Abstract
Spontaneous coronary artery dissection is a rare cause of acute coronary syndrome. It is most commonly seen in young women, without atherosclerosis, in the peripartum period. Management options include conservative medical treatment, percutaneous coronary intervention or a surgical approach depending on the presentation, extent of dissection and luminal stenosis. We describe three unusual cases of spontaneous coronary artery dissection occurring in young/middle-aged men—the first in association with heavy mechanical work, the second in association with high levels of anxiety and emotional distress and the third in association with intense physical exercise. In each case we report the use of coronary angiography and intravascular ultrasound in the diagnosis of the condition, and their successful management using percutaneous coronary intervention.
Background
Acute coronary syndrome is a commonly encountered diagnosis on the acute medical take. However, the underlying pathology, as seen in our case series, may not always be secondary to ischaemic heart disease and plaque rupture. Although spontaneous coronary artery dissection (SCAD) is well described in the literature, it is mostly seen in a different subset of patients from those that we encountered. This case series therefore highlights the need to consider the possibility of SCAD in young men presenting with acute coronary syndrome in the absence of factors for ischaemic heart disease.
Case presentation
Case 1
A 49-year-old man presented to the emergency department of our district general hospital with a history of severe, sudden onset chest pain. On further questioning, it became evident that the chest pain had begun following lifting of heavy machinery at work. The patient had a history of hypercholesterolaemia, and was a current cigarette smoker. There was also a positive family history of ischaemic heart disease. He had no history of recreational drug use. There were no significant findings on physical examination, and no evidence of vasculitides or connective tissue disease.
Case 2
A 53-year-old man attended the medical admissions unit of our district general hospital reporting intermittent chest pain for several weeks. His initial experience was of a tearing central chest pain 3 weeks prior to his admission. Since then, he had experienced intermittent chest pain and dyspnoea on exertion. He had been fit and well until this episode, with no significant medical history or relevant family history. Of note, he described recent anxiety and emotional distress associated with a highly stressful and demanding job, and had been feeling particularly anxious around the time of the initial chest pain. He was a smoker of 10 cigarettes a day, had no history of recreational drug use and was taking no medications. On examination, he was found to have a weak radial pulse with a rate of 100 bpm and a blood pressure of 159/125 mm Hg in his right arm, with a similar finding on the left side. His jugular venous pressure was elevated to 6 cm above the clavicle; heart sounds were normal and auscultation of his chest revealed bibasal inspiratory crackles. There was no evidence of connective tissue disease.
Case 3
A 40-year-old man presented to the emergency department of our district general hospital with a history of chest pain, associated with a period of intense physical exercise. The patient gave a history of sudden onset severe, central chest pain after 2 h of vigorous exercise. The symptoms began to resolve with rest. However, further chest pain was experienced after resuming exercise, prompting the patient to attend the local hospital. The patient had no significant medical history and no relevant family history. He was a non-smoker with no history of recreational drug use, and was taking no medications. He had no risk factors for ischaemic heart disease. There were no abnormal findings on clinical examination, and no evidence of vasculitides or connective tissue disease.
Investigations
Case 1
A 12-lead ECG revealed changes consistent with an anterior ST elevation myocardial infarction (ST elevation in leads V2–4). Based on the history and ECG changes the patient was thrombolysed, with resolution of the ST segment elevation.
Case 2
A 12-lead ECG showed T wave inversion in leads II, III, aVF, V5 and V6, along with left ventricular hypertrophy by Sokolow-Lyon index. The admission troponin I was mildly elevated at 0.15 μg/L (normal range 0–0.08 μg/L). An echocardiogram showed severely impaired left ventricular systolic function, with an estimated ejection fraction of 20%; moderate-to-severe dilation of the left ventricle and severe biatrial dilation.
Case 3
The 12-lead ECG performed on admission showed sinus rhythm, with no significant ST segment deviation. The troponin I level on admission was increased at 0.26 μg/L, and at 12 h after the onset of chest pain was further elevated at 8.78 μg/L. His echocardiogram showed hypokinesia of the anteroseptal wall of the left ventricle.
Treatment
In each of the three cases, the patients proceeded to coronary angiography, with a view to performing percutaneous coronary intervention (PCI).
