Abstract
The inflammasome is an intracellular multiprotein complex involved in the activation of caspase-1 and the processing of the proinflammatory cytokines interleukin-1β (IL-1β) and IL-18. The inflammasome in the central nervous system (CNS) is involved in the generation of an innate immune inflammatory response through IL-1 cytokine release and in cell death through the process of pyroptosis. In this review, we consider the different types of inflammasomes (NLRP1, NLRP2, NLRP3, and AIM2) that have been described in CNS cells, namely neurons, astrocytes, and microglia. Importantly, we focus on the role of the inflammasome after brain and spinal cord injury and cover the potential activators of the inflammasome after CNS injury such as adenosine triphosphate and DNA, and the therapeutic potential of targeting the inflammasome to improve outcomes after CNS trauma.
Keywords: brain injury, caspase-1, IL-1, inflammasome, spinal cord injury, stroke
Introduction
The innate immune response has a critical role in the pathology after injury to the central nervous system (CNS). Activation of pattern recognition receptors (PRRs) such as toll-like receptors, NOD-like receptors, or RIG-like receptors by danger-associated molecular patterns (DAMPs) such as adenosine triphosphate (ATP) or heat shock proteins results in production of inflammatory cytokines such as interleukin (IL)-1β, IL-18, tumor necrosis factor, or type I interferons that subsequently activate the adaptive immune response. A key component of the innate immune response is the inflammasome, an NLR-based multiprotein complex responsible for the activation of caspase-1 after infections, injury, and in autoimmune diseases. The present review addresses the role of the inflammasome in the innate immune response after injury to the brain and spinal cord. This review covers the different inflammasomes that have been described in neurons, astrocytes, and microglia as well as the mechanism of activation of the inflammasome (Table 1) in the CNS and the different therapeutic interventions that target inflammasomes after CNS trauma.
Table 1. Inflammasomes in the CNS and their activators.
| Inflammasome | Activators | Components | References |
|---|---|---|---|
| NLRP1 | High extracellular potassium | NLRP1, caspase-1, caspase-11, ASC, XIAP | de Rivero Vaccari et al.,6 Silverman et al.18 |
| NLRP2 | ATP | NLRP2, caspase-1, ASC | Minkiewicz et al.7 |
| NLRP3 | ATP, β-amyloid | NLRP3, caspase-1, ASC | Halle et al.,8 Mariathasan et al.60 |
| AIM2 | DNA | AIM2, caspase-1, ASC | Adamczak SE et al.11 |
Abbreviations: CNS, central nervous system; XIAP, X-linked inhibitor of apoptosis protein.
Inflammasome Expression in the Central Nervous System
The inflammasome was first described as a multiprotein complex comprising caspase-1, the adaptor protein apoptosis-associated speck-like protein containing a caspase-activating recruitment domain protein (ASC), and the sensor NLR.1 Several NOD-like receptors have been shown to form inflammasomes as well as the ALR protein AIM2. Most of the inflammasomes described to date contain an NLR protein, namely NLRP1, NLRP2, NLRP3, NLRP6, NLRP7, NLRP12, or NLRC4 (NLR-and caspase-activating recruitment domain-containing 4).2, 3 However, only few inflammasomes have been described and characterized in the CNS: The NLRP1 inflammasome in neurons,4, 5, 6 the NLRP2 inflammasome in astrocytes,7 the NLRP3 inflammasome in microglia,8, 9, 10 and the absent in melanoma-2 (AIM2) inflammasome in neurons (Figure 1).11 However, other inflammasome sensors (including NLRP3, NLRP6, NLRP12, and interferon-γ-inducible protein 16) may form inflammasomes in the CNS, but convincing data have not been generated to support this idea. Notable among the NLR sensors is NLRP3 that can be induced by amyloid-β through TLR activation and has been associated with the pathogenesis of Alzheimer's disease.8, 12 However, in the CNS, it has not been clearly demonstrated that NLRP3 forms protein–protein interactions to form a multiprotein complex with other inflammasome components such as capase-1 or ASC in the CNS.
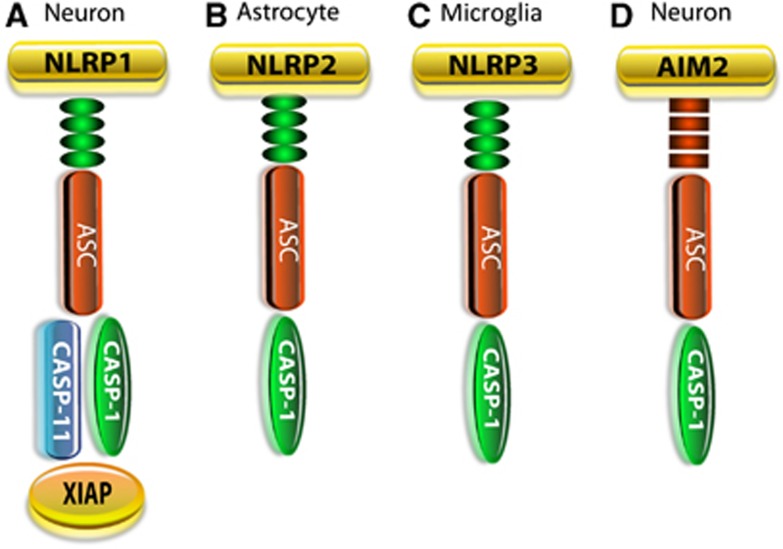
Figure 1.
Inflammasome structure. The sensory component of NOD-like receptors (NLRs) or AIM2-like receptors interacts with the adaptor protein ASC and caspase-1. (A) The NLRP1 inflammasome in neurons differs from that in macrophages in that it also contains X-linked inhibitor of apoptosis protein (XIAP) and caspase-11. (B) The NLRP2 inflammasome is present in astrocytes and comprises caspase-1, ASC, and NLRP2. (C) In microglia, the NLRP3 inflammasome comprises NLRP3, ASC, and caspase-1, whereas (D) in neurons the AIM2 inflammasome comprises caspase-1, ASC, and AIM2.
