Abstract
When encountering emotional events, memory for those events is typically enhanced. But it has been unclear how emotionally arousing events influence memory for preceding information. Does emotional arousal induce retrograde amnesia or retrograde enhancement? The current study revealed that this depends on the top-down goal relevance of the preceding information. Across three studies, we found that emotional arousal induced by one image facilitated memory for the preceding neutral item when people prioritized that neutral item. In contrast, an emotional image impaired memory for the preceding neutral item when people did not prioritize that neutral item. Emotional arousal elicited by negative and positive pictures both showed this pattern of enhancing or impairing memory for the preceding stimulus depending on its priority. These results indicate that emotional arousal amplifies the effects of top-down priority in memory formation.
Keywords: Emotions, Memory
Decades of research indicate that emotionally arousing events or items are more likely to be remembered than neutral events or items (for a review see LaBar & Cabeza, 2006). But there is surprisingly little consensus about another important aspect of emotional memory: how do arousing stimuli influence memory for neutral stimuli around them?
For instance, animal research reveals that emotional arousal after training enhances memory of the training experiences (for reviews see McGaugh, 2000; 2004). Studies in humans also demonstrate enhanced memory for neutral stimuli preceding emotional items (Anderson, Wais, & Gabrieli, 2006; Finn & Roediger, 2011; Knight & Mather, 2009; Nielson & Arentsen, 2012; Nielson & Powless, 2007; Nielson, Yee, & Erickson, 2005). However, other studies reveal the opposite pattern: impaired memory for neutral stimuli preceding emotional items (Bornstein, Liebel, & Scarberry, 1998; Hurlemann et al., 2005; Knight & Mather, 2009; Miu, Heilman, Opre, & Miclea, 2005; Strange, Hurlemann, & Dolan, 2003; Strange, Kroes, Fan, & Dolan, 2010).
How can the same types of emotional stimuli lead to opposite effects on preceding neutral information in different contexts? Arousal-biased competition (ABC) theory (Mather & Sutherland, 2011) proposes a simple mechanism to explain these contradictory results. The theory posits that emotional arousal modulates the strength of competing mental representations, enhancing memory for high priority items while inhibiting memory for low priority items. The priority of an item can be determined by either its bottom-up perceptual salience or its top-down goal relevance (Fecteau & Munoz, 2006). Thus, according to ABC theory, experiencing emotional arousal should enhance later memory for stimuli that are currently the target of one’s top-down goal or perceptually conspicuous, but inhibit memory for other stimuli. Initial research testing ABC theory demonstrates that perceptual saliency helps determine whether emotional arousal enhances or impairs attention and perceptual learning of particular stimuli (Lee, Itti, & Mather, 2012; Sutherland & Mather, 2012).
In the current study, we examined the role of top-down goal relevance. In particular, we investigated whether goal relevance determines whether emotional arousal induces retrograde amnesia or enhancement. Following previous research (Hurlemann et al., 2005), participants learned image sequences that included several neutral objects and one perceptual oddball (either emotional or neutral). We manipulated participants’ goal when encoding the image sequence; some participants were told to focus on objects that appeared right before the oddball (oddball-1 objects) in each sequence, whereas other participants were told to focus on another image. We predicted that memory for oddball-1 objects would be enhanced by the emotional arousal induced by oddball images when participants prioritized oddball-1 objects, but impaired by that same arousing content when participants prioritized other images. We tested this prediction both with negative and positive emotionally arousing oddball images.
The current study also examined the effects of goal-relevance on memory for subsequent neutral stimuli: objects that followed oddball images (oddball+1 objects). One possibility is that top-down priority modulates the effects of arousal similarly irrespective of whether arousal occurs before or after encountering a to-be-prioritized stimulus. Thus, memory for oddball+1 objects should show emotion-induced impairment when they are not prioritized, but show emotion-induced facilitation when they are prioritized. However, previous research shows emotion-induced memory enhancement predominantly for preceding items, but not for subsequent items (e.g., Knight & Mather, 2009). In addition, emotionally arousing stimuli can impair perceptual processing of stimuli presented immediately afterwards in the same modality (Bocanegra & Zeelenberg, 2009). Thus, an alternative possibility is that emotional arousal interferes with encoding representations of subsequent stimuli, hindering execution of goals about which items to prioritize, leading to weaker interactions between priority and arousal on memory for subsequent items.
Study 1
In Study 1, we tested our hypothesis that top-down goal relevance can determine whether emotionally negative oddballs lead to retrograde amnesia or enhancement. Some participants were asked to prioritize oddball-1 objects (prioritize-oddball-1 condition), whereas the other participants were asked to prioritize oddball images (prioritize-oddball condition). We predicted that memory for oddball-1 objects would be enhanced by negative oddballs when those objects were prioritized, but impaired by the same oddballs when the oddballs were prioritized.
