Abstract
In stroke, there is an imperative need to develop disease-modifying drugs able to (1) induce neuroprotection and vasculoprotection, (2) modulate recovery and brain plasticity, and (3) limit the short-term motor and cognitive consequences. We hypothesized that fenofibrate, a peroxisome proliferator-activated receptor-α (PPAR-α) agonist, could exert a beneficial effect on immediate and short-term poststroke consequences related to its pleiotropic mechanisms. Rats or mice were subjected to focal ischemia to determine the effects of acute treatment by fenofibrate on (i) motor and memory impairment, (2) both cerebral and vascular compartments, (3) inflammation, (4) neurogenesis, and (5) amyloid cascade. We show that fenofibrate administration results in both neuronal and vascular protection and prevents the short-term motor and cognitive poststroke consequences by interaction with several mechanisms. Modulation of PPAR-α generates beneficial effects in the immediate poststroke consequences by mechanisms involving the interactions between polynuclear neutrophils and the vessel wall, and microglial activation. Fenofibrate modulates mechanisms involved in neurorepair and amyloid cascade. Our results suggest that PPAR-α agonists could check the key points of a potential disease-modifying effect in stroke.
Keywords: brain ischemia, inflammation, neuroprotection, peroxisome proliferator-activated receptor-alpha
Introduction
Of the pharmacological targets likely to induce a pleiotropic brain effect after stroke, the three isoforms (α, β/δ, γ) of peroxisome proliferator-activated receptors (PPARs) are prime candidates to induce a disease-modifying effect with pleiotropic mechanisms that target the whole neurovascular unit:1 (1) to induce a protective effect on brain, (2) to induce brain repair processes favoring short- and long-term functional recovery, and (3) to modulate the molecular pathways that may contribute to the occurrence of postischemic cognitive impairment (such as the amyloid cascade).2, 3, 4 As nuclear receptors, they upregulate or downregulate genes that in turn influence many of the pathophysiological pathways involved in ischemic damage, neurodegeneration, and brain repair processes.5, 6, 7, 8
The three isoforms have distinct physiological functions and we particularly focused on the PPAR-α isoform because of its potential pharmacological properties on oxidative stress, inflammation, leukocyte–endothelium interactions, stem cells, and amyloid cascade.9, 10, 11 Indeed fenofibrate, a direct synthetic ligand of PPAR-α isoform, showed a preventive neuroprotective effect decreasing oxidative stress depending on the increase in numerous antioxidant enzyme activities, and preventing ischemia-induced expression of vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 (ICAM-1) in cerebral vessels.12 Furthermore, PPAR-α has been described to be involved in pathways present also in the control of proliferation, migration, and differentiation of neural stem cells (Wnt, STAT3, and NF-κB pathways).9
Peroxisome proliferator-activated receptor-α isoform is expressed by all three cell compartments in the neurovascular unit.13 Therefore, this receptor may be an interesting target able to modulate the immediate consequences of stroke (in particular, vascular dysfunction and cell necrosis) and potentially further modify the short- and long-term consequences of stroke (in particularly, functional recovery, neurogenesis, and stroke-induced memory impairment). Fenofibrate has a long-term clinical experience for its use as a lipid-lowering agent. In this study, we investigated whether fenofibrate administered during the acute phase of stroke could exert a beneficial effect on immediate and short-term poststroke lesional, functional consequences and stroke-induced memory alterations.
Materials and methods
Animals
All animal protocols were performed in strict accordance with the Ethical Committee in Animal Experimentation of Nord-Pas-de-Calais (C2EA-75) and the European community legislation (2010/63/UE). The experiments are reported in accordance with the ARRIVE guidelines. Male Wistar rats (mean weight 300 g) (Elevage Janvier, Le Genest Saint-Isle, France) or male C57BL/6 wild-type mice (mean weight 25 g) (Elevage Janvier) were used. Animals were housed in a light- and temperature-controlled environment with unlimited access to food and water. Animals were randomly assigned to the different groups. Experimental data were monitored by blinded investigator for group allocation. The study included all animals that underwent the whole protocol (72 hours or 7 days) and excluded from results nonischemic animals or animals with subcortical infarcts (10% to 15% per group).
Drug
Fenofibrate (F-6020; Sigma-Aldrich, Chimie Lyon, France) was dissolved in vehicle (water; 0.1% Tween-80; 0.5% carboxymethylcellulose) and orally administered poststroke by gavage twice a day during 72 hours or 7 days.
Experimental Design
Two separate protocols were performed, the first concerning the early administration of fenofibrate and the dose-response effect, the second concerning the delayed administration of fenofibrate and the short-term consequences (details of protocols in Supplementary Data). STAIR recommendations were considered to design the two protocols.14
Protocol 1: Early 72 hours fenofibrate treatment
To assess the effect of PPAR-α activation on the acute phase of cerebral ischemia, male Wistar rats (6 to 12 animals per group) underwent a 1-hour middle cerebral artery occlusion (MCAo), followed by 72-hour reperfusion. Fenofibrate (50 or 200 mg/kg per day) or vehicle was administered by gavage twice a day up to 72 hours after onset of ischemia, with the first dose given at 1 hour after ischemia. Sham-operated animals were treated identically, except that the middle cerebral arteries were not occluded. Assessments included motor behavior and infarct volume at 24 and 72 hours (6 to 12 animals per group). Plasmatic dosages of fenofibric acid (FFA) were performed at 24 and 72 hours.
Vascular reactivity was assessed in vitro in rats (4 to 5 animals per group) 72 hours after the surgery. Markers of the inflammation (myeloperoxidase (MPO), Ox-42, and ICAM-1) were studied at 24 and 72 hours after ischemia by immunohistochemistry and western blotting.
For methodological reasons (intravital microscopy limited to the mouse), we acquired additional data in mice submitted or not to MCAo, with the observation of in vivo microcirculation in arterioles and venules at 24 and 72 hours (10 animals per group) and the study of in vivo vascular reactivity at 72 hours (8 animals per group).
Protocol 2: Delayed 7 days fenofibrate treatment
We studied the effect of a delayed poststroke administration of fenofibrate on motor recovery, neurogenesis, and stroke-induced memory impairment. Fenofibrate (200 mg/kg per day) or vehicle was administered by gavage twice a day during 7 days, with the first dose given only 8 hours after onset of ischemia or sham surgery. The animals were killed 7 days after MCAo. The hepatic weight was measured at 72 hours and 7 days.
Neurologic score, grip strength test (24 hours, 72 hours, and 7 days) and infarct volume (7 days) were assessed (6 to 14 animals per group).
Neurogenesis was evaluated at 7 days (4 animals per group) and PPAR-α expression was studied 7 days after ischemia by immunohistochemistry (8 to 12 animals per group).
Stroke-induced memory impairment was assessed by cognitive test (Y-maze; 16 to 25 animals per group), immunochemistry with amyloid deposition (4 animals per group) and quantitative PCR with amyloid-related secretases (3 animals per group) at 7 days after ischemia.
