Abstract
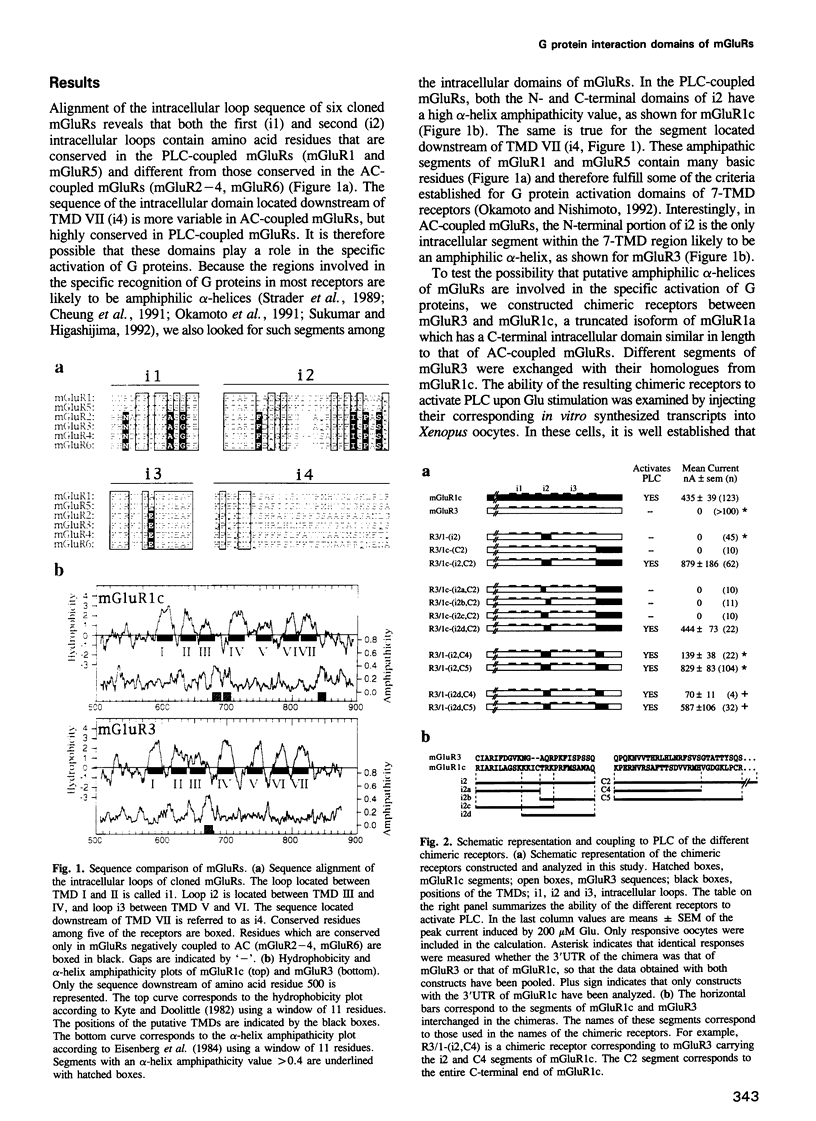
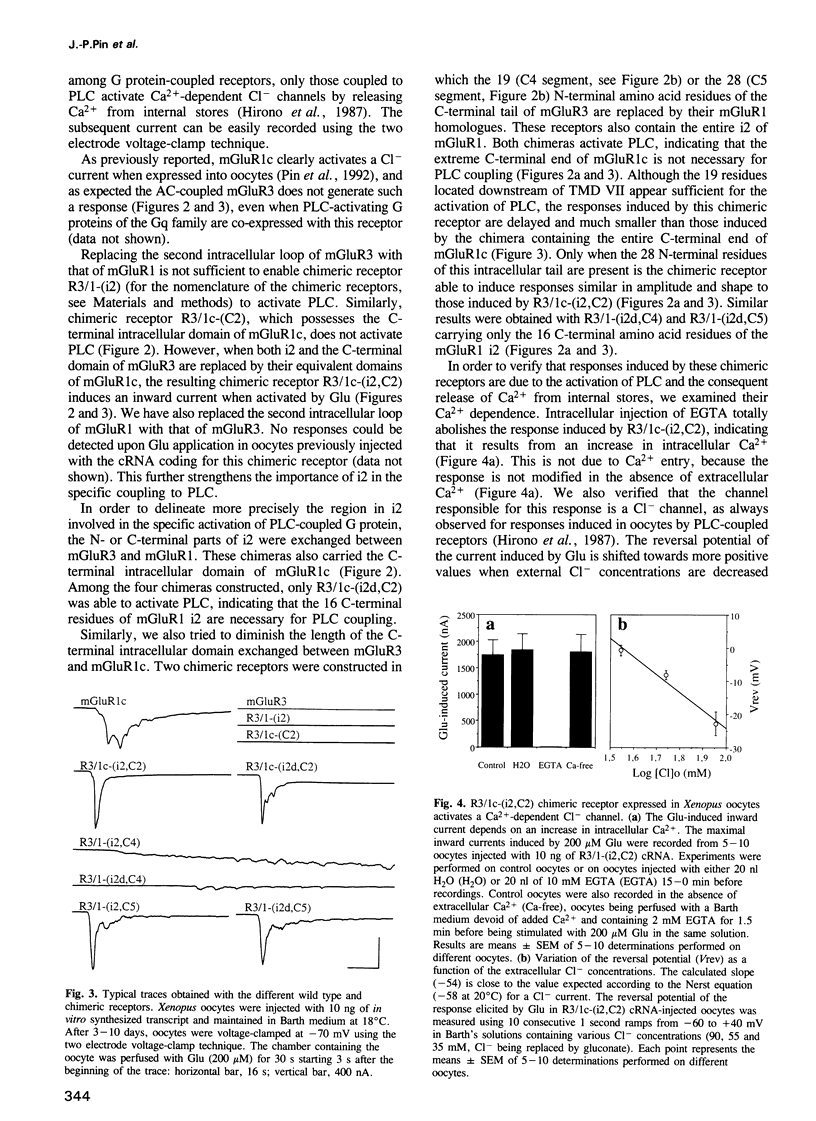
G protein-coupled glutamate receptors (mGluR) have recently been characterized. These receptors have seven putative transmembrane domains, but display no sequence homology with the large family of G protein-coupled receptors. They constitute therefore a new family of receptors. Whereas mGluR1 and mGluR5 activate phospholipase C (PLC), mGluR2, mGluR3, mGluR4 and mGluR6 inhibit adenylyl cyclase (AC) activity. The third putative intracellular loop, which determines the G protein specificity in many G protein-coupled receptors, is highly conserved among mGluRs, and may therefore not be involved in the specific recognition of G proteins in this receptor family. By constructing chimeric receptors between the AC-coupled mGluR3 and the PLC-coupled mGluR1c, we report here that both the C-terminal end of the second intracellular loop and the segment located downstream of the seventh transmembrane domain are necessary for the specific activation of PLC by mGluR1c. These two segments are rich in basic residues and are likely to be amphipathic alpha-helices, two characteristics of the G protein interacting domains of all G protein-coupled receptors. This indicates that whereas no amino acid sequence homology between mGluRs and the other G protein-coupled receptors can be found, their G protein interacting domains have similar structural features.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe T., Sugihara H., Nawa H., Shigemoto R., Mizuno N., Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992 Jul 5;267(19):13361–13368. [PubMed] [Google Scholar]
- Charpak S., Gähwiler B. H., Do K. Q., Knöpfel T. Potassium conductances in hippocampal neurons blocked by excitatory amino-acid transmitters. Nature. 1990 Oct 25;347(6295):765–767. doi: 10.1038/347765a0. [DOI] [PubMed] [Google Scholar]
- Chazenbalk G. D., Nagayama Y., Russo D., Wadsworth H. L., Rapoport B. Functional analysis of the cytoplasmic domains of the human thyrotropin receptor by site-directed mutagenesis. J Biol Chem. 1990 Dec 5;265(34):20970–20975. [PubMed] [Google Scholar]
- Cheung A. H., Huang R. R., Graziano M. P., Strader C. D. Specific activation of Gs by synthetic peptides corresponding to an intracellular loop of the beta-adrenergic receptor. FEBS Lett. 1991 Feb 25;279(2):277–280. doi: 10.1016/0014-5793(91)80167-2. [DOI] [PubMed] [Google Scholar]