We also examined memory for oddball+1 objects. Since the oddball+1 objects were not relevant to participants’ goals in either priority condition, in both conditions we expected impaired memory for oddball+1 objects following negative compared with neutral oddballs.
Methods
Participants
Participants were 146 volunteers who found our study on lists of online psychology experiments (Mage = 24.12, SD = 6.27, range 18–59; 62 males). They were randomly assigned to the prioritize-oddball-1 (N = 77) or the prioritize-oddball condition (N = 69). To ensure data quality, we had several exclusion criteria: those whose false alarm rates in a memory test were 100% or were defined as outliers (more than three times the interquartile range higher than the third quartile; Tukey 1977) within their condition, those who used only one option for all old responses during the memory test (see Procedure), those who repeatedly took part in the study, and those who participated in the study from the same organization as another participant within 30 min. Seven participants were excluded, leaving 139 people (Mage = 24.30, SD = 6.27, range 18–59; 59 males; prioritize-oddball-1: N = 74; prioritize-oddball: N = 65).
Stimuli
We constructed six lists of ten semantically different items. Each list involved nine non-oddball stimuli (photo objects shown with black labels without any frame) and one perceptual oddball (photo pictures shown with a thick black frame with white labels) that appeared at the 4th, 5th or 6th position in the list; the serial position of the oddball was randomly determined for each list.
Oddballs
Oddball stimuli included six picture pairs obtained from a previous study (Mather & Nesmith, 2008); in each pair, a negative picture was yoked with a less arousing neutral picture which was similar in appearance, complexity, content and focus of interest, and had the same verbal label (S-Table 1). Each participant was shown one of the pictures from each pair; whether they saw the negative or neutral version was counterbalanced across participants.
Non-oddballs
Photographs of neutral objects obtained from previous research (Kensinger, Garoff-Eaton, & Schacter, 2006) were used as non-oddball items.
Eighteen pairs of photo objects were used as oddball-1 and oddball+1 objects. Each pair consisted of two photo objects that shared the same verbal label but had different perceptual features (e.g., color, orientation, and shape). One-third of the pairs were assigned to oddball-1 objects, another third were assigned to oddball+1 objects, and the remaining third were used as distractors in the final memory test. The assignment of object pairs to the three item types and the additional assignment to negative and neutral conditions was counterbalanced across participants.
From each pair assigned to oddball-1 and oddball+1 objects, one of the two photo objects was randomly chosen and shown during the encoding phase; the one not chosen served as the foil shown with the old item in the memory test. An additional 42 photo objects were shown in the remaining list positions during the encoding phase.
Procedure
Participants first completed the encoding phase, in which they viewed six lists of ten images (each shown for 1 sec with its title). Prior to the encoding phase, they were told to remember as many images as possible for a later memory test while focusing especially on one image. Participants in the prioritize-oddball condition were told to focus on the oddball picture, whereas participants in the prioritize-oddball-1 condition were told to focus on the oddball-1 object. To ensure that participants focused on the to-be-prioritized items, after each list, participants were prompted to type the name of the target picture (Figure 1).
Figure 1.
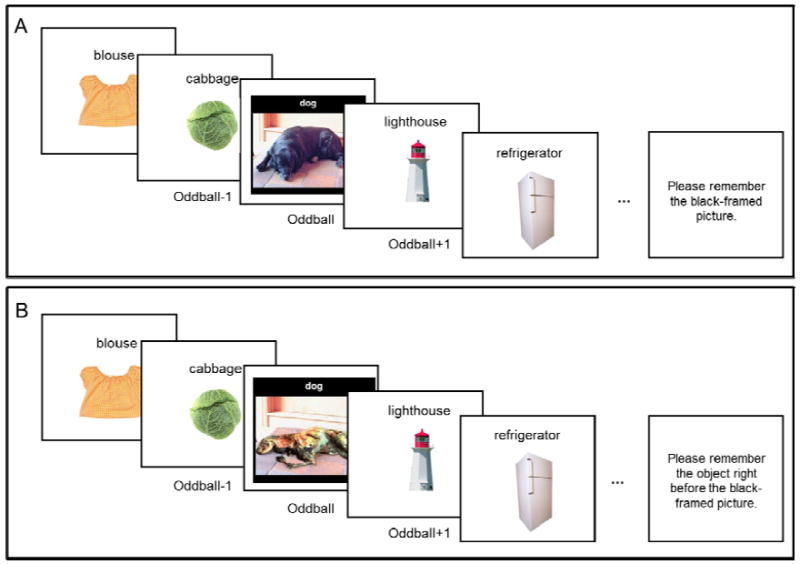
A) Schematic representations of a neutral trial in the prioritize-oddball condition, and B) a negative trial in the prioritize-oddball-1 condition.