Middle Cerebral Artery Occlusion Model
Cerebral infarct was induced by intraluminal MCAo as previously described.15 Anesthesia was induced by intraperitoneal chloral hydrate administration (300 mg/kg). A rectal probe was inserted and body temperature was maintained at 37±0.5°C with a heating lamp. The caudal artery was exposed, cannulated with a 24 G polyethylene catheter and connected to a blood pressure monitor. The mean arterial blood pressure (mm Hg) was monitored throughout the experiment and blood samples were taken before, during, and after ischemia, to measure blood pH, arterial PaO2 (mm Hg), and arterial PaCO2 (mm Hg). Briefly, the right carotid arteries were exposed through a midline cervical incision and the common carotid and external carotid arteries were ligated with a silk suture. An aneurysm clip was placed across the internal carotid artery and an arteriotomy was made in the common carotid artery stump, allowing the introduction of a monofilament nylon suture with its tip rounded by flame heating. The suture was gently advanced into the internal carotid artery and passed into the intracranial circulatory system as far as in the narrow lumen at the start of the middle cerebral artery. After 30 minutes for mice or 60 minutes for rats, the suture was carefully removed until its tip was blocked by a ligature placed on the common carotid artery (to allow reperfusion). As in index of the reliability of the MCAo model, in vivo magnetic resonance imaging was performed in a set of randomly selected animals (n=3 per group) 24 hours after the surgery in a 7-Tesla, narrow-bore small animal imaging system (Biospec 70/20 USR; Bruker Biospin, Wissembourg, France) to document infarct. We acquired two-dimensional, T2-weighted images using a turboRARE pulse sequence (relaxation time: 2,500 ms; echo time: 65 ms; field of view: 4 × 4 cm; matrix: 256 × 256, RARE factor: 8).
Neurologic Assessment
Neurologic function was measured at 24 hours, 72 hours, and 7 days after MCAo using the Bederson score:16 an animal with no apparent deficits obtained=0; the presence of forelimb flexion=1; decreased resistance to push=2; and circling=3.
Behavioral Assessment
Rotarod test
Motor coordination was evaluated 24 and 72 hours after the transient MCAo or sham procedure. The rats were placed on an accelerating rotarod.17 The speed of the rotarod was increased from 4 to 40 rotations per minute in 2 minutes. The latency to fall off the rotarod was recorded. Each rat was tested once. All rats were trained for 5 continuous days before the formal tests.
Grip strength test
Forelimb grip strength was determined by an automated grip strength meter at 24 hours, 72 hours, and 7 days after the transient MCAo or sham procedure. The experimenter grasped the rat by the tail and suspended it above a grip ring.18 After 3 seconds, the animal was gently lowered toward the grip ring and allowed to grasp the ring with its forepaws. The experimenter then quickly lowered the remainder of the animal's body to a horizontal position and tugged the animal's tail until its grasp of the ring was broken. The mean force in Newtons was determined with a computerized electronic pull strain gauge.
Y-maze test
The rats were evaluated for spatial memory in one wooden Y-maze before killing at 7 days after the transient MCAo. The maze was lacquered black, and consisted of three arms with an angle of 120° between each two arms.19 The three identical arms were randomly designated: Start arm, in which the rats started to explore (always open); Novel arm, which was blocked at the first trial, but open at the second trial; and Other arm (always open). The Y-maze test consisted of two trials separated by an intertrial interval to assess spatial recognition memory. The first trial (training) lasted 10 minutes and allowed the rat to explore only two arms (Start arm and Other arm) of the maze, with the third arm (Novel arm) being blocked. After a 1-hour intertrial interval, the second trial (retention) was conducted. The rat was placed back in the maze in the same starting arm, with free access to all three arms for 300 seconds. The first arm visited, the number and duration of explorations of each arm were recorded during the second trial.
Infarct Volume Measurement
The animals were killed with an overdose of intraperitoneal pentobarbital (200 mg/kg) 24 hours, 72 hours, or 7 days after reperfusion. Brains were removed, frozen and coronally dissected into 50-μm-thick slices on a cryostat at 12 levels in 1 mm steps. Sections were stained by cresyl fast violet. The unstained area of the brain was defined as the infarct zone. Infarcted cortical and subcortical areas and hemispheric areas were calculated separately for each coronal slice using the image analysis software Color Image 1.32 (NIMH, Bethesda, MD, USA) after digitization.
Quantification of Plasmatic Fenofibric Acid
The major metabolite of fenofibrate is fenofibric acid. Quantification of plasmatic FFA will be performed by liquid chromatography and MS/MS method.20 Fenofibric acid and carbamazepine (internal standard) were prepared in methanol. Calibration curve of FFA was realized at the following concentrations: 100 μg/mL; 200 μg/mL; 500 μg/mL; 1,000 μg/mL; and 2,000 μg/mL. Internal standard (carbamazepine 0.5 μg/mL) was added. In all, 100 μL of plasma sample was obtained from rats receiving fenofibrate 50 or 200 mg/kg per day (n=5 animals for each dose) after induction of cerebral ischemia at 24 and 72 hours. After addition of ethyl acetate 3 mL and acetate buffer (500 μL, pH 1), the mixtures were vortexed, followed by centrifugation at 2,200 g. The organic layer was separated and evaporated to dryness using a gentle stream of nitrogen. The residue was reconstituted in 100 μL of buffer ammonium formiate 5 mmol/L, pH 3/methanol (20/80 v-v). The LC system is a Perkin Acquity UPLC-TQDetector (Waters, Guyancourt, France). In all, 15 μL of the samples was injected on Acquity UPLC HSS C18 column (150 × 2.1 × 1.8 μm). Detection of the ion was performed in the multiple reaction-monitoring mode, monitoring the transition of the m/z 319.3 uma precursor ion to the m/z 233.1 product ion for FFA and m/z 237.2 precursor ion to the 194 product ion for internal standard. The data analysis is processed by the Analyst software (version 1.4.1, AB SCIEX, Les Ulis, France). Results are expressed in ratio of areas under the curve of FFA and internal standard carbamazepine.
Vascular Reactivity
In vitro vasoreactivity analysis (in rats)
Endothelium-dependent and -independent relaxations were assessed in a Halpern arteriograph (Living Systems Instrumentation, Burlington, VT, USA). We used a proximal segment of the right middle cerebral artery perfused with oxygenated Krebs solution and maintained at 37°C and pH 7.4. The lumen diameter was measured using image analysis.21 The relaxant dose-response curve for acetylcholine (ACh) was determined by stepwise, cumulative addition (from 10−9 mol/L to 3.10−5 mol/L ACh) after artery preconstriction with 5-hydroxytryptamine (10−6 mol/L induced 90% of the maximum constriction). To test nitric oxide-mediated smooth muscle relaxation, a single concentration of sodium nitroprusside (10−5 mol/L) was added to the bath after artery preconstriction. Relaxant responses were expressed as the percent increase in the preconstricted artery diameter.
Intravital Microscopy (In Mice)
Animals were anesthetized by intraperitoneal injection of 300 mg/kg chloral hydrate. The head of each mouse was placed in a stereotaxic frame. A craniotomy was performed with high-speed drill (S791, Bien-Air, Bienne, Switzerland) in the right parietal bone. The dura matter was retracted, and the surface of exposed brain was continuously superfused with artificial cerebrospinal fluid at 37°C and pH 7.4.
The cerebral microcirculation was observed with the use of an intravital microscope. An Eclipse 50i Nikon microscope (Nikon, Champigny-Sur Marne, France) fitted with a Xenon light source and epi-fluorescence assembly was used with filter sets for acridine orange (excitation: 470 nm, full width at half maximum 40; emission 540 nm, full width at half maximum 40). A video-camera (Model E2v, L3Vision, Bievres, France) mounted on the microscope projected the image onto a monitor.
