45 ng/mL recombinant PTX3 (R&D Systems). Secondary antibodies were conjugated with Alexa 488 or 594 fluorochromes (Invitrogen, Paisley, UK) to visualize the antigens. Sections were mounted onto gelatin-coated slides and coverslipped with Prolong Gold antifade with or without 4′,6-diamidino-2-phenylindole (Invitrogen). Images were acquired using Olympus BX51 upright microscope and captured using a Coolsnap ES camera (Photometrics, Tucson, AZ, USA) through MetaVue Software (Molecular Devices, Sunnyvale, CA, USA). A similar protocol was used for immunocytochemical staining on cell cultures.
Image Analysis
All measurements were conducted in a masked manner in randomly selected images within corresponding regions of the ipsi- and contralateral hemispheres. Total hemispheric counts were calculated from overlapping images covering all regions of interest within the hemisphere. Glial scar integrity was estimated on GFAP-immunostained brain sections by measuring the perimeters of the damaged area and of the surrounding glial scar. The percentage of infarct area surrounded by GFAP-positive scar was calculated. ImageJ software (National Institutes of Health, USA) was used for image analysis and quantification.
Cell Cultures
Primary neuronal cell cultures were prepared from the brains of mice embryos at 14 to 16 days of gestation as described previously.26 Cultures were used for experiments at day in vitro 12. Primary mixed glial cultures were prepared from the brains of 1 to 3-day-old mice as described previously27 and grown until confluency (day in vitro 14 to day in vitro 20).
Cell Treatments for Pentraxin-3 Expression Experiments
To study PTX3 expression, neuronal, and glial cultures were treated with vehicle (0.1% low-endotoxins bovine serum albumin diluted in 0.9% NaCl), recombinant rat IL-1α or IL-1β (National Institute for Biological Standards and Control, NIBSC, UK) (0.03–30 IU/mL diluted in vehicle) in the presence or absence of IL-1Ra (NIBSC) (10 μg/mL for glia and 1 μg/mL for neurons) for 24 hours. Cultures were also treated with IL-1α or IL-1β (0.3 or 3 IU/mL) heated at 95°C for 30 minutes, N-methyl-D-aspartate (100 μmol/L and 500 μmol/L, Tocris Bioscience), CoCl2 (500 μmol/L), 2-deoxyglucose (2DG, 15 mmol/L), and H2O2 (50 μmol/L or 500 μmol/L) for 24 hour.
Glial Proliferation Assays
To study the effect of PTX3 on glial proliferation in vitro, cells were trypsinized, centrifuged, resuspended and counted, and 10,000 cells were seeded per well (in 96-well plates). Cells were kept in serum-free media for 2 hours before being treated for 48 hours with one single dose of PTX3 (0.3 ng/mL, R&D Systems), FGF2 (100 ng/mL, Invitrogen), or cytosine arabinoside (10 μmol/L, Sigma-Aldrich). Cell proliferation was assessed using a commercial BrdU cell proliferation kit (Calbiochem/Merck, Nottingham, UK). Briefly, cells were incubated with BrdU for 48 hours before 1-hour incubation with anti-BrdU antibody. Horseradish peroxidase-conjugated secondary antibody was added, and a colorimetric assay was performed. Percentage of proliferation was calculated in comparison with the control well.
Statistical Analysis
Animals were randomized for in vivo experiments and all quantitative measurements were performed in a masked manner. Data were analyzed with GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). Comparisons between two groups were made using Student's t-tests. For multiple groups comparisons, one-way analysis of variance followed by Bonferroni's (to evaluate differences between all groups) or Dunnett's (to compare other groups with a control/vehicle group) post hoc tests were used. For multiple groups and serial dilutions (NSC proliferation assays) analysis, two-way analysis of variance was used.
Results
Cerebral Ischemia Induces Pentraxin-3 Expression in the Brain
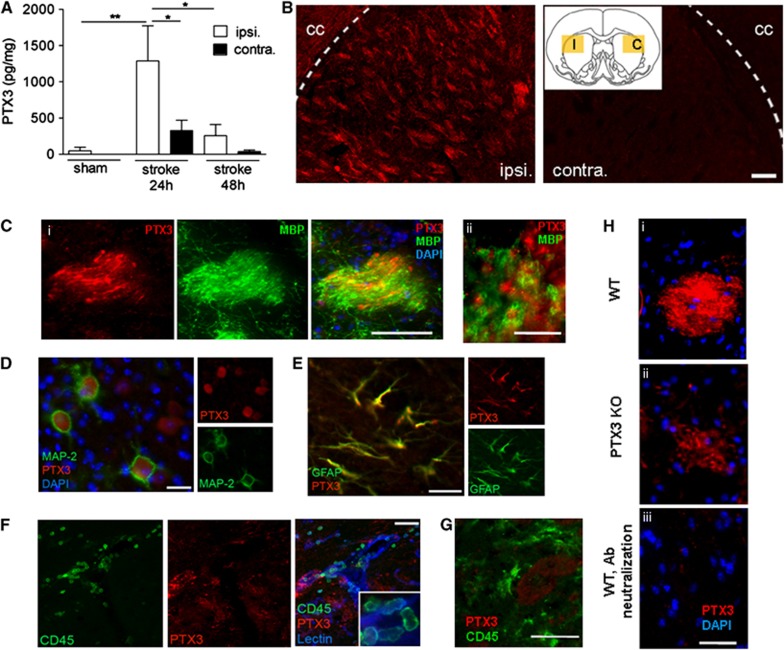