After the encoding phase, participants completed a demographic questionnaire and then a memory test. On each memory test trial, participants saw two different photographs of the same object: one labeled “A” and the other labeled “B.” Which version was shown as “A” was randomized. Participants’ task was to indicate whether both images were new or which image they saw during the encoding phase if they were familiar with the objects (Figure 2). The memory test included oddball-1, oddball+1 and new objects (distractors).
Figure 2.

Schematic representation of the memory test.
Response coding
The memory test was designed to obtain more detailed information about participants’ memory compared with a standard old-new recognition memory test. Following past studies (Kensinger, Garoff-Eaton, & Schacter, 2007), each response for old objects was coded as a correct response for specific recognition when the participants selected the exact same object as the one shown during the encoding phase. Old responses given to either the identical or the other version of the same object were also obtained as a measure for the general memory of an item. However, performance was at ceiling in this measure; for example, the correct general recognition rate for oddball-1 objects in the prioritize-oddball-1 condition was .98, .97, and .96 in Studies 1-3 respectively. Therefore, in the current paper, we focused on the specific recognition measure. To control for the false alarm rates, we analyzed the corrected recognition rate (correct specific recognition rate - false alarm rate).
Results and Discussion
Encoding phase
Performance was better in the prioritize-oddball (Mneg = .98, Mneut = .98) than in the prioritize-oddball-1 condition (Mneg = .95, Mneut = .94), F(1, 137) = 6.82, ηP 2 = .05, p < .05, with no other significant effects (ps > .60).
Memory for oddball-1 objects
A 2 (valence: negative vs. neutral) × 2 (priority: oddball-1 vs. oddball) analysis-of-variance (ANOVA) on the corrected recognition rate for oddball-1 objects revealed better memory in the prioritize-oddball-1 than the prioritize-oddball condition, F(1, 137) = 11.75, ηP 2 = .08, p < .01. This indicates that participants prioritized oddball-1 objects more in the prioritize-oddball-1 condition, as expected. In addition, we found a significant valence-by-priority interaction (Figure 3A), F(1, 137) =10.16, ηP 2 = .07, p < .01. Consistent with our prediction, when participants prioritized oddball-1 objects, their memory for oddball-1 objects was more accurate in the negative than in the neutral condition (Mneg = .71, Mneut = .64), F(1, 137) = 4.43, p < .05. Also as predicted, the prioritize-oddball condition showed the opposite pattern: worse memory for oddball-1 objects in the negative than in the neutral condition (Mneg = .54, Mneut = .46), F(1, 137) = 5.74, p < .05. Thus, depending on the top-down goal, seeing negative pictures produced either retrograde amnesia or retrograde enhancement for preceding neutral information.
Figure 3.
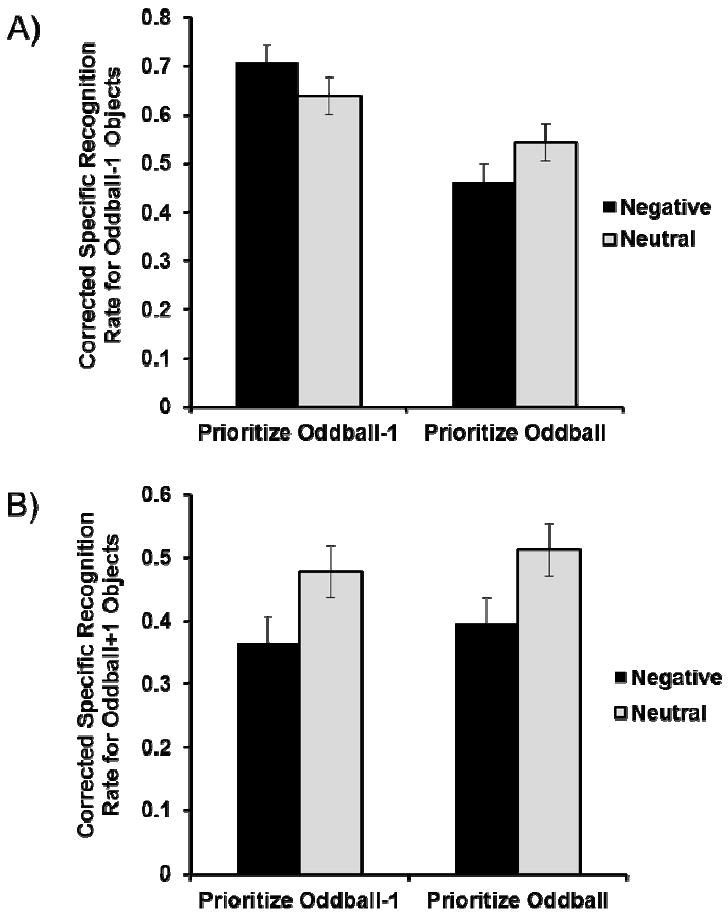
Corrected specific recognition rates in the memory test in Study 1. A) Memory for oddball-1 objects. B) Memory for oddball+1 objects. Error bars represent standard error.