Case 1
Coronary angiography was performed via the right radial route, revealing a normal, dominant right coronary artery (RCA); normal left main stem (LMS) artery and only minor irregularities in the mid-left anterior descending artery (LAD; figure 1). There was no significant disease in the first diagonal. The lack of obvious coronary artery disease was puzzling due to the significant ST elevation on presentation that had resolved with thrombolysis. An intravascular ultrasound (IVUS) was therefore performed to further evaluate the LAD. This revealed a very characteristic spiral dissection of the LAD (figure 2), originating from just distal to the diagonal over a 30 mm segment. In addition there was significant plaque, with a vessel diameter of only 2 mm2 in the middle of the disrupted artery. Interestingly, the angiogram had failed to reveal this due to the spiral dissection, which concealed the true nature of the disease. Follow-on PCI was performed to this vessel under IVUS-guidance. A 3 mm×33 mm everolimus-eluting stent was deployed in the proximal end and postdilated using a non-compliant balloon at 16 atmospheres (figure 3), with excellent apposition of the stent and resolution of the dissection and plaque confirmed by IVUS.
Figure 1.
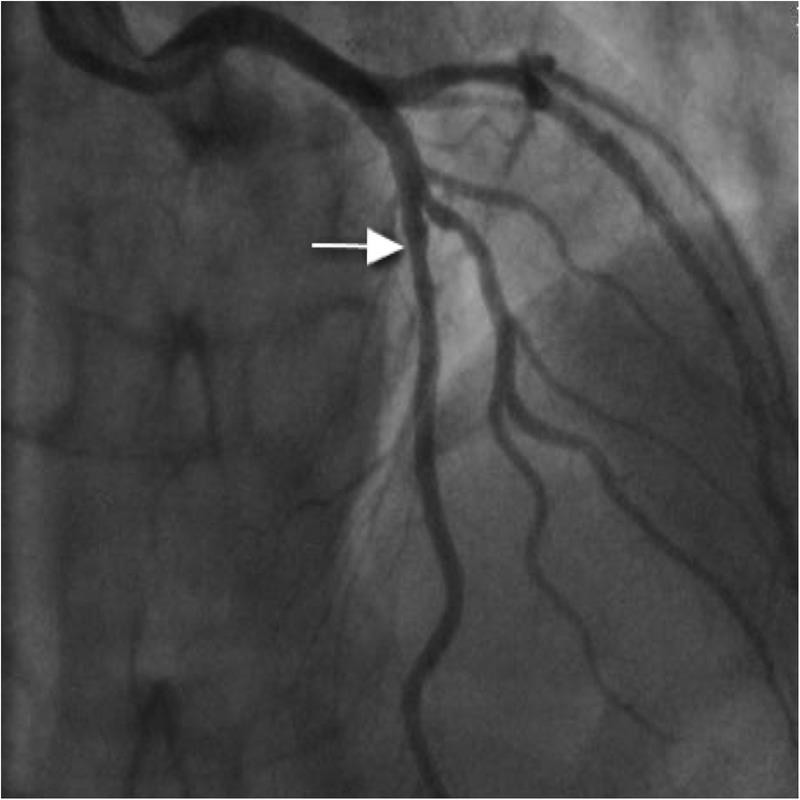
Minor irregularities in left anterior descending artery (arrow).
Figure 2.
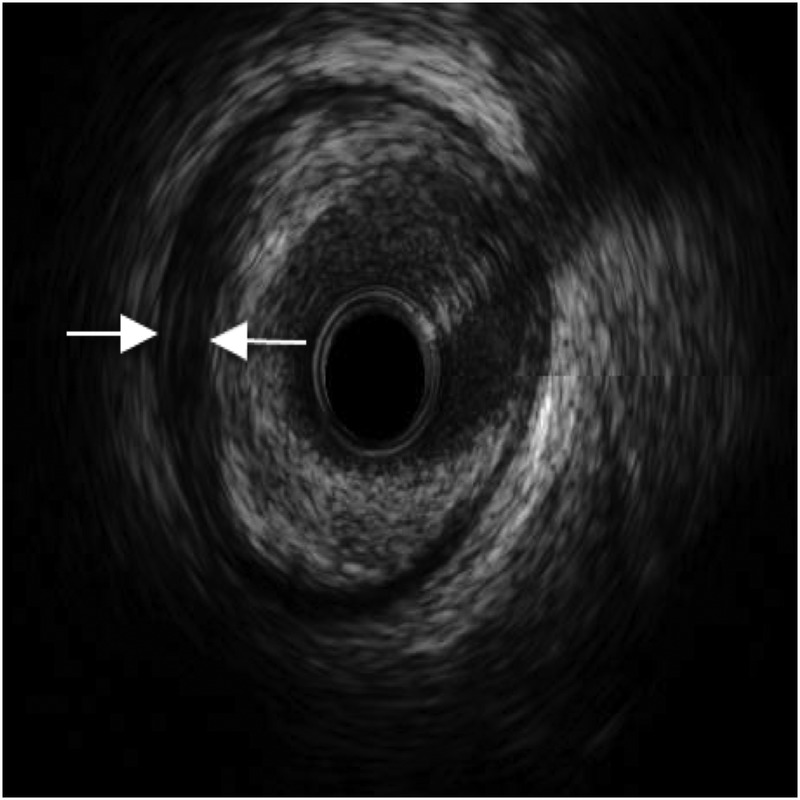
Intravascular ultrasound showing spontaneous dissection in left anterior descending artery (arrows).