Observation of cerebral microcirculation
Leukocyte accumulation was assessed after 24 and 72 hours of reperfusion. For each mouse, five randomly selected venular and arteriolar segments, 25 to 50 μm in diameter and 100 μm long, were evaluated for 1 minute for leukocyte recruitment. The mean for each parameter was calculated. The leukocyte behavior was observed after the intraperitoneal injection of 0.3 mL of 1% acridine orange (Molecular Probes, Invitrogen, France). Rolling leukocytes were defined as cells crossing the 100 μm vascular segment at a significantly lower velocity than centerline velocity of leukocytes. Leukocytes were considered adherent to the endothelium if they stuck and remained stationary for at least 30 seconds.
Acetylcholine-induced vasodilatation of pial arterioles
The arteriolar diameter was measured using a calibrated reticule in the Metamorph software (Molecular Imaging, Roper Scientific, Evry, France) 30 minutes after surgery of the skull (basal diameter) and 5 minutes after topical administration of ACh (10−5 mol/L) (diluted in the artificial cerebrospinal fluid).22 The response to ACh is expressed as the percent change in diameter compared with the basal arteriolar diameter. Endothelial function is evaluated as in the four groups of mice at 72 hours of reperfusion.
Immunohistochemistry
After 24 hours, 72 hours, or 7 days of reperfusion, the rats were anesthetized and perfused through the heart with cold saline and 4% paraformaldehyde. After 24 hours of fixation in 4% paraformaldehyde at 4 °C, the brains were cryoprotected in 20% and 30% sucrose solutions in phosphate-buffered saline at 4 °C. Parallel sets of brain sections (20-μm thick) were immnunostained with antibodies against Ox-42 (a marker of microglial activation), MPO, ICAM-1, PPAR-α, and amyloid-β to assess the effect of fenofibrate.23 After fixation, brain sections were blocked with 10% normal goat serum and incubated overnight at 4°C with mouse anti-rat Ox-42 (1:500; MCA275R, Serotec, Colmar, France), rabbit polyclonal anti-MPO (1:500; A0398, DAKO, Les Ulis, France), mouse anti-rat ICAM-1 (1:500; MCA773, Serotec), mouse anti-rat PPAR-α (1:100; Affinity Bioreagents, Golden, CO, USA) and rabbit anti-rodent amyloid-β (1:3,000, Signet, Covance, Rueil-Malmaison, France) antibodies. Next, sections were incubated with biotinylated horse anti-mouse IgG for Ox-42, ICAM-1, and PPAR-α, and anti-rabbit IgG for MPO and amyloid-β for 3 hours at room temperature, followed by treatment with an avidin-biotinylated enzyme complex (ABC kit; Vector Laboratories, Eurobio/Abcys, Les Ulis, France) and finally diaminobenzidine tetrahydrochloride. Neutrophil infiltration, microglial activation, PPAR-α, and amyloid-β were quantified at one coronal level (+0.48 mm relative to Bregma) counting positive cells on six adjacent fields in the ischemic zone. As controls, we used brain sections of sham rats, submitted to surgery for MCAo, without advancing the intraluminal filament into the internal carotid artery.
Bromodeoxyuridine Administration and Immunofluorescence
Bromodeoxyuridine (BrdU; Sigma-Aldrich) was administered intraperitoneally in two injections of 30 mg/kg per day between the third and sixteenth days preceding the week of behavioral tests, and a remainder 30 mg/kg 24 hours before killing.24 A double labelling BrdU-NeuN or BrdU-PPAR-α was performed on the brains sections. Sections of 14 μm were taken along eight defined stages. The sections were kept at −80°C until use. For labelling, the nonspecific sites were blocked (Normal Goat Serum 2%, Triton X-100 0.3%) for 10 minutes. Finally, the primary antibodies were deposited in the recommended dilution for each antibody in the serum blocking solution at 4°C overnight (mouse anti-rat anti-NeuN, 1:200, VMA377, Eurobio/Abcys, Les Ulis, France; rat anti-BrdU, 1:100, MCA2060, Serotec; mouse anti-rat PPAR-α, 1:100, Affinity Bioreagents). Next, brain sections were incubated with secondary antibodies (AlexaFluor 488 and 568, 1:400) prepared in blocking serum at room temperature incubation for 1 hour. The slides were then placed in the presence of VectaShield. Fluorescent images were obtained using confocal microscopy. Specific laser and channels for excitation and emission of the fluorescence were used, as appropriated for each fluorescent compound. Colabelled cells by NeuN and BrdU were quantified at one coronal level (+0.48 mm relative to Bregma) counting positive cells on four adjacent fields in the ischemic zone (cortex and striatum).
Western Blotting
Western blot analysis was performed on cortex homogenates. We analyzed the expression of ICAM-1 72 hours after cerebral ischemia in control, ischemic and treated animals (anti-ICAM-1, 1:500; MCA773, Serotec). For the experiment, blots were probed with β-actin (1:500; Sigma-Aldrich) as an internal control. After digitization, densitometric values were evaluated using the Image Analysis software.
RNA Extraction and Reverse Transcription
Total RNA was extracted from dissected brains with Extract-all (Eurobio, Les Ulis, France) according to the kit manufacturer protocol. Quantitative PCR was performed using a LightCycler system according to the manufacturer's instructions with LightCycler-FastStartDNA Master SYBR Green mix (Roche Applied Science, Meylan, France). Protocol consisted in a hot start step (8 minutes at 95 °C) followed by 50 cycles including a 10-second denaturation step (95°C), a 10-second annealing step (60°C), and a 10-second elongation step at 72°C. PCR efficiencies were optimized according to Roche Applied Science's recommendations on a standard sample expressing all studied genes. To confirm amplification specificity, PCR products were subjected to a melting curve analysis. Quantification data represent the mean of three experiments from four animals in each group. Relative quantification analyses were performed by the RelQuant 1.01 Software (Roche Applied Science). The sequences for the primers used for each of the genes analyzed are the following: Actin Up 5′-agccatgtacgtagccatcc-3′, Low 5′-ctctcagctgtggtggtgaa-3′ A Disintegrin And Metalloproteinase-10 (ADAM-10) Up 5′-gaggaaaaagcgcacaactc-3′, Low 5′-tgttacggattccggagaag-3′ Amyloid Precursor Protein (APP) Up 5′-gcggacacagactatgctga-3′, Low 5′-ctctgtggcctcttcgtagg-3′ Beta-site APP Cleaving Enzyme-1 (BACE-1) Up 5′-gctgcagtcaagtccatcaa-3′, Low 5′-attgctgaggaaggatggtg-3′, Presenilin-1 (PS-1) Up 5′-ggtccacttcgtatgctggt-3′, Low 5′-ctcccactcctcactgaagc-3′ Presenilin-2 (PS-2) Up 5′-ccctgatgctcctcttcttg-3′, Low 5′-agtgcgctgatcacaatgag-3′.
Statistical Analysis
All values were expressed as mean±standard error mean (s.e.m.). Statistical analysis was performed using the SPSS 12.0 software (IBM, Boigny-sur Bionne, France). Comparisons among multiple groups were performed using one-way analysis of variance followed by a post hoc Protected Least Significant Difference Fisher test if variance analysis was significant post hoc. Comparisons between two groups were achieved using Mann–Whitney test. A value of P<0.05 was considered to indicate statistical significant.