Memory for oddball+1 objects
The corrected recognition rates for oddball+1 objects revealed a significant valence effect, F(1,137) = 10.63, ηP 2 = .07, p < .01, with no other significant effects (ps > .50). As expected, in both conditions, participants showed worse memory for oddball+1 objects when the objects followed negative than neutral pictures (Figure 3B; oddball–1: Mneg = .36, Mneut= .48; oddball: Mneg = .39, Mneut = .51).
Study 2
Study 2 extended Study 1 in two ways. First, we examined whether similar effects were obtained with positive emotions. Second, we added a condition in which participants prioritized oddball+1 objects (prioritize-oddball+1 condition). Thus, we manipulated the goal-relevance of oddball+1 objects as well, to examine the role of top-down priority on memory for subsequent information.
Methods
Participants
Participants were 227 volunteers recruited via lists of online psychology experiments (Mage = 26.41, SD = 8.94, range 18–62; 62 males). None of their IP addresses matched those of participants in Study 1. They were randomly assigned to prioritize-oddball-1 (N = 68), prioritize-oddball (N = 75) or prioritize-oddball+1 condition (N = 84). We applied the same exclusion criteria as Study 1. In addition, we excluded three participants in the prioritize-oddball+1 condition who typed not only the title of the oddball+1 objects, but also all other objects following oddballs during the encoding phase. The remaining participants included 205 people (Mage = 26.78, SD = 9.23, range 18–62; 52 males, prioritize-oddball-1: N = 64; prioritize-oddball: N = 67; prioritize-oddball+1: N = 74).
Stimuli and Procedure
The procedures were identical to Study 1, except for two modifications. First, oddball pictures were replaced by six positive pictures and their yoked visually similar, but less arousing neutral pictures with the same labels (S-Table 1; Mather & Nesmith, 2008). Second, we included a prioritize-oddball+1 condition, where participants were told to focus on the oddball+1 object and type its name after each list.
Results and Discussion
Encoding phase
Neither the priority condition, nor valence influenced performance in the encoding phase (ps > .10; prioritize-oddball: Mpos = .97, Mneut = .97; prioritize-oddball-1: Mpos = .93, Mneut = .94; prioritize-oddball+1: Mpos = .94, Mneut = .97).
Memory for oddball-1 objects
Oddball-1 objects were not relevant to participants’ goals in either the prioritize-oddball+1 or the prioritize-oddball condition, and as expected, we found a similar valence effect in these two conditions without any interaction (p > .30). Therefore, these two conditions were combined into one “prioritize-another-item” group. A 2 (valence: positive vs. neutral) × 2 (priority: oddball-1 vs. another) ANOVA was then performed on the corrected recognition rate. This ANOVA revealed a significant priority effect, F(1, 203) = 7.58, ηP 2 = .04, p < .01, and a significant valence-by-priority interaction (Figure 4A), F(1, 203) = 8.91, ηP 2 = .04, p < .01. Consistent with our prediction, memory for oddball-1 objects was enhanced by positive oddballs in the prioritize-oddball-1 condition (Mpos = .72, Mneut = .64), F(1, 203) = 4.20, p < .05, but impaired by positive oddballs in the prioritize-another-item group (Mpos = .53, Mneut = .59), F(1, 203) = 5.29, p < .05.
Figure 4.
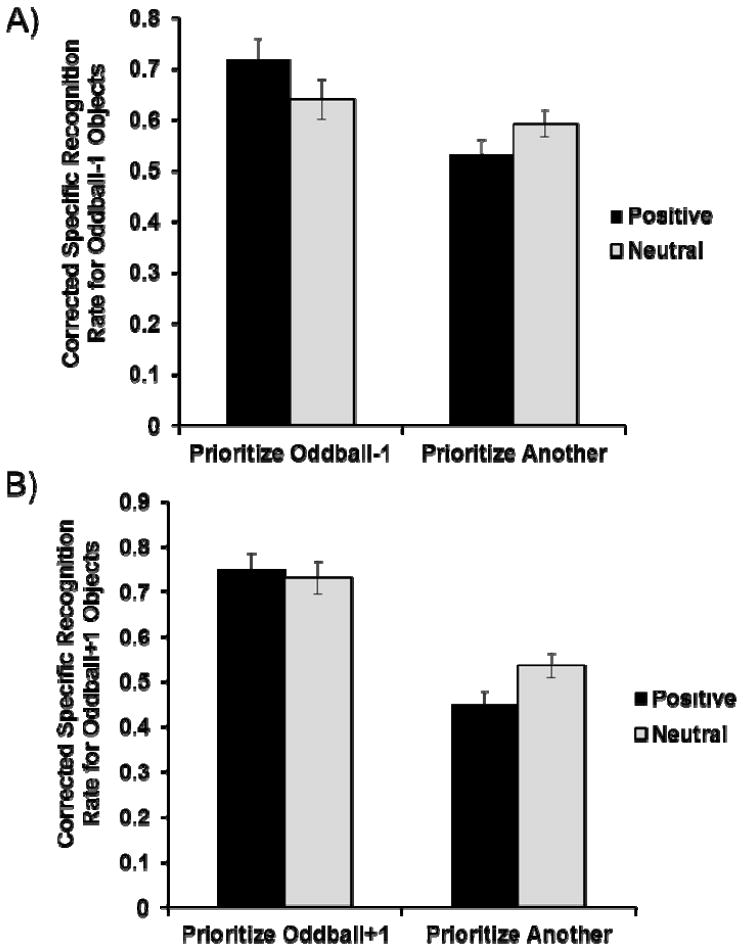
Corrected specific recognition rates in the memory test in Study 2. A) Memory for oddball-1 objects. B) Memory for oddball+1 objects. Error bars represent standard error.