- Cotecchia S., Exum S., Caron M. G., Lefkowitz R. J. Regions of the alpha 1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2896–2900. doi: 10.1073/pnas.87.8.2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotecchia S., Ostrowski J., Kjelsberg M. A., Caron M. G., Lefkowitz R. J. Discrete amino acid sequences of the alpha 1-adrenergic receptor determine the selectivity of coupling to phosphatidylinositol hydrolysis. J Biol Chem. 1992 Jan 25;267(3):1633–1639. [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagni L., Bossu J. L., Bockaert J. Activation of a Large-conductance Ca2+-Dependent K+ Channel by Stimulation of Glutamate Phosphoinositide-coupled Receptors in Cultured Cerebellar Granule Cells. Eur J Neurosci. 1991;3(8):778–789. doi: 10.1111/j.1460-9568.1991.tb01674.x. [DOI] [PubMed] [Google Scholar]
- Franke R. R., König B., Sakmar T. P., Khorana H. G., Hofmann K. P. Rhodopsin mutants that bind but fail to activate transducin. Science. 1990 Oct 5;250(4977):123–125. doi: 10.1126/science.2218504. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Tanabe Y., Aramori I., Masu M., Shimamoto K., Ohfune Y., Nakanishi S. Agonist analysis of 2-(carboxycyclopropyl)glycine isomers for cloned metabotropic glutamate receptor subtypes expressed in Chinese hamster ovary cells. Br J Pharmacol. 1992 Oct;107(2):539–543. doi: 10.1111/j.1476-5381.1992.tb12780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima T., Uzu S., Nakajima T., Ross E. M. Mastoparan, a peptide toxin from wasp venom, mimics receptors by activating GTP-binding regulatory proteins (G proteins). J Biol Chem. 1988 May 15;263(14):6491–6494. [PubMed] [Google Scholar]
- Hirono C., Ito I., Sugiyama H. Neurotensin and acetylcholine evoke common responses in frog oocytes injected with rat brain messenger ribonucleic acid. J Physiol. 1987 Jan;382:523–535. doi: 10.1113/jphysiol.1987.sp016382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houamed K. M., Kuijper J. L., Gilbert T. L., Haldeman B. A., O'Hara P. J., Mulvihill E. R., Almers W., Hagen F. S. Cloning, expression, and gene structure of a G protein-coupled glutamate receptor from rat brain. Science. 1991 May 31;252(5010):1318–1321. doi: 10.1126/science.1656524. [DOI] [PubMed] [Google Scholar]
- Kobilka B. K., Kobilka T. S., Daniel K., Regan J. W., Caron M. G., Lefkowitz R. J. Chimeric alpha 2-,beta 2-adrenergic receptors: delineation of domains involved in effector coupling and ligand binding specificity. Science. 1988 Jun 3;240(4857):1310–1316. doi: 10.1126/science.2836950. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- König B., Arendt A., McDowell J. H., Kahlert M., Hargrave P. A., Hofmann K. P. Three cytoplasmic loops of rhodopsin interact with transducin. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6878–6882. doi: 10.1073/pnas.86.18.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechleiter J., Girard S., Clapham D., Peralta E. Subcellular patterns of calcium release determined by G protein-specific residues of muscarinic receptors. Nature. 1991 Apr 11;350(6318):505–508. doi: 10.1038/350505a0. [DOI] [PubMed] [Google Scholar]