Results
Physiological Parameters
There were no differences in physiologic parameters (weight, blood pressure, temperature, pH, PaCO2, and PaO2) between vehicle and fenofibrate-treated groups before, during, and after the surgical procedure (data not shown).
Fenofibrate Induces an Acute Neuroprotective Effect in a Dose-Dependent Manner
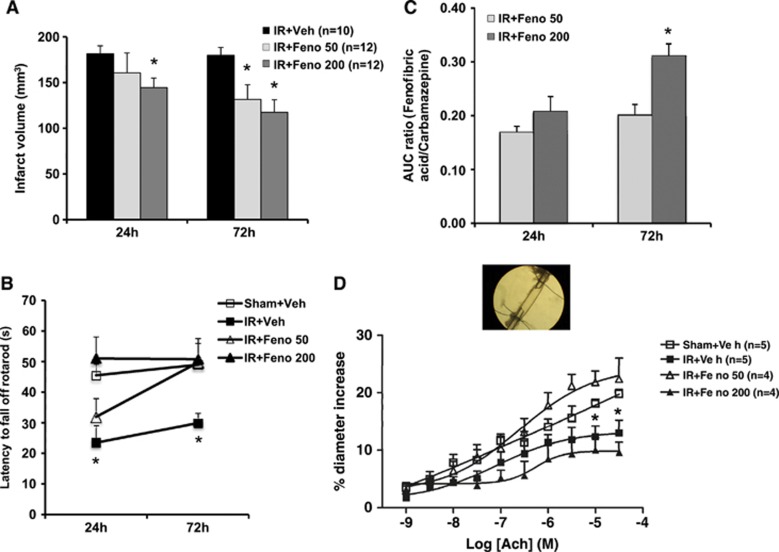
Fenofibrate reduced the size of the infarct (Figure 1A) in a dose-dependent manner. The decrease in infarct size was significant at 72 hours for 50 and 200 mg/kg per day of fenofibrate, with a higher neuroprotective effect at 200 mg/kg per day. On the motor level, early administration (1 hour after the onset ischemia) of fenofibrate improved postischemia performances evaluated in the rotarod test at 24 and 72 hours (Figure 1B). The functional recovery was faster at the dose of 200 mg/kg per day than 50 mg/kg per day. These effects were parallel to the FFA plasmatic concentration (Figure 1C).
Figure 1.
Dose-effect study of fenofibrate. (A) Infarct volumes determined 24 and 72 hours after ischemia were lower in fenofibrate-treated rats (n=12) than in control rats (n=10). *P<0.05 vs. IR+Veh group. (B) The postischemia functional impairment (as evaluated in the rotarod test) was smaller in fenofibrate-treated rats (n=12) than in ischemic animals (n=10) 24 and 72 hours after the induction of cerebral ischemia. *P<0.05 vs. Sham+Veh group. (C) Plasmatic fenofibric acid increased in a dose-dependent manner at 24 and 72 hours. *P<0.05 vs. IR+Feno 50 group. (D) In vitro, the application of increasing concentrations of acetylcholine (ACh) to the middle cerebral artery led to endothelium-dependent relaxation, which was impaired in the aftermath of IR. This dysfunction was abolished in fenofibrate-treated rats at 50 mg/kg per day (n=4). *P<0.05 vs. Sham+Veh group. AUC, area under the curve; IR, ischemia/reperfusion.
Fenofibrate Prevents Postischemic Endothelial Dysfunction
In the aftermath of cerebral ischemia, the vascular wall could participate in the pathophysiology of postischemic impairment.25 The ischemia-reperfusion process altered ACh-induced endothelium-dependent relaxation at 72 hours in vitro (Figure 1D). Administration of fenofibrate after ischemia prevented this endothelial dysfunction at 50 mg/kg per day but not at 200 mg/kg per day. Endothelium-independent relaxing responses to sodium nitroprusside were similar in all groups.
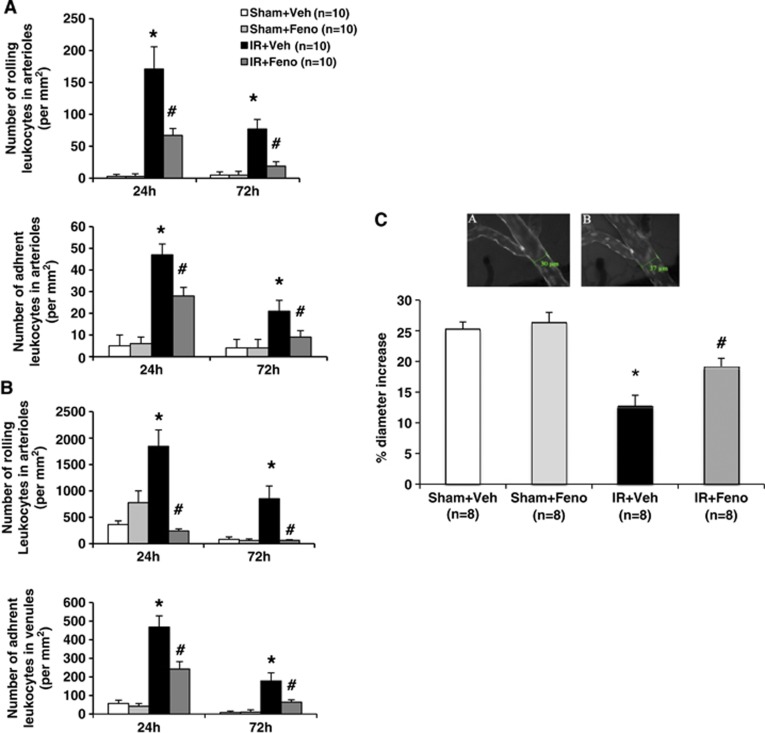
Fenofibrate Induces an Acute Protective Effect on Leukocyte Rolling and Adhesion
To explore the protective effect of fenofibrate on vascular compartment at 50 mg/kg per day, we studied in vivo their effects on leukocyte rolling and adhesion by videomicroscopy in mice as neutrophils could contribute to the endothelial dysfunction.21 Cerebral ischemia induced an increase in rolling and adherent leukocytes in arterioles (Figure 2A) and veins (Figure 2B) at 24 and 72 hours. Fenofibrate prevented the leukocyte rolling and adhesion otherwise evidenced 24 and 72 hours after the ischemia in arterioles (Figure 2A) and veins (Figure 2B). In vivo fenofibrate induced also a partial vasculoprotective effect (Figure 2C) when it is administered immediately after experimental brain ischemia in mice.
Figure 2.
Effects of treatment with the peroxisome proliferator-activated receptor-α (PPAR-α) agonist fenofibrate on interaction leukocyte–endothelium during the acute phase of cerebral ischemia and vascular reactivity in vivo. (A) In murine venules, fenofibrate treatment induced from 24 to 72 hours a significant decrease in leukocyte rolling and adhesion (n=10 per group); *P<0.05 vs. Sham+Veh group; #P<0.001 vs. IR+Veh group. (B) In murine arterioles, fenofibrate treatment induced from 24 to 72 hours a significant decrease in leukocyte rolling and adhesion (n=10 per group); *P<0.05 vs. Sham+Veh group; #P<0.001 vs. IR+Veh group. (C) In vivo, acetylcholine (ACh)-induced endothelium-dependent relaxation was determined by videomicroscopy of the murine pial arteries (A: the absence of ACh; B: the presence of ACh). The dysfunction in relaxation usually seen under ischemic conditions was abolished by fenofibrate treatment (n=8 per group). *P<0.05 vs. Sham+Veh group; #P<0.05 vs. IR+Veh group. Scale bar, 100 μm. IR, ischemia/reperfusion.
