Abstract
G protein-mediated signal transduction is essential for the regulation of cardiovascular function, including heart rate, growth, contraction, and vascular tone. Regulators of G protein Signaling (RGS proteins) fine-tune G protein-coupled receptor-induced signaling by regulating its magnitude and duration through direct interaction with the α subunits of heterotrimeric G proteins. Changes in the RGS protein expression and/or function in the heart often lead to pathophysiological changes and are associated with cardiac disease in animals and humans, including hypertrophy, fibrosis development, heart failure, and arrhythmias.
This article focuses on Regulator of G protein Signaling 2 (RGS2), which is widely expressed in many tissues and is highly regulated in its expression and function. Most information to date has been obtained in biochemical, cellular, and animal studies, but data from humans is emerging. We review recent advances on the functional role of cardiovascular RGS2 and the mechanisms that determine its signaling selectivity, expression, and functionality. We highlight key unanswered questions and discuss the potential of RGS2 as a therapeutic target.
Introduction
Cells are continuously barraged with a multitude of extracellular signals that must be properly received, integrated, and processed to ensure proper function. The majority of signals are received by seven-transmembrane-spanning G protein-coupled receptors (GPCRs), which represent the largest and most ubiquitous family of membrane receptors. Activated GPCRs catalyze guanine nucleotide exchange on the α subunit of heterotrimeric GTP-binding proteins (G proteins), which are divided into four subfamilies according to structural and functional similarities in their α subunits. Sixteen different Gα subunits are associated with Gβγ complexes that are assembled from 5β and 12γ subunits (reviewed in Wettschureck and Offermanns, 2005). When bound to GTP, Gα and its cognate Gβγ subunit elicit cellular responses by activating (or inhibiting) downstream signaling molecules. The specificity with which G proteins relay the information from GPCRs to intracellular effectors (e.g., enzymes, protein kinases, and ion channels) primarily determines the range of responses a cell is able to make to a particular external signal, although G protein-independent effects can also occur (see below). The magnitude and duration of cellular responses depend on how long G proteins remain activated, which is determined by the relatively slow intrinsic GTPase activity of Gα subunits. After GTP hydrolysis, inactive GDP-bound Gα reassociates with Gβγ, and both can then be ready for a new activation cycle. RGS proteins accelerate the deactivation of select G proteins by serving as GTPase-activating proteins (GAPs) and, in some instances, can also block signal generation (Berman et al., 1996; Hepler et al., 1997).
Twenty canonical RGS proteins have been identified since their discovery 2 decades ago (reviewed in Hollinger and Hepler, 2002); they are divided into four subfamilies based on the sequence homology of their ~120 amino acid RGS homology (RH) domain that interacts with one or more G protein α subunits, and the size and organization of the additional flanking domains that facilitate additional protein–protein (or protein–lipid) interactions. More than half of them bind to and regulate Gαi/o and Gαq/11, which mediate GPCR-dependent inhibition of adenylate cyclases (AC) and activation of phospholipase C β (PLCβ), respectively (Fig. 1); the other RGS protein isoforms are selective for Gαi/o proteins.
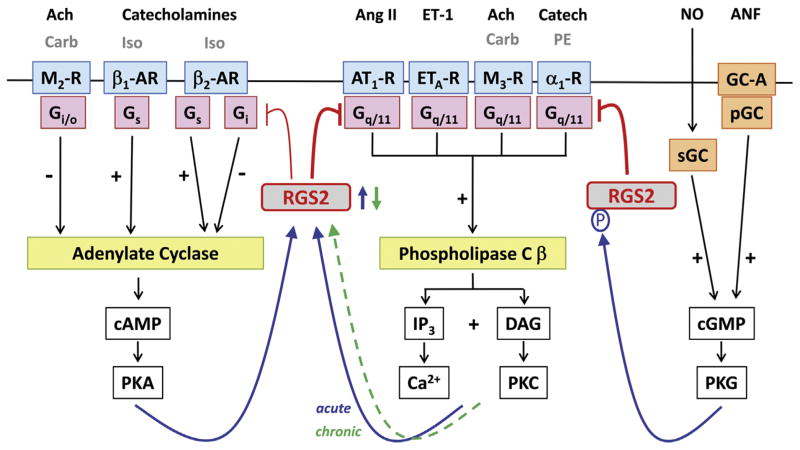
Fig. 1.