- Lechleiter J., Hellmiss R., Duerson K., Ennulat D., David N., Clapham D., Peralta E. Distinct sequence elements control the specificity of G protein activation by muscarinic acetylcholine receptor subtypes. EMBO J. 1990 Dec;9(13):4381–4390. doi: 10.1002/j.1460-2075.1990.tb07888.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester R. A., Jahr C. E. Quisqualate receptor-mediated depression of calcium currents in hippocampal neurons. Neuron. 1990 May;4(5):741–749. doi: 10.1016/0896-6273(90)90200-y. [DOI] [PubMed] [Google Scholar]
- Liggett S. B., Caron M. G., Lefkowitz R. J., Hnatowich M. Coupling of a mutated form of the human beta 2-adrenergic receptor to Gi and Gs. Requirement for multiple cytoplasmic domains in the coupling process. J Biol Chem. 1991 Mar 15;266(8):4816–4821. [PubMed] [Google Scholar]
- Masu M., Tanabe Y., Tsuchida K., Shigemoto R., Nakanishi S. Sequence and expression of a metabotropic glutamate receptor. Nature. 1991 Feb 28;349(6312):760–765. doi: 10.1038/349760a0. [DOI] [PubMed] [Google Scholar]
- Moffett S., Mouillac B., Bonin H., Bouvier M. Altered phosphorylation and desensitization patterns of a human beta 2-adrenergic receptor lacking the palmitoylated Cys341. EMBO J. 1993 Jan;12(1):349–356. doi: 10.1002/j.1460-2075.1993.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan D. T., Bridges R. J., Cotman C. W. The excitatory amino acid receptors: their classes, pharmacology, and distinct properties in the function of the central nervous system. Annu Rev Pharmacol Toxicol. 1989;29:365–402. doi: 10.1146/annurev.pa.29.040189.002053. [DOI] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992 Oct 23;258(5082):597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- Nicoletti F., Wroblewski J. T., Novelli A., Alho H., Guidotti A., Costa E. The activation of inositol phospholipid metabolism as a signal-transducing system for excitatory amino acids in primary cultures of cerebellar granule cells. J Neurosci. 1986 Jul;6(7):1905–1911. doi: 10.1523/JNEUROSCI.06-07-01905.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Dowd B. F., Hnatowich M., Caron M. G., Lefkowitz R. J., Bouvier M. Palmitoylation of the human beta 2-adrenergic receptor. Mutation of Cys341 in the carboxyl tail leads to an uncoupled nonpalmitoylated form of the receptor. J Biol Chem. 1989 May 5;264(13):7564–7569. [PubMed] [Google Scholar]
