Abstract
How does an organism’s internal state direct its actions? At one moment an animal forages for food with acrobatic feats such as tree climbing and jumping between branches. At another time, it travels along the ground to find water or a mate, exposing itself to predators along the way. These behaviors are costly in terms of energy or physical risk, and the likelihood of performing one set of actions relative to another is strongly modulated by internal state. For example, an animal in energy deficit searches for food and a dehydrated animal looks for water. The crosstalk between physiological state and motivational processes influences behavioral intensity and intent, but the underlying neural circuits are poorly understood. Molecular genetics along with optogenetic and pharmacogenetic tools for perturbing neuron function have enabled cell type-selective dissection of circuits that mediate behavioral responses to physiological state changes. Here, we review recent progress into neural circuit analysis of hunger in the mouse by focusing on a starvation-sensitive neuron population in the hypothalamus that is sufficient to promote voracious eating. We also consider research into the motivational processes that are thought to underlie hunger in order to outline considerations for bridging the gap between homeostatic and motivational neural circuits.
Interoceptive neural circuits sense physiological signals of basic survival needs (e.g. energy or water) and influence the selection of the actions that result in the satisfaction of those needs. Food intake is obviously essential for survival, and the neural processes mediating hunger are under strong selective pressure. In light of this, these circuits are expected to be developmentally controlled with some hardwired components. One group of such specialized circuit elements is the interoceptive neuron populations that monitor circulating levels of the nutrients and hormones that reflect the energetic status of an animal [1].
Starvation-sensing neural circuits
A number of starvation-sensitive neuronal cell types are located in the hypothalamus, a conserved forebrain region most widely associated with maintaining physiological homeostasis. The gene Agouti related protein (Agrp) is specifically expressed in a small population of neurons [2,3] within the arcuate nucleus, which is a part of the hypothalamus that samples circulating factors transported from blood into the brain. AGRP neurons are electrically activated by hormone signals of energy deficit [4] and are inhibited by signals of energy surfeit [5,6]. Manipulation of electrical activity in these neurons has striking behavioral consequences. Rapid ablation of AGRP neurons in adult mice leads to aphagia [7], and inhibition of their electrical activity suppresses feeding [8•]. Conversely, application of optogenetic [9•] and pharmacogenetic [8•] tools for rapid cell type-specific neuron activation showed that these neurons were sufficient to evoke voracious eating in well-fed mice within minutes and without training. AGRP neuron-evoked feeding is specific for food in the presence of water [9•], indicating behavioral specificity for an explicit consummatory goal. In addition, the magnitude of feeding behavior is closely tied to the level of activity in AGRP neurons, where strong activation results in consumption similar to 24-hour food deprivation and lower levels of activity result in intermediate food intake [9•]. These experiments indicate that AGRP neurons are a sensory neuron population that transduces the level of a physiological need into an appropriate behavioral response by acting as a gas pedal controlling appetite.
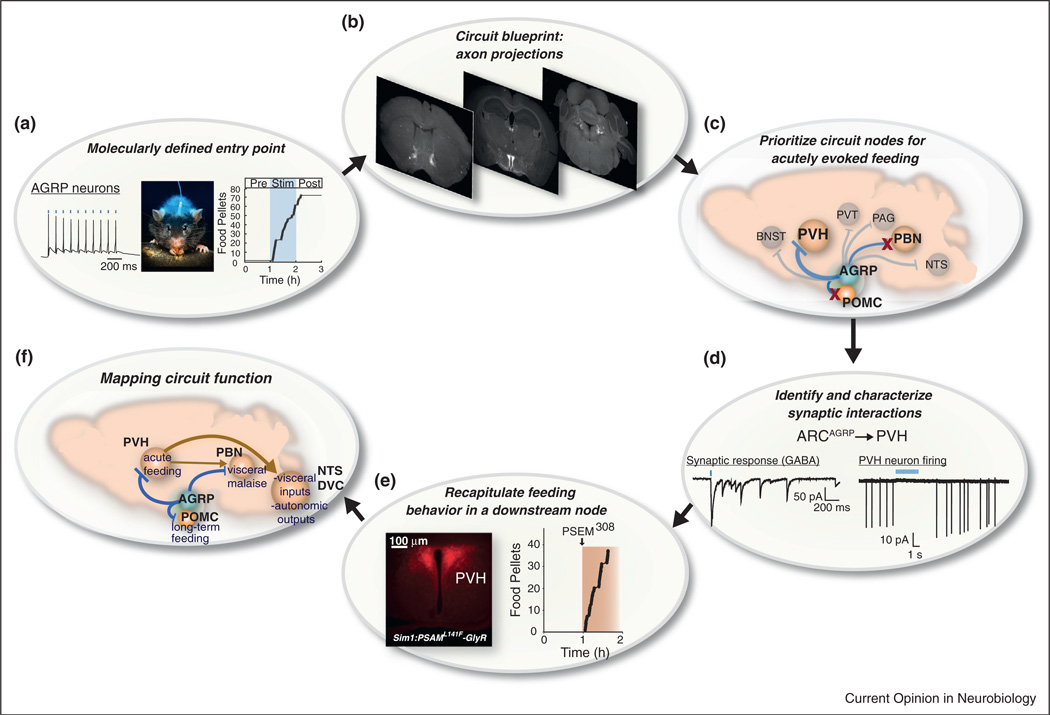
AGRP neurons, which mediate behavioral responses for food seeking and consumption, can serve as an entry point to neural circuits for these complex motivated behaviors associated with hunger (Figure 1a). AGRP neuron axonal projections provide an anatomical map of downstream brain regions mediating feeding behavior (Figure 1b), and the circuits that lead to voracious feeding during AGRP neuron activation can be examined node-by-node using optogenetic and pharmacogenetic circuit manipulation techniques [10••]. An important first step is that these tools enable the functional significance of downstream populations targeted by AGRP neurons to be directly compared. By activating specific AGRP neuron axon projections, it was found that only a subset of these projections can evoke voracious feeding. In particular, photostimulation of AGRP axons targeting the paraventricular hypothalamic nucleus (PVH) acutely elicited feeding behavior but projections to the parabrachial nucleus or to POMC neurons in the arcuate nucleus did not [10••] (Figure 1c). Cell type-specific channelrhodopsin-assisted circuit mapping [11] revealed that AGRP neurons inhibit specific postsynaptic targets due to asynchronous synaptic release of gamma-aminobutyric acid (GABA) [10••] (Figure 1d). Therefore, AGRP neuron projections inhibit PVH neurons (Figure 1d), and consistent with this, pharmacogenetic silencing of PVH neurons recapitulates the evoked feeding response from activating AGRP neurons [10••] (Figure 1e). The general approach outlined here uses cell type-specific circuit mapping to establish functional synaptic connectivity between cell types, and the measured circuit operations (e.g. excitation or inhibition) guide electrical manipulations of the postsynaptic population in behaving animals (Figure 1f). The methodology applied for ARCAGRP→PVH circuitry is also suitable for extending the analysis of feeding circuits downstream of the PVH to higher order circuit nodes, such as hindbrain regions implicated in appetite [12].