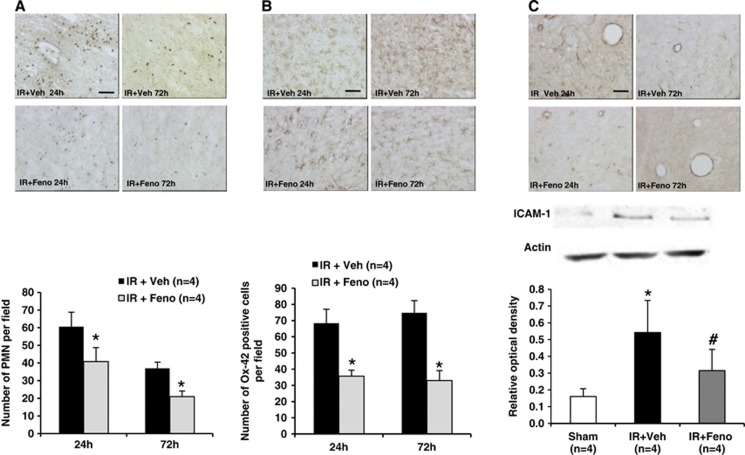
Fenofibrate Prevents Both Parenchymatous and Vascular Inflammation
On the tissue level, postischemic inflammation resulted in tissue expression of MPO (a marker of polymorphonuclear leukocytes (PMNs) infiltration) and Ox-42 (a marker of microglial activation). Ischemia induced an increase in PMNs infiltration in the ischemic area (Figure 3A) as well as microglial activation (Figure 3B) at 24 and 72 hours. Fenofibrate administration at 50 mg/kg per day significantly reduced parenchymatous infiltration by circulating PMNs (Figure 3A) and in situ microglial activation (Figure 3B) at 24 and 72 hours in the ischemic area. Furthermore, fenofibrate administration prevented the postischemia increase in the expression of endothelial adhesion proteins ICAM-1 (Figure 3C).
Figure 3.
Effects of treatment with the peroxisome proliferator-activated receptor-α (PPAR-α) agonist fenofibrate on postischemic inflammation in rats. (A) Neutrophil infiltration was decreased after the induction of ischemia by fenofibrate treatment (n=4 per group); *P<0.05 vs. IR+Veh group. Scale bar, 100 μm. (B) Immunohistochemical quantification of the expression of Ox-42 revealed a significant decrease after fenofibrate treatment (n=4 per group); *P<0.05 vs. IR+Veh group. Scale bar, 25 μm. (C) Quantitative evaluation of the intercellular adhesion molecule-1 (ICAM-1) (using immunohistochemistry and western blotting) revealed a significant decrease in the expression of this protein during fenofibrate treatment (n=4 per group) after the induction of ischemia; *P<0.05 vs. Sham group; #P<0.05 vs. IR+Veh group. Scale bar, 100 μm. IR, ischemia/reperfusion.
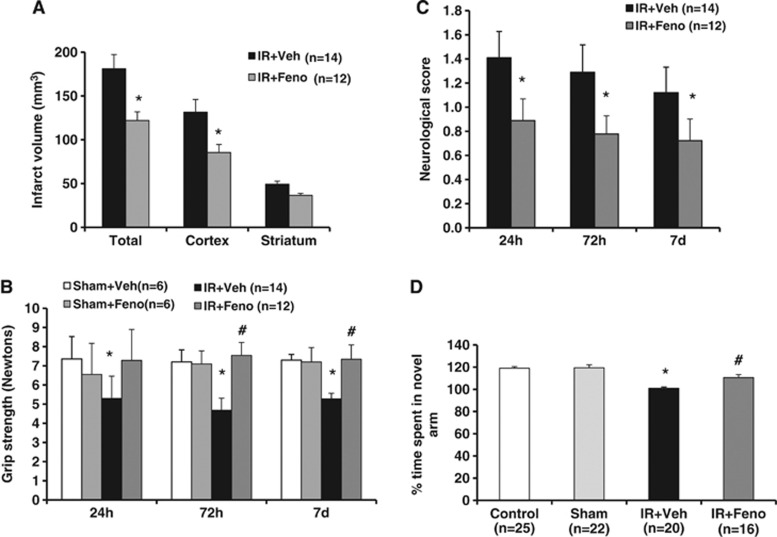
Fenofibrate Induces a Delayed Protective Effect on Infarct Volume, Motor Function, Neurologic Deficit, and Memory Recovery
Delayed administration of fenofibrate 200 mg/kg per day (8 hour after the start of reperfusion) also enabled a reduction in infarct size after 7 days of treatment (Figure 4A). Fenofibrate increased gradually liver weights of animals at 72 hours and 7 days compared with vehicle-treated animals. At 24 hours, mortality was more important in vehicle-treated group as compared with fenofibrate-treated group. Then, at 72 hours and 7 days, this difference was not observed between the treated animals and the control group (data not shown).
Figure 4.
Effects of delayed treatment with the peroxisome proliferator-activated receptor-α (PPAR-α) agonist fenofibrate on infarct volume, motor level, neurologic deficit, and memory recovery. (A) Delayed administration of fenofibrate enabled a reduction in infarct size. *P<0.05 vs. IR+Veh group. (B) Delayed and prolonged administration of fenofibrate induced motor improvements in grip strength test. *P<0.05 vs. Sham+Veh group; #P<0.05 vs. IR+Veh group. (C) Fenofibrate prevented neurologic deficits induced by ischemia. *P<0.05 vs. IR+Veh group. (D) The postischemia visuo-spatial memory impairment (as evaluated in Y-maze test) was prevented in fenofibrate-treated rats (n=16) than in ischemic animals (n=20). *P<0.05 vs. Sham group; #P<0.05 vs. IR+Veh group. IR, ischemia/reperfusion.
The neurologic deficit at 24 hours, 72 hours, and 7 days after ischemia onset was decreased in the fenofibrate-treated group (Figure 4C). Delayed and prolonged administration of fenofibrate produced motor improvement until 7 days after ischemia in the grip strength test (Figure 4B).
In the Y-maze test, the total number of visits in the three arms did not differ between the sham and ischemic groups (data not shown). Differences in the time spent in the new arm of the Y-maze observed among sham and ischemic groups were due to ischemic memory deficits and not locomotor deficits. On the cognitive level, a 7-day course of fenofibrate prevented the postischemic visuo-spatial memory impairment. Postischemic rats experienced a memory deficiency in the Y-maze test since they spend as much time in the new arm of the labyrinth as in the arm already explored. This defect in memory-based learning is partially prevented by fenofibrate administration (Figure 4D).