Regulation of G protein-mediated signaling by RGS2 in the heart. Inhibitory effects of RGS2 on G protein-mediated signaling are indicated in red. Opposing regulation of adenylate cyclase by Gs (stimulatory) and Gi/o (inhibitory), and Gq/11-mediated activation of phospholipase Cβ are depicted to the left and middle, respectively. G protein-coupled receptors are shaded in blue, G proteins in pink, and effector enzymes in yellow. Endogenous neurotransmitters and peptide hormones are indicated above their respective receptors in black font, while additional experimentally used receptor agonists are in gray font. All 2nd messengers and downstream protein kinases are depicted in open boxes. Generation of cGMP via soluble or particulate guanylate cyclases (sGC and pGC, respectively) is shown to the right. Blue arrows indicate upregulation of RGS2 expression (in response to enhanced signaling via the adenylate cyclase and phospholipase C β pathways) or enhanced functionality (via cGMP-mediated phosphorylation). In contrast to acute stimulation of the Gq/11-PLCβ signaling pathway, chronically enhanced Gq/11-mediated signaling leads to RGS2 downregulation (green dashed arrow). See text for details on RGS2 as signal integrator between the Gs/Gi/o and the Gq/11 signaling pathways as well as the non-GPCR cGMP signaling pathway. Abbreviations: Ach, acetylcholine; ANF, atrial natriuretic factor; Ang II, angiotensin II; AT1-R, angiotensin II receptor type 1; α1-AR, α1-adrenergic receptor; β1-AR, β1-adrenergic receptor; β2-AR, β2-adrenergic receptor; Carb, carbachol; Catech, catecholamines; cAMP, cyclic adenosine monophosphate; cGMP, cyclic guanosine monophosphate; DAG, diacylglycerol; ET-1, endothelin 1; ETA-R, endothelin receptor type A; GC-A, guanylate cyclase-coupled receptor A; IP3, inositol trisphosphate; Iso, isoproterenol; M2-R, M2 muscarinic Ach receptor; M3-R, M3 muscarinic Ach receptor; NO, nitric oxide; P, phosphorylation; PE, phenylephrine; PKA, protein kinase A; PKC, protein kinase C; PKG, protein kinase G.
Expression profiles for RGS proteins have been reported for many tissues and cell types, mostly based on mRNA data due to low cellular levels of RGS proteins and limited availability of antibodies that can unequivocally recognize individual endogenous RGS isoforms. Several canonical RGS proteins are expressed in the mammalian and human myocardium, with overlapping but distinct expression patterns between the two major cell types (myocytes and fibroblasts) and between cardiac regions (atria and ventricles) (reviewed in Zhang and Mende, 2011).
This review article focuses on RGS2, which is widely expressed in many tissues and has been linked to the regulation of cardiovascular function, immune responses, reward behavior, anxiety, schizophrenia, weight gain, bone formation, and cancer (reviewed in Bansal et al., 2007). It is expressed in all cardiovascular cell types, with comparable mRNA levels in myocytes and fibroblasts (Zhang and Mende, 2011). RGS2, which is highly regulated in its expression and function, is a much more potent and selective negative regulator of Gq/11 signaling than other RGS isoforms, although recent data suggest some effect on Gi/o as well. For information on other RGS proteins that are involved in regulating cardiovascular signaling and function, the reader is referred to other reviews (Gu et al., 2009; Hendriks-Balk et al., 2008; Zhang and Mende, 2011).
Functional importance of RGS2 in cardiovascular disease
Gq/11-mediated signal transduction is well recognized for its role in regulating both vascular tone and cardiac remodeling in response to hemodynamic stress, which entails changes in cardiac structure, including myocyte hypertrophy and excess extracellular matrix deposition, and subsequent cardiac dysfunction. Cell culture studies provided first clues as to the importance of RGS2 as a regulator of Gq/11-mediated function in cardiovascular cells. For example, gain-of-function studies in neonatal and adult rat ventricular myocytes (Hao et al., 2006; Zou et al., 2006) and fibroblasts (Zhang et al., 2011) showed RGS2 expression level-dependent inhibition of Gq/11-mediated PLCβ activation along with inhibition of myocyte hypertrophy, fibroblast proliferation, and total collagen production. Conversely, loss-of-function studies, in which RGS2 expression was markedly reduced via RNA interference, showed the opposite phenotype in both cell types despite the presence of several other Gq/11-regulating RGS proteins (such as RGS3, RGS4, and RGS5) (Zhang et al., 2006; Zhang et al., 2011).
RGS2 and cardiac remodeling
Strong evidence for a central role of endogenous RGS2 in mitigating the cardiac remodeling in response to pressure overload in vivo was provided by a mouse model with global deletion of endogenous RGS2 (Takimoto et al., 2009). In the absence of RGS2, transverse aortic constriction induced enhanced Gq/11-mediated signaling and exacerbated cardiac hypertrophy, fibrosis, and dysfunction, particularly in the early phase, and led to increased mortality. The involvement of the Gq/11/PLCβ signaling pathway was indicated by worsening of the hypertrophic response and dilatation in transgenic mice overexpressing Gαq and lacking RGS2, and by inhibition of rapid hypertrophy development and functional deterioration in the presence of a PLCβ inhibitor. Physiological hypertrophy in response to exercise, which is not related to Gq/11 signaling (Maillet et al., 2013), was not affected, nor was a cardiac phenotype observed under basal conditions despite mild hypertension. Surprisingly, in spite of ample evidence for a role of RGS2 in negatively regulating Gq/11 signaling and hypertrophy in myocytes, myocyte-specific RGS2 overexpression in transgenic mice in vivo did not attenuate ventricular Gq/11-mediated signaling and hypertrophy in response to pressure overload or Angiotensin II (Ang II) infusion, irrespective of the duration of the hypertrophic stimulus (acute vs. prolonged) and the timing of RGS2 expression (conditional vs. constitutive) (Park-Windhol et al., 2012). Differences of RGS2 functionality between ventricles and atria, where transgenic RGS2 markedly inhibited Gq/11 signaling (Park-Windhol et al., 2012), warrant further investigation into the underlying mechanisms of chamber-specific regulation of RGS2 functionality in this model.