Memory for oddball+1 objects
Next, we examined memory for oddball+1 objects. Since oddball+1 objects were not relevant to participants’ goals in either the prioritize-oddball-1 or the prioritize-oddball condition, these two conditions were combined into one group (“prioritize-another-item” group); indeed, these two conditions showed a similar valence effect without any interaction (p > .20). The corrected recognition rate was then submitted to a 2 (valence) × 2 (priority: oddball+1 vs. another) ANOVA. This ANOVA revealed a significant priority effect, F(1, 203) = 39.00, ηP 2 = .16, p < .01, and a valence-by-priority interaction (Figure 4B), F(1, 203) = 4.43, ηP 2 = .02, p < .05. Replicating Study 1’s results, positive oddballs impaired memory for subsequent objects when participants did not prioritize those objects (Mpos = .45, Mneut = .54), F(1, 203) = 8.41, p < .01. In contrast, this emotion-induced impairment did not occur in the prioritize-oddball+1 condition (p > .60; Mpos = .75, Mneut = .73), though we did not see the emotion-induced memory facilitation.
Study 3
Study 3 fulfilled three objectives. The first goal was to confirm our results from Studies 1 and 2 for preceding information while including positive and negative emotions within a single study. We expected to replicate that goal-relevance of preceding items determines whether emotional images (irrespective of valence) induce retrograde amnesia or enhancement. Second, we aimed to further examine the effects of goal-relevance on memory for subsequent information. In Study 2, positive oddballs enhanced memory for goal-relevant preceding information but not subsequent information. In Study 3, we examined whether this diminished effect occurred with negative emotion or was specific to positive emotion. Lastly, we addressed whether the effects of emotional oddballs are attributable to arousal or valence by using a trial-by-trial analysis.
Methods
Participants
Sixty-four undergraduates and graduate students (Mage = 20.71, SD = 2.92, range 18-34; 22 males) were randomly assigned to the prioritize-oddball-1 (N = 32), or prioritize-oddball+1 condition (N = 32).
Stimuli
We increased the number of lists to 42; each list included seven non-oddball objects and one oddball that appeared at the 3rd, 4th, 5th or 6th position. Oddballs were 42 pictures (14 positive, 14 negative and 14 neutral) obtained from the International Affective Picture System (S-Table 1; Lang, Bradley, & Cuthbert, 2008).
Non-oddballs included 112 pairs of photo objects obtained from previous research (Kensinger et al., 2006) and other resources (e.g., Internet). They were randomly assigned to oddball-1 or oddball+1 objects and further assigned to one of the four conditions (positive, negative, neutral or distractors in a memory test); assignment was counterbalanced across participants. During the encoding phase, one of the photo objects was shown from each pair; which version was shown was counterbalanced across participants. The one not shown served as a foil in the memory test. An additional 210 photo objects were shown in the remaining list positions during the encoding phase.
Procedure
The procedure was based on Study 2 with several modifications. First, we had participants complete the study in the lab. Second, we omitted the prioritize-oddball condition. Third, we increased the total number of trials to 42 (14 trials per condition), divided into seven blocks, each of which included six trials (2 positive, 2 negative and 2 neutral).
In each block, participants first underwent the encoding phase, where they saw six lists of seven images (each image shown for 1.2 sec followed by a 500-ms blank screen; each list followed by a cue to retrieve the prioritized item). After the encoding phase, participants were given a three-digit number and asked to count back from the number by 3s for 1 min. Participants then completed a memory test on oddball-1 and oddball+1 objects. To prevent participants from selectively remembering oddball-1 and oddball+1 objects, the memory test also included other objects randomly chosen from half of the lists in each block (filler items).
After all blocks, participants viewed each oddball picture and rated it on arousal (1: least arousing -9: most arousing) and valence (1: extremely negative-9: extremely positive).