Figure 3.
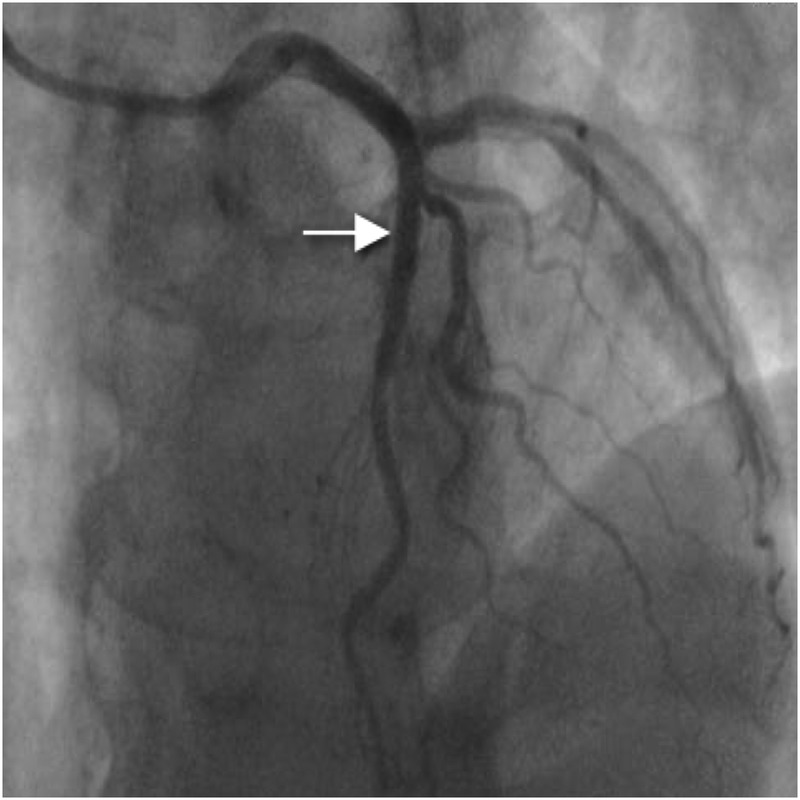
Left anterior descending artery poststent insertion (arrow).
Case 2
Coronary angiography was performed via the right radial route. Findings revealed a normal LMS, atheromatous mid-LAD (40–50% stenosis), mild atheroma in the RCA and the appearance of spontaneous dissection in the dominant mid-left circumflex artery (LCx; figure 4). IVUS of the LCx was performed and showed a huge plaque burden in the proximal and middle portions of the vessel. There was also ruptured plaque and spontaneous dissection in the mid-LCx (figure 5). There was a minimum luminal cross-sectional area of 3.8 mm2. PCI was then performed to the proximal and middle segments of the LCx. Overlapping 4×24 mm and 4×20 mm everolimus-eluting stents were placed respectively, and postdilated using a non-compliant balloon at 16 atmospheres. Good flow in the distal circumflex territory was observed following stent insertion (figure 6), with good stent deployment confirmed by IVUS.
Figure 4.
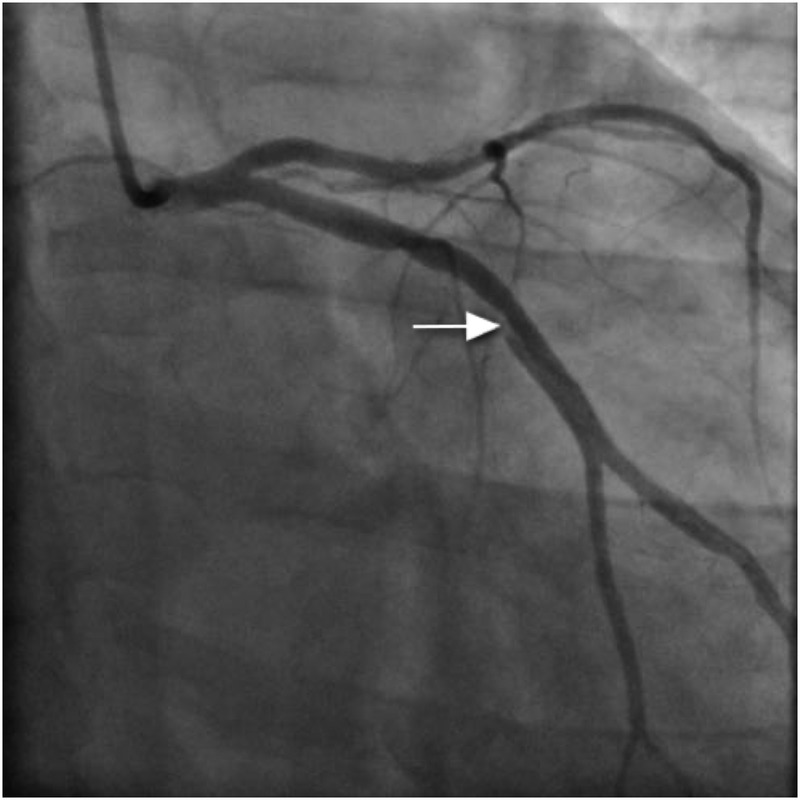
Spontaneous dissection in mid-left circumflex artery (arrow).