Pentraxin-3 protein levels increased significantly by 26-fold (P<0.01) in the ipsilateral hemisphere compared with the ipsilateral hemisphere of sham-operated animals, and were higher by fourfold (P<0.05) in the ipsilateral hemisphere compared with the contralateral side, 24 hours after cerebral ischemia (Figure 1A). Pentraxin-3 expression strongly declined in the ipsilateral hemisphere (by fivefold, P<0.05) but remained higher than the contralateral side, 48 hour after cerebral ischemia (Figure 1A). Immunofluorescent staining revealed expression of PTX3 in the ipsilateral, but not in the contralateral hemisphere (Figure 1B). Pentraxin-3 immunostaining was particularly intense in white matter bundles of the striatum, in fine nerve fibers surrounded by myelin basic protein-positive structures (Figure 1C). We detected PTX3 expression in MAP-2-positive neurons in the ipsilateral cortex (Figure 1D) and in GFAP-positive astrocytes within the ipsilateral corpus callosum (Figure 1E). We did not find any recruited CD45-positive leukocytes and macrophages/microglia containing PTX3 24 hours (Figures 1F and 1G, respectively), 48 hours or 6 days (Supplementary Figure 4) after MCAo. In WT mice, immunoneutralization of PTX3 antibodies with recombinant PTX3 protein fully eliminated immunostaining (Figure 1H, i and iii). However, some PTX3 KO mice still showed a pale staining (Figure 1H, ii). Residual PTX3 immunostaining detected in PTX3 KO mice might be because of a low level of cross-reactivity with other pentraxins known to be expressed in the brain.28
Figure 1.
Cerebral ischemia drives pentraxin-3 (PTX3) expression in the brain. (A) Cerebral ischemia results in PTX3 production in the ipsilateral (ipsi.) hemisphere 24 hours after experimental stroke compared with the contralateral (contra.) hemisphere and sham surgery as measured by enzyme-linked immunosorbent assay. (B) Immunohistochemistry reveals PTX3 staining in the ischemic (I, ipsi.) but not on the contralateral (C, contra.) striatum. cc, corpus callosum. Yellow rectangles in the insert show the place of the fluorescent micrographs. (C) Pentraxin-3 and myelin basic protein (MBP) staining in white matter tracts of the ipsilateral striatum (i). High magnification shows the proximity of myelin and PTX3 profiles (ii). (D, E) MAP-2-positive neurons (D) and glial fibrillary acidic protein (GFAP)-positive astrocytes (E) (in the ipsilateral cortex and cc, respectively) show PTX3 staining. (F, G) CD45-positive neutrophils recruited to the ischemic striatum (F) and macrophages (G) do not show PTX3 staining 24 hours after middle cerebral artery occlusion. (H) Striatal PTX3 staining along with 4′,6-diamidino-2-phenylindole (DAPI) in wild-type (WT) (i) and PTX3 knockout (KO) (ii) animals. Pentraxin-3 staining is abolished by PTX3 antibody neutralization (preincubation with recombinant PTX3 peptide, iii). Scale bars, 100 mm (B), 40 μm (C i) 10 μm (C ii), 20 μm (D, E), 50 μm (F), 50 μm (G), 100 μm (H). *P<0.05, **P<0.01, one-way analysis of variance followed by Bonferroni's post hoc test (n=3 to 6). Error bars show s.e.m.
Central, but not Peripheral Expression of Pentraxin-3 is Dependent on Interleukin-1 after Cerebral Ischemia
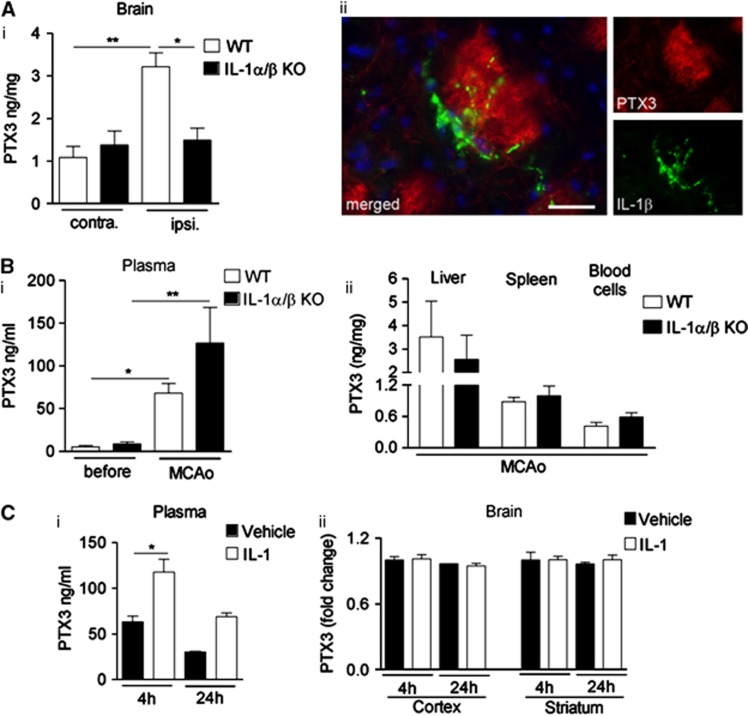
To investigate whether endogenous IL-1 drives PTX3 expression after cerebral ischemia, we measured PTX3 levels in the brain of WT or IL-1α/β KO mice after MCAo. Interleukin-1α/β KO mice showed no increase in brain PTX3 levels after MCAo compared with WT mice (Figure 2A, i), suggesting that IL-1 is essential for central PTX3 production. In WT mice, IL-1β immunostaining was observed in close proximity to PTX3-positive bundles (Figure 2A, ii). In marked contrast with the brain, plasma PTX3 levels were increased in both WT and IL-1α/β KO mice (Figure 2B, i), and similar levels of PTX3 were found in liver, spleen, and circulating blood cells in both genotypes (Figure 2B, ii). These data indicate that central, but not systemic, expression of PTX3 is dependent on IL-1 after acute brain injury. The fact that IL-1α/β KO mice have high plasma-derived but not brain-derived PTX3 after MCAo suggests that peripheral PTX3 does not substantially contribute to central PTX3 levels even after stroke.