- Okamoto T., Katada T., Murayama Y., Ui M., Ogata E., Nishimoto I. A simple structure encodes G protein-activating function of the IGF-II/mannose 6-phosphate receptor. Cell. 1990 Aug 24;62(4):709–717. doi: 10.1016/0092-8674(90)90116-v. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Murayama Y., Hayashi Y., Inagaki M., Ogata E., Nishimoto I. Identification of a Gs activator region of the beta 2-adrenergic receptor that is autoregulated via protein kinase A-dependent phosphorylation. Cell. 1991 Nov 15;67(4):723–730. doi: 10.1016/0092-8674(91)90067-9. [DOI] [PubMed] [Google Scholar]
- Okamoto T., Nishimoto I. Analysis of stimulation-G protein subunit coupling by using active insulin-like growth factor II receptor peptide. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8020–8023. doi: 10.1073/pnas.88.18.8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T., Nishimoto I. Detection of G protein-activator regions in M4 subtype muscarinic, cholinergic, and alpha 2-adrenergic receptors based upon characteristics in primary structure. J Biol Chem. 1992 Apr 25;267(12):8342–8346. [PubMed] [Google Scholar]
- Ostrowski J., Kjelsberg M. A., Caron M. G., Lefkowitz R. J. Mutagenesis of the beta 2-adrenergic receptor: how structure elucidates function. Annu Rev Pharmacol Toxicol. 1992;32:167–183. doi: 10.1146/annurev.pa.32.040192.001123. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov YuA, Abdulaev N. G., Bogachuk A. S. Two adjacent cysteine residues in the C-terminal cytoplasmic fragment of bovine rhodopsin are palmitylated. FEBS Lett. 1988 Mar 28;230(1-2):1–5. doi: 10.1016/0014-5793(88)80628-8. [DOI] [PubMed] [Google Scholar]
- Pin J. P., Waeber C., Prezeau L., Bockaert J., Heinemann S. F. Alternative splicing generates metabotropic glutamate receptors inducing different patterns of calcium release in Xenopus oocytes. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10331–10335. doi: 10.1073/pnas.89.21.10331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savarese T. M., Fraser C. M. In vitro mutagenesis and the search for structure-function relationships among G protein-coupled receptors. Biochem J. 1992 Apr 1;283(Pt 1):1–19. doi: 10.1042/bj2830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoepp D. D., Johnson B. G., Salhoff C. R., McDonald J. W., Johnston M. V. In vitro and in vivo pharmacology of trans- and cis-(+-)-1-amino-1,3-cyclopentanedicarboxylic acid: dissociation of metabotropic and ionotropic excitatory amino acid receptor effects. J Neurochem. 1991 May;56(5):1789–1796. doi: 10.1111/j.1471-4159.1991.tb02082.x. [DOI] [PubMed] [Google Scholar]
- Schoepp D., Bockaert J., Sladeczek F. Pharmacological and functional characteristics of metabotropic excitatory amino acid receptors. Trends Pharmacol Sci. 1990 Dec;11(12):508–515. doi: 10.1016/0165-6147(90)90052-a. [DOI] [PubMed] [Google Scholar]
- Sladeczek F., Pin J. P., Récasens M., Bockaert J., Weiss S. Glutamate stimulates inositol phosphate formation in striatal neurones. Nature. 1985 Oct 24;317(6039):717–719. doi: 10.1038/317717a0. [DOI] [PubMed] [Google Scholar]