Figure 1.
Node-by-node deconstruction of neural circuits for hunger. (a) AGRP neurons are a molecularly defined, starvation-sensitive circuit entry point for which external activation (e.g. using optogenetics- blue ticks and shading) is sufficient to induce voracious food consumption. Photo from Igor Siwanowicz. (b) The axon projections of AGRP neurons reveal a map of the downstream second-order regions potentially mediating the evoked feeding response. (c) The functional influence of specific AGRP neuron axonal projections can be prioritized by cell type-specific neuron and axon activation or silencing methods. Three AGRP neuron projections to regions previously associated with feeding behavior (POMC, PVH and PBN neurons — a subset of the total) have been examined. Projections to the PVH were sufficient to recapitulate the effect of AGRP neuron somatic activation. (d) Cell type-specific functional circuit mapping revealed the inhibitory interaction of AGRP neurons with PVH neurons (blue ticks are 1-ms light pulses). (e) This inhibitory synaptic operation could be independently recapitulated by neuronal silencing in molecularly defined PVHSIM1 neurons using neuronal silencers. (f) Circuit analysis indicates that distinct projections of AGRP neurons have different functions. ARCAGRP→ARCPOMC connections influence long-term regulation of food intake and other aspects of energy homeostasis. ARCAGRP→PBN connections are involved in suppressing visceral malaise. ARCAGRP→PVH connections regulate acute food intake. PVH neurons in turn communicate with brainstem regions associated with satiety (NTS: nucleus of the solitary tract, DVC: dorsal vagal complex).
Motivational properties of AGRP neuron activity
A key characteristic of hunger is the willingness to work for food. However, neuronal perturbations that increase food consumption vary in their capacity to increase motivation. Animals in a state of energy deficit will work much harder for food in an operant task than animals fed ad libitum [13]. In contrast, rats with ventromedial hypothalamus lesions or chemical silencing of the nucleus accumbens shell do not overeat if required to work for food [14–16]. As with food deprivation, AGRP neuron activation influences both food seeking and consumption. During AGRP neuron stimulation, mice work for food on a progressive ratio by nose-poking [8•] or lever-pressing [10••], and they devote nearly as much effort to the task as 24-hour food deprived animals. Furthermore, using the circuit analysis techniques mentioned above, it was found that silencing the PVH recapitulates the motivational, food-seeking effects of activating the presynaptic AGRP neurons [10••]. Thus, AGRP neurons are also a sensory neuron entry point to motivational processes, and their downstream circuit connections allow identification of motivationally important brain regions not previously associated with instrumental responding (e.g. the PVH). This framework highlights the potential, using modern circuit mapping and manipulation methods in the mouse, to map these motivational processes associated with homeostatically sensitive neurons to successive downstream circuit nodes that regulate behavioral intent and intensity.
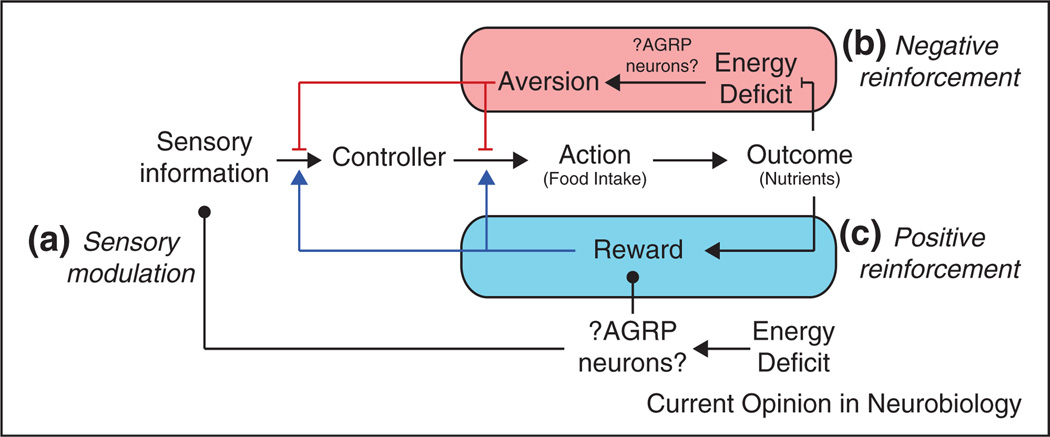
What motivational processes might be engaged by AGRP neurons and other cell types that regulate energy balance? AGRP neuron activity is expected to induce some, but not necessarily all, of the motivational characteristics of deprivation-induced hunger. As a framework for future investigation, we have broadly outlined here three mechanisms that have been examined for deprivation-induced hunger: firstly, enhancement of sensitivity to food-related sensory cues; secondly, negative reinforcement processes where actions that lead to eating shut off an unpleasant need state; thirdly, positive reinforcement, where deprivation increases the incentive value of food by acting on either the perceived pleasantness or post-ingestive qualities of nutrients.