Figure 2.
Central, but not peripheral pentraxin-3 (PTX3) expression is interleukin-1 (IL-1)-dependent after cerebral ischemia. (A) Enzyme-linked immunosorbent assay (ELISA) analyses of brain from wild type (WT) and IL-1α/β knockout (KO) mice 24 hours after middle cerebral artery occlusion (MCAo) indicates that brain PTX3 protein levels are elevated in the ipsilateral hemisphere of WT, but not of IL-1α/β KO mice (i). IL-1β-positive (green) cells are found in close vicinity of PTX3-positive structures (red) within the ipsilateral striatum (ii). (B) Plasma PTX3 increases 24 hours after MCAo in both WT and IL-1α/β KO mice (i), and PTX3 levels in liver, spleen, and blood cells do not vary between genotypes (ii), as measured by ELISA. (C). Peripheral IL-1β injection (i.p.) results in an upregulation of PTX3 in plasma (i), but not in cerebral cortex or striatum (ii). Scale bar, 25 μm. *P<0.05, **P<0.01, one-way analysis of variance followed by Bonferroni's post hoc test (A, B), n=3; (C), i n=3, ii n=2 to 3). Error bars show s.e.m.
Peripheral IL-1 injection nearly doubled plasma PTX3 levels (Figure 2C, i), with no effect on PTX3 expression in the cortex or striatum (Figure 2C, ii). These results suggest that peripheral PTX3 does not cross the BBB in the absence of brain injury.
Interleukin-1, but not Injury-Driven Stimuli, Induces Pentraxin-3 Expression in Brain Cells
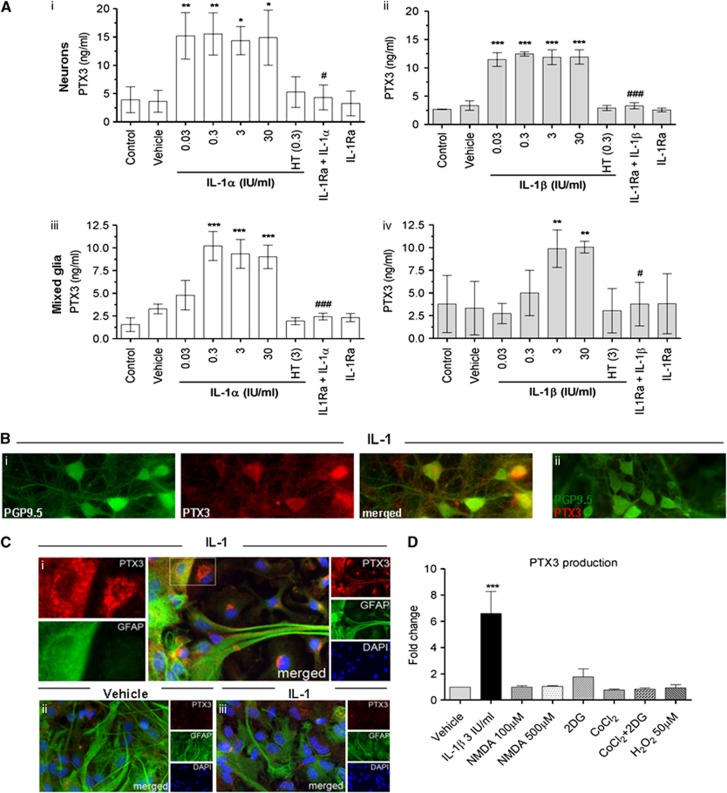
To confirm the role of IL-1 as key driver of PTX3 expression in the brain, we treated neuronal (Figure 3A, i and ii) and mixed glial (Figure 3A, iii and iv) cultures with IL-1α or IL-1β, and we measured PTX3 release. Low basal levels of PTX3 protein were detected in untreated or vehicle-treated cultures. In contrast, both IL-1 isoforms induced a marked increase in PTX3 synthesis and release, which was abrogated by heat inactivation of IL-1, or in the presence of the naturally occurring IL-1Ra, while IL-1Ra alone had no effect on PTX3 expression (Figure 3A). These findings were also confirmed by immunocytochemistry. Neurons (Figure 3B, i) and astrocytes (Figure 3C, i) showed a marked increase in PTX3 staining after treatment with IL-1 compared with basal conditions (Figure 3C, ii). Interleukin-1-induced PTX3 immunostaining was absent when the PTX3 antibody was immunoneutralized with recombinant PTX3 (Figures 3B and 3C, ii and iii).