Delayed Treatment by Fenofibrate Modulates Postischemic Neurogenesis
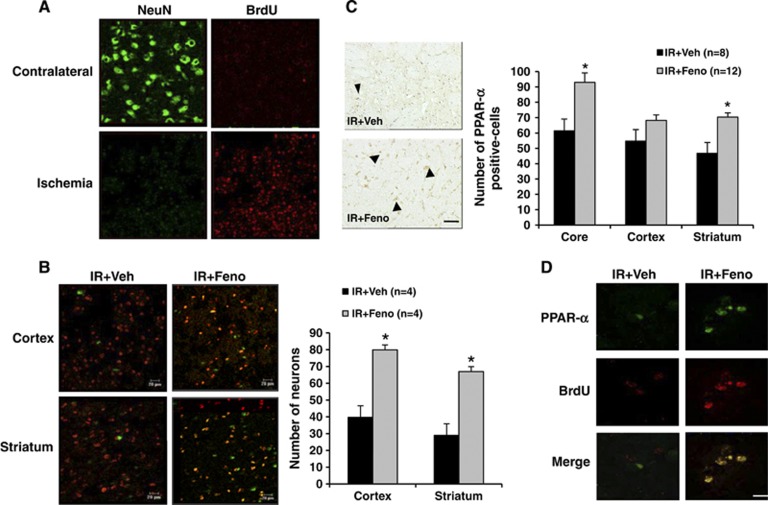
We showed that some of BrdU-labelled cells co-express PPAR-α. In the aftermath of brain ischemia, cell proliferation occurred in the ischemic focus, as evidenced by a significant increase in the number of cells incorporating BrdU (Figure 5A). Some of these cells differentiate into neurons, as shown by BrdU and NeuN colabelling (Figure 5B). Fenofibrate administration significantly increased cell proliferation and the number of cells colabelled by BrdU and NeuN both in the striatum and in the cortex. In the striatal or cortical ischemic focus, there was evidence of a significant increase in the number of NeuN-labelled cells (Figure 5B). After 7 days of fenofibrate treatment, the number of PPAR-α-expressing cells was also significantly higher (Figure 5C). The number of cells coexpressing PPAR-α and BrdU or PPAR-α and NeuN were significantly higher than in vehicle-treated rats. Fenofibrate administration significantly increased cell proliferation of cells colabelled by BrdU and PPAR-α (Figure 5D).
Figure 5.
Evaluation 7 days after the induction of cerebral ischemia of the peroxisome proliferator-activated receptor-α (PPAR-α) agonist fenofibrate effects on neurogenesis in rat. (A) Cell proliferation occurred in the ischemic area as evidenced by a significant increase in the number of cells incorporating bromodeoxyuridine (BrdU) compared with contralateral area of the lesion induced by ischemia. Scale bar, 20 μm. (B) Some of these cells differentiate into neurons, as shown by BrdU and NeuN colabelling in the ischemic area. Fenofibrate administration significantly increased cell proliferation and the number of cells colabelled by BrdU and NeuN both in the striatum and in the cortex; *P<0.05 vs. IR+Veh group. Scale bar, 20 μm. (C) After 7 days of fenofibrate treatment, the number of PPAR-α-expressing cells was also significantly higher in the core of ischemia and in the striatum; *P<0.05 vs. IR+Veh group. (D) Fenofibrate administration significantly increased cell proliferation of cells colabelled by bromodeoxyuridine (BrdU) and PPAR-α. Scale bar, 10 μm.
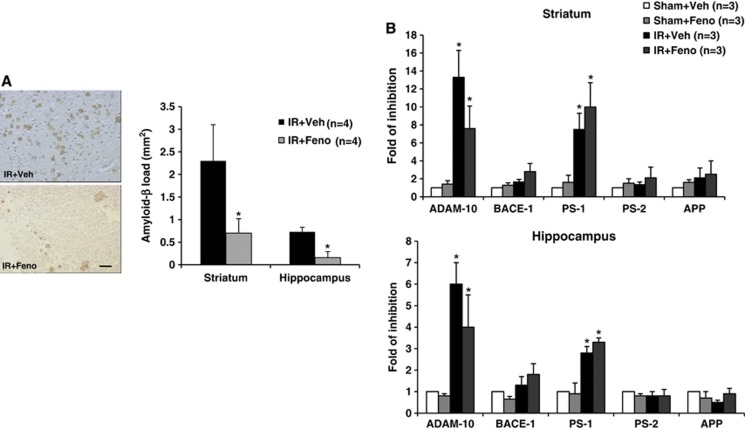
Fenofibrate Prevents the Postischemic Memory Expression of Amyloid-β Peptide
During the 7 days after brain ischemia, depositions of amyloid-β peptide appeared around the boundary of the ischemic focus (whether in the striatum or in the hippocampus) (Figure 6A). Analysis of ADAM-10, BACE-1, PS-1, PS-2, and APP mRNA levels in whole brain lysates from the striatum or the hippocampus regions showed that ischemia/reperfusion induced a decrease in ADAM-10 and PS-1 mRNA level (Figure 6B). In fenofibrate-treated animals, the expression of amyloid-β peptide during the first week postischemia was significantly lower than in controls (Figure 6A). Nevertheless, fenofibrate administration did not significantly downregulate ADAM-10 and PS-1 mRNA while ADAM-10 inhibition was less important in fenofibrate-treated group (Figure 6B).
Figure 6.
Effects of treatment with the peroxisome proliferator-activated receptor-α (PPAR-α) agonist fenofibrate during the acute phase of cerebral ischemia on stroke-induced amyloid deposition and amyloid-related secretases. (A) Amyloid-β peptide deposition around the boundary of the ischemic focus was decreased 7 days after the induction of ischemia by fenofibrate treatment in the rat (n=4 per group); *P<0.05 vs. IR+Veh group. Scale bar, 25 μm. (B) Analysis of A Disintegrin And Metalloproteinase-10 (ADAM-10), BACE-1, PS-1, PS-2, and APP mRNA levels in whole brain lysates from the striatum or the hippocampus regions showed that ischemia/reperfusion induced a decrease in ADAM-10 and PS-1 mRNA level; *P<0.05 vs. Sham+Veh group. Nevertheless, fenofibrate administration did not significantly downregulate ADAM-10 and PS-1 mRNA while ADAM-10 inhibition was less important in fenofibrate-treated group. IR, ischemia/reperfusion.
Discussion
We show for the first time that fenofibrate administration during the acute phase of experimentally induced brain ischemia has beneficial immediate and short-term effects. Early and delayed administration of fenofibrate (relative to the onset of ischemia) induces a significant decrease in the infarct size and countered functional impairments in brain ischemia model. This effect appears to be dependent on the dose of fenofibrate. In parallel, and according to the dose of fenofibrate, we observe an associated vasculoprotective effect. The prevention of the endothelial dysfunction observed in the first 72 hours is accompanied by (1) the prevention of anomalies in leukocyte-endothelium interactions induced by the ischemic process, and (2) a decrease in the tissue inflammation resulting from microglial activation and parenchymatous infiltration by polynuclear neutrophils. Short-term monitoring of ischemic animals shows that fenofibrate increases postischemic neurogenesis, favors functional recovery, prevents the appearance of postischemic amyloid deposition, and has a beneficial effect on ischemia-induced learning impairments.