The NLRP1 Inflammasome
The NLRP1 inflammasome in the brain and spinal cord comprises NLRP1, caspase-1, ASC, caspase-11, and the inhibitor of apoptosis proteinX-linked inhibitor of apoptosis protein (XIAP)6 (Figure 1). This neuronal NLRP1 inflammasome differs in structure from the NLRP1 inflammasome described in peripheral macrophages in a number of ways: First, the NLRP1 inflammasome complex in neurons is preassembled in unstimulated neurons or in normal CNS tissues. In contrast, it appears that in unstimulated macrophages NLRP1 components are not preassembled in a multiprotein complex, but rapidly form protein–protein associations upon stimulation by muramyl dipeptide.13 In this regard, immunoprecipitation experiments indicate protein–protein interactions are made between NLRP1, ASC, caspase-1, caspase-11, and XIAP in neuronal lysates of sham and spinal cord-injured and brain-injured rodents.5, 6 These studies indicate that the inflammasome components in the CNS are preassembled before activation; however, it is possible that additional inflammasome proteins are recruited into inflammasome complexes as the inflammatory response becomes exacerbated. It has been suggested that preassembly of inflammasome complexes in CNS cells facilitates a rapid triggering of the innate immune response after CNS trauma. However, it is also possible that CNS cells maintain low levels of IL-1β in the brain and spinal cord that is regulated by preassembled inflammasomes. Preassembly of NLRP1 inflammasomes has also been noted in melanoma cells that constitutively secrete IL-1β.14
In the CNS, caspase-11 forms protein–protein interactions with other inflammasome proteins; whether caspase-11 forms part of the same inflammasome with caspase-1 or whether caspase-11 forms its own inflammasome, independent of caspase-1, in the CNS remains to be investigated. Importantly, caspase-11 is involved in the processing of IL-1β, and recent evidence has emerged indicating that caspase-11 has a role in noncanonical inflammasome activation after bacterial infections.15
The NLRP1 inflammasome is associated with the inhibitor of apoptosis protein XIAP that may keep inflammasome expression in check (Figure 1A).6 Moreover, spinal cord injury (SCI)-induced activation of the inflammasome was found to be associated with cleavage of XIAP into smaller fragments. Cleavage of XIAP produces an N-terminal BIR1-2 fragment with reduced ability to inhibit caspases.16 Therefore, SCI-induced XIAP cleavage may reduce the threshold for activation of caspase-1, leading to processing and secretion of IL-1β and IL-18 (Figure 2). We suggest that XIAP in the NLRP1 inflammasome complex may serve to inhibit caspase-1 activity preventing the activation and processing of IL-1β and IL-18. In addition, XIAP has been shown to regulate innate immune signaling by interactions with NOD1 and NOD2 through an interaction with RIP2,17 but XIAP has not been shown to be associated with the NLRP1 inflammasome expressed in macrophages.
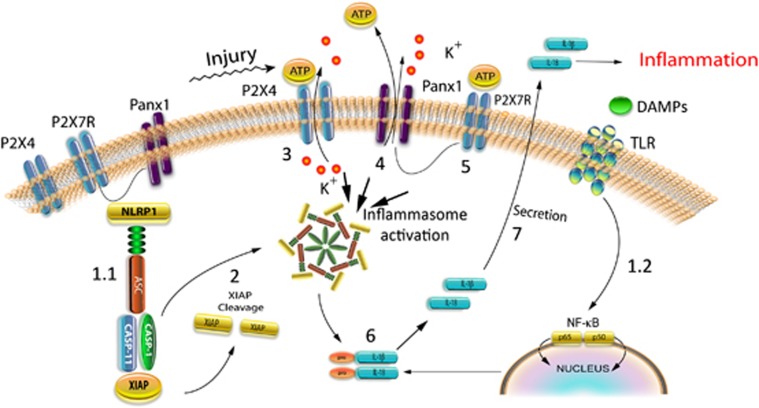
Figure 2.
Mechanism of inflammasome activation involving P2X4, P2X7, and the pannexin-1 channel. (1.1) In the uninjured central nervous system (CNS), the neuronal NLRP1 inflammasome is a multiprotein complex consisting of inflammatory caspase-1, caspase-11, NLRP1, the adaptor protein ASC, and the inhibitor of apoptosis protein X-linked inhibitor of apoptosis protein (XIAP) that are linked to P2X7 and pannexin-1. (1.2) Danger/damage-associated molecular patterns (DAMPs) activate distinct toll-like receptors (TLRs), which initiate a complex sequence of signaling events leading to synthesis of pro-IL-1β. This step is referred to as priming. (2) Upon inflammasome activation, XIAP is cleaved, thus decreasing its inhibitory capacity on caspase-1. (3) Adenosine triphosphate (ATP) released from dying cells activates P2X4. (4) P2X4 then facilitates K+ efflux, resulting in opening of the pannexin-1 channels. High extracellular potassium opens the pannexin-1 channel that results in ATP release. Adenosine triphosphate released from pannexin-1 further increases the extracellular levels of ATP. (5) High extracellular levels of ATP activate P2X7. (6) Upon inflammasome activation, pro-IL-1β and pro-IL-18 are cleaved into their respective active forms, IL-1β and IL-18. (7) Mature (bioactive) IL-1β and IL-18 are secreted into the extracellular space by an unknown release mechanism. Panx-1, pannexin-1.
Coimmunoprecipitation of neuronal lysates indicates that components of the NLRP1 multiprotein inflammasome complex interact with pannexin-1 and the purinergic P2X7 receptor (Figure 2).18 Pannexin-1 appears to be part of a large protein-signaling complex that links P2X7 receptors to the components of the inflammasome, resulting in caspase-1 activation and eventual release of IL-1 cytokines.19 In addition, deletion of P2X4 in mice results in decreased innate immune responses after SCI compared with wild-type animals.20 This decreased inflammatory response involves a significant reduction in inflammasome activation in neurons as determined by decreased cleavage of caspase-1 and production of IL-1β, as well as decreased infiltration of immune cells into the injury site acutely after SCI. Pannexin-1, P2X4, and P2X7 have not been linked to NLRP1 inflammasomes in hematopoietic cells, yet pannexin-1 and P2X7 may influence NLRP3 inflammasome activity.21, 22
In macrophages, NLRP1 expression is governed by several regulatory mechanisms and putative protein interactions.23 The sterol regulatory element binding protein-1a basic helix-loop-helix zipper transcription factor differentially regulates the expression of Nlrp1a in hematopoietic cells.24 At the posttranslational level, NLRP1 undergoes autocleavage at Ser 123 in the FIIND domain that is required for function.25, 26, 27 Prosurvival Bcl-2 and Bcl-xL bind NLRP1 and inhibit NLRP1 activation28 before formation of the inflammasome complex. Finally, NLRP1 forms an inflammasome in vivo, independently of ASC and caspase-11 that triggers an IL-1β-dependent inflammatory disease that is negatively regulated by IL-18.29 At present, it is unclear whether neuronal NLRP1 inflammasome activation is controlled in a similar fashion as the NLRP1a inflammasome in macrophages.