Figure 3.
Interleukin-1 (IL-1) but not ischemic, excitotoxic, or oxidative stimuli induces PTX3 release in vitro. (A) Primary neuronal (i and ii) and mixed glial (iii and iv) cultures were treated with IL-1α or IL-1β (0.03 IU/mL to 30 IU/mL), heat treated (HT) IL-1α/IL-1β (0.3 or 3 IU/mL, respectively), IL-1Ra (10 μg/mL) plus IL-1α/IL-1β (0.3 or 3 IU/mL, respectively) or IL-1Ra alone for 24 hours, and PTX3 release was measured in the culture supernatants by enzyme-linked immunosorbent assay (ELISA). Both IL-1α (i and iii) and IL-1β (ii and iv) significantly induced release of PTX3 from neurons and mixed glial cells, which was completely inhibited by heat treatment of IL-1 or co-incubation with IL-1Ra. (B) Pentraxin-3 is expressed by cultured neurons (PGP9.5-possitive) treated with IL-1 (i). Pentraxin-3 staining is abolished by PTX3 antibody neutralization (preincubation with recombinant PTX3 peptide, ii). (C) Astrocytes (glial fibrillary acidic protein (GFAP)-positive) treated with IL-1 (i) express PTX3, whereas vehicle-treated astrocytes do not express PTX3 (ii). Pentraxin-3 staining is abolished by PTX3 antibody neutralization (iii). (D) Pentraxin-3 ELISA of supernatants of neuronal cultures treated with IL-1β (3 IU/mL), N-methyl-D-aspartate (NMDA) (100 or 500 μmol/L), CoCl2 (500 μmol/L) with or without 2-deoxyglucose (2DG) (1500 μmol/L) or H2O2 (50 μmol/L) for 24 hours. Only IL-1β induced PTX3 production in vitro. *P<0.05, **P<0.01, ***P<0.001 IL-1 treated versus vehicle. #P<0.05, ### P<0.001 IL-1+IL-1Ra versus IL-1 alone, using one-way analysis of variance and Bonferroni's (A) n=3 to 5) or Dunnett's (D) n=3) post hoc tests. Error bars show s.e.m. DAPI, 4′,6-diamidino-2-phenylindole.
We next investigated whether PTX3 expression is induced primarily by inflammatory (IL-1 dependent) mechanisms, or can be induced by other (injury dependent) stimuli present in vivo after cerebral ischemia such as excitotoxicity, hypoxia, glucose depletion, or oxidative stress.29 Pentraxin-3 (PTX3) production was not induced by treatment of neuronal cultures with excitotoxic (N-methyl-D-aspartate), hypoxic (activation of HIF-1 by CoCl2)30 stimuli, after glucose depletion with 2-deoxyglucose (2DG) or after oxidative stress (H2O2) (Figure 3D), suggesting that the main inducer of PTX3 in ischemic injury is IL-1.
Pentraxin-3 Deficiency Affects Delayed but not Acute Brain Damage after Middle Cerebral Artery Occlusion
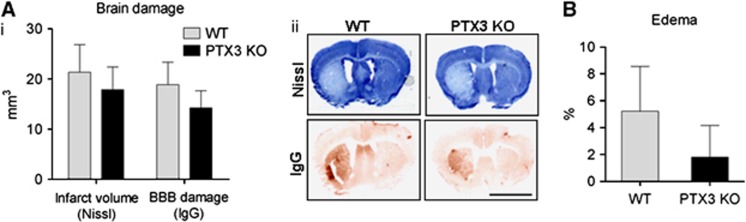
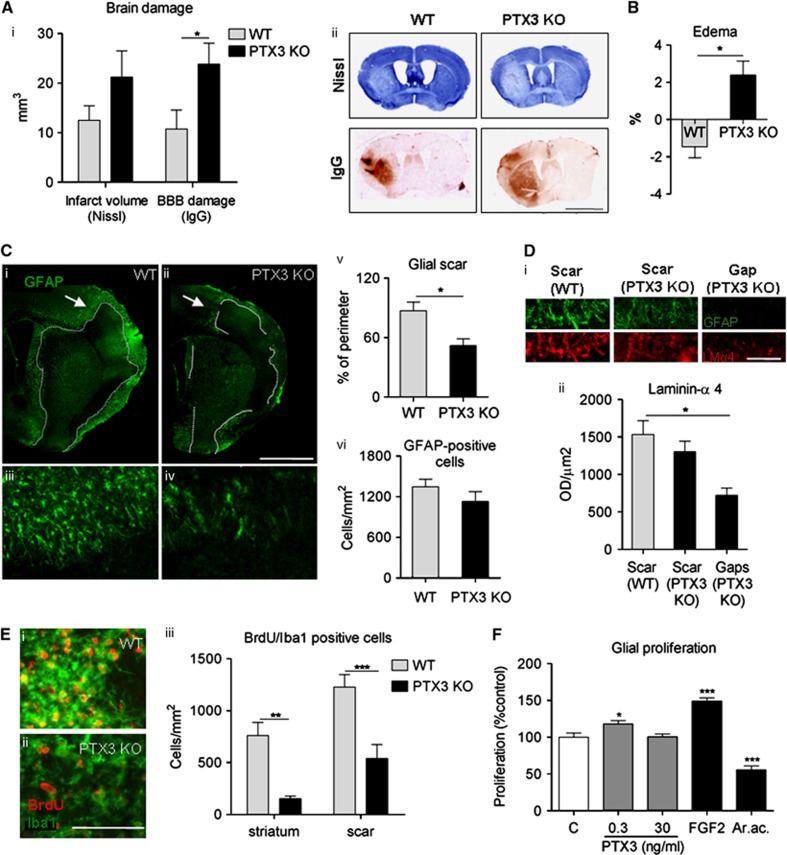
To explore whether PTX3 influences neuronal death, brain damage was assessed in WT and PTX3 KO mice early (48 hours) and late (6 days) after acute ischemic brain injury. No significant differences were found in infarct volume, BBB damage (Figure 4A), or brain edema (Figure 4B) between WT and PTX3 KO mice 48 hours after MCAo, although a trend for reduced edema (2±2% in PTX3 KO versus 5±3% in WT; mean±s.e.m.) in PTX3 KO mice was observed (Figure 4B). The absence of a significant effect may be due to the high variation. In contrast, 6 days after MCAo, BBB damage was significantly higher (Figure 5A) and resolution of edema did not occur (Figure 5B) in PTX3 