The study of dose-effect response to fenofibrate treatment showed different actions on neuronal and vascular compartments. We observed a dose-dependent effect on infarct volume in parallel to an improvement in motor function. Preventive neuroprotective effect of the fenofibrate has already been shown (i.e., administration preceding the induction of brain ischemia).12, 26, 27 These effects of fibrates are completely absent in PPARα-KO mice, underlying the role of this nuclear receptor in this neuroprotective effect.12 But this is the first study to show a neuroprotective effect during the acute-phase administration of this drug. Our observations agree with recent work showing that preventive neuroprotection is partially lost if long-term fenofibrate administration is stopped 3 days before ischemia,23 suggesting a relationship to the drug's presence in the body at the time of ischemia induction. These data are supported by FFA assays showing a greater neuroprotective effect parallel to an increase in the plasma concentration of FFA. However, fenofibrate prevented ischemia-induced endothelial dysfunction only at a dose of 50 mg/kg per day and not at 200 mg/kg per day. Even if PPAR-α isoform is well expressed by all cell compartments in the neurovascular unit,7 these results suggest that the protective mechanisms of fenofibrate are different according to the cellular compartment. Our demonstration of the neuroprotective effect of delayed postischemia administration suggests that clinically relevant translation of these results is feasible because of compatibility with clinical practice. The demonstration of a reduction in infarct size and a concomitant impact on functional performance also fits with the STAIR guidelines on the preclinical development of neuroprotective stroke drugs.28
Fenofibrate's neuroprotective effect is associated with modulation of the vascular consequences of brain ischemia, in terms of endothelial function anomalies and leukocyte–endothelium interactions. This modulation of the vascular consequences of ischemia fits with the known vascular expression of PPAR-α within the neurovascular unit.13 The fact that this effect can be observed in vitro and in vivo in two different species emphasizes its reproducibility. The endothelial dysfunction that occurs in the aftermath of brain ischemia is explained by PMN–endothelium interactions.21 It results from the leukocyte rolling and adhesion prompted by the postischemic elevation in adhesion proteins (such as ICAM-1).29 We have shown directly that fenofibrate is able to reduce these leukocyte–endothelium interactions by preventing the postischemic overexpression of ICAM-1, related to the PPAR-α effect on gene expression of adhesion proteins via NF-κB inhibition.30 However, the modulation of oxidative stress could also explain the effects of fenofibrate, by mechanism involving an increase in nitric oxide availability.12 The intimate mechanisms of this mobilization of neutrophils remain to be explored.
The mobilization of neutrophils actively participates in the deleterious effects of systemic inflammation in cerebral ischemia.31 Inhibition of leukocyte–endothelium interactions also accounts for some of the parenchymatous effects of fenofibrate—particularly the infiltration of the ischemic area by the PMNs responsible for local inflammation or oxidant release. However, fenofibrate seems also to exert a direct effect on the microglial activation in the brain parenchyma, related to glial expression of PPAR-α. The existence of a potentially direct, parenchymatous effect of fenofibrate is at least partially substantiated by the demonstration of a significant neuroprotection after stereotaxic injection of a controlled-release fenofibrate formulation into the brain parenchyma.32 This further confirms the value of an agent such as fenofibrate, whose ubiquitous action within the neurovascular unit increases its therapeutic potential.
Early administration of fenofibrate improves postischemia motor performances. This improvement is maintained even after a delayed and prolonged administration of fenofibrate. Moreover on the cognitive level, treatment by fenofibrate during 7 days after ischemia prevents the postischemic memory impairment. Functional recovery is associated with an infarct volume decrease. The potential value of fenofibrate is further reinforced by our demonstration of the drug's short-term beneficial effects. In addition to effects in the early phases of ischemia, fenofibrate is able to upmodulate the brain tissue reorganization responsible for functional recovery and brain repair. It is known that spontaneous, postischemic neurogenesis results from the activation and differentiation of stem cells of neural or hematopoietic cells. This process is partly mediated by neurovascular interactions but has a low yield. Fenofibrate increases the neurogenesis observed well after ischemia. A PPAR-α modulation effect is suggested by the fact that the new cells express this receptor. This correlates with more fundamental work implicating PPAR-α in the physiology of stem cells9 related to PPAR-α expression by neural stem cell that we show here. The mobilization by fenofibrate of new cells expressing PPAR-α may also have a synergistic effect on functional recovery by neurorepair mechanism. It remains to characterize the mechanisms of mobilization of endogenous stem cells, in particular the role of endothelial, neuronal, or glial involved signals. Even though motor handicap is often the most obvious immediate consequence of ischemic cerebrovascular events, it is nevertheless the case that poststroke dementia probably creates the greatest burden in medical, social, and economic terms.33 Poststroke dementia may solely be a consequence of vascular lesions or can also result from the intermeshing of vascular damage and Alzheimer's-type degenerative lesions, since brain ischemia induces experimentally amyloid deposition or tau phosphorylation.2, 33, 34 We showed here that ischemia-induced amyloid deposition is associated with a decrease in ADAM-10 and PS-1. This effect is not explained by inhibition of the secretases involved in the amyloid cascade, as demonstrated by our results suggesting the involvement of another mechanism of fenofibrate. It would be interesting to study the effects of fenofibrate on other pathophysiological pathways like oxidative stress, brain inflammation, and cholinergic deficit involved in neurovascular impairments and cognitive deficit. The associated, fenofibrate-induced reduction in ischemic and amyloid lesions may explain the observed reduction in the intensity of postischemia memory disorders. To assess cognitive function, we used Y-maze test but other tests may be relevant (such as the Morris water maze test, passive avoidance test). The other important issue is to evaluate the effect of fenofibrate at long term on the amyloid cascade.
In summary, we have shown for the first time that acute administration of fenofibrate exerts several different effects in the aftermath of brain ischemia. In the early phase, fenofibrate reduces infarct size. At later time points, the drug favors brain repair and minimizes the impact of brain ischemia on the amyloid cascade that contributes to the occurrence of poststroke dementia. All these properties suggest that fenofibrate and PPAR-α activation could exert disease-modifying effect in stroke. The clinical translation of these preclinical results is likely to be rapid, since fenofibrate has been used safety for over 40 years to treat certain vascular risk factors associated with stroke.
Acknowledgments
The authors thank the platform of Imagerie et Biophotonique Cellulaire Fonctionnelle (Interdisciplinary Research Institute, Parc de la Haute Borne, Villeneuve d'Ascq, France) and Dave Trinel. The authors are grateful to the imaging platform of the Institut de Medecine Predictive et de Recherche Thérapeutique (IFR114) and to Florent Auger. The authors thank Dr Luc Humbert and Dr Jean-François Wiart (engineers from the Laboratoire de Toxicologie and Génopathies, Centre de Biologie, Bd du Professor J Leclercq 59037 LILLE) for the fenofibric acid assays.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Journal of Cerebral Blood Flow & Metabolism website (http://www.nature.com/jcbfm)
This work was supported, in part, by Conseil Régional du Nord-Pas-de-Calais.