Importantly, the role of ASC in inflammasome regulation is controversial. Based upon in vitro reconstitution experiments of inflammasome components, it has been suggested that ASC is not essential for inflammasome activation, but acts as an amplifier of inflammasome activity to enhance the innate immune response.30 In support of this idea is the observation that human NLRP1 forms protein–protein interactions with caspase-1 through its PYD domain in the absence of ASC.31 However, the NLRP1 protein in mice lacks a functional PYD and therefore, homodimeric interaction between NLRP1 and ASC is not expected. However, it is possible that other yet unidentified proteins mediate interactions between ASC and NLRP1 in rodents. Is ASC needed in all inflammasomes? Some argue that ASC acts as an enhancer of the inflammatory response and that it is not necessary in all inflammasomes to activate caspase-1. For instance, NLRP1 in humans can form protein–protein interactions with caspase-1 through its caspase-activating recruitment domain, in the absence of ASC. In the presence of ASC, such interaction can occur through the PYD domain. In contrast, NLRP1 in mice lacks a functional PYD domain. As a result, in mice, a homodimeric interaction between NLRP1 and ASC through its PYD is not expected.31 There are three polymorphic paralogs of the NLRP1 gene: Nlrp1a, Nlrp1b, and Nlrp1c each of which is activated by different ligands.32 Whether other proteins are involved in the interaction between ASC and NLRP1 in rodents remains unknown. Moreover, there is always a possibility of heterodimeric interactions between the different protein domains.
The effects of knocking out NLRP1 or ASC in mice after SCI remains to be explored, yet P2X4 knockout mice, show decreased inflammasome activation after SCI, consistent with improved histopathological and functional outcomes.20 Importantly, P2X4 was present in neurons of the spinal cord. Therefore, these data corroborate the therapeutic potential of targeting the inflammasome in neurons to improve outcomes after SCI.
The NLRP2 Inflammasome
NLRP2 is a cytoplasmic protein also known as: NALP2, NBS1, PAN1, and PYPAF2. NLRP2 is expressed in a variety of tissues including the human brain.33 NLRP2 associates with ASC and caspase-1 and inhibits NF-κB activation.33, 34, 35 An allelic variant of NLRP2 that fails to inhibit the NF-κB transcriptional pathway contributes to the pathology of chronic inflammatory disorders such as Muckle–Wells syndrome.36
Recently, we reported that human astrocytes express the NLRP2 protein that forms a functional inflammasome consisting of NLRP2, ASC, and caspase-17 (Figure 1B). The NLRP2 inflammasome complex in astrocytes interacts with the P2X7 receptor and pannexin-1. Exogenous application of ATP activates the NLRP2 inflammasome, leading to rapid processing of caspase-1 and production of mature IL-1β. Moreover, the pannexin-1 channel blocker probenecid and the P2X7 receptor inhibitor Brilliant Blue G reduced ATP-induced NLRP2 activation. Silencing of NLRP2 with small interfering RNA reduced NLRP2 protein levels and attenuated caspase-1 activation by ATP, thus demonstrating that NLRP2, which is activated by ATP, has a critical role in the astrocyte innate immune response.
The NLRP3 Inflammasome
To date, the NLRP3 inflammasome remains the most studied inflammasome. The availability of NLRP3 knockout animals has resulted in a tremendous amount of studies focusing on this inflammasome, particularly in the peripheral immune system. Generally, two stimulating signals are needed to activate the NLRP3 inflammasome. One signal represents the priming step2 that produces pro-IL-1β and the second signal is directly responsible for the activation of caspase-1 and the processing of pro-IL-1β by active caspase-1 (Figure 2).37, 38 It is unclear if a similar NLRP3 activation scheme is operative in CNS cells. In addition, many studies have been carried in vitro using cells that are deficient in NLRP3 inflammasome components.39, 40, 41 The availability of NLRP3 knockout mice has provided an animal model to study regulation of NLRP3 during infections42, 43 and in autoimmune diseases.44, 45 There are a few studies that suggest that the NLRP3 inflammasome has a role in the CNS. These studies have employed the use of NLRP3 knockout mouse lines to suggest that the NLRP3 inflammasome is activated in microglia after Mycobacterium tuberculosis infection46 as well as by β-amyloid; the latter linking the NLRP3 inflammasome with Alzheimer's disease,12 but whether the NLRP3 inflammasome components form protein–protein associations with each other in the CNS needs to be determined (Figure 1C).
The AIM2 Inflammasome
AIM2 is a member of the hemopoietic interferon-inducible nuclear 200 (HIN200) family of proteins and was recently discovered to form an inflammasome.47, 48, 49, 50 AIM2 is activated by viral, bacterial, and host ectopic double-stranded DNA and induces cleavage of pro-caspase-1, maturation of pro-IL-1β, and pyroptosis.47, 48, 49, 50, 51, 52, 53 Although it has been known that AIM2 is expressed in tissues outside the immune system such as spleen, small intestine, and peripheral blood leukocytes,54 its functional role in either the recognition of pathogenic DNA or autoimmune reactions to host nucleic acids has only been recently established in innate immune cells.48, 55, 56 Recent work from our laboratory indicates that AIM2 associates with ASC and caspase-1 to form a DNA-responsive inflammasome in cortical neurons11 (Figure 1D). Further characterization of the neuronal AIM2 inflammasome will provide important information that will guide us in the development of therapies to treat inflammasomes activated by DNA after injury or viral infections.
Inflammasome Activators in the CNS
Activation of inflammasomes is complex and regulated at different levels through protein interactions that regulate the overall response of these important signaling platforms. A detailed account of the multifactorial regulatory mechanisms that govern inflammasomes can be found in a recent review.2 Our purpose is to discuss the regulatory mechanisms that facilitate a balance but effective inflammasome-mediated immune response during CNS injury and disease. As a result of cellular damage or infection to the host, there is a release of molecules that are recognized by PRRs such as NLRP1.57, 58, 59 These molecules may be exogenous such as those found in bacterial infections (e.g. lipopolysaccharides, muramyl dipeptide, or flagellin), or endogenous such as those produced during sterile inflammation and in necrosis. Exogenous ligands that activate PRRs are referred to as PAMPs (pathogen-associated molecular patterns), whereas DAMPs (danger/damage-associated molecular patterns) correspond to endogenous ligands that are released by dying or damaged cells after cellular stress. Of interest to the area of CNS injury are DAMPS that can be recognized by PRRs such as toll-like receptors or NOD-like receptors (Figure 3).