Endogenous RGS2 also serves as a mediator of anti-hypertrophic and cardioprotective effects of cGMP, which is generated by guanylate cyclases (GC) and degraded by phosphodiesterases (PDEs). To inhibit pressure overload-induced Gq/11 signaling and hypertrophy, the cGMP-selective PDE5 inhibitor sildenafil requires RGS2, which was indicated by its lack of effect in RGS2 knockout mice (Takimoto et al., 2009). The underlying mechanism involves cGMP-induced RGS2 phosphorylation that enhances RGS2 functionality as a negative regulator of Gq/11 signaling (see below for more detail).
Furthermore, RGS2 likely participates in the molecular signaling changes that ensue in response to cardiac resynchronization therapy (CRT), in which both ventricles are paced to recoordinate contraction in failing hearts that are dyssynchronous due to conduction delays. Marked upregulation of RGS2 and RGS3 at mRNA and protein levels was reported in myocytes from dogs with tachypacing-induced dyssynchronous heart failure that had been subjected to CRT; their mRNAs were also found to be upregulated in left ventricular biopsy samples from patients that responded to CRT (compared to non-responders) (Chakir et al., 2012). Enhanced CRT-induced β-adrenergic responsiveness in myocytes, which improved calcium handling and contraction, was attributed to increased β1-adrenoreceptor expression and AC activity and reduced Gi-mediated signaling, which was associated with upregulation of RGS2 and RGS3 (Chakir et al., 2009, 2012). CRT studies in the absence of RGS2 (alone or in combination with RGS3) are required to establish a causal link between RGS2/RGS3 upregulation and CRT efficacy, along with further investigations into the timeline of events and the precise mechanisms.
RGS2 and arrhythmia
As recently reviewed (Stewart et al., 2012), a requirement for RGS proteins for heart rate regulation was initially shown by a knock-in strategy, in which RGS protein-insensitive Gαi2 or Gαo subunits were expressed in place of endogenous protein. RGS4 and RGS6 were subsequently identified to participate in parasympathetic heart rate control, at least in part, by inhibiting M2 receptor-induced Gi/o protein activation and subsequent Gβγ-mediated activation of inwardly rectifying K+ channels (Stewart et al., 2012). Enhanced susceptibility to atrial tachycardia/fibrillation has been reported in mice with global targeted deletion of RGS5 (Qin et al., 2013) or RGS2 (Tuomi et al., 2010). In RGS2 knockout mice, atrial tachycardia/fibrillation induced by vagal or programmed stimulation could be blocked with a M3 receptor selective antagonist leading the authors to speculate that the absence of RGS2 may lead to enhanced M3 receptor-induced Gq/11 activation and potentially the associated IK,M3 current they previously described (reviewed in Jones et al., 2012). Although the mechanisms are still poorly understood, enhanced Gq/11 signaling is believed to participate in the pathogenesis of atrial arrhythmogenic remodeling and fibrillation in both mice and humans. Targeting Gq/11 signaling in the atria has been proposed as a potential therapeutic strategy (Nattel, 2009). RGS2 transgenic mice with markedly inhibited Gq/11 signaling in the atria but not ventricles (Park-Windhol et al., 2012) could be a valuable model to address this question.
RGS2 and blood pressure regulation
RGS2 also plays an important role in maintaining normal vascular tone and blood pressure: mice with global RGS2 deletion have a hypertensive phenotype, which was initially attributed to enhanced and prolonged Gq/11-mediated vascular pressor responses (Heximer et al., 2003; Tang et al., 2003) and impaired nitric oxide (NO)-induced, cGMP-mediated relaxation responses (Sun et al., 2005; Tang et al., 2003). In vitro studies were first performed in aortic rings (Tang et al., 2003) and later extended to vascular resistance vessels (Sun et al., 2005). These studies supported the notion that RGS2 regulates vascular smooth muscle by acting on Gαq/11 and thereby blunting Gq/11-mediated vasoconstriction. In addition, RGS2 serves as a mediator for vasodilatory cGMP effects by a mechanism that involves cGMP-dependent protein kinase G (PKG)-induced RGS2 phosphorylation, which enhances the functional activity of RGS2, and thereby mitigates Gq/11-mediated vasoconstriction (Tang et al., 2003). More recently, RGS2 was shown to also act in the endothelium of resistance arteries (Osei-Owusu et al., 2012), where it promotes endothelial-derived hyperpolarizing factor-dependent vasodilation via a Gi/o-dependent mechanism. A critical role of RGS2 expressed in the kidney for blood pressure regulation was demonstrated by kidney transplantation from a mouse lacking RGS2 into a wild-type mouse, which was sufficient to induce hypertension (Gurley et al., 2010). Importantly, a role of RGS2 in regulating vascular tone in humans has been suggested by observations of increased and decreased RGS2 expression in patients with reduced or elevated blood pressure, respectively, and by identification of several single-nucleotide polymorphisms (SNPs) in RGS2 in hypertensive patients from different ethnic populations (reviewed in Gu et al., 2009). The frequency of RGS2 mutations in hypertensive patients is generally low and if, how, and to what extent they influence blood pressure regulation and play a pathophysiological role requires further investigations. For example, the gene product from one of the candidate hypertension allele in RGS2 showed less efficient membrane association and weaker inhibition of receptor-induced Gq/11 signaling in vitro, which suggests that it may potentially contribute to hypertension development (Gu et al., 2008b). This particular SNP (R44H) was originally identified in a Japanese hypertension cohort (Yang et al., 2005) (see below for other examples). It is located in the N-terminal amphiphatic α-helix of RGS2, which interacts with the inner leaflet of the plasma membrane and enhances the inhibitory effect of RGS2 on Gq/11-coupled GPCR signaling (Gu et al., 2007).