Supplementary Material
References
- del Zoppo GJ. Stroke and neurovascular protection. N Engl J Med. 2006;354:553–555. doi: 10.1056/NEJMp058312. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Gregory J, Kuchibhotla KV, Fine S, Wei Y, Ayata C, et al. Cerebrovascular lesions induce transient β-amyloid deposition. Brain. 2011;134:3697–3707. doi: 10.1093/brain/awr300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C. Neurovascular regulation in the normal brain and in Alzheimer's disease. Nat Rev Neurosci. 2005;5:347–360. doi: 10.1038/nrn1387. [DOI] [PubMed] [Google Scholar]
- Pluta R, Ułamek M, Jablonski M. Consideration of the ischemic basis and treatment of Alzheimer's disease. Folia Neuropathol. 2010;48:11–26. [PubMed] [Google Scholar]
- Collino M, Patel NS, Thiemermann C. PPARs as new therapeutic targets for the treatment of cerebral ischaemia/reperfusion injury. Ther Adv Cardiovasc Dis. 2008;2:179–197. doi: 10.1177/1753944708090924. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Landreth GE. PPARs in the brain. Biochim Biophys Acta. 2007;1771:1031–1045. doi: 10.1016/j.bbalip.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Nicolakakis N, Hamel E. The nuclear receptor PPARγ as a therapeutic target for cerebrovascular and brain dysfunction in Alzheimer's disease. Front Aging Neurosci. 2010;2:1–10. doi: 10.3389/fnagi.2010.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouk T, Potey C, Gautier S, Bastide M, Deplanque D, Staels B, et al. PPARs: a potential target for a disease-modifying strategy in stroke. Curr Drug Targets. 2013;14:752–767. doi: 10.2174/1389450111314070005. [DOI] [PubMed] [Google Scholar]
- Cimini A, Cerù MP. Emerging roles of peroxisome proliferator-activated receptors (PPARs) in the regulation of neural stem cells proliferation and differentiation. Stem Cell Rev. 2008;4:293–303. doi: 10.1007/s12015-008-9024-2. [DOI] [PubMed] [Google Scholar]
- Cimini A, Benedetti E, D'Angelo B, Cristiano L, Falone S, Di Loreto S, et al. Neuronal response of peroxisomal and peroxisome-related proteins to chronic and acute Abeta injury. Curr Alzheimer Res. 2009;6:238–251. doi: 10.2174/156720509788486518. [DOI] [PubMed] [Google Scholar]
- Gervois P, Fruchart JC, Staels B. Drug Insight: mechanisms of action and therapeutic applications for agonists of peroxisome proliferator-activated receptors. Nat Clin Pract Endocrinol Metab. 2007;3:145–156. doi: 10.1038/ncpendmet0397. [DOI] [PubMed] [Google Scholar]
- Deplanque D, Gelé P, Pétrault O, Six I, Furman C, Bouly M, et al. Peroxisome proliferator-activated receptor-alpha activation as a mechanism of preventive neuroprotection induced by chronic fenofibrate treatment. J Neurosci. 2003;23:6264–6271. doi: 10.1523/JNEUROSCI.23-15-06264.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Farioli-Vecchioli S, Cerù MP. Immunolocalization of peroxisome proliferator-activated receptors and retinoid X receptors in the adult rat CNS. Neuroscience. 2004;123:131–145. doi: 10.1016/j.neuroscience.2003.08.064. [DOI] [PubMed] [Google Scholar]
- Stroke Therapy Academic Industry Roundtable (STAIR) Recommendations for standards regarding preclinical neuroprotective and restorative drug development. Stroke. 1999;30:2752–2758. doi: 10.1161/01.str.30.12.2752. [DOI] [PubMed] [Google Scholar]
- Bastide M, Gelé P, Pétrault O, Pu Q, Caliez A, Robin E, et al. Delayed cerebrovascular protective effect of lipopolysaccharide in parallel to brain ischemic tolerance. J Cereb Blood Flow Metab. 2003;23:399–405. doi: 10.1097/01.WCB.0000050064.57184.F2. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]
- Zhang L, Chen J, Li Y, Zhang ZG, Chopp M. Quantitative measurement of motor and somatosensory impairments after mild (30 min) and severe (2 h) transient middle cerebral artery occlusion in rats. J Neurol Sci. 2000;174:141–146. doi: 10.1016/s0022-510x(00)00268-9. [DOI] [PubMed] [Google Scholar]
- Zadrozniak A, Wojda E, Wlaź A, Łuszczki JJ. Characterization of acute adverse-effect profiles of selected antiepileptic drugs in the grip-strength test in mice. Pharmacol Rep. 2009;61:737–742. doi: 10.1016/s1734-1140(09)70128-8. [DOI] [PubMed] [Google Scholar]
- Dellu F, Mayo W, Cherkaoui J, Le Moal M, Simon H. A two-trial memory task with automated recording: study in young and aged rats. Brain Res. 1992;588:132–139. doi: 10.1016/0006-8993(92)91352-f. [DOI] [PubMed] [Google Scholar]
- Trivedi RK, Kallem RR, Mullangi R, Srinivas NR. Simultaneous determination of rosuvastatin and fenofibric acid in human plasma by LC-MS/MS with electrospray ionization: assay development, validation and application to a clinical study. J Pharm Biomed Anal. 2005;39:661–669. doi: 10.1016/j.jpba.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Pétrault O, Ouk T, Gautier S, Laprais M, Gelé P, Bastide M, et al. Pharmacological neutropenia prevents endothelial dysfunction but not smooth muscle functions impairment induced by middle cerebral artery occlusion. Br J Pharmacol. 2005;144:1051–1058. doi: 10.1038/sj.bjp.0706124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didion SP, Ryan MJ, Didion LA, Fegan PE, Sigmund CD, Faraci FM. Increased superoxide and vascular dysfunction in CuZn SOD-deficient mice. Circ Res. 2002;91:938–944. doi: 10.1161/01.res.0000043280.65241.04. [DOI] [PubMed] [Google Scholar]
- Ouk T, Laprais M, Bastide M, Mostafa K, Gautier S, Bordet R. Withdrawal of fenofibrate treatment partially abrogates preventive neuroprotection in stroke via loss of vascular protection. Vascul Pharmacol. 2009;51:323–330. doi: 10.1016/j.vph.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Ling L, Zeng J, Pei Z, Cheung RT, Hou Q, Xing S, et al. Neurogenesis and angiogenesis within the ipsilateral thalamus with secondary damage after focal cortical infarction in hypertensive rats. J Cereb Blood Flow Metab. 2009;29:1538–1546. doi: 10.1038/jcbfm.2009.76. [DOI] [PubMed] [Google Scholar]
- Fisher M. Injuries to the vascular endothelium: vascular wall and endothelial dysfunction. Rev Neurol Dis. 2008;5:S4–S11. [PubMed] [Google Scholar]
- Guo Q, Wang G, Namura S. Fenofibrate improves cerebral blood flow after middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2010;30:70–78. doi: 10.1038/jcbfm.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Jiang XF, Katayama T, Osada S, Umesono K, Namura S. Brain protection by resveratrol and fenofibrate against stroke requires peroxisome proliferator-activated receptor alpha in mice. Neurosci Lett. 2003;352:203–206. doi: 10.1016/j.neulet.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, et al. Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke. 2009;40:2244–2250. doi: 10.1161/STROKEAHA.108.541128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang RL, Chopp M, Zaloga C, Zhang ZG, Jiang N, Gautam SC, et al. The temporal profiles of ICAM-1 protein and mRNA expression after transient MCA occlusion in the rat. Brain Res. 1995;682:182–188. doi: 10.1016/0006-8993(95)00346-r. [DOI] [PubMed] [Google Scholar]
- Delerive P, De Bosscher K, Besnard S, Vanden Berghe W, Peters JM, Gonzalez FJ, et al. Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem. 1999;274:32048–32054. doi: 10.1074/jbc.274.45.32048. [DOI] [PubMed] [Google Scholar]
- McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms. J Neurosci. 2007;27:4403–4412. doi: 10.1523/JNEUROSCI.5376-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klose D, Laprais M, Leroux V, Siepmann F, Deprez B, Bordet R, et al. Fenofibrate-loaded PLGA microparticles: effects on ischemic stroke. Eur J Pharm Sci. 2009;37:43–52. doi: 10.1016/j.ejps.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120:287–296. doi: 10.1007/s00401-010-0718-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluta R. Alzheimer lesions after ischemia-reperfusion brain injury. Folia Neuropathol. 2004;42:181–186. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.