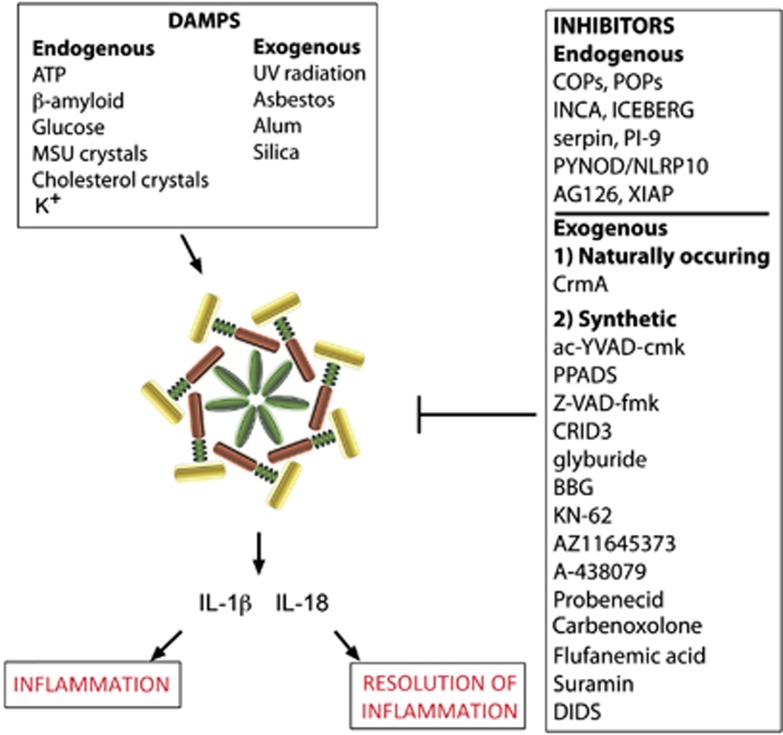
Figure 3.
Activators and inhibitors of the inflammasome. Inflammasomes are activated by danger-associated molecular patterns (DAMPS) that result in caspase-1 activation and processing of the proinflammatory cytokines IL-1β and IL-18. Resolution of inflammation results in tissue homeostasis, but chronic inflammatory response may result in cell death. Inhibitors of the inflammasome are grouped endogenous such as ICEBERG, INCA, or POP or exogenous such as probenecid, carbenoxolone, glyburide, or YVAD. MSU, monosodium urate; UV, ultraviolet.
Theoretically, any molecule that is found in a ‘foreign' site within a cell or in the extracellular matrix may induce an immune response after recognition by PRRs and subsequent production of inflammatory cytokines. For example, adenosine triphosphate (ATP)60 or high mobility group box 1 are not normally present in the cytoplasm but are released into the cytoplasm and extracellular matrix after trauma and thus behave as DAMPs to initiate an innate immune response (Figure 3).61 Activation of the inflammasome occurs through binding of DAMPs or PAMPs. There are several mechanisms that have been described to be involved in the activation of the inflammasome outside the CNS such as an efflux of potassium ions62 or activation of purinergic receptors by ATP (Figure 2).63 Other inflammasome activators are listed in Figure 3; however, little is known about the signaling pathways required for triggering inflammasome activation in the CNS. Recently, we provided evidence that high extracellular K+ opens the pannexin-1 channel and activates the inflammasome in neurons and astrocytes, but not THP-1 cells.18 This signaling pathway in neurons is mediated through protein interactions between pannexin-1 and inflammasome proteins.18 We also provide evidence that ATP acting on P2X7 induces rapid cell death and that antibody neutralization of ASC blocks cell death.18 Thus, contrary to the widely accepted view in macrophages and monocytes that low intracellular K+ triggers NLRP1b and NLRP3 inflammasome activation,62, 64 high extracellular K+ surrounding cells such as neurons and astrocytes opens pannexin-1 channels and induces processing of caspase-1 (Figure 2). It is possible that this discrepancy for K+ activation is due to the expression of pannexin-1 channels between the two disparate cell populations. Supporting this idea is our recent observation that THP-1 cells do not contain detectable levels of pannexin-1 (unpublished data) whereas this protein is abundant in neurons and astrocytes. Moreover, it is unclear whether K+ activation of pannexin-1 channels occurs in vivo. The high concentration of K+ ions required for activation of pannexin-1 (>20 mM) probably only occurs near dying cells. Another possibility is that, K+ efflux through pannexin-1 may produce a concentration of K+ that is high enough to activate the channel.19 Pannexin-1 channels serve as ATP release channels. When co-expressed with P2Y or with P2X7, extracellular ATP results in pannexin-1-mediated membrane currents.65, 66 This type of positive feedback is consistent with the observation of ATP-induced ATP release.67 Sustained application of ATP to cells co-expressing P2X7 with pannexin-1 results in cell death.66 Thus, there must be regulatory mechanisms that keep this deadly channel in check. Indeed, ATP has also recently been identified as a pannexin-1 channel inhibitor, so it regulates its own permeation pore.68 The molecular mechanism by which pannexin-1 regulates inflammasome activation is not well understood. Studies employing pannexin-1 knockout cells or pannexin-1 knockout mice report that deletion of the pannexin-1 gene improved functional outcomes after stroke, but there was little effect on IL-1β release.69 Importantly, pannexin-1 knockout mice present a hypomorphic phenotype;70 thus, these animals have reduced pannexin-1 function. Therefore, this finding calls for a precise quantification of pannexin-1 expression in its relation to function to provide a better understanding of the role of pannexin-1 in inflammasome activation. In addition, inflammasome activation has been suggested to have a role in the development of seizures in a pannexin-1-dependent mechanism.71 Accordingly, kainic acid induces seizures via a mechanism involving opening of pannexin-1 and release of ATP. Moreover, knocking out of pannexin-1 decreases kainic acid-induced seizure duration.71
Besides high extracellular potassium, ATP (1 mmol/L) has been recently shown to activate the NLRP2 inflammasome in astrocytes.7 In this scenario, ATP most likely acts on the purinergic receptor P2X7 (Figure 2). These findings are consistent with immunoprecipitation experiments demonstrating that P2X7 and the pannexin-1 channel form protein–protein interactions with the inflammasome.18 Moreover, the purinergic receptor P2X4 also contributes to inflammasome activation after injury to the spinal cord.20 Accordingly, P2X4 knockout mice showed decreased caspase-1 and IL-1β processing in neurons and improved histopathological and functional improvement after SCI in rodents, indicating an involvement of P2X4 in the activation of the inflammasome.20 In addition, the P2X4 knockout animals showed decreased infiltration of neutrophils and M1 macrophages into the spinal cord after injury.20 Interestingly, it has been proposed that ATP released after injury first activates the P2X4 receptor (<100 μmol/L), which is more sensitive to ATP than the P2X7 receptor.72 Activation of P2X4 results in release of K+. This increase in K+ results in opening of the pannexin-1 channel, which then releases ATP, thus increasing the levels of ATP and activating the P2X7 receptor, resulting in inflammasome activation73 (Figure 2).