RGS2 in cell signaling regulation
RGS2 as a GAP for select G proteins
RGS2 is well known to negatively regulate Gq/11-mediated signaling by accelerating signal termination via direct binding of its RH domain to activated GTP-bound Gαq/11 subunits and converting them to their inactive GDP-bound form (see below). In addition, as originally shown for RGS4 and RGS19 (or GAIP) (Hepler et al., 1997), RGS2 can block signal generation by blocking the effector phospholipase Cβ from access to Gαq/11 (Anger et al., 2004).
Structural and functional analyses of the RGS2 RH domain in a complex with Gαq have provided important mechanistic clues (Nance et al., 2013). RGS2 binds to Gαq in a tilted orientation that is different from how other RGS proteins bind to Gαi/o proteins. This is due to the interaction of two evolutionary, highly conserved amino acids in the RGS2 RH domain (C106, N184) with one of the three switch regions in Gαq, which are intimately associated with the binding and hydrolysis of GTP, and to more extensive interaction between RGS2 and the α-helical domain of Gαq. The exact role of a third unique and highly conserved amino acid in RGS2 (E191) is not fully understood and may perhaps help RGS2 select against Gαi/o.
Based on its unique structure compared to other closely related RGS isoforms, RGS2 can act as a very potent negative regulator of Gq/11 signaling and was long believed to be highly selective. More recent evidence suggests that RGS2 can also regulate Gi/o signaling in some settings, although the potential significance in the cardiovascular system is not yet fully understood. Initial studies with recombinant RGS2 and Gαi/o subunits showed no interaction or increase in GTPase activity (Heximer et al., 1997). The ability of RGS2 to accelerate GTP hydrolysis and inhibit Gαi signaling under certain experimental conditions was first revealed in reconstituted phospholipid vesicles and transfected COS-7 cells and shown to require M2 receptor activation (Ingi et al., 1998). However, in adult ventricular myocytes, RGS2 was found to have no effect on M2 receptor-induced Gi/o-mediated inhibition of cAMP, while other cardiac RGS proteins (RGS3–RGS5) were potent Gi/o inhibitors (Hao et al., 2006). Recent data suggest a novel role of RGS2 as a terminator of β2 adrenoreceptor-coupled Gi signaling in cultured mouse ventricular myocytes (Chakir et al., 2011). In the endothelium of resistance arteries, RGS2 has been implicated to promote EDHF-dependent vasodilation by blunting pertussis toxin-sensitive Gi/o-mediated signaling (Osei-Owusu et al., 2012). Whether RGS2 serves similar roles in other cardiovascular cell types and in vivo remains to be further investigated.
While the type of interactions between the RGS RH domains and Gα subunits are important determinants of the signaling selectivity of RGS proteins in biochemical experiments, association with other proteins (e.g., GPCRs, scaffold proteins, and effectors), subcellular compartmentalization, and spatiotemporal expression contribute in the cellular context as well.
RGS2 interactions with other proteins
The N-terminus of RGS2 can bind to several other proteins, depending on cell type and context. For example, RGS2 can bind to GPCRs via its N-terminus, either directly (e.g., Bernstein et al., 2004) or via the scaffold protein spinophilin (Wang et al., 2005). Interaction with the third intracellular loop of select GPCRs (including Gq/11-coupled M1,3,5 receptors and α1a receptors as well as Gs- and Gi-coupled β2-adrenoceptors) has been observed in pull-down assays with recombinant proteins; functional RGS2–GPCR interactions have been suggested by RGS2 recruitment to the plasma membrane upon GPCR co-expression in the absence of agonist stimulation in cellular studies (reviewed in Neitzel and Hepler, 2006). Furthermore, RGS2 was shown to interact with AC and negatively regulate its activity in some cell types (Sinnarajah et al., 2001). This interaction also occurs at the N-terminus (Salim et al., 2003) but via residues that are distinct from the membrane-binding sequence (Heximer et al., 2001). Importantly, in adult rat ventricular myocytes and fibroblasts, RGS2 does not seem to inhibit AC directly, since RGS2 overexpression did not affect cAMP generation upon stimulation with isoproterenol or forskolin, a direct activator of AC (Hao et al., 2006; Zhang et al., 2008). Additional proteins that were reported to interact with the RGS2 N-terminus, albeit only in non-cardiac cells so far, are the cation channel TRPV6 (Schoeber et al., 2006) and tubulin (Heo et al., 2006).
Taken together, the N-terminus of RGS2 facilitates plasma membrane targeting and can bind to a number of proteins. In addition, it contains the phosphorylation site for PKG (see Regulation of RGS2 functionality section below). The RH domain appears to have the capacity to bind to proteins other than Gα subunits as well: although the functional significance is still unclear, RGS2 was reported to bind via its RH domain to the rate-controlling enzyme in protein translation (eukaryotic initiation factor 2Bε) and inhibit it (Nguyen et al., 2009), which may contribute to the anti-hypertrophic effect of RGS2. Further investigations are needed to fully delineate which of these events occur in the cardiovascular system and whether they are cell type-dependent. It also remains to be determined how the different interactions and events are orchestrated, facilitate or compete with each other, and what their functional relevance is in health and disease.