Sensory sensitivity in hunger
One view is that hunger modulates the sensitivity to sensory cues associated with food such as taste and smell and that this greater sensitivity influences behavioral responding [17] (Figure 2a). Experiments in invertebrates provide the clearest examples of deprivation state modulating sensory signaling. In flies, olfactory signals in the presynaptic terminals of olfactory receptor neurons were enhanced in hunger, which was mediated by the neuropeptide sNPF receptor (a neuropeptide Y analog) under regulation by insulin receptor signaling [18•]. Also in flies, gustatory responses to sugar were increased in hunger by a dopamine-modulated process [19]. Similarly in worms, food removal activates calcium signals in the AWC exteroceptive chemosensory neuron in an insulin-signaling inhibited process that is important for food seeking [20]. In each of these studies, a mechanistic understanding of the molecular pathways controlling the alteration in sensory neuron response amplitudes was extended through genetic manipulation to determine the influence on feeding behavior.
Figure 2.
Control processes for food seeking and consumption behaviors that may be modulated by AGRP neurons activated during energy deficit. Sensory information is integrated by multiple brain systems that influence control of motor actions (these systems are represented generically in the diagram as ‘Controller’), including those involved in food seeking or consumption. (a) AGRP neurons, which are activated by energy deficit, could increase the sensitivity to sensory stimuli associated with food. (b) Negative reinforcement. For a food deprived animal, sensory information or actions that lead to nutrient ingestion could be reinforced by suppressing an aversive energy deficit state, possibly mediated by AGRP neurons. Red T-arrows represent a state-dependent aversion signal whose offset increases the propensity to respond to sensory cues that predict food or to select actions that result in nutrient consumption. (c) Positive reinforcement. Nutrients are rewarding, even in the absence of a need state, and energy deficit, possibly acting through AGRP neurons, may increase the reward value of food. Blue arrows represent a signal that reinforces responding to sensory information or action selection that results in nutrient ingestion.
Whether this modulation of sensory sensitivity extends to rodents, primates, and humans is less clear. Some evidence shows modulation of olfactory and gustatory signaling in mammalian organisms [21–23]. In humans, the experimental results are mixed: some experiments show no influence of hunger or satiety state on perceived intensity of odors [24] or tastes [25], while others suggest a shift [26] or showed inconsistent effects [27,28]. Psychophysical studies in rats found that taste sensitivity in a discriminative responding (go/no-go) experiment for salt taste is not altered by a different homeostatic need, sodium deprivation [29]. Moreover, in primates electrophysiological recordings from the nucleus of the solitary tract (a hindbrain gustatory relay nucleus) and primary gustatory cortex, indicate that the intensity of response to tastants is not altered by hunger to satiety transitions [30,31]. Additional work will be needed to examine the influence of hunger processes on sensory sensitivity in mammals, but, currently, this mechanism is not well supported.
Reinforcement processes in hunger
In behavioral analysis, the term ‘reinforcement’ reflects an internal process by which a particular outcome of behavior increases the probability of an action that preceded it either by acting as a reward (positive reinforcement) or by removing an unpleasant state (negative reinforcement). The reinforcing properties of food have been assessed through various behavioral tasks such as conditioned place preference for a chamber previously associated with food [32,33], conditioned flavor preference [34], and elevated lever pressing [13]. Multiple mechanisms have been identified, likely operating in concert, underlying the reinforcing properties of food in hungry animals.
Negative reinforcement
Negative reinforcement was classically viewed as a mechanism through which hunger could influence motivation for food. According to this view, actions are reinforced (their probability increased) if they result in elimination of unpleasant need states [35,36] (Figure 2b). The aversive motivating condition has been called ‘drive’, which was used to explain how a need state energizes behavior and reinforces the actions that led to drive reduction [36]. A key test of this theory was performed by analyzing affective properties of lateral hypothalamus electrical stimulation. Lateral hypothalamus activation was thought to recapitulate the underlying processes of deprivation-induced hunger based on observations that electrical stimulation in this area could evoke feeding behavior [37] and also that some lateral hypothalamic neurons are inhibited by nutrient signals [38]. According to drive reduction theory, lateral hypothalamus-mediated feeding behavior would be a reaction to an aversive internal need state that the animal would normally eliminate by eating nutrients previously associated with reducing the activity of these putative need-sensing neurons. The affective consequences of activating the lateral hypothalamus could be assayed by testing whether the animal would engage in lever pressing to shut off electrical stimulation in the absence of food. In contrast to predictions from this theory, electrodes implanted in the lateral hypothalamus led to vigorous self-stimulation [39,40]. This result was used to argue against negative reinforcement mechanisms of hunger, at least with respect to lateral hypothalamic stimulation [41]. More recently, negative reinforcement mechanisms have been emphasized for the motivation to reuse drugs of abuse to alleviate the intense discomfort of withdrawal symptoms [42]. Negative reinforcement has also been associated with the relief from pain by an analgesic [43]. Lateral hypothalamus-evoked and AGRP neuron-evoked feeding show different characteristics [1], so whether negative reinforcement contributes to the behavioral motivation during energy deficit or during AGRP neuron stimulation remains an area for consideration.