KO mice. Infarct volume was not significantly different between WT and PTX3 KO mice 6 days after MCAo. To understand the reason for differences in resolution of brain edema between WT and PTX3 KO mice after cerebral ischemia, we measured expression of the tight junction proteins occludin and zonula occludens-1, the matrix metalloproteinase-9 and the water channel aquaporin-4. Zonula occludens-1 expression was higher in PTX3 KO compared with WT mice, but we did not find differences in the expression of other markers assessed (Supplementary Figure 5).
Figure 4.
Lack of pentraxin-3 (PTX3) does not affect brain damage acutely after middle cerebral artery occlusion (MCAo). (A) Forty-eight hours after MCAo, no significant difference is observed in infarct size or blood–brain barrier (BBB) damage between wild-type (WT) and PTX3 knockout (KO) mice (i), as measured by Nissl (cresyl violet) staining and immunoglobulin G (IgG) infiltration (ii). (B) No significant difference is observed in edema between WT and PTX3 KO mice, 48 hours after MCAo. Scale bar, 3 mm. Students t-test (A, B) n=6 to 7). Error bars show s.e.m.
Figure 5.
Pentraxin-3 (PTX3) knockout (KO) mice show increased blood–brain barrier (BBB) damage and edema, and impaired glial responses compared with wild type (WT) mice, 6 days after middle cerebral artery occlusion (MCAo). (A) Blood–brain barrier breakdown, but not overall infarct size is significantly increased in PTX3 KO mice compared with WT mice, as measured on Nissl (cresyl violet) and immunoglobulin G (IgG) infiltration. (B) Edema is not resolved in PTX3 KO mice whereas it is reversed in WT mice. (C) Lack of PTX3 disrupts astrocytic scar formation as observed by glial fibrillary acidic protein (GFAP) staining in the ipsilateral hemisphere of PTX3 KO mice. Arrows in ‘i' and ‘ii' correspond to the inserts shown in ‘iii' and ‘iv'. The length of injured area surrounded by astrocytic scar is delineated with a dotted line. In average, GFAP-positive scar covers only 52% of the perimeter of the injured area in PTX3 KO mice, whereas in WT mice, it covers 87% (v). The total amount of astrocytic cells is not different between genotypes (vi). (D) The gaps in the glial scar of PTX3 KO mice (areas where GFAP-positive astrocytes are absent surrounding the infarct) showed significantly less extracellular matrix production identified by laminin α-4 staining compared with that of WT mice where astrocytic scar formation was not impaired around the infarct. (E) Immunohistochemical staining reveals a profound decrease in proliferating (bromodeoxyuridine (BrdU)-positive) microglia (Iba1-positive) in the striatum and around the glial scar of PTX3 KO mice compared with WT mice. (F) Addition of low (0.3 ng/mL) but not high (30 ng/mL) concentration of PTX3 to WT mixed glial cultures induces significant proliferation within 48 hours. LMα-4, laminin α-4, AraC, cytosine arabinoside. Scale bar, 3 mm (A), 1 mm (C), 50 μm (D), 100 μm (E) Students t-test (A–C, E), n=5 to 6) or one-way analysis of variance followed by Bonferroni's (D), n=3 to 4) or Dunnett's post hoc tests (F), n=9) were used. *P<0.05, **P<0.01, ***P<0.001. Error bars show s.e.m.