Potential role of RGS2 in non-canonical GPCR signaling
The traditional concept of GPCRs initiating G protein-dependent signaling and only at the plasma membrane has been expanded significantly in recent years. (i) Several GPCRs are now known to also elicit cellular effects by recruitment, activation, and scaffolding of cytoplasmic signaling complexes, with β-arrestins promoting alternate downstream signaling pathways while simultaneously inhibiting upstream G protein-dependent signaling (reviewed in Shukla et al., 2011). Novel GPCR ligands that can selectively activate either G protein- or β-arrestin-mediated pathways are in development for heart failure and other diseases, particularly in settings where one pathway is maladaptive, whereas the other may be beneficial (reviewed in Violin et al., 2013). Further investigations are needed to determine if and how RGS2 (and other RGS proteins) affect β-arrestin-mediated GPCR signaling. By accelerating Gα inactivation and thereby facilitating the reassociation of Gα and Gβγ subunits, RGS proteins could potentially affect Gβγ-dependent recruitment of G protein-coupled receptor kinases, a crucial first step in β-arrestin-mediated signaling. (ii) Evidence is mounting that GPCRs and G proteins are not limited to the plasma membrane and can exert functional effects in nuclear membranes (reviewed in Tadevosyan et al., 2012) and endosomes (reviewed in Lohse and Calebiro, 2013). RGS2 (and other RGS proteins) can be located in the nucleus as well (reviewed in Sethakorn et al., 2010) and have been linked to Golgi membranes and intracellular transport (Sullivan et al., 2000). The potential (patho)physiological role(s) of RGS2 in regulating β-arrestin-mediated and/or intracellular GPCR signaling, along with the respective underlying mechanisms, remain to be investigated.
Regulation of RGS2 expression and functionality
Both deficiency and overabundance of RGS2 are associated with dysregulation of vascular tone and blood pressure in mice and man, indicating the importance of precise control of RGS2 protein expression level and function to maintain normal regulation (reviewed in Gu et al., 2009). The expression and function of RGS2 in the myocardium are also dynamically regulated and tightly controlled. Expression studies for RGS2 (and other cardiac RGS proteins) in animal models of cardiac hypertrophy and failure as well as in human myocardium yielded heterogeneous findings, dependent on the species, specific model, and RGS protein investigated (reviewed in Hendriks-Balk et al., 2008). Importantly, RGS2 expression can be altered by clinical therapies, as shown for CRT (see RGS2 and cardiac remodeling section above) and drugs at therapeutic doses (see Regulation of RGS2 expression and Conclusions and clinical perspective sections below).
Regulation of RGS2 expression
Mechanistic studies in various cell types showed that RGS2 expression is regulated by many different stimuli (reviewed in Kach et al., 2012). Cardiovascular cells show a biphasic regulation, in which a transient rise upon short-term stimulation of the Gq/11 signaling pathway is followed by a persistent decline in response to sustained stimulation (Fig. 1). For example, both RGS2 mRNA and protein levels are markedly upregulated in adult rat ventricular myocytes that were either infected with adenovirus encoding constitutively active GαqQ209L or stimulated with a Gq/11-coupled receptor agonist or a PKC activator (Hao et al., 2006). Similarly, RGS2 mRNA was markedly upregulated in response to stimulation with Ang II in cultured vascular smooth muscle cells (VSMC) (Grant et al., 2000) and cardiac fibroblasts (Zhang et al., 2011). RGS2 upregulation was also observed in freshly isolated cardiac fibroblasts from rats subjected to short-term Ang II infusion (less than 3 days) (Zhang et al., 2011). Given that RGS2 functions as a potent GAP for Gαq/11 and accelerates its inactivation, transient upregulation of RGS2 in response to acute activation of the Gq/11-mediated signaling pathway likely represents a negative feedback mechanism. In contrast, prolonged stimulation leads to marked RGS2 downregulation, as shown in pressure overload-induced hypertrophic myocardium, in myocytes from mice expressing GαqQ209L (Zhang et al., 2006), and in myocytes and fibroblasts from rats subjected to prolonged Ang II infusion in vivo (Zhang et al., 2011). These data strongly suggest that RGS2 downregulation in the stressed or injured hearts may contribute to the pathogenesis of cardiac remodeling.
In both myocytes and fibroblasts, RGS2 mRNA is also significantly upregulated in response to acute β-adrenergic stimulation (in the absence of any regulatory effects of RGS2 on Gs-coupled cAMP accumulation) (Hao et al., 2006; Zhang et al., 2008), suggesting potential cross-regulation between Gs- and Gq/11-mediated signaling pathways (Fig. 1). RGS2 upregulation was more pronounced in fibroblasts than in myocytes, indicating cell type-specific differences and that Gs-mediated regulation of RGS2 might be of particular importance in cardiac fibroblasts. Functional importance of Gs-induced, RGS2-mediated cross-regulation on Gq/11 signaling remains to be investigated in the heart, but has been suggested in other cell types, such as osteoblasts (Roy et al., 2006) and primary human airway smooth muscle cells, in which RGS2 expression is upregulated by long-acting β2-adrenoceptor agonists (LABAs) and synergistically enhanced by glucocorticoids (Holden et al., 2011).