Results and Discussion
Encoding phase
Performance was better in the prioritize-oddball-1 than in the prioritize-oddball+1 condition, F(1, 62) = 4.72, ηP 2 = .05, p < .05. In addition, the valence-by-priority interaction was significant, F(1, 124) = 4.46, ηP 2 = .09, p < .05; performance in the prioritize-oddball+1 condition was better in the negative than in the neutral condition (Mneg = .98, Mneut = .94, Mpos = .97), ts(62) = 2.75, ds = 0.54, p < .05 (Tukey), while the valence effect was not significant in the prioritize-oddball-1 condition (p > .50; Mneg = .97, Mneut = .99, Mpos = .98). Given this significant interaction and that our main interest is on the effects of emotion on memory retention (rather than encoding performance), in subsequent analyses on memory tests, we focused on trials where participants made a correct response during the encoding phase. However, results were similar when including items from incorrect encoding phase trials.
Oddball picture rating
Negative pictures (M = 2.14) were rated more negatively than neutral pictures (M = 5.09), and positive pictures (M = 6.85) were rated more positively than neutral pictures, ts(124) = 25.67, 15.37, ds = 4.17, 2.04, ps < .01 (Tukey). In addition, negative pictures (M = 6.79) were rated as more arousing than positive pictures (M = 5.09) that were rated as more arousing than neutral pictures (M = 2.10), ts(124) = 5.67, 15.05, ds = 0.97, 3.46, ps < .01 (Tukey). The priority conditions did not significantly affect the valence and arousal ratings (ps > .15).
Memory for oddball-1 objects
A planned-contrast test (Rosenthal & Rosnow, 1985) on the corrected recognition rates confirmed the results from Studies 1 and 2 (Figure 5A), F(1, 124) = 3.93, p < .05, as memory for oddball-1 objects was enhanced by positive and negative oddballs in the prioritize-oddball-1 condition (Mneg = .73, Mneut = .69, Mpos = .72), but impaired by positive and negative oddballs in the prioritize-oddball+1 condition (Mneg = .54, Mneut = .57, Mpos = .54).
Figure 5.
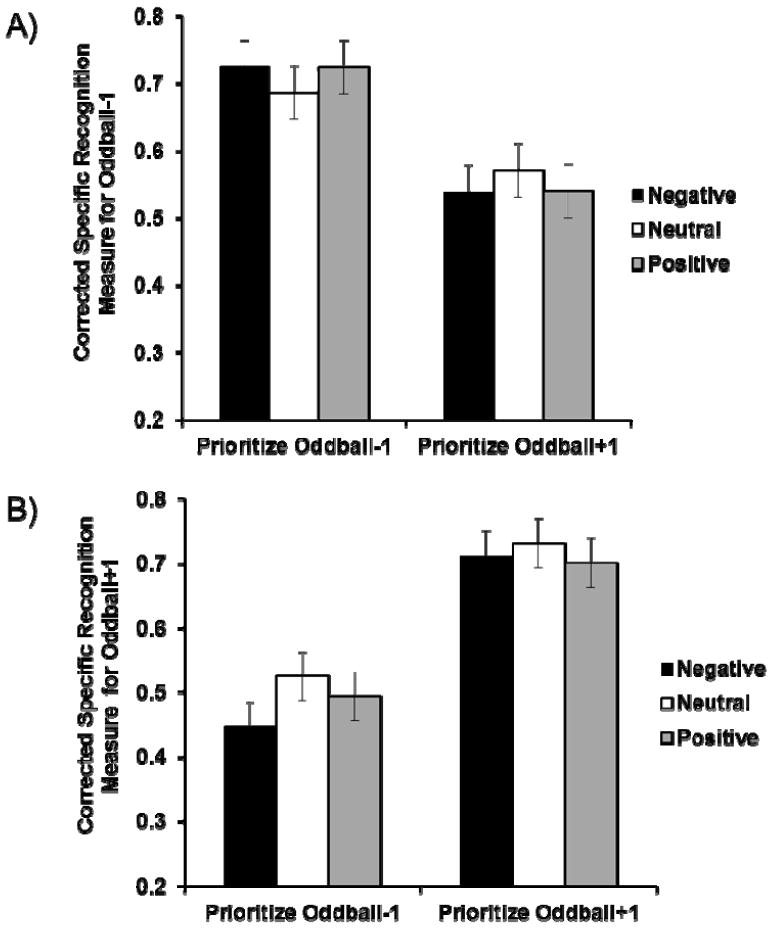
Corrected specific recognition rates in the memory test in Study 3. A) Memory for oddball-1 objects. B) Memory for oddball+1 objects. Error bars represent standard error.