Figure 5.
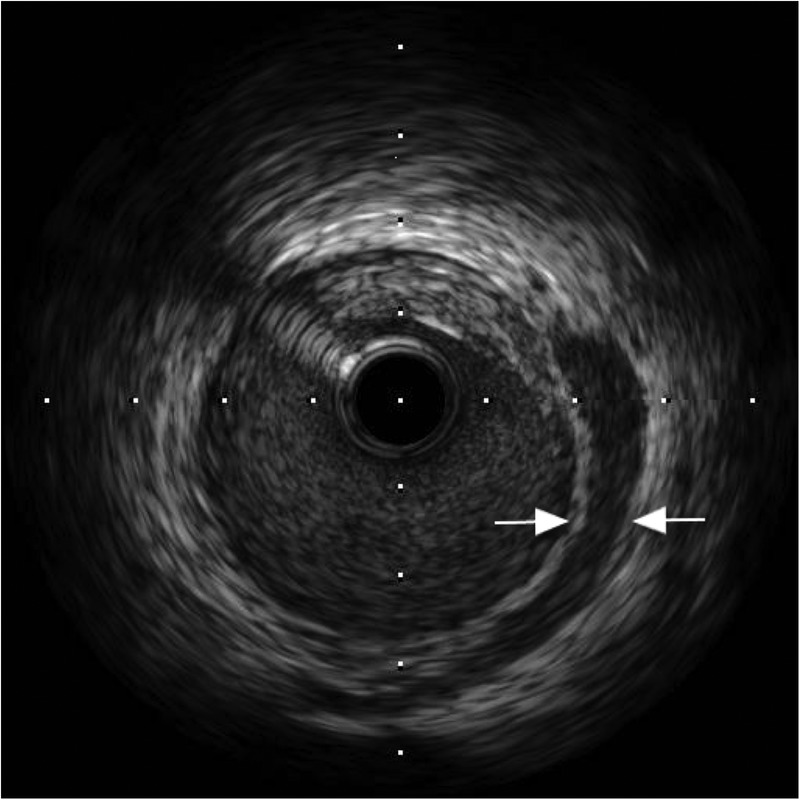
Intravascular ultrasound showing spontaneous dissection in mid-left circumflex artery (arrows).
Figure 6.
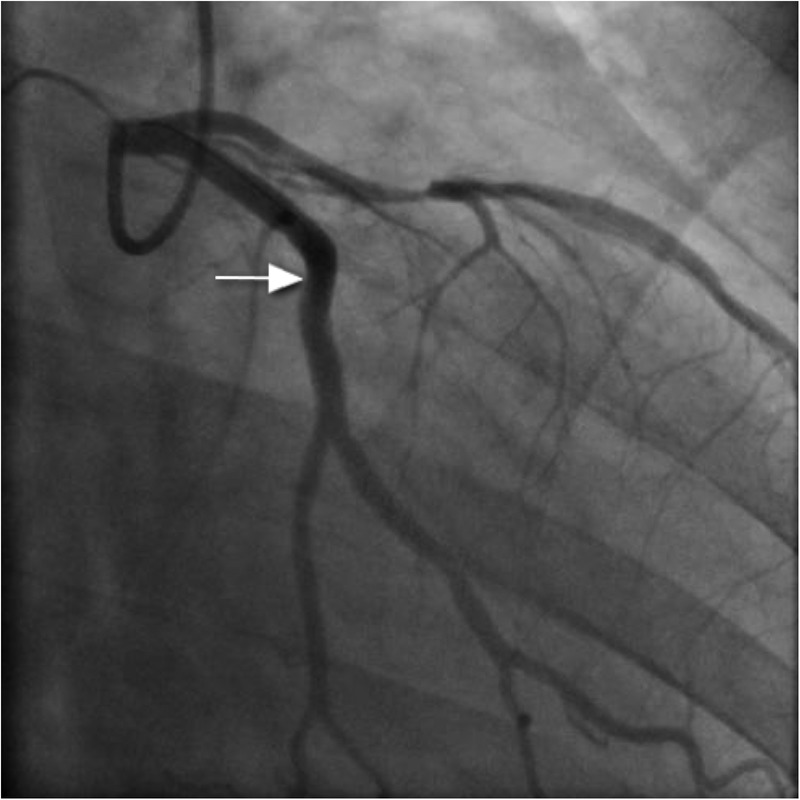
Left circumflex artery poststent insertion (arrow).