Positive reinforcement
Positive reinforcement mechanisms reflect the pursuit of rewards from the environment. Outcomes that increase positive affect (i.e. feel good) have the potential to be reinforcing (Figure 2c). Unlike negative reinforcement, need states are not required for this, which greatly increases the generality of the concept [44]. In the case of food seeking and consumption behaviors, hunger has been investigated as increasing the incentive value from both the sensory and the post-ingestive/caloric qualities of food.
Palatability
Hunger states influence the incentive value of food, in part, by changing the hedonic qualities of food-associated sensory cues. This is embodied in the separate concepts of alliesthesia [45] and sensory-specific satiety [46]. Alliesthesia refers to the change in perceived pleasantness of external sensory cues associated with changes in internal state. Examples include differences in the reported pleasantness of exposure to warm solutions under conditions of bodily hyperthermia or hypothermia and also the flavor cues associated with sweet solutions in hunger versus satiety [45]. A key challenge is assessing the hedonic qualities of sensory cues without relying on a motivational readout, such as level of consumption, which can result from other processes [47]. Therefore, much of the evidence for hunger modulation of palatability is based on human studies, which take advantage of subjective self-reporting of internal feelings. Shifts in pleasantness of odors [24,28], taste [27], and visual [27,48] cues are found when comparing subjects in hunger and satiety states. Opioid pathways appear to be especially important for selectively controlling ratings of food pleasantness without affecting subjective ratings of hunger level [49]. The hormone ghrelin, an energy deficit signal from the gut, also increases the reported palatability of food [50].
Evaluating pleasantness separately from other motivational factors is substantially more challenging in non-human organisms, which cannot report subjective ratings. Taste-reactivity facial expressions are the primary objective method used to evaluate pleasantness (also referred to as ‘liking’). Manipulation of hunger state in rats is associated with taste reactivity scores that suggest increased liking of a sucrose–quinine mixture only after the fairly extreme condition of 48-hour of food deprivation (but not 24-hour deprivation) [51]. In non-human primates, the firing rate of neurons in the orbitofrontal cortex is both taste responsive and is sensitive to the hunger or satiety states and the tendency to accept or reject a particular food, in contrast to responses from primary gustatory cortical neurons, which are not found to be modulated by hunger [52]. In consideration of the influence of AGRP neurons on food seeking and consumption, one possibility is that AGRP neurons act on brain regions that increase the pleasantness of sensory cues, such as taste and smell, associated with food.
Post-ingestive effects
The reinforcing properties of food can also be mediated through post-ingestive/caloric processes. Rodents develop a preference for a non-nutritive flavor paired with intragastric infusions of caloric sugars, fats, and amino acids [34,53,54]. Nutrient reinforcement does not require food deprivation [54]. Acquisition of a preference for sucrose can also be developed in mice genetically engineered to lack sweet taste sensation [55•], indicating the important role for post-ingestive reinforcement by nutrients. Systemic injections of a dopamine D1 receptor antagonist during training blocks flavor preference learning with intragastric glucose infusions [56], and the post-ingestive consequences of nutrients have been found to include elevation of dopamine levels in the striatum [55•]. This is consistent with other experiments showing that the reinforcing properties of food are mediated, in part, by the midbrain dopaminergic system, and manipulation of dopamine levels and dopamine receptor activity are capable of enhancing or eliminating food reinforcement effects [57–59].
Hormonal modulation
Hormones signaling physiological energy balance modulate the incentive value of food. Intracerebroventricular administration of the hormone ghrelin, which signals energy deficit, increases lever pressing of non-food deprived rats for sucrose solution to levels similar to those of animals under 24-hour food deprivation [60]. Conversely, the hormone leptin, which signals fat reserves, suppresses conditioned place preference for sucrose in food deprived mice [61]. In addition, acute administration of leptin or insulin to hungry animals decreases lever pressing for sucrose solution in a progressive ratio task [62].
Hormonal modulation of the incentive value of food is mediated, at least in part, though dopamine neurons. Ghrelin increases the expression of tyrosine hydroxylase, dopamine turnover, and electrical activity of dopamine neurons [63–65]. Therefore, physiological signals of energy state appear capable of modulating the incentive value of food, in part, through upregulation of the dopaminergic system. Leptin is reported to reduce the electrical activity of ventral tegmental area dopamine neurons in brain slices and in vivo, and knockdown of the leptin receptors in ventral tegmental area dopamine neurons increase the incentive salience of sucrose in a preference test [66–70].