Inflammasome Activation after Brain and Spinal Cord Injury
In acute CNS injury models, a spectrum of inflammatory events have been reported including activation and recruitment of inflammatory cells, increased gene expression and protein levels of proinflammatory cytokines as well as a host of cell signaling cascades that lead to both detrimental and reparative processes.
IL-1β has been widely implicated in the pathophysiology of SCI, traumatic brain injury, and stroke.4, 5, 6 However, it is not until recently that the inflammasome, a main upstream activator of IL-1β, has been described to be involved in the regulation of inflammation after SCI.6 Accordingly, caspase-1 is activated after injury when compared with sham/uninjured animals. The protein levels of caspase-1 in the spinal cord are elevated late up to 3 days after trauma.6 Similarly, the protein levels of the adaptor protein ASC were increased after SCI, whereas the levels of NLRP1 did not change at any of the time points tested. Of importance is the finding that the NLRP1 inflammasome was present in motor neurons of the spinal cord, indicating that neurons express an inflammatory complex.6 Similar findings regarding inflammasome activation in neurons have been obtained after traumatic brain injury5, 74 and stroke.4 Increased levels of NLRP1, ASC, and caspase-1 have also been reported in the CSF of traumatic brain injury patients and are associated with poor prognosis.75 Thus, inflammatory innate immune responses, especially in the early postinjury period, are important therapeutic targets for reducing the detrimental effects of secondary injury mechanisms. However, more experiments are needed to show a direct effect between inflammasome proteins and poor outcomes after CNS injury.
Clinical Implications of Targeting the Inflammasome after Central Nervous System Injury
Although several therapeutic approaches targeting early inflammatory process have been tested in the clinic, there are no therapies that have therapeutic benefit in the treatment of inflammation after CNS injury. Blocking inflammasome activation appears to offer an ideal approach to decrease inflammation and improve histopathological and behavioral outcomes after CNS injury. Inhibition of the inflammasome with neutralizing antibodies against inflammasome proteins such as ASC or NLRP1 has been successfully used in preclinical models of brain injury5, 74 and SCI6 in rodents. This approach resulted in decreased caspase-1 activation, IL-1β processing, histopathological damage, while improving behavioral outcomes.6 However, the immune mechanism of antibody neutralization of inflammasome activation has yet to be reported.
In addition to neutralizing antibodies against inflammasome proteins, other drugs have been shown to block inflammasome activation. For example, probenecid, an FDA-approved drug for the treatment of gout and hyperuricemia, inhibits inflammasome activation by high extracellular K+ in cultured neurons and astrocytes18 and has been shown to decrease caspase-1 in the brain of aged rats.76 Moreover, glyburide, typically used in the treatment of diabetes mellitus, inhibits NLRP3 inflammasome activation;77 however, to date, glyburide has not been shown to inhibit inflammasome activation in the CNS. Other substances such as YVAD, PPADs, KN-62, and antibiotics (Figure 3) have been suggested as possible therapeutics to block inflammasome activation, but these substances have not been successfully tested in the CNS. Finally, Anakinra/Kineret has been tested in CNS injury models, but this drug targets IL-1β signaling downstream of the inflammasome.78
Conclusions and Future Perspectives
In this review, we have summarized the current understanding and gaps in our knowledge regarding regulation of inflammasomes in the CNS after injury. Although detailed information has been gained about many aspects of inflammasome regulation in peripheral tissues, many important unresolved questions regarding inflammasome expression and regulation in the CNS remain unanswered or poorly understood (Figure 4).
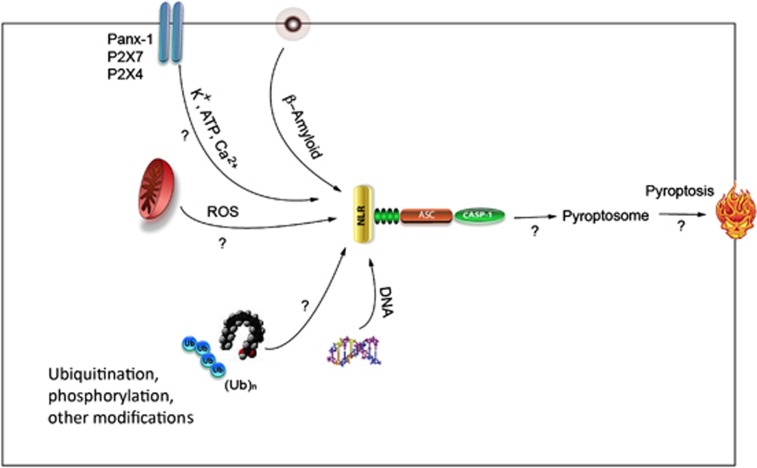
Figure 4.
Unsolved problems in inflammasome regulation in the central nervous system (CNS). How are pannexin-1, P2X4, and P2X7 are involved in inflammasome activation through potassium and ATP? Does calcium have a role on inflammasome activation in the CNS? Does β-amyloid activate other inflammasomes in addition to NLRP3? How does reactive oxygen species (ROS) have a role in inflammasome activation? What is the role of mitochondria in inflammasome activation? How does the inflammasome activation lead to pyroptosis? Are there additional activators and inhibitors of inflammasomes? Do protein modifications such as phosphorylation or ubiquitination have a role on regulating activation of the inflammasome? How much inflammasome activation is beneficial and when does it become detrimental?
One main question regarding inflammasome expression that remains unanswered is whether oligodendroglia, or ependymal cells harbor inflammasomes, and whether they are similar in composition to defined inflammasomes in peripheral immune cells. In addition, it is not clear what inflammasome triggers activate CNS cells after injury and whether CNS inflammasomes require multiple activation pathways. Moreover, future research should focus on identification of host molecules that function upstream of NOD-like receptors to regulate inflammasome activity.