Case 3
Coronary angiography, performed via the right radial route, revealed normal LMS, LCx and RCAs. However, a clot was seen in the LAD artery adjacent to the first diagonal, with a hazy appearance (figure 7). IVUS was performed and confirmed dissection in the LAD with adjacent clot (figure 8). Follow-on PCI was performed to the LAD. A 3.5 mm×20 mm everolimus-eluting stent was deployed and postdilated with a non-compliant balloon at 16 atmospheres (figure 9). There was an excellent result with thrombolysis in myocardial infarction flow 3 acquired. IVUS poststent placement confirmed good stent deployment (figure 10).
Figure 7.
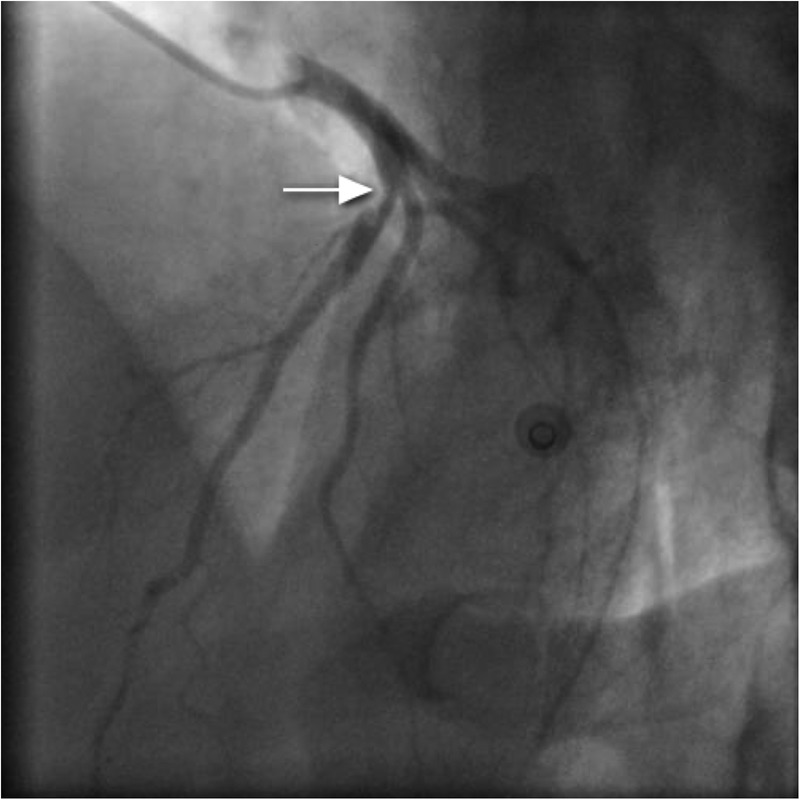
Dissection flap with clot (hazy appearance of left anterior descending artery; arrow).
Figure 8.