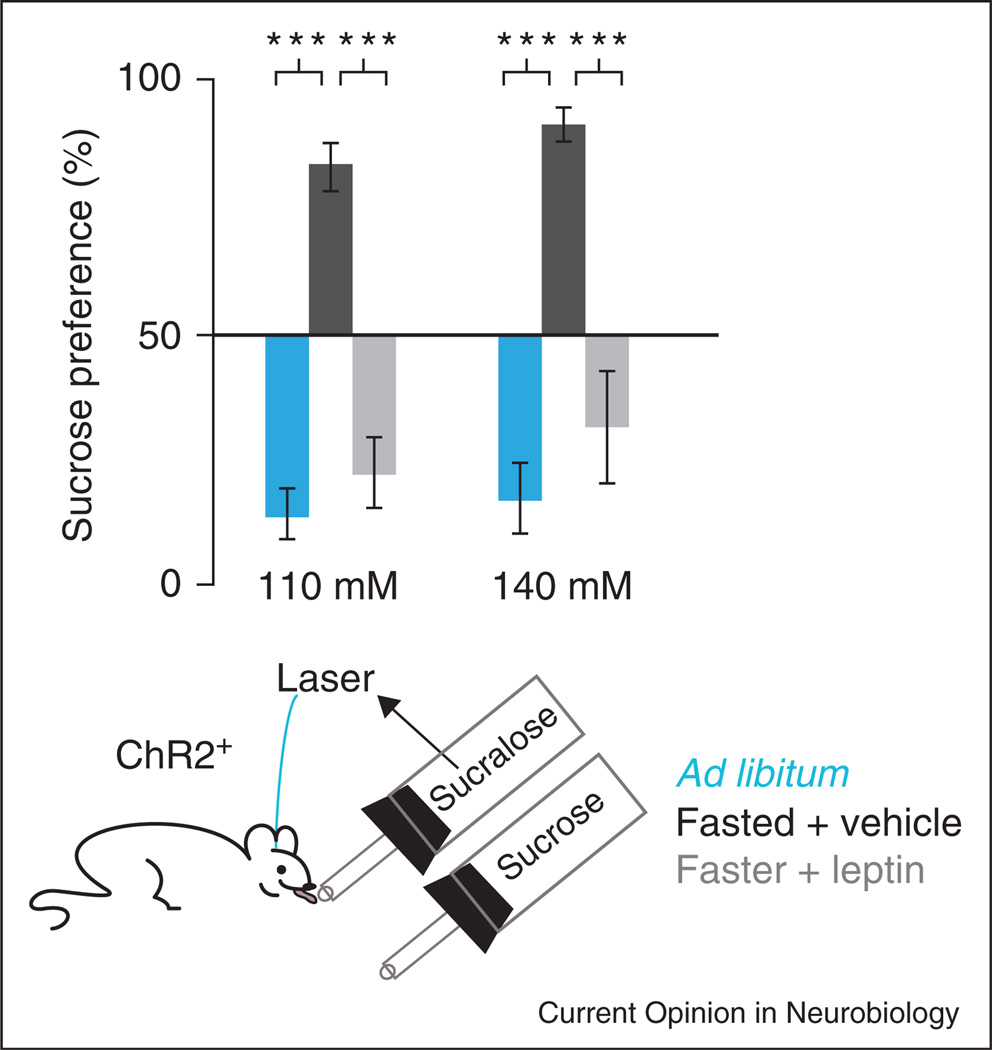
Recent experiments have shown that optogenetic activation of dopamine neurons concurrently with consumption of a non-nutritive sweetener is a combination preferred over the nutritive sweetener, sucrose, alone [71••] (Figure 3). This further highlights the capability for dopamine neurons to mediate the reinforcing qualities of sweet flavors. Importantly though, in food deprived mice the preference for a non-nutritive sweetener paired with dopamine neuron activation was reversed to sucrose preference, and this preference was switched back to the artificial sweetener + dopamine neuron activation in food deprived mice treated with the hormone leptin (Figure 3). The processes underlying these preference switches are unclear, but these experiments indicate an important influence of physiological state and hormones on the reinforcement qualities of taste, nutrients, and dopamine neuron activity.
Figure 3.
Reversible modulation of consummatory preference for sweet solutions dependent on nutrient content, energy deficit, and leptin. In ad libitum (freely) fed mice, optogenetic co-activation of dopamine neurons with consumption of the non-nutritive sweetener sucralose induced a preference for sucralose solution over a nutritive sucrose solution. Food deprivation (24-hour) reversed this preference, but food-restricted mice given leptin switched their preference back to sucralose. Reproduced from [72•].
The relative influence of physiological signals acting directly on dopamine neurons or influencing these circuits by way of starvation-sensing neurons, such as AGRP neurons, is an area for further examination. For example, recent work suggests that AGRP neurons play a developmental role in modulating dopamine neuron function [72•]. A direct projection of AGRP neuron axons is apparent in the vicinity of midbrain dopamine neurons, although the projection is very sparse in adult mice [72•] and has not been functionally characterized. It is not yet known whether AGRP neurons influence dopamine neuron responsiveness during food-deprivation. Given the motivational consequences of AGRP neuron activation, the downstream circuits are likely to reveal important motivational circuit processes associated with fundamental need states.
Conclusions
Here, we have outlined some considerations for investigating motivational mechanisms that underlie the voracious food seeking and consumption associated with hunger. AGRP neurons facilitate circuit analysis of hunger circuits from cell types and synaptic physiology to animal behavior. Rapid manipulation of AGRP neurons offers a means to control and examine behavioral and circuit mechanisms underlying the motivational processes that mediate goal-directed behaviors. The interaction between AGRP neurons and motivationally important brain regions is a key area for exploration. Investigation of the components that mediate the homeostatic response to energetic need, will be the key to understanding the needs and desires associated with essential physiological requirements for survival.