To examine the effects of arousal and valence, a hierarchical generalized linear model analysis (HGML; Raudenbush & Bryk, 2002) was performed with each trial as a level-1 unit and each participant as a level-2 unit. The dependent variable was specific memory performance for an oddball-1 object from each trial (1: correct; 0: incorrect). Predictors included the participant’s arousal and valence ratings for the oddball picture from that trial, the priority condition (oddball-1 vs. oddball+1), an arousal-by-priority interaction term, and a valence-by-priority interaction term. This analysis revealed a significant priority effect, z = 3.91, p < .01, reflecting better memory for oddball-1 objects when these objects were prioritized than when oddball+1 objects were prioritized, as expected. In addition, there was a significant arousal-by-priority interaction, z = 2.41, p < .05, with no other significant effects (ps > .15). These results indicate that arousal, rather than valence, plays a crucial role in modulating the effects of priority on memory for preceding information.
Memory for oddball+1 objects
A planned-contrast test on the corrected recognition rates did not show the pattern expected by ABC theory (p > .30; Figure 5B). To explore the results, a separate contrast analysis was performed for each priority condition. Replicating Study 2’s results, memory for oddball+1 objects was impaired by positive and negative oddballs when the objects were not prioritized (Mneg = .45, Mneut = .53, Mpos = .49), F(1, 124) = 5.72, p < .05. In contrast, emotional oddballs did not enhance memory for oddball+1 objects when they were prioritized (p = .20; Mneg = .71, Mneut = .73, Mpos = .70).
To examine the role of arousal and valence, a HGLM analysis (equivalent to the one used for oddball-1 objects) was performed on memory for oddball+1 objects. There was a significant effect of priority, z = -5.65, p < .01, reflecting better memory for oddball+1 objects when these objects were prioritized than when oddball-1 objects were prioritized, as expected. But neither the effects of arousal nor the arousal-by-priority interaction was significant (ps > .20). Instead, we found a valence-by-priority interaction, z = 1.94, p = .055. These results indicate that negative pictures, relative to positive pictures, impaired memory for the subsequent object less when that object was prioritized than when the preceding object was prioritized.
General Discussion
Previous research presents a conflicting set of findings in which emotionally arousing stimuli sometimes enhance and sometimes impair memory for preceding neutral stimuli (for a review see Mather & Sutherland, 2011). The current study tested the hypothesis that top-down stimuli priority helps determine how arousal modulates memory. Consistent with this hypothesis, emotional images (either positive or negative) enhanced memory for preceding neutral objects when people prioritized those objects, but impaired memory for preceding objects when people did not prioritize the objects. Thus, depending on the focus of current goals, emotional images produced either retrograde amnesia or retrograde enhancement for preceding neutral information.
In contrast, memory for subsequent neutral information showed a weaker interaction between arousal and priority. Emotional images impaired memory for subsequent neutral objects when those objects were not prioritized. This emotion-induced impairment was diminished when people prioritized the subsequent objects, though we did not find emotion-induced memory enhancement for goal-relevant subsequent objects. Furthermore, the selective impairment for subsequent neutral objects appeared stronger for negative than positive emotion.
The weaker nature of the post-arousal effects might be mediated by two different effects of emotion on perception. First, encountering emotional stimuli can impair perceptual processing of subsequent stimuli in the same modality for at least 500 ms (Bocanegra & Zeelenberg, 2009), making it harder to prioritize subsequent stimuli under arousal. Second, positive emotional arousal might broaden attentional focus (Fredrickson & Branigan, 2005) and enhance attention to subsequent stimuli even when they are not the target of goals (e.g., Waring & Kensinger, 2009). Because of these effects on perception (in addition to memory), emotional arousal may affect processing of subsequent information in a more complex manner than preceding information.
Prioritizing goal-relevant information and ignoring irrelevant stimuli is crucial in human memory. This prioritization process is particularly important under emotional arousal; to increase future survival, one needs to learn the most important aspects of emotional events without being distracted by other details. The current findings indicate that emotional arousal amplifies the impact of top-down stimuli priority on memory, making it even more likely that people remember what was most important while forgetting the rest. These findings not only inform the basic mechanisms of emotion-memory interaction, but also have practical implications for eyewitness memory and students’ learning in classroom.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute on Aging (R01AG025340, and K02AG032309).
Footnotes
Authorship
M.S. and M.M developed the study concept. All authors contributed to the study design. Data collection were performed by K.F. M.S. performed the data analysis. M.S. drafted the paper, and M.M. and K.F provided critical revisions. All authors approved the final version of the paper for submission.