Although the precise mechanisms that regulate RGS2 expression have not yet been fully delineated, transcriptional, posttranscriptional, and posttranslational mechanisms are involved. Protein kinase C (PKC)-dependent and Ca2+-dependent changes participate in Gq/11-mediated RGS2 mRNA regulation (Grant et al., 2000), while cAMP and protein kinase A (PKA) are involved in mediating Gs-mediated mRNA upregulation (Tsingotjidou et al., 2002). One study showed Ang II-induced RGS2 mRNA upregulation via sequential activation of PKC, iPLA2β, PKA, and cAMP-response element-binding protein (CREB) in cultured VSMC and identified a conserved cAMP-response element (CRE) in the murine RGS2 promoter that is critical for CREB binding and RGS2 promoter activation (Xie et al., 2011). Importantly, three SNPs identified in hypertensive patients are located within the CRE1 sequence of the human RGS2 promoter, and three mutant RGS2 promoter constructs that correspond to the identified human SNPs exhibit a greatly diminished or absent response to forskolin, suggesting that these three human SNPs may negatively affect RGS2 mRNA expression and contribute to the elevated blood pressure in human (Xie et al., 2011). Recent evidence also suggests epigenetic regulation of the RGS2 promoter and a potential link to prostate cancer (Wolff et al., 2012).
MicroRNAs, a class of endogenous, evolutionarily conserved, small (~22-nucleotide) non-coding RNAs, generally negatively regulate gene expression by repressing mRNA translation and/or promoting mRNA degradation via binding to the 3′ untranslated regions of mRNAs (Van Rooij et al., 2008). Several miRNAs (i.e., miRNA-22, -96, and -1271) have been suggested to target RGS2 via these posttranscriptional mechanisms (Jensen and Covault, 2011; Muinos-Gimeno et al., 2011), but the functional relevance is not yet understood.
Posttranslational mechanisms that affect RGS2 expression include regulation of protein stability and degradation. It was recently reported that, without change in RGS2 mRNA levels, cardiotonic steroids (such as digoxin) selectively increased RGS2 (but not RGS4) protein levels in cultured VSMC and in mouse heart and kidney in vivo (Sjögren et al., 2012). Although the precise mechanism is unclear, Na+/K+-ATPase was required for cardiotonic steroid-induced stabilization of RGS2 protein (Sjögren et al., 2012). Regulation of RGS2 protein degradation also alters its expression. For example, RGS2 protein degradation via the proteasome can be blunted by PKG, which does not depend on RGS2 phosphorylation (Osei-Owusu et al., 2007). The N-end rule pathway, which is based on destabilizing residues in the N-terminus of a protein, is another important regulatory mechanism for proteosomal degradation. While several RGS proteins including RGS2 have potentially destabilizing N-terminal residues and are predicted to be degraded by the N-end rule pathway, only RGS4, RGS5, and RGS16 have been confirmed thus far (Sjögren and Neubig, 2010). Detection of two potentially destabilizing mutations of RGS2 in a group of hypertensive individuals from Japan highlighted the potential clinical relevance (Yang et al., 2005). In particular, one of the mutations (Q2L) showed much reduced protein expression in HEK293 cells that was markedly enhanced by pretreatment with a proteasome inhibitor (Bodenstein et al., 2007).
Regulation of RGS2 functionality
Posttranslational phosphorylation and lipid modifications of RGS proteins contribute to the regulation of RGS protein activity and subcellular localization (reviewed in Zhang and Mende, 2011). For example, phosphorylation of RGS2 by PKC was reported to decrease its GAP activity in COS-7 cells (Cunningham et al., 2001), although the residue and functional significance are still unknown. In contrast, phosphorylation of RGS2 at N-terminal S46 and S64 by PKG increases RGS2 GAP activity in human VSMC and causes translocation of RGS2 to the membrane (Tang et al., 2003). Similarly, in mouse VSMC, association of RGS2 with the plasma membrane is promoted by activation of PKG, and RGS2 is required for cGMP-mediated inhibition of vasoconstrictor-elicited PLCβ activation, Ca2+ store release, and capacitative Ca2+ entry (Osei-Owusu et al., 2007). In adult mouse ventricular myocytes, atrial natriuretic factor, which counteracts hypertrophy via guanylyl cyclase-A receptor (GC-A) activation and cGMP production, suppresses Ca2+ currents and transients in response to Ang II (but not β-adrenergic) stimulation in a PKG- and RGS2-dependent manner (Klaiber et al., 2010). Furthermore, in normal mice (but not RGS2 knockout mice), PKG activation by chronic inhibition of cGMP-selective PDE5 suppressed maladaptive cardiac hypertrophy (Takimoto et al., 2009). These findings demonstrate the importance of PKG activation and RGS2 phosphorylation in inhibiting Gq/11-coupled stimuli in the heart and thereby place RGS2 as a link between non-GPCR and GPCR signaling pathways (Fig. 1). Furthermore, they highlight the potential relevance of using sildenafil in the treatment of heart failure with normal ejection fraction.
Palmitoylation, another common posttranslational modification for RGS proteins that often serves as a membrane-targeting signal, can also alter the GAP activity of RGS2 in a residue-dependent manner (reviewed in Zhang and Mende, 2011). Palmitoylation of C106 and C199 leads to an increase of RGS2 GAP activity, while palmitoylation of C116 causes a decrease of RGS2 GAP activity (Ni et al., 2006). The authors propose that change of RGS2 GAP activity might be related to a conformational change of RGS2 upon palmitoylation.
Another mechanism to regulate RGS2 function is via alternate translation initiation sites, which in human RGS2 can lead to four different RGS2 proteins of distinct size and function (Gu et al., 2008a). Since the four sites identified are located in the N-terminus upstream of the Gα-interacting RH domain, the functional effect of RGS2 as negative regulator of G protein-mediated signal transfer was preserved, whereas AC inhibition was impaired once the interaction domain between RGS2 and AC was missing or incomplete.