Acknowledgements
S.M.S.’s research is supported by the Howard Hughes Medical Institute.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Sternson SM. Hypothalamic survival circuits: blueprints for purposive behaviors. Neuron. 2013;77:810–824. doi: 10.1016/j.neuron.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS. Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science. 1997;278:135–138. doi: 10.1126/science.278.5335.135. [DOI] [PubMed] [Google Scholar]
- 3.Broberger C, Johansen J, Johansson C, Schalling M, Hokfelt T. The neuropeptide y/agouti gene-related protein (agrp) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc Natl Acad Sci U S A. 1998;95:15043–15048. doi: 10.1073/pnas.95.25.15043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cowley MA. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 5.Fioramonti X, Contie S, Song Z, Routh VH, Lorsignol A, Penicaud L. Characterization of glucosensing neuron subpopulations in the arcuate nucleus: integration in neuropeptide y and proopio melanocortin networks? Diabetes. 2007;56:1219–1227. doi: 10.2337/db06-0567. [DOI] [PubMed] [Google Scholar]
- 6.van den Top M, Lee K, Whyment AD, Blanks AM, Spanswick D. Orexigen-sensitive npy/agrp pacemaker neurons in the hypothalamic arcuate nucleus. Nat Neurosci. 2004;7:493–494. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 7.Luquet S, Perez FA, Hnasko TS, Palmiter RD. Npy/agrp neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 8. Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of agrp neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. Pharmacogenetic techniques were used to show bidirectional modulation of feeding behavior by acutely activating or silencing AGRP neurons.
- 9. Aponte Y, Atasoy D, Sternson SM. Agrp neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. Optogenetic techniques were used to show that AGRP neurons were sufficient to rapidly evoke eating in sated animals. These experiments found a relationship between the level of AGRP neuron activity and the magnitude of the food intake response.
- 10. Atasoy D, Betley JN, Su HH, Sternson SM. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. Circuit mapping techniques were used in AGRP neuron circuits to prioritize the relative contribution of multiple circuit projections for their role in feeding behavior. Circuit operations that led to food seeking and consumption were recapitulated in a second-order circuit node.
- 11.Petreanu L, Huber D, Sobczyk A, Svoboda K. Channelrhodopsin-2-assisted circuit mapping of long-range callosal projections. Nat Neurosci. 2007;10:663–668. doi: 10.1038/nn1891. [DOI] [PubMed] [Google Scholar]
- 12.Kirchgessner AL, Sclafani A. Pvn-hindbrain pathway involved in the hypothalamic hyperphagia-obesity syndrome. Physiol Behav. 1988;42:517–528. doi: 10.1016/0031-9384(88)90153-9. [DOI] [PubMed] [Google Scholar]
- 13.Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 14.Miller NE, Bailey CJ, Stevenson JA. Decreased “hunger” but increased food intake resulting from hypothalamic lesions. Science. 1950;112:256–259. doi: 10.1126/science.112.2905.256. [DOI] [PubMed] [Google Scholar]
- 15.Teitelbaum P. Random and food-directed activity in hyperphagic and normal rats. J Comp Physiol Psychol. 1957;50:486–490. doi: 10.1037/h0048419. [DOI] [PubMed] [Google Scholar]
- 16.Zhang M, Balmadrid C, Kelley AE. Nucleus accumbens opioid, gabaergic, and dopaminergic modulation of palatable food motivation: contrasting effects revealed by a progressive ratio study in the rat. Behav Neurosci. 2003;117:202–211. doi: 10.1037/0735-7044.117.2.202. [DOI] [PubMed] [Google Scholar]
- 17.Sengupta P. The belly rules the nose: feeding state-dependent modulation of peripheral chemosensory responses. Curr Opin Neurobiol. 2012;23:68–75. doi: 10.1016/j.conb.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Root CM, Ko KI, Jafari A, Wang JW. Presynaptic facilitation by neuropeptide signaling mediates odor-driven food search. Cell. 2011;145:133–144. doi: 10.1016/j.cell.2011.02.008. An insulin receptor-dependent process for regulating the magnitude of olfactory signaling in hungry flies.
- 19.Inagaki HK, Ben-Tabou de-Leon S, Wong AM, Jagadish S, Ishimoto H, Barnea G, Kitamoto T, Axel R, Anderson DJ. Visualizing neuromodulation in vivo: Tango-mapping of dopamine signaling reveals appetite control of sugar sensing. Cell. 2012;148:583–595. doi: 10.1016/j.cell.2011.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chalasani SH, Kato S, Albrecht DR, Nakagawa T, Abbott LF, Bargmann CI. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–621. doi: 10.1038/nn.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin YK, Egan JM. Roles of hormones in taste signaling. Results Probl Cell Differ. 2010;52:115–137. doi: 10.1007/978-3-642-14426-4_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savigner A, Duchamp-Viret P, Grosmaitre X, Chaput M, Garcia S, Ma M, Palouzier-Paulignan B. Modulation of spontaneous and odorant-evoked activity of rat olfactory sensory neurons by two anorectic peptides, insulin and leptin. J Neurophysiol. 2009;101:2898–2906. doi: 10.1152/jn.91169.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci U S A. 2000;97:11044–11049. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeomans MR, Mobini S. Hunger alters the expression of acquired hedonic but not sensory qualities of food-paired odors in humans. J Exp Psychol Anim Behav Process. 2006;32:460–466. doi: 10.1037/0097-7403.32.4.460. [DOI] [PubMed] [Google Scholar]
- 25.Pasquet P, Monneuse MO, Simmen B, Marez A, Hladik CM. Relationship between taste thresholds and hunger under debate. Appetite. 2006;46:63–66. doi: 10.1016/j.appet.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 26.Zverev YP. Effects of caloric deprivation and satiety on sensitivity of the gustatory system. BMC Neurosci. 2004;5:5. doi: 10.1186/1471-2202-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rolls ET, Rolls BJ, Rowe EA. Sensory-specific and motivation-specific satiety for the sight and taste of food and water in man. Physiol Behav. 1983;30:185–192. doi: 10.1016/0031-9384(83)90003-3. [DOI] [PubMed] [Google Scholar]