Other important questions that remain unsolved are shown in Figure 4: Are there additional upstream sensors or adaptors that regulate inflammasome activation? How do high extracellular K+, Ca2+, and ATP in the extracellular space regulate Panx-1 and P2X receptors to cause inflammasome activation? How are these channels linked to the inflammasome and what signaling intermediates are crucial of inflammasome activation? Is mitochondrial localization essential for inflammasome formation and how does ROS regulate inflammasome formation? Does β-Amyloid activate other inflammasomes besides NLRP3? How do posttranslational modifications regulate activation? Does DNA activate other inflammasomes besides AIM2? How does inflammasome activation induce pyroptosis?
It will also be significant to define the contribution of CNS inflammasome-independent pathways to the innate inflammatory response in vivo after injury. For example, caspase-8 has recently been identified to be recruited to the inflammasome to process pro-IL-1β,79 but the relative contribution of caspase-8 to inflammation processes elicited by CNS injury has not been studied.
In addition, it is possible that signals emanating from organelles like mitochondria, endosomes, and lysosomes may yield information about the way inflammasome effector proteins are tightly controlled, and it may be possible to compare these regulatory mechanisms with those that govern inflammatory cell death processes such as pyroptosis (Figure 4). Clearly, inflammasomes have emerged to have a broad spectrum of controls regulating inflammatory responses in CNS injury and disease. Therefore, inhibitors of inflammation and drugs that target inflammasome functions are poised to have a major role in treating neurologic diseases and conditions. However, significant work is required to better understand activation and regulation of components of the inflammasome in different cells.
The authors declare no conflict of interest.
Footnotes
Grant sponsor: National Institute of Health; Grant numbers: NS059836 and NS042133; Grant sponsor: Craig Nielsen Foundation; Grant number: 221346.
References
- Martinon F, Burns K, Tschopp J. The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Latz E, Xiao TS, Stutz A. Activation and regulation of the inflammasomes. Nat Rev Immunol. 2013;13:397–411. doi: 10.1038/nri3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vladimer GI, Marty-Roix R, Ghosh S, Weng D, Lien E. Inflammasomes and host defenses against bacterial infections. Curr Opin Microbiol. 2013;16:23–31. doi: 10.1016/j.mib.2012.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Alonso OF, Bramlett HM, Dietrich WD, Keane RW. Therapeutic neutralization of the NLRP1 inflammasome reduces the innate immune response and improves histopathology after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29:1251–1261. doi: 10.1038/jcbfm.2009.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Lotocki G, Marcillo AE, Dietrich WD, Keane RW. A molecular platform in neurons regulates inflammation after spinal cord injury. J Neurosci. 2008;28:3404–3414. doi: 10.1523/JNEUROSCI.0157-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkiewicz J, de Rivero Vaccari JP, Keane RW. Human astrocytes express a novel NLRP2 inflammasome. Glia. 2013;61:1113–1121. doi: 10.1002/glia.22499. [DOI] [PubMed] [Google Scholar]
- Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T, et al. The NALP3 inflammasome is involved in the innate immune response to amyloid-beta. Nat Immunol. 2008;9:857–865. doi: 10.1038/ni.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanamsagar R, Torres V, Kielian T. Inflammasome activation and IL-1beta/IL-18 processing are influenced by distinct pathways in microglia. J Neurochem. 2011;119:736–748. doi: 10.1111/j.1471-4159.2011.07481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi F, Yang Y, Kouadir M, Fu Y, Yang L, Zhou X, et al. Inhibition of phagocytosis and lysosomal acidification suppresses neurotoxic prion peptide-induced NALP3 inflammasome activation in BV2 microglia. J Neuroimmunol. 2013;260:121–125. doi: 10.1016/j.jneuroim.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Adamczak SE, de Rivero Vaccari JP, Dale G, Brand F, Nonner D, Bullock R, et al. Pyroptotic neuronal cell death mediated by the AIM2 inflammasome J Cereb Blood Flow Metab 2013(e-pub ahead of print). [DOI] [PMC free article] [PubMed]
- Tan MS, Yu JT, Jiang T, Zhu XC, Tan L. The NLRP3 inflammasome in Alzheimer's disease. Mol Neurobiol. 2013;48:875–882. doi: 10.1007/s12035-013-8475-x. [DOI] [PubMed] [Google Scholar]
- Hsu LC, Ali SR, McGillivray S, Tseng PH, Mariathasan S, Humke EW, et al. A NOD2-NALP1 complex mediates caspase-1-dependent IL-1beta secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc Natl Acad Sci USA. 2008;105:7803–7808. doi: 10.1073/pnas.0802726105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Liu W, Luo Y, Tanaka A, Cai X, Norris DA, et al. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J Biol Chem. 2010;285:6477–6488. doi: 10.1074/jbc.M109.064907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigano E, Mortellaro A. Caspase-11: the driving factor for noncanonical inflammasomes. Eur J Immunol. 2013;43:2240–2245. doi: 10.1002/eji.201343800. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC. Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J. 1999;18:5242–5251. doi: 10.1093/emboj/18.19.5242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A, Correa RG, Garrison JB, Le Negrate G, Welsh K, Huang Z, et al. XIAP mediates NOD signaling via interaction with RIP2. Proc Natl Acad Sci USA. 2009;106:14524–14529. doi: 10.1073/pnas.0907131106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, et al. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl G, Keane RW. Pannexin: from discovery to bedside in 11+/−4 years. Brain Res. 2012;1487:150–159. doi: 10.1016/j.brainres.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rivero Vaccari JP, Bastien D, Yurcisin G, Pineau I, Dietrich WD, De Koninck Y, et al. P2X4 receptors influence inflammasome activation after spinal cord injury. J Neurosci. 2012;32:3058–3066. doi: 10.1523/JNEUROSCI.4930-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marina-Garcia N, Franchi L, Kim YG, Miller D, McDonald C, Boons GJ, et al. Pannexin-1-mediated intracellular delivery of muramyl dipeptide induces caspase-1 activation via cryopyrin/NLRP3 independently of Nod2. J Immunol. 2008;180:4050–4057. doi: 10.4049/jimmunol.180.6.4050. [DOI] [PubMed] [Google Scholar]
- Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Resp Crit Care Med. 2010;182:774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- Kummer JA, Broekhuizen R, Everett H, Agostini L, Kuijk L, Martinon F, et al. Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J Histochem Cytochem. 2007;55:443–452. doi: 10.1369/jhc.6A7101.2006. [DOI] [PubMed] [Google Scholar]
- Im SS, Yousef L, Blaschitz C, Liu JZ, Edwards RA, Young SG, et al. Linking lipid metabolism to the innate immune response in macrophages through sterol regulatory element binding protein-1a. Cell Metab. 2011;13:540–549. doi: 10.1016/j.cmet.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Osualdo A, Reed JC. NLRP1, a regulator of innate immunity associated with vitiligo. Pigment Cell Melanoma Res. 2012;25:5–8. doi: 10.1111/j.1755-148X.2011.00942.x. [DOI] [PubMed] [Google Scholar]
- Finger JN, Lich JD, Dare LC, Cook MN, Brown KK, Duraiswami C, et al. Autolytic proteolysis within the function to find domain (FIIND) is required for NLRP1 inflammasome activity. J Biol Chem. 2012;287:25030–25037. doi: 10.1074/jbc.M112.378323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinsohn JL, Newman ZL, Hellmich KA, Fattah R, Getz MA, Liu S, et al. Anthrax lethal factor cleavage of Nlrp1 is required for activation of the inflammasome. PLoS Pathog. 2012;8:e1002638. doi: 10.1371/journal.ppat.1002638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruey JM, Bruey-Sedano N, Luciano F, Zhai D, Balpai R, Xu C, et al. Bcl-2 and Bcl-XL regulate proinflammatory caspase-1 activation by interaction with NALP1. Cell. 2007;129:45–56. doi: 10.1016/j.cell.2007.01.045. [DOI] [PubMed] [Google Scholar]
- Masters SL, Gerlic M, Metcalf D, Preston S, Pellegrini M, O'Donnell JA, et al. NLRP1 inflammasome activation induces pyroptosis of hematopoietic progenitor cells. Immunity. 2012;37:1009–1023. doi: 10.1016/j.immuni.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faustin B, Lartigue L, Bruey JM, Luciano F, Sergienko E, Bailly-Maitre B, et al. Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol Cell. 2007;25:713–724. doi: 10.1016/j.molcel.2007.01.032. [DOI] [PubMed] [Google Scholar]
- Schroder K, Tschopp J. The inflammasomes. Cell. 2010;140:821–832. doi: 10.1016/j.cell.2010.01.040. [DOI] [PubMed] [Google Scholar]
- Boyden ED, Dietrich WF. Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat Genet. 2006;38:240–244. doi: 10.1038/ng1724. [DOI] [PubMed] [Google Scholar]
- Bruey JM, Bruey-Sedano N, Newman R, Chandler S, Stehlik C, Reed JC. PAN1/NALP2/PYPAF2, an inducible inflammatory mediator that regulates NF-kappa B and caspase-1 activation in macrophages. J Biol Chem. 2004;279:51897–51907. doi: 10.1074/jbc.M406741200. [DOI] [PubMed] [Google Scholar]
- Conti BJ, Davis BK, Zhang JH, O'Connor W, Williams KL, Ting JPY. Caterpiller 16.2 (Clr16.2), a novel Nbd/Lrr family member that negatively regulates T Cell function. J Biol Chem. 2005;280:18375–18385. doi: 10.1074/jbc.M413169200. [DOI] [PubMed] [Google Scholar]
- Lich JD, Ting JPY. Monarch-1/PYPAF7 and other CATERPILLER (CLR, NOD, NLR) proteins with negative regulatory functions. Microbes Infect. 2007;9:672–676. doi: 10.1016/j.micinf.2007.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontalba A, Gutierrez O, Fernandez-Luna JL. NLRP2, an inhibitor of the NF-kappa B pathway, is transcriptionally activated by NF-kappa B and exhibits a nonfunctional allelic variant. J Immunol. 2007;179:8519–8524. doi: 10.4049/jimmunol.179.12.8519. [DOI] [PubMed] [Google Scholar]
- Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, et al. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol. 2009;183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Eigenbrod T, Nunez G. Cutting edge: TNF-alpha mediates sensitization to ATP and silica via the NLRP3 inflammasome in the absence of microbial stimulation. J Immunol. 2009;183:792–796. doi: 10.4049/jimmunol.0900173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer C, Duewell P, Mayer C, Lehr HA, Fitzgerald KA, Dauer M, et al. Colitis induced in mice with dextran sulfate sodium (DSS) is mediated by the NLRP3 inflammasome. Gut. 2010;59:1192–1199. doi: 10.1136/gut.2009.197822. [DOI] [PubMed] [Google Scholar]
- Jin C, Frayssinet P, Pelker R, Cwirka D, Hu B, Vignery A, et al. NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc Natl Acad Sci USA. 2011;108:14867–14872. doi: 10.1073/pnas.1111101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu X, Ma J, Wu J, Wang Y, Zhou R, et al. Gene deletion of Gabarap enhances Nlrp3 inflammasome-dependent inflammatory responses. J Immunol. 2013;190:3517–3524. doi: 10.4049/jimmunol.1202628. [DOI] [PubMed] [Google Scholar]
- Costa A, Gupta R, Signorino G, Malara A, Cardile F, Biondo C, et al. Activation of the NLRP3 inflammasome by group B streptococci. J Immunol. 2012;188:1953–1960. doi: 10.4049/jimmunol.1102543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eitel J, Meixenberger K, van Laak C, Orlovski C, Hocke A, Schmeck B, et al. Rac1 regulates the NLRP3 inflammasome which mediates IL-1beta production in Chlamydophila pneumoniae infected human mononuclear cells. PloS ONE. 2012;7:e30379. doi: 10.1371/journal.pone.0030379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- Martinon F, Tschopp J. Inflammatory caspases: linking an intracellular innate immune system to autoinflammatory diseases. Cell. 2004;117:561–574. doi: 10.1016/j.cell.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Dorhoi A, Nouailles G, Jorg S, Hagens K, Heinemann E, Pradl L, et al. Activation of the NLRP3 inflammasome by Mycobacterium tuberculosis is uncoupled from susceptibility to active tuberculosis. Eur J Immunol. 2012;42:374–384. doi: 10.1002/eji.201141548. [DOI] [PubMed] [Google Scholar]
- Burckstummer T, Baumann C, Bluml S, Dixit E, Durnberger G, Jahn H, et al. An orthogonal proteomic-genomic screen identifies AIM2 as a cytoplasmic DNA sensor for the inflammasome. Nat Immunol. 2009;10:266–272. doi: 10.1038/ni.1702. [DOI] [PubMed] [Google Scholar]