Conclusions and clinical perspective
Chronic heart failure remains a major cause of illness and death; many different strategies have been pursued to tackle heart failure by inhibiting and/or reversing cardiac hypertrophy and fibrosis (Koitabashi and Kass, 2012). While the hypertrophic response to pathological stress was long viewed as a necessary adaptive effect at least initially, it is not necessarily required to preserve heart function (Koitabashi and Kass, 2012). Selective antagonists to GPCRs, the most common targets of therapeutic drugs, have been widely used in clinical therapies for patients with cardiac hypertrophy, heart failure, and hypertension (e.g., Ang II receptor and β adrenoceptor blockers). However, given that (i) the number of GPCRs (>200 in the heart) far exceeds the number of G proteins (<11 in any given cell) and therefore many different GPCRs couple to the same type of G protein and (ii) several GPCR agonists are often elevated in pathological settings (e.g., Ang II and ET-1 are both elevated in heart failure and activate their respective Gq/11-coupled receptors), targeting at the G protein level via RGS proteins is viewed as a promising different therapeutic approach. The potential of RGS proteins as therapeutic targets was recognized for many different pathophysiological states and organs soon after their discovery almost two decades ago; the progress made and challenges that need to be overcome are reviewed in detail elsewhere (Sjögren et al., 2010; Sjögren and Neubig, 2010).
RGS2 has emerged as a functionally important, unique regulator of cell signaling and as a therapeutic target. Evidence from in vitro biochemical and cellular studies and in vivo animal models and human patients indicates that RGS2 plays a prominent role in regulating blood pressure and the cardiac remodeling response to hemodynamic stress and may potentially be involved in atrial fibrillation as well. RGS2 is widely expressed both during development and adulthood and regulates cell signaling and functional behavior of cardiovascular cells (myocytes, fibroblasts, VSMC, and endothelial cells) and other cell types. Mechanistically, as recently highlighted by Osei-Owusu et al. (2012), it will be important to tease apart the functional contribution of RGS2 in individual cell types to the cardiovascular phenotype and to avoid potential confounding contributions from gene targeting during development, which can be achieved by cell type-selective and inducible genetic modification of RGS2 in vivo, respectively. In addition, further studies (especially in large animal models and humans) are needed to fully appreciate the functional relevance of RGS2 in the heart, vasculature and beyond both under physiological and pathophysiological conditions, and to fully understand the mechanisms of its action and regulation.
Therapeutically, modulating the expression and/or activity of RGS2 in vivo may eventually lead to novel strategies to treat patients with cardiac hypertrophy, heart failure, and/or hypertension. For example, downregulation of RGS2 expression in response to prolonged Gq/11 signaling is believed to exacerbate the pathophysiology of hypertension, hypertrophy, and fibrosis. Thus, enhancing RGS2 expression or function could have beneficial therapeutic effects via enhanced repression of cardiovascular pathological Gq/11 signaling. In principle, strategies to enhance RGS2 expression or function include altering its GAP activity, steady-state expression, protein and lipid interactions, posttranslational modifications, and/or subcellular localization. The recently identified unique structure of a complex of the RGS2 RH domain and the Gαq subunit offers new structural information that may allow for the identification of compounds that can selectively stabilize this interaction.
Recent studies suggest that the steady-state level of RGS2 expression can be altered by existing clinical device or drug therapies, which may have significant therapeutic potential for cardiovascular diseases and beyond. As described, RGS2 protein expression was found to be upregulated in response to cardiac synchronization therapy in dogs and humans with heart failure, and it has been proposed that upregulation of RGS2 protein upon restoration of synchronous contraction in the failing hearts might contribute to Gαs-biased β2-adrenergic receptor signaling and its beneficial effects by mitigating β2 adrenoceceptor/Gi signaling (Chakir et al., 2012). Commonly used drugs can also upregulate RGS2 protein expression at therapeutic doses, which raises the possibility that their effects might in part be mediated by RGS2 and could open novel avenues for pharmacological regulation of RGS2 expression. One example is cardiotonic steroids(CTS), which selectively enhanced RGS2 (but not RGS4) protein expression via slowing its degradation in VSMC and in vivo (Sjögren et al., 2012). CTS-induced RGS2 was functional and reduced Gq/11-mediated signaling, which might potentially contribute to the beneficial effects of low-dose digoxin treatment in heart failure. The synergistic effect of β2 adrenoceptor agonists and glucocorticoids, is another example of marked drug-induced RGS2 protein upregulation, albeit by a different genomic mechanism (Holden et al., 2011). It has so far been shown in human airway smooth muscle cells, but may have clinical implications in the cardiovascular system as well.
In addition to its important role of a potent negative regulator of Gq/11-mediated signaling, RGS2 is now also recognized for its apparent capacity to modulate Gi/o signaling and to interact with other proteins. While the functional significance of these additional interactions are not yet well understood, they may offer additional therapeutic benefits, but at the same time raise the level of complexity for the design of novel, safe, and effective future therapies involving RGS2. Recent studies suggest a potential link between RGS2 SNPs and hypertension. Future in-depth investigations will likely yield more insights into the identity and abundance of RGS2 SNPs, the link between specific mutations and cardiovascular disease phenotypes, and their pathophysiological significance in different patient populations. This could provide additional mechanistic insights, which may further facilitate therapeutic development. The expression of RGS2 in non-cardiac cell and tissues must be taken into account when regulation of RGS2 is considered and developed as a therapeutic approach in heart disease.