References
- Anderson AK, Wais PE, Gabrieli JDE. Emotion enhances remembrance of neutral events past. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(5):1599–1604. doi: 10.1073/pnas.0506308103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocanegra BR, Zeelenberg R. Dissociating emotion-induced blindness and hypervision. Emotion. 2009;9:865–873. doi: 10.1037/a0017749. [DOI] [PubMed] [Google Scholar]
- Bornstein BH, Liebel LM, Scarberry NC. Repeated testing in eyewitness memory: a means to improve recall of a negative emotional event. Applied Cognitive Psychology. 1998;12(2):119–131. doi: 10.1002/(sici)1099-0720(199804)12:2<119∷aid-acp500>3.0.co;2-4. [DOI] [Google Scholar]
- Fecteau JH, Munoz DP. Salience, relevance, and firing: a priority map for target selection. Trends in Cognitive Sciences. 2006;10(8):382–390. doi: 10.1016/j.tics.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Finn B, Roediger HL. Enhancing retention through reconsolidation: Negative emotional arousal following retrieval enhances later recall. Psychological Science. 2011;22:781–786. doi: 10.1177/0956797611407932. [DOI] [PubMed] [Google Scholar]
- Fredrickson BL, Branigan C. Positive emotions broaden the scope of attention and thought-action repertoires. Cognition and Emotion. 2005;19(3):313–332. doi: 10.1080/02699930441000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlemann R, Hawellek B, Matusch A, Kolsch H, Wollersen H, Madea B, et al. Noradrenergic modulation of emotion-induced forgetting and remembering. Journal of Neuroscience. 2005;25:6343–6349. doi: 10.1523/JNEUROSCI.0228-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Memory for specific visual details can be enhanced by negative arousing content. Journal of Memory and Language. 2006;54(1):99–112. doi: 10.1016/j.jml.2005.05.005. [DOI] [Google Scholar]
- Kensinger EA, Garoff-Eaton RJ, Schacter DL. Effects of emotion on memory specificity: Memory trade-offs elicited by negative visually arousing stimuli. Journal of Memory and Language. 2007;56(4):575–591. [Google Scholar]
- Knight M, Mather M. Reconciling findings of emotion-induced memory enhancement and impairment of preceding items. Emotion. 2009;9(6):763–781. doi: 10.1037/a0017281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, Cabeza R. Cognitive neuroscience of emotional memory. Nature Review Neuroscience. 2006;7(1):54–64. doi: 10.1038/nrn1825. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-8. University of Florida; Gainesville, FL: 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. [Google Scholar]
- Lee T-H, Itti L, Mather M. Evidence for arousal-biased competition in perceptual learning. Frontiers in Psychology. 2012;3 doi: 10.3389/fpsyg.2012.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Nesmith K. Arousal-enhanced location memory for pictures. Journal of Memory and Language. 2008;58:449–464. doi: 10.1016/j.jml.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Sutherland M. Arousal-biased competition in perception and memory. Perspectives on Psychological Science. 2011;6:114–133. doi: 10.1177/1745691611400234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaugh JL. Memory--A century of consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience. 2004;27(1):1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- Miu AC, Heilman RM, Opre A, Miclea M. Emotion-induced retrograde amnesia and trait anxiety. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31(6):1250–1257. doi: 10.1037/0278-7393.31.6.1250. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Arentsen TJ. Memory modulation in the classroom: Selective enhancement of college examination performance by arousal induced after lecture. Neurobiology of Learning and Memory. 2012;98(1):12–16. doi: 10.1016/j.nlm.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Powless M. Positive and negative sources of emotional arousal enhance long-term word-list retention when induced as long as 30 min after learning. Neurobiology of Learning and Memory. 2007;88(1):40–47. doi: 10.1016/j.nlm.2007.03.005. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Yee D, Erickson KI. Memory enhancement by a semantically unrelated emotional arousal source induced after learning. Neurobiology of Learning and Memory. 2005;84(1):49–56. doi: 10.1016/j.nlm.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models. Second edition. Thousand Oaks: Sage Publications; 2002. [Google Scholar]
- Rosenthal RA, Rosnow RL. Contrast analysis: Focused comparisons in the analysis of variance. New York: Cambridge University Press; 1985. [Google Scholar]
- Strange BA, Hurlemann R, Dolan RJ. An emotion-induced retrograde amnesia in humans is amygdala- and beta-adrenergic-dependent. Proceedings of the National Academy of Sciences. 2003;100:13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange BA, Kroes MC, Fan J, Dolan RJ. Emotion causes targeted forgetting of established memories. Frontiers in Behavioral Neuroscience. 2010;4 doi: 10.3389/fnbeh.2010.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland MR, Mather M. Negative arousal amplifies the effects of saliency in short-term memory. Emotion. 2012;12(6):1367–1372. doi: 10.1037/a0027860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tukey JW. Exploratory data analysis. Reading, MA: Addison-Wesley; 1977. [Google Scholar]
- Waring JD, Kensinger EA. Effects of emotional valence and arousal upon memory trade-offs with aging. Psychology and Aging. 2009;24(2):412–422. doi: 10.1037/a0015526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