Pentraxin-3 Deficiency Compromises Glial Scar Formation after Cerebral Ischemia
We then investigated the role of PTX3 in glial responses that are involved in brain repair after injury. Astrocytic scar formation (GFAP-positive cells surrounding the infarct) was markedly impaired in mice lacking PTX3 (Figure 5C): only 52% of the perimeter of the infarct was covered by astrocytic scar in PTX3 KO mice versus almost complete coverage (87%) in WT mice (Figure 5C, v). Although scar formation was profoundly affected, the overall number of astrocytes was similar between both genotypes in the ipsilateral hemisphere (Figure 5C, vi). Astrocytic proliferation was quantified to explore whether it could account for deficient scar formation, but no significant difference was observed (data not shown). As PTX3 is involved in the stabilization of the extracellular matrix in peripheral tissues, we assessed the levels of laminin α-4 in the astrocytic scar and in the gaps of the scar. Both genotypes had a similar amount of laminin α-4 within the scar; however, over 50% of laminin α-4 staining was lost in the gaps (seen in PTX3 KO mice) compared with the scar of WT mice (Figure 5D), indicating that the absence of PTX3 compromises glial responses and extracellular matrix deposition after brain injury. Microglial proliferation, which is also important for glial scar formation, was profoundly reduced in PTX3 KO compared with WT mice both in the scar surrounding the infarct and in the core of the infarct in the striatum (Figure 5E). We have shown earlier that proliferation of microglia in the striatum after MCAo is not affected significantly by the size of infarct or the amount of neuronal injury, unlike in the cerebral cortex;31 therefore, our data indicate that PTX3 is involved in glial proliferative responses in response to brain injury. To study if PTX3 could directly affect glial proliferation, BrdU incorporation was quantified in WT mixed glial cultures treated with PTX3. Low concentration of PTX3 (0.3 ng/mL) was effective at inducing glial proliferation although a higher concentration of PTX3 (30 ng/ml) was not effective (Figure 5F). As expected, FGF2 (used as a positive control) strongly induced proliferation, whereas cytosine arabinoside (used as a negative control) inhibited it (Figure 5F).
Discussion
The present study is the first to show that the acute-phase protein PTX3, lately associated with severity of vascular diseases,10, 11, 12, 13, 15 is expressed in the brain after acute ischemic brain injury. Our data demonstrate that IL-1, which has acute detrimental effects in brain injury,16 induces PTX3 after cerebral ischemia, and that PTX3 contributes to brain recovery by promoting repair mechanisms such as glial scar formation, resolution of edema.
Here we show that not only circulating (as shown by Ryu et al 15) but also local PTX3 expression takes place in the brain in response to ischemic damage. Pentraxin-3 is reportedly expressed by macrophages, but we found no PTX3-positive leukocytes or microglia/macrophages in the brain. We cannot exclude the possibility that PTX3 is released very quickly by macrophages, and could also contribute to elevated PTX3 levels in the brain after cerebral ischemia. As PTX3 levels correlate positively with worse outcome in stroke patients,15 further investigation is needed to reveal how brain-derived PTX3 contributes to circulating PTX3 levels in experimental models. We demonstrate that IL-1 produced locally after cerebral ischemia32 is the key driver of brain PTX3 expression after MCAo. It has been shown that IL-1 injection into the brain can induce PTX3 expression,19 but we show that the increase in endogenous IL-1 after MCAo induces PTX3 expression in the brain after MCAo. Also in vitro, IL-1 (but not other ischemia-associated stimuli) greatly enhances PTX3 expression. Pentraxin-3 levels are thus likely to be increased in many CNS inflammatory disorders, since most of them present high levels of IL-1.32
Although IL-1 also enhances circulating PTX3 levels, peripheral PTX3 expression can occur in an IL-1-independent manner (as observed in IL-1α/β KO mice), highlighting a different regulation of PTX3 expression in the CNS. Pentraxin-3 can be induced by other proinflammatory cytokines such as TNF-α19, which could account for the increase in peripheral PTX3 after MCAo. Furthermore, circulating IL-1 did not drive central PTX3 expression in our model, demonstrating the specific role for centrally expressed IL-1 in brain PTX3 production. Other cytokines may contribute to brain PTX3 production although to a lower extent than IL-1, as even IL-1α/β KO mice show an increase in PTX3 levels after MCAo compared with sham.
Interestingly, PTX3 deletion did not affect brain damage early after the ischemic event (48 hours after MCAo), but we found that PTX3 is a critical mediator of brain responses 6 days after ischemic brain injury. In particular, lack of PTX3 impaired glial scar formation and low levels of PTX3 promoted glial proliferation. The glial scar is fundamental to limit the spread of the damage and may also help the recovery of vascular functions.33, 34 Indeed, we found that PTX3 KO mice had higher level of BBB damage and delayed resolution of brain edema. Surprisingly, PTX3 KO mice had higher expression of zonula occludens-1, which could suggest more endothelial tight junctions and hence a less-damaged BBB. However, our data indicate increased BBB permeability and edema, implying that tight junctions in PTX3 KO mice were less functionally intact than in WT animals at 6 days reperfusion. Therefore, it is possible that zonula occludens-1 upregulation is a compensatory response in PTX3 KO mice to counterbalance the increased BBB damage. Extracellular matrix instability in the areas of the scar that were depleted of astrocytes was suggested by the decrease in levels of laminin α-4, which is essential for stability of the basal lamina and for the formation of new blood vessels.35 Furthermore, we also found impaired microglial proliferation in PTX3 KO mice. There is increasing evidence that microglial activation and proliferation can promote repair.31, 36 Further research is needed to reveal the molecular mechanisms whereby PTX3 exerts its actions on glial responses and extracellular matrix deposition. We observed a nonsignificant trend for smaller infarct size (41%±28%) in WT mice compared with PTX3 KO mice at 6 days reperfusion, but the robust reduction of glial proliferation (which was also confirmed in vitro), reduced scar formation, and increased edema in PTX3 KO mice cannot be explained by variable differences in infarct size. We demonstrated earlier that microglial proliferation after cerebral ischemia in the striatum is largely independent from infarct size.37 In addition, PTX3 KO mice showed no significant difference in the infarct volume from WT mice at 48-hour reperfusion, arguing against the contribution of the primary ischemic injury to later inflammatory actions in these experiments.