- Fernandes-Alnemri T, Yu JW, Datta P, Wu J, Alnemri ES. AIM2 activates the inflammasome and cell death in response to cytoplasmic DNA. Nature. 2009;458:509–513. doi: 10.1038/nature07710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ablasser A, Charrel-Dennis M, Bauernfeind F, Horvath G, Caffrey DR, et al. AIM2 recognizes cytosolic dsDNA and forms a caspase-1-activating inflammasome with ASC. Nature. 2009;458:514–518. doi: 10.1038/nature07725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinam VA, Jiang Z, Waggoner SN, Sharma S, Cole LE, Waggoner L, et al. The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat Immunol. 2010;11:395–402. doi: 10.1038/ni.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi: 10.1111/j.1600-065X.2011.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng K, Broz P, Jones J, Joubert LM, Monack D. Elevated AIM2-mediated pyroptosis triggered by hypercytotoxic Francisella mutant strains is attributed to increased intracellular bacteriolysis. Cell Microbiol. 2011;13:1586–1600. doi: 10.1111/j.1462-5822.2011.01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao EA, Leaf IA, Treuting PM, Mao DP, Dors M, Sarkar A, et al. Caspase-1-induced pyroptosis is an innate immune effector mechanism against intracellular bacteria. Nat Immunol. 2010;11:1136–1142. doi: 10.1038/ni.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeYoung KL, Ray ME, Su YA, Anzick SL, Johnstone RW, Trapani JA, et al. Cloning a novel member of the human interferon-inducible gene family associated with control of tumorigenicity in a model of human melanoma. Oncogene. 1997;15:453–457. doi: 10.1038/sj.onc.1201206. [DOI] [PubMed] [Google Scholar]
- Kopfnagel V, Wittmann M, Werfel T. Human keratinocytes express AIM2 and respond to dsDNA with IL-1beta secretion. Exp Dermatol. 2011;20:1027–1029. doi: 10.1111/j.1600-0625.2011.01382.x. [DOI] [PubMed] [Google Scholar]
- Dombrowski Y, Peric M, Koglin S, Kammerbauer C, Goss C, Anz D, et al. Cytosolic DNA triggers inflammasome activation in keratinocytes in psoriatic lesions. Sci Transl Med. 2011;3:82ra38. doi: 10.1126/scitranslmed.3002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi ME. DAMPs, PAMPs and alarmins: all we need to know about danger. J Leuk Biol. 2007;81:1–5. doi: 10.1189/jlb.0306164. [DOI] [PubMed] [Google Scholar]
- Said-Sadier N, Ojcius DM. Alarmins, inflammasomes and immunity. Biomed J. 2012;35:437–449. doi: 10.4103/2319-4170.104408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012;249:158–175. doi: 10.1111/j.1600-065X.2012.01146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariathasan S, Weiss DS, Newton K, McBride J, O'Rourke K, Roose-Girma M, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440:228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- Lu B, Nakamura T, Inouye K, Li J, Tang Y, Lundback P, et al. Novel role of PKR in inflammasome activation and HMGB1 release. Nature. 2012;488:670–674. doi: 10.1038/nature11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchi L, Kanneganti TD, Dubyak GR, Nunez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem. 2007;282:18810–18818. doi: 10.1074/jbc.M610762200. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F. Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci. 2007;28:465–472. doi: 10.1016/j.tips.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Petrilli V, Papin S, Dostert C, Mayor A, Martinon F, Tschopp J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Diff. 2007;14:1583–1589. doi: 10.1038/sj.cdd.4402195. [DOI] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X7 receptor death complex. FEBS lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodin P, Burnstock G. ATP-stimulated release of ATP by human endothelial cells. J Cardiovasc Pharmacol. 1996;27:872–875. doi: 10.1097/00005344-199606000-00015. [DOI] [PubMed] [Google Scholar]
- Qiu F, Dahl GP. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2008. [DOI] [PMC free article] [PubMed]
- Bargiotas P, Krenz A, Hormuzdi SG, Ridder DA, Herb A, Barakat W, et al. Pannexins in ischemia-induced neurodegeneration. Proc Natl Acad Sci USA. 2011;108:20772–20777. doi: 10.1073/pnas.1018262108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanstein R, Negoro H, Patel NK, Charollais A, Meda P, Spray DC, et al. Promises and pitfalls of a Pannexin1 transgenic mouse line. Front Pharmacol. 2013;4:61. doi: 10.3389/fphar.2013.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago MF, Veliskova J, Patel NK, Lutz SE, Caille D, Charollais A, et al. Targeting pannexin1 improves seizure outcome. PloS ONE. 2011;6:e25178. doi: 10.1371/journal.pone.0025178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North RA, Surprenant A. Pharmacology of cloned P2X receptors. Ann Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- Bernier LP. Purinergic regulation of inflammasome activation after central nervous system injury. J Gen Phys. 2012;140:571–575. doi: 10.1085/jgp.201210875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomura S, de Rivero Vaccari JP, Keane RW, Bramlett HM, Dietrich WD. Effects of therapeutic hypothermia on inflammasome signaling after traumatic brain injury. J Cereb Blood Flow Metab. 2012;32:1939–1947. doi: 10.1038/jcbfm.2012.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamczak S, Dale G, de Rivero Vaccari JP, Bullock MR, Dietrich WD, Keane RW. Inflammasome proteins in cerebrospinal fluid of brain-injured patients as biomarkers of functional outcome: clinical article. J Neurosurg. 2012;117:1119–1125. doi: 10.3171/2012.9.JNS12815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawhinney LJ, de Rivero Vaccari JP, Dale GA, Keane RW, Bramlett HM. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci. 2011;12:123. doi: 10.1186/1471-2202-12-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, et al. Glyburide inhibits the Cryopyrin/Nalp3 inflammasome. J Cell Biol. 2009;187:61–70. doi: 10.1083/jcb.200903124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll M, Kuemmerle-Deschner JB. Inflammasome and cytokine blocking strategies in autoinflammatory disorders. Clin Immunol. 2013;147:242–275. doi: 10.1016/j.clim.2013.04.008. [DOI] [PubMed] [Google Scholar]
- Man SM, Tourlomousis P, Hopkins L, Monie TP, Fitzgerald KA, Bryant CE. Salmonella infection induces recruitment of Caspase-8 to the inflammasome to modulate IL-1beta production. J Immunol. 2013;191:5239–5246. doi: 10.4049/jimmunol.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]