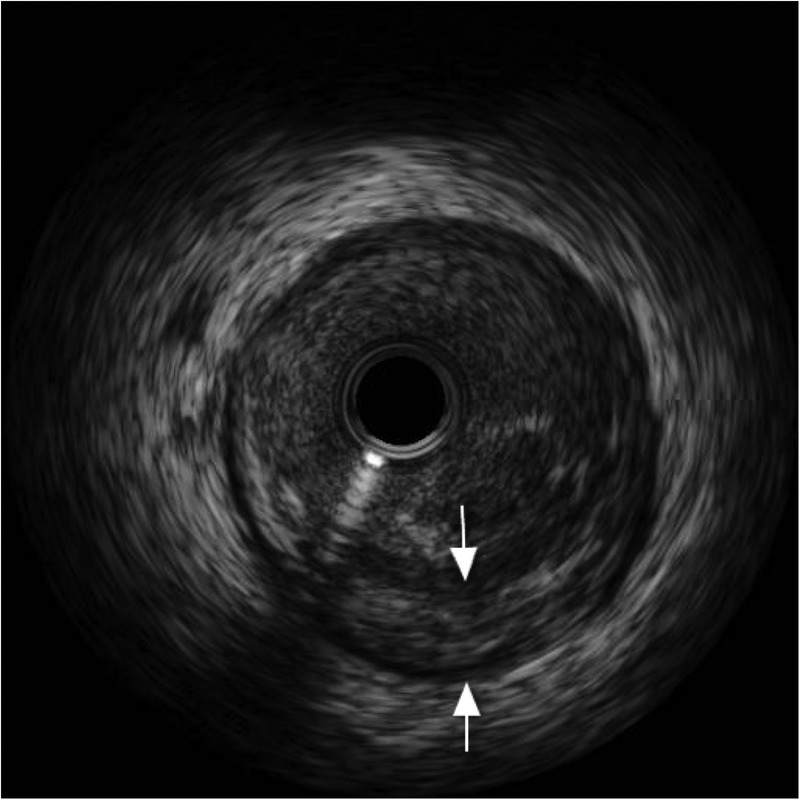
Intravascular ultrasound of left anterior descending artery showing dissection (arrows).
Figure 9.
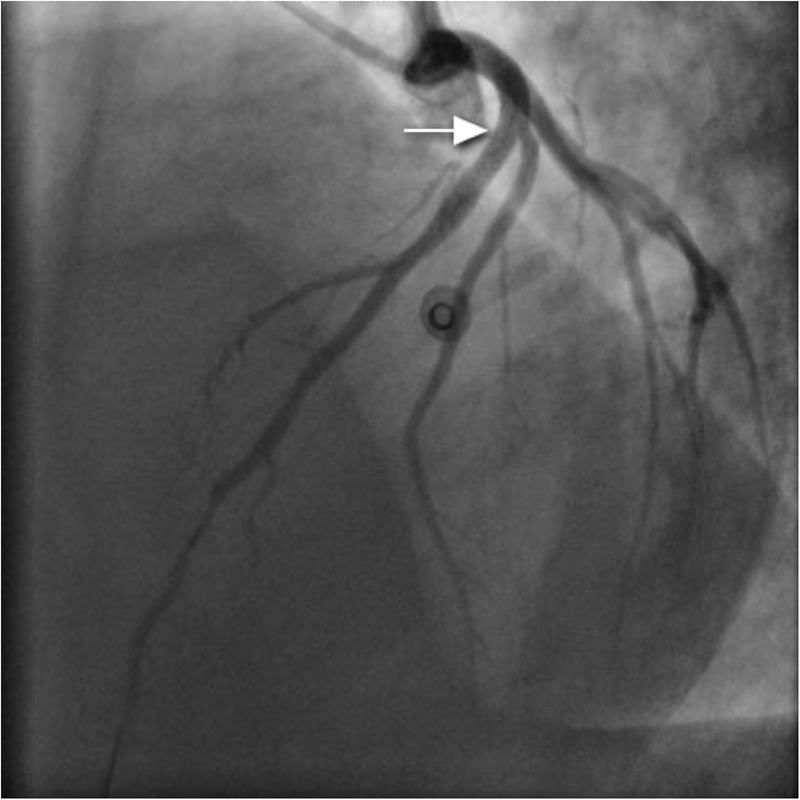
Left anterior descending artery poststent insertion (arrow).
Figure 10.
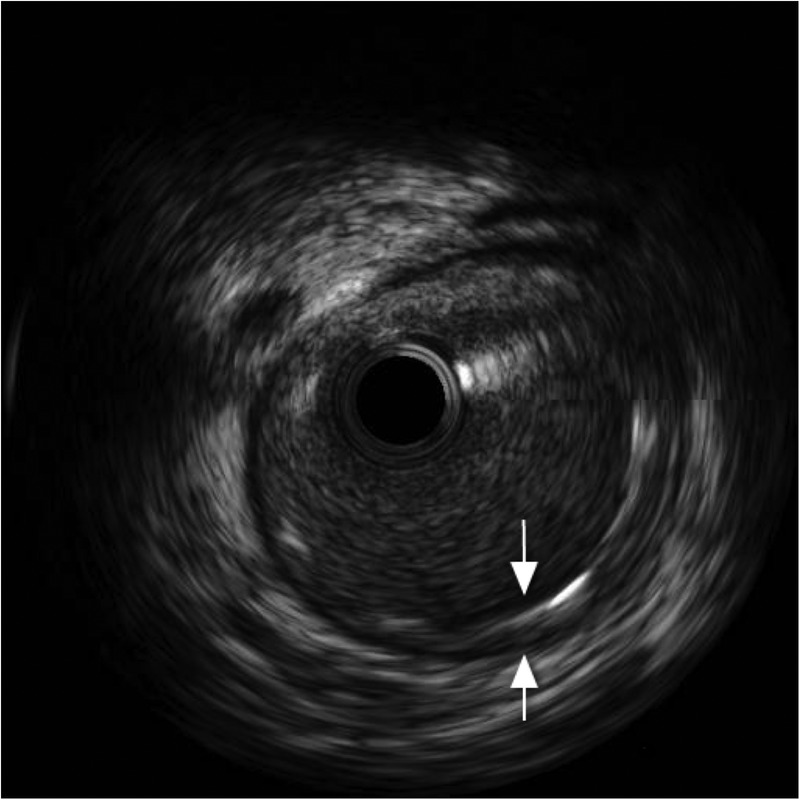
Intravascular ultrasound poststent insertion (arrows).