In summary, our results demonstrate for the first time that IL-1 induces PTX3 expression in the brain after cerebral ischemia, and that PTX3 modulates glial responses and resolution of edema. Understanding the effects of IL-1 at different stages after brain injury could allow selective blockade of the detrimental acute effects of IL-1 actions while allowing its potential role in repair during the recovery phase. The exact mechanisms by which IL-1-induced PTX3 contributes to brain recovery need further investigation, but the current results highlight PTX3 as a new possible target for CNS inflammatory disorders.
Acknowledgments
We would like to thank Professor Alberto Mantovani and Professor Richard Grencis for critically reviewing the manuscript, Ms Catherine Smedley for genotyping and Mr Jordon Tolbot for aquaporin-4 measurement.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
Funding was provided by the Medical Research Council (Grant No. G0801296) the European Union's Seventh Framework Programme (FP7/2008–2013) under Grant agreement Nos. 201024 and 202213 (European Stroke Network) and the Simon and Simone Collins studentship.
Supplementary Material
References
- Denes A, Thornton P, Rothwell NJ, Allan SM. Inflammation and brain injury: acute cerebral ischaemia, peripheral and central inflammation. Brain, Behav Immun. 2010;24:708–723. doi: 10.1016/j.bbi.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-K, Tran T, Tansey M. Neuroinflammation in Parkinson's disease. J Neuroimmune Pharmacol. 2009;4:419–429. doi: 10.1007/s11481-009-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaull BBA, Todd S, McGuinness B, Passmore AP. Inflammation and anti-inflammatory strategies for Alzheimers disease. A mini-review. Gerontology. 2010;56:3–14. doi: 10.1159/000237873. [DOI] [PubMed] [Google Scholar]
- Manfredi AA, Rovere-Querini P, Bottazzi B, Garlanda C, Mantovani A. Pentraxins, humoral innate immunity and tissue injury. Curr Opin Immunol. 2008;20:538–544. doi: 10.1016/j.coi.2008.05.004. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Garlanda C, Bottazzi B, Peri G, Doni A, Martinez de la Torre Y, et al. The long pentraxin PTX3 in vascular pathology. Vascul Pharmacol. 2006;45:326–330. doi: 10.1016/j.vph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Salustri A, Garlanda C, Hirsch E, De Acetis M, Maccagno A, Bottazzi B, et al. PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development. 2004;131:1577–1586. doi: 10.1242/dev.01056. [DOI] [PubMed] [Google Scholar]
- Scarchilli L, Camaioni A, Bottazzi B, Negri V, Doni A, Deban L, et al. PTX3 interacts with inter-α-trypsin inhibitor. J Biol Chem. 2007;282:30161–30170. doi: 10.1074/jbc.M703738200. [DOI] [PubMed] [Google Scholar]
- Tranguch S, Chakrabarty A, Guo Y, Wang H, Dey SK. Maternal pentraxin 3 deficiency compromises implantation in mice. Biol Reprod. 2007;77:425–432. doi: 10.1095/biolreprod.107.062414. [DOI] [PubMed] [Google Scholar]
- Latini R, Maggioni AP, Peri G, Gonzini L, Lucci D, Mocarelli P, et al. Prognostic significance of the long pentraxin PTX3 in acute myocardial infarction. Circulation. 2004;110:2349–2354. doi: 10.1161/01.CIR.0000145167.30987.2E. [DOI] [PubMed] [Google Scholar]
- Kaess BM, Vasan RS. Heart failure: pentraxin 3-a marker of diastolic dysfunction and HF. Nat Rev Cardiol. 2011;8:246–248. doi: 10.1038/nrcardio.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara J, Sugiyama S, Nozaki T, Sugamura K, Konishi M, Ohba K, et al. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J Am Coll Cardiol. 2011;57:861–869. doi: 10.1016/j.jacc.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Jenny NS, Arnold AM, Kuller LH, Tracy RP, Psaty BM. Associations of pentraxin 3 with cardiovascular disease and all-cause death: the cardiovascular health study. Arterioscler Thromb Vasc Biol. 2009;29:594–599. doi: 10.1161/ATVBAHA.108.178947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciacca P, Betta P, Mattia C, Li Volti G, Frigiola A, Curreri S, et al. Pentraxin-3 in late-preterm newborns with hypoxic respiratory failure. Front Biosci (Elite Ed) 2010;2:805–809. doi: 10.2741/e141. [DOI] [PubMed] [Google Scholar]
- Ryu W-S, Kim CK, Kim BJ, Kim C, Lee S-H, Yoon B-W. Pentraxin 3: a novel and independent prognostic marker in ischemic stroke. Atherosclerosis. 2012;220:581–586. doi: 10.1016/j.atherosclerosis.2011.11.036. [DOI] [PubMed] [Google Scholar]
- Allan SM, Tyrrell PJ, Rothwell NJ. Interleukin-1 and neuronal injury. Nat Rev Immunol. 2005;5:629–640. doi: 10.1038/nri1664. [DOI] [PubMed] [Google Scholar]
- Relton JK, Rothwell NJ. Interleukin-1 receptor antagonist inhibits ischaemic and excitotoxic neuronal damage in the rat. Brain Res Bull. 1992;29:243–246. doi: 10.1016/0361-9230(92)90033-t. [DOI] [PubMed] [Google Scholar]