- 28.Rolls ET, Rolls JH. Olfactory sensory-specific satiety in humans. Physiol Behav. 1997;61:461–473. doi: 10.1016/s0031-9384(96)00464-7. [DOI] [PubMed] [Google Scholar]
- 29.Brosvic GM, Hoey NE. Dietary sodium deprivation does not alter taste sensitivity in the rat. Physiol Behav. 1990;47:881–888. doi: 10.1016/0031-9384(90)90013-t. [DOI] [PubMed] [Google Scholar]
- 30.Yaxley S, Rolls ET, Sienkiewicz ZJ, Scott TR. Satiety does not affect gustatory activity in the nucleus of the solitary tract of the alert monkey. Brain Res. 1985;347:85–93. doi: 10.1016/0006-8993(85)90891-1. [DOI] [PubMed] [Google Scholar]
- 31.Yaxley S, Rolls ET, Sienkiewicz ZJ. The responsiveness of neurons in the insular gustatory cortex of the macaque monkey is independent of hunger. Physiol Behav. 1988;42:223–229. doi: 10.1016/0031-9384(88)90074-1. [DOI] [PubMed] [Google Scholar]
- 32.Spyraki C, Fibiger HC, Phillips AG. Attenuation by haloperidol of place preference conditioning using food reinforcement. Psychopharmacology (Berl) 1982;77:379–382. doi: 10.1007/BF00432775. [DOI] [PubMed] [Google Scholar]
- 33.Papp M. Different effects of short- and long-term treatment with imipramine on the apomorphine- and food-induced place preference conditioning in rats. Pharmacol Biochem Behav. 1988;30:889–893. doi: 10.1016/0091-3057(88)90115-3. [DOI] [PubMed] [Google Scholar]
- 34.Ackroff K, Dym C, Yiin YM, Sclafani A. Rapid acquisition of conditioned flavor preferences in rats. Physiol Behav. 2009;97:406–413. doi: 10.1016/j.physbeh.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freud S. Instincts and their vicissitudes. In: Strachey J, editor. Standard edition of the complete psychological works of sigmund freud. Vol. 14. London: Hogarth Press; 2001. pp. 111–140. [Google Scholar]
- 36.Hull CL. Principles of behavior. New York: D. Appleton-Century Company; 1943. [Google Scholar]
- 37.Delgado JM, Anand BK. Increase of food intake induced by electrical stimulation of the lateral hypothalamus. Am J Physiol. 1953;172:162–168. doi: 10.1152/ajplegacy.1952.172.1.162. [DOI] [PubMed] [Google Scholar]
- 38.Oomura Y, Ooyama H, Sugimori M, Nakamura T, Yamada Y. Glucose inhibition of the glucose-sensitive neurone in the rat lateral hypothalamus. Nature. 1974;247:284–286. doi: 10.1038/247284a0. [DOI] [PubMed] [Google Scholar]
- 39.Hoebel BG, Teitelbaum P. Hypothalamic control of feeding and self-stimulation. Science (New York, N Y) 1962;135:375–377. doi: 10.1126/science.135.3501.375. [DOI] [PubMed] [Google Scholar]
- 40.Margules DL, Olds J. Identical “feeding” and “rewarding” systems in the lateral hypothalamus of rats. Science (New York, N Y) 1962;135:374–375. doi: 10.1126/science.135.3501.374. [DOI] [PubMed] [Google Scholar]
- 41.Olds J. The discovery of reward systems in the brain. In: Valenstein ES, Scott, editors. Brain stimulation and motivation: research and commentary. Glenview, IL: Foresman and Company; 1973. pp. 81–99. [Google Scholar]
- 42.Koob GF. Neural mechanisms of drug reinforcement. Ann N Y Acad Sci. 1992;654:171–191. doi: 10.1111/j.1749-6632.1992.tb25966.x. [DOI] [PubMed] [Google Scholar]
- 43.Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, Ossipov MH, King T, Fields HL, Porreca F. Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proc Natl Acad Sci U S A. 2012;109:20709–20713. doi: 10.1073/pnas.1214605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 45.Cabanac M. Physiological role of pleasure. Science. 1971;173:1103–1107. doi: 10.1126/science.173.4002.1103. [DOI] [PubMed] [Google Scholar]
- 46.Rolls BJ, Rolls ET, Rowe EA, Sweeney K. Sensory specific satiety in man. Physiol Behav. 1981;27:137–142. doi: 10.1016/0031-9384(81)90310-3. [DOI] [PubMed] [Google Scholar]
- 47.Berridge KC. Measuring hedonic impact in animals and infants: microstructure of affective taste reactivity patterns. Neurosci Biobehav Rev. 2000;24:173–198. doi: 10.1016/s0149-7634(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 48.Stoeckel LE, Cox JE, Cook EW, 3rd, Weller RE. Motivational state modulates the hedonic value of food images differently in men and women. Appetite. 2007;48:139–144. doi: 10.1016/j.appet.2006.07.079. [DOI] [PubMed] [Google Scholar]
- 49.Yeomans MR, Gray RW. Opioid peptides and the control of human ingestive behaviour. Neurosci Biobehav Rev. 2002;26:713–728. doi: 10.1016/s0149-7634(02)00041-6. [DOI] [PubMed] [Google Scholar]
- 50.Druce MR, Neary NM, Small CJ, Milton J, Monteiro M, Patterson M, Ghatei MA, Bloom SR. Subcutaneous administration of ghrelin stimulates energy intake in healthy lean human volunteers. Int J Obes (Lond) 2006;30:293–296. doi: 10.1038/sj.ijo.0803158. [DOI] [PubMed] [Google Scholar]
- 51.Berridge KC. Modulation of taste affect by hunger, caloric satiety, and sensory-specific satiety in the rat. Appetite. 1991;16:103–120. doi: 10.1016/0195-6663(91)90036-r. [DOI] [PubMed] [Google Scholar]
- 52.Rolls ET, Sienkiewicz ZJ, Yaxley S. Hunger modulates the responses to gustatory stimuli of single neurons in the caudolateral orbitofrontal cortex of the macaque monkey. Eur J Neurosci. 1989;1:53–60. doi: 10.1111/j.1460-9568.1989.tb00774.x. [DOI] [PubMed] [Google Scholar]
- 53.Sclafani A, Nissenbaum JW. Robust conditioned flavor preference produced by intragastric starch infusions in rats. Am J Physiol. 1988;255:R672–R675. doi: 10.1152/ajpregu.1988.255.4.R672. [DOI] [PubMed] [Google Scholar]
- 54.Yiin YM, Ackroff K, Sclafani A. Flavor preferences conditioned by intragastric nutrient infusions in food restricted and free-feeding rats. Physiol Behav. 2005;84:217–231. doi: 10.1016/j.physbeh.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 55. de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. Mice that lack an ion channel for sweet taste transduction still show a strong preference for sugar due to post-ingestive effects.