Outcome and follow-up
Each of the patients made a good recovery and were discharged home with dual antiplatelet therapy (aspirin 75 mg daily for life and clopidogrel 75 mg daily for 1 year)
Discussion
SCAD is a rare but important cause of acute coronary syndrome.1 The exact pathogenesis has not been established to date. Essentially there is forced separation (dissection plane) of the layers of the coronary artery wall, with the creation of a false lumen. Either the intima-media or media-adventitia interfaces can be involved. The resulting effect is blood tracking into the false lumen, which can compromise the true lumen and hence lead to features of myocardial ischaemia or infarction.
With developments in coronary imaging, the diagnosis and management of this potentially life-threatening condition has been greatly improved.2 IVUS is now readily available in most interventional units and CT of coronary arteries is fast becoming widespread. With the option of these modalities to augment findings from readily available coronary angiography, SCAD is increasingly being recognised.
Patients with SCAD present with a variety of features including arrhythmias, unstable angina, acute myocardial infarction, cardiogenic shock, pericardial effusion and tamponade, or sudden cardiac death.3 The most commonly affected subset of patients is women, especially during the peripartum period. This is due to the various hormonal, immunological and haemodynamic changes occurring in maternal physiology during pregnancy.4–6 In non-pregnancy associated cases of SCAD, postmortem analysis and IVUS have shown that the vast majority have underlying atherosclerosis.7 8 IVUS can distinguish between intramural haematoma from atherosclerotic plaques, it can localise the dissection layer, and it can identify the false and true lumens.9 10 Other risk factors involved in SCAD are hypertension, connective tissue diseases, vasculitides, drug usage (recreational and medicinal), aortic dissection, prolonged sneezing and excessive exercise.1 Although any artery can be involved, the LAD artery is the commonest vessel to be affected in both pregnancy and non-pregnancy associated cases.11 12
There have only been a small number of cases reported of SCAD occurring due to strenuous exercise and most of these have involved either female patients or male patients who were not athletic.13–16 The mechanism for SCAD during intense exercise is not yet defined but probably relates to increased blood pressure and raised coronary blood flow during exercise, leading to increased shear stress on the vessel and subsequent dissection.
Psychological distress has also been linked to SCAD.17 18 The role of psychological stress in causing SCAD is even less clear than that of physical exertion. One possibility is that during psychological distress, the coronary arteries undergo intense spasm, which has been linked to causing SCAD.19
Learning points.
In younger men presenting with chest pain associated with exertion or stress, consideration should be given to a diagnosis of spontaneous coronary artery dissection (SCAD) if there is absence of risk factors for ischaemic heart disease.
Management options for patients with SCAD include conservative management, percutaneous coronary intervention (PCI) or cardiac surgery, depending on the presentation, extent of dissection and luminal stenosis. It is well recognised that conservative management can lead to full recovery without the associated risks of interventional procedures.1 12 20 However, we have demonstrated the value of intravascular ultrasound (IVUS) and PCI in the management of such patients presenting with acute coronary syndrome with significant luminal stenosis confirmed by IVUS.
In these patients who have associated ST segment elevation, the risks of thrombolysis should be avoided by proceeding to coronary angiography and IVUS-guided PCI.
SCAD is mostly associated with younger women, without atherosclerosis in the peripartum period. However, as our case series has demonstrated, patients may not always fit the “usual’ profile for SCAD and this diagnosis should be kept in mind by physicians.
Footnotes
Contributors: BNT is the main author participated in drafting the manuscript. SA participated in drafting of the manuscript. JC participated in conception of case report. RA participated in final approval of the manuscript
Competing interests: None.