- Andre R, Moggs JG, Kimber I, Rothwell NJ, Pinteaux E. Gene regulation by IL-1β independent of IL-1R1 in the mouse brain. Glia. 2006;53:477–483. doi: 10.1002/glia.20302. [DOI] [PubMed] [Google Scholar]
- Polentarutti N, Bottazzi B, Di Santo E, Blasi E, Agnello D, Ghezzi P, et al. Inducible expression of the long pentraxin PTX3 in the central nervous system. J Neuroimmunol. 2000;106:87–94. doi: 10.1016/s0165-5728(00)00214-9. [DOI] [PubMed] [Google Scholar]
- Schwartz M. Macrophages and microglia in central nervous system injury: are they helpful or harmful. J Cereb Blood Flow Metab. 2003;23:385–394. doi: 10.1097/01.WCB.0000061881.75234.5E. [DOI] [PubMed] [Google Scholar]
- Rolph MS, Zimmer S, Bottazzi B, Garlanda C, Mantovani A, Hansson GK. Production of the long pentraxin PTX3 in advanced atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2002;22:e10–e14. doi: 10.1161/01.atv.0000015595.95497.2f. [DOI] [PubMed] [Google Scholar]
- Wheeler RD, Boutin H, Touzani O, Luheshi GN, Takeda K, Rothwell NJ. No role for interleukin-18 in acute murine stroke-induced brain injury. J Cereb Blood Flow Metab. 2003;23:531–535. doi: 10.1097/01.WCB.0000059587.71206.BA. [DOI] [PubMed] [Google Scholar]
- Chapman KZ, Dale VQ, Denes A, Bennett G, Rothwell NJ, Allan SM, et al. A rapid and transient peripheral inflammatory response precedes brain inflammation after experimental stroke. J Cereb Blood Flow Metab. 2009;29:1764–1768. doi: 10.1038/jcbfm.2009.113. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Humphreys N, Lane TE, Grencis R, Rothwell N. Chronic systemic infection exacerbates ischemic brain damage via a CCL5 (regulated on activation, normal T-cell expressed and secreted)-mediated proinflammatory response in mice. J Neurosci. 2010;30:10086–10095. doi: 10.1523/JNEUROSCI.1227-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Rothwell NJ, Pinteaux E, Boutin H. Contribution of interleukin-1 receptor accessory protein b to interleukin-1 actions in neuronal cells. Neurosignals. 2011;19:222–230. doi: 10.1159/000330803. [DOI] [PubMed] [Google Scholar]
- Pinteaux E, Parker LC, Rothwell NJ, Luheshi GN. Expression of interleukin-1 receptors and their role in interleukin-1 actions in murine microglial cells. J Neurochem. 2002;83:754–763. doi: 10.1046/j.1471-4159.2002.01184.x. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Bottazzi B, Bastone A, Mantovani A. Pentraxins at the crossroads between inate immunity, inflammation, matrix deposition, and female fertility. Anu Rev Immunol. 2005;23:337–366. doi: 10.1146/annurev.immunol.23.021704.115756. [DOI] [PubMed] [Google Scholar]
- Moskowitz MA, Lo EH, Iadecola C. The Science of stroke: mechanisms in search of treatments. Neuron. 2010;67:181–198. doi: 10.1016/j.neuron.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz E, Carlin S, Dyomina K, Burke S, Thaler HT, Blasberg R, et al. Real-time imaging of HIF-1α stabilization and degradation. PLoS ONE. 2009;4:e5077. doi: 10.1371/journal.pone.0005077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denes A, Vidyasagar R, Feng J, Narvainen J, McColl BW, Kauppinen RA, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–1953. doi: 10.1038/sj.jcbfm.9600495. [DOI] [PubMed] [Google Scholar]
- Allan S, Pinteaux E. The interleukin-1 system: an attractive and viable therapeutic target in neurodegenerative disease. Curr Drug Targets CNS Neurol Disord. 2003;2:293–302. doi: 10.2174/1568007033482742. [DOI] [PubMed] [Google Scholar]
- Kawano H, Kimura-Kuroda J, Komuta Y, Yoshioka N, Li H, Kawamura K, et al. Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res. 2012;349:1–12. doi: 10.1007/s00441-012-1336-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, Schwartz M. The bright side of the glial scar in CNS repair. Nat Rev Neurosci. 2009;10:235–241. doi: 10.1038/nrn2591. [DOI] [PubMed] [Google Scholar]
- Thyboll J, Kortesmaa J, Cao R, Soininen R, Wang L, Iivanainen A, et al. Deletion of the lamininα 4 chain leads to impaired microvessel maturation. Mol Cell Biol. 2002;22:1194–1202. doi: 10.1128/MCB.22.4.1194-1202.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polazzi E, Monti B. Microglia and neuroprotection: from in vitro studies to therapeutic applications. Prog Neurobiol. 2010;92:293–315. doi: 10.1016/j.pneurobio.2010.06.009. [DOI] [PubMed] [Google Scholar]
- Denes A, Ferenczi S, Kovacs KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood-brain barrier damage and brain oedema independently of infarct size. J Neuroinflammation. 2011;8:164. doi: 10.1186/1742-2094-8-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.