- Strader C. D., Sigal I. S., Dixon R. A. Structural basis of beta-adrenergic receptor function. FASEB J. 1989 May;3(7):1825–1832. doi: 10.1096/fasebj.3.7.2541037. [DOI] [PubMed] [Google Scholar]
- Strittmatter S. M., Valenzuela D., Kennedy T. E., Neer E. J., Fishman M. C. G0 is a major growth cone protein subject to regulation by GAP-43. Nature. 1990 Apr 26;344(6269):836–841. doi: 10.1038/344836a0. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Ito I., Hirono C. A new type of glutamate receptor linked to inositol phospholipid metabolism. Nature. 1987 Feb 5;325(6104):531–533. doi: 10.1038/325531a0. [DOI] [PubMed] [Google Scholar]
- Sukumar M., Higashijima T. G protein-bound conformation of mastoparan-X, a receptor-mimetic peptide. J Biol Chem. 1992 Oct 25;267(30):21421–21424. [PubMed] [Google Scholar]
- Tanabe Y., Masu M., Ishii T., Shigemoto R., Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992 Jan;8(1):169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Nomura A., Masu M., Shigemoto R., Mizuno N., Nakanishi S. Signal transduction, pharmacological properties, and expression patterns of two rat metabotropic glutamate receptors, mGluR3 and mGluR4. J Neurosci. 1993 Apr;13(4):1372–1378. doi: 10.1523/JNEUROSCI.13-04-01372.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen C., Kristensen P., Mulvihill E., Haldeman B., Suzdak P. D. L-2-amino-4-phosphonobutyrate (L-AP4) is an agonist at the type IV metabotropic glutamate receptor which is negatively coupled to adenylate cyclase. Eur J Pharmacol. 1992 Nov 2;227(3):361–362. doi: 10.1016/0922-4106(92)90018-q. [DOI] [PubMed] [Google Scholar]
- Trombley P. Q., Westbrook G. L. L-AP4 inhibits calcium currents and synaptic transmission via a G-protein-coupled glutamate receptor. J Neurosci. 1992 Jun;12(6):2043–2050. doi: 10.1523/JNEUROSCI.12-06-02043.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wess J., Bonner T. I., Dörje F., Brann M. R. Delineation of muscarinic receptor domains conferring selectivity of coupling to guanine nucleotide-binding proteins and second messengers. Mol Pharmacol. 1990 Oct;38(4):517–523. [PubMed] [Google Scholar]
- Winder D. G., Conn P. J. Activation of metabotropic glutamate receptors increases cAMP accumulation in hippocampus by potentiating responses to endogenous adenosine. J Neurosci. 1993 Jan;13(1):38–44. doi: 10.1523/JNEUROSCI.13-01-00038.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong S. K., Parker E. M., Ross E. M. Chimeric muscarinic cholinergic: beta-adrenergic receptors that activate Gs in response to muscarinic agonists. J Biol Chem. 1990 Apr 15;265(11):6219–6224. [PubMed] [Google Scholar]