- 56.Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D1 but not d2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2001;68:709–720. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 57.Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- 58.Salamone JD, Cousins MS, Maio C, Champion M, Turski T, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in a concurrent lever pressing and feeding procedure. Psychopharmacology (Berl) 1996;125:105–112. doi: 10.1007/BF02249408. [DOI] [PubMed] [Google Scholar]
- 59.Zweifel LS, Parker JG, Lobb CJ, Rainwater A, Wall VZ, Fadok JP, Darvas M, Kim MJ, Mizumori SJ, Paladini CA, Phillips PE, et al. Disruption of nmdar-dependent burst firing by dopamine neurons provides selective assessment of phasic dopamine-dependent behavior. Proc Natl Acad Sci U S A. 2009;106:7281–7288. doi: 10.1073/pnas.0813415106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Overduin J, Figlewicz DP, Bennett-Jay J, Kittleson S, Cummings DE. Ghrelin increases the motivation to eat, but does not alter food palatability. Am J Physiol Regul Integr Comp Physiol. 2012;303:R259–R269. doi: 10.1152/ajpregu.00488.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Figlewicz DP, Higgins MS, Ng-Evans SB, Havel PJ. Leptin reverses sucrose-conditioned place preference in food-restricted rats. Physiol Behav. 2001;73:229–234. doi: 10.1016/s0031-9384(01)00486-3. [DOI] [PubMed] [Google Scholar]
- 62.Figlewicz DP, Bennett JL, Naleid AM, Davis C, Grimm JW. Intraventricular insulin and leptin decrease sucrose self-administration in rats. Physiol Behav. 2006;89:611–616. doi: 10.1016/j.physbeh.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 63.Jerlhag E, Egecioglu E, Dickson SL, Andersson M, Svensson L, Engel JA. Ghrelin stimulates locomotor activity and accumbal dopamine-overflow via central cholinergic systems in mice: implications for its involvement in brain reward. Addict Biol. 2006;11:45–54. doi: 10.1111/j.1369-1600.2006.00002.x. [DOI] [PubMed] [Google Scholar]
- 64.Jerlhag E, Egecioglu E, Dickson SL, Douhan A, Svensson L, Engel JA. Ghrelin administration into tegmental areas stimulates locomotor activity and increases extracellular concentration of dopamine in the nucleus accumbens. Addict Biol. 2007;12:6–16. doi: 10.1111/j.1369-1600.2006.00041.x. [DOI] [PubMed] [Google Scholar]
- 65.Abizaid A. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. J Clin Invest. 2006;116:3229–3239. doi: 10.1172/JCI29867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hommel JD, Trinko R, Sears RM, Georgescu D, Liu ZW, Gao XB, Thurmon JJ, Marinelli M, DiLeone RJ. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron. 2006;51:801–810. doi: 10.1016/j.neuron.2006.08.023. [DOI] [PubMed] [Google Scholar]
- 67.Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and gabaergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- 68.Fulton S. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron. 2006;51:811–822. doi: 10.1016/j.neuron.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 69.Trinko R, Gan G, Gao XB, Sears RM, Guarnieri DJ, DiLeone RJ. Erk1/2 mediates leptin receptor signaling in the ventral tegmental area. PLoS ONE. 2011;6:e27180. doi: 10.1371/journal.pone.0027180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brunetti L, Michelotto B, Orlando G, Vacca M. Leptin inhibits norepinephrine and dopamine release from rat hypothalamic neuronal endings. Eur J Pharmacol. 1999;372:237–240. doi: 10.1016/s0014-2999(99)00255-1. [DOI] [PubMed] [Google Scholar]
- 71. Domingos AI, Vaynshteyn J, Voss HU, Ren X, Gradinaru V, Zang F, Deisseroth K, de Araujo IE, Friedman J. Leptin regulates the reward value of nutrient. Nat Neurosci. 2011;14:1562–1568. doi: 10.1038/nn.2977. Reversible modulation of consummatory preference for sweet solutions was dependent on the interactions between dopamine neuron activity, nutrient content, energy deficit, and leptin.
- 72. Dietrich MO, Bober J, Ferreira JG, Tellez LA, Mineur YS, Souza DO, Gao XB, Picciotto MR, Araujo I, Liu ZW, Horvath TL. Agrp neurons regulate development of dopamine neuronal plasticity and nonfood-associated behaviors. Nat Neurosci. 2012;15:1108–1110. doi: 10.1038/nn.3147. Mice with AGRP neuron dysfunction or ablation show enhanced synaptic plasticity on dopamine neurons and increased sensitivity to cocaine.