Patient consent: None.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Vrints CJ. Spontaneous coronary artery dissection. Heart 2010;96:801–8 [DOI] [PubMed] [Google Scholar]
- 2.Porto I, Banning AP. Intravascular ultrasound imaging in the diagnosis and treatment of spontaneous coronary dissection with drug-eluting stents. J Invasive Cardiol 2004;16:78–80 [PubMed] [Google Scholar]
- 3.Dhawan R, Singh G, Fesniak H. Spontaneous coronary artery dissection: the clinical spectrum. Angiology 2002;53:89–93 [DOI] [PubMed] [Google Scholar]
- 4.Slight R, Behranwala AA, Nzewi O, et al. Spontaneous coronary artery dissection: a report of two cases occurring during menstruation. N Z Med J 2003;116:U585. [PubMed] [Google Scholar]
- 5.Bac DJ, Lotgering FK, Verkaaik AP, et al. Spontaneous coronary artery dissection during pregnancy and postpartum. Eur Heart J 1995;16:136–8 [DOI] [PubMed] [Google Scholar]
- 6.Thompson EA, Ferraris S, Gress T, et al. Gender differences and predictors of mortality in spontaneous coronary artery dissection: a review of reported cases. J Invasive Cardiol 2005;17:59–61 [PubMed] [Google Scholar]
- 7.De Maio SJ, Jr, Kinsella SH, Silverman ME. Clinical course and long-term prognosis of spontaneous coronary artery dissection. Am J Cardiol 1989;64:471–4 [DOI] [PubMed] [Google Scholar]
- 8.Maehara A, Mintz GS, Castagna MT, et al. Intravascular ultrasound assessment of spontaneous coronary artery dissection. Am J Cardiol 2002;89:466–8 [DOI] [PubMed] [Google Scholar]
- 9.Arnold JR, West NE, van Gaal WJ, et al. The role of intravascular ultrasound in the management of spontaneous coronary artery dissection. Cardiovasc Ultrasound 2008;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deftereos S, Giannopoulos G, Mavrogianni A, et al. Role of grey-scale intravascular ultrasound and ChromaFlo in deciding on treatment approach for spontaneous coronary artery dissection in a young woman. Hellenic J Cardiol 2011;52:364–6 [PubMed] [Google Scholar]
- 11.Jorgensen MB, Aharonian V, Mansukhani P, et al. Spontaneous coronary dissection: a cluster of cases with this rare finding. Am Heart J 1994;127: 1382–7 [DOI] [PubMed] [Google Scholar]
- 12.Vanzetto G, Berger-Coz E, Barone-Rochette G, et al. Prevalence, therapeutic management and medium-term prognosis of spontaneous coronary artery dissection: results from a database of 11,605 patients. Eur J Cardiothorac Surg 2009;35:250–4 [DOI] [PubMed] [Google Scholar]
- 13.Umman S, Olcay A, Sezer M, et al. Exercise-induced coronary artery dissection treated with an anticoagulant and antiaggregants. Anadolu Kardiyol Derg 2006;6:385–6 [PubMed] [Google Scholar]
- 14.Ellis CJ, Haywood GA, Monro JL. Spontaneous coronary artery dissection in a young woman resulting from an intense gymnasium “work-out”. Int J Cardiol 1994;47:193–4 [DOI] [PubMed] [Google Scholar]
- 15.Almahmeed WA, Haykowski M, Boone J, et al. Spontaneous coronary artery dissection in young women. Cathet Cardiovasc Diagn 1996;37:201–5 [DOI] [PubMed] [Google Scholar]
- 16.Taçoy G, Sahinarslan A, Timurkaynak T. Spontaneous multivessel coronary artery dissection in a wrestler. Anadolu Kardiyol Derg 2007;7:193–5 [PubMed] [Google Scholar]
- 17.Karabag T, Dogan SM. A case of spontaneous multivessel coronary artery dissection presenting with acute myocardial infarction and ventricular tachycardia. Catheter Cardiovasc Interv 2011;79:113–16 [DOI] [PubMed] [Google Scholar]
- 18.Mayr A, Klug G, Jaschke W, et al. Persistent spontaneous dissection of the left anterior descending coronary artery after emotional pressure. Wien Klin Wochenschr 2010;122:515–17 [DOI] [PubMed] [Google Scholar]
- 19.Choi SG, Jeong MH, Bak SW, et al. Successful management of spontaneous dissection with spasm in both coronary arteries. Chonnam Med J 2010;46:112–16 [Google Scholar]
- 20.Tweet MS, Hayes SN, Pitta SR, et al. Clinical features, management and prognosis of spontaneous coronary artery dissection. Circulation 2012;126:579–88 [DOI] [PubMed] [Google Scholar]


