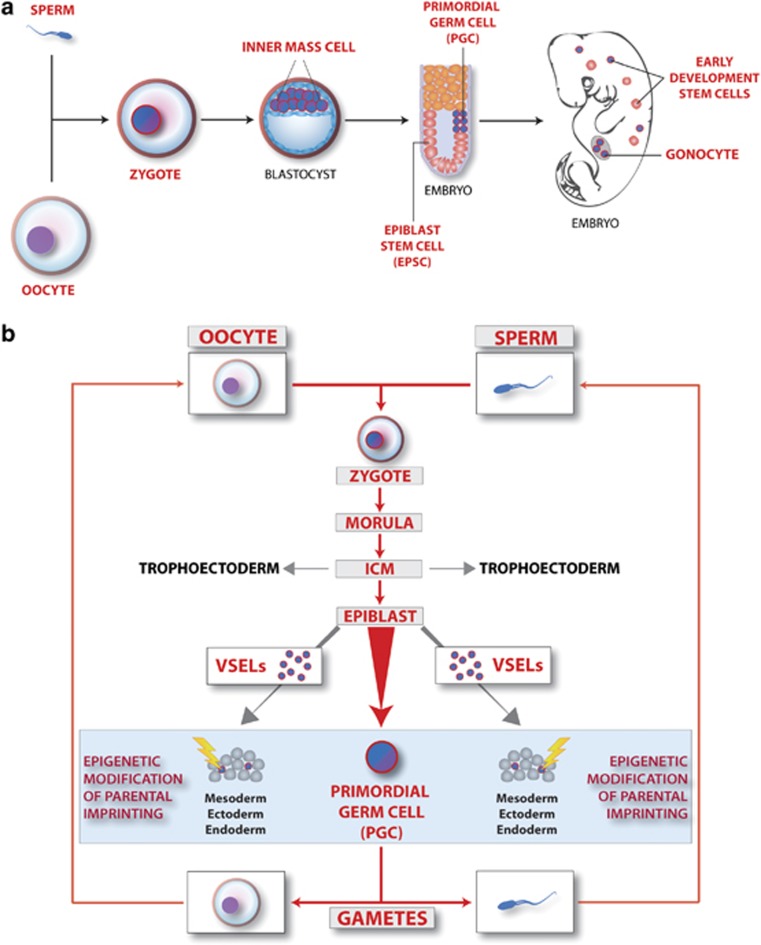
Figure 1.
The overall concept of a presence of developmental early epiblast/germline-derived stem cells in adult tissues. (a) Retention of germline potential during ontogenesis. Cells with germline potential are shown in blue. The earliest and the most primitive cell in the germline is the totipotent zygote. The germline potential is subsequently retained during development in Oct-4+ cells located in the inner cell mass cells (ICMs) of the developing blastocyst, epiblast stem cells (EPSCs), primordial germ cells (PGCs) and gonocytes in gonads. Epiblast/germline potential could also be retained in rare early-development small VSELs deposited during development in peripheral tissues as founders for more differentiated monopotent tissue-committed stem cells. Some of these small cells express Oct-4 (shown in blue). (b) The cycle of life—from zygote to germ cells. From a developmental and evolutionary point of view, the germline (shown by red arrows) carries the genome (nuclear and mitochondrial DNA) from one generation to the next, and all somatic cell lines bud out during ontogenesis from the germline to help germline cells accomplish this mission effectively. The germline potential is established in the fertilized oocyte (zygote) and subsequently retained in the morula, inner cell mass of the blastocyst (ICM), EPSC, PGCs and mature germline cells (oocytes and sperm). The first cells that bud out from the germ lineage are trophoectodermal cells, which give rise to the placenta. Subsequently, during gastrulation, EPSCs are a source of PSCs for all three germ layers (meso-, ecto- and endo-derm) and PGCs. We hypothesize that at this stage some EPSCs and PGCs are deposited as Oct-4+ VSELs in tissues developing from meso-, ecto- and endo-derm (blue circles). Blue box and yellow arrows pointing at VSELs indicate mechanism based on epigenetic modification of parentally imprinted genes (for example, at Igf2–H19 and KCNQ1p57Kip2 loci) that keeps these early-development cells quiescent in adult tissues. A similar mechanism based on erasure of imprinting also regulates the quiescent state of PGCs.