Acknowledgments
This work was supported by grants from the National Heart Lung and Blood Institute (HL80127 and HL113918) and from the American Heart Association (Established Investigator Award 0740098N) to UM. PZ has been supported by the Rhode Island Foundation (Grant no. 20092936), a Junior Faculty COBRE Pilot Project Award from the National Center for Research Resources and the National Institute of General Medical Sciences (5P20 RR018728-10/8P20 GM103537-10) and a National Scientist Development Grant (13SDG14370008) from the American Heart Association.
References
- Anger T, Zhang W, Mende U. Differential contribution of GTPase activation and effector antagonism to the inhibitory effect of RGS proteins on Gq-mediated signaling. Journal of Biological Chemistry. 2004;276:3906–15. doi: 10.1074/jbc.M309496200. [DOI] [PubMed] [Google Scholar]
- Bansal G, Druey KM, Xie Z. R4 RGS proteins: regulation of G-protein signaling and beyond. Pharmacology and Therapeutics. 2007;116:473–95. doi: 10.1016/j.pharmthera.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman DM, Wilkie TM, Gilman AG. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell. 1996;86:445–52. doi: 10.1016/s0092-8674(00)80117-8. [DOI] [PubMed] [Google Scholar]
- Bernstein LS, Ramineni S, Hague C, Cladman W, Chidiac P, Levey AI, et al. RGS2 binds directly and selectively to the M1 muscarinic acetylcholine receptor third intracellular loop to modulate Gq/11α signaling. Journal of Biological Chemistry. 2004;279:21248–56. doi: 10.1074/jbc.M312407200. [DOI] [PubMed] [Google Scholar]
- Bodenstein J, Sunahara RK, Neubig RR. N-terminal residues control proteasomal degradation of RGS2, RGS4, and RGS5 in human embryonic kidney 293 cells. Molecular Pharmacology. 2007;71:1040–50. doi: 10.1124/mol.106.029397. [DOI] [PubMed] [Google Scholar]
- Chakir K, Daya SK, Aiba T, Tunin RS, Dimaano VL, Abraham TP, et al. Mechanisms of enhanced β-adrenergic reserve from cardiac resynchronization therapy. Circulation. 2009;119:1231–40. doi: 10.1161/CIRCULATIONAHA.108.774752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakir K, Zhu W, Tsang S, Woo AY, Yang D, Wang X, et al. RGS2 is a primary terminator of β2-adrenergic receptor-mediated Gi signaling. Journal of Molecular and Cellular Cardiology. 2011;50:1000–7. doi: 10.1016/j.yjmcc.2011.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakir K, Depry C, Dimaano VL, Zhu WZ, Vanderheyden M, Bartunek J, et al. Gαs-biased β2-adrenergic receptor signaling from restoring synchronous contraction in the failing heart. Science Translational Medicine. 2012;3:100ra88. doi: 10.1126/scitranslmed.3001909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham ML, Waldo GL, Hollinger S, Hepler JR, Harden TK. Protein kinase C phosphorylates RGS2 and modulates its capacity for negative regulation of Gα11 signaling. Journal of Biological Chemistry. 2001;276:5438–44. doi: 10.1074/jbc.M007699200. [DOI] [PubMed] [Google Scholar]
- Grant SL, Lassegue B, Griendling KK, Ushio-Fukai M, Lyons PR, Alexander RW. Specific regulation of RGS2 messenger RNA by angiotensin II in cultured vascular smooth muscle cells. Molecular Pharmacology. 2000;57:460–7. doi: 10.1124/mol.57.3.460. [DOI] [PubMed] [Google Scholar]
- Gu S, He J, Ho WT, Ramineni S, Thal DM, Natesh R, et al. Unique hydrophobic extension of the RGS2 amphipathic helix domain imparts increased plasma membrane binding and function relative to other RGS R4/B subfamily members. Journal of Biological Chemistry. 2007;282:33064–75. doi: 10.1074/jbc.M702685200. [DOI] [PubMed] [Google Scholar]
- Gu S, Anton A, Salim S, Blumer KJ, Dessauer CW, Heximer SP. Alternative translation initiation of human regulators of G-protein signaling-2 yields a set of functionally distinct proteins. Molecular Pharmacology. 2008a;73:1–11. doi: 10.1124/mol.107.036285. [DOI] [PubMed] [Google Scholar]
- Gu S, Tirgari S, Heximer SP. The RGS2 gene product from a candidate hypertension allele shows decreased plasma membrane association and inhibition of Gq. Molecular Pharmacology. 2008b;73:1037–43. doi: 10.1124/mol.107.044214. [DOI] [PubMed] [Google Scholar]
- Gu S, Cifelli C, Wang S, Heximer SP. RGS proteins: identifying new GAPs in the understanding of blood pressure regulation and cardiovascular function. Clinical Science (London, England: 1979) 2009;116:391–9. doi: 10.1042/CS20080272. [DOI] [PubMed] [Google Scholar]
- Gurley SB, Griffiths RC, Mendelsohn ME, Karas RH, Coffman TM. Renal actions of RGS2 control blood pressure. Journal of the American Society of Nephrology. 2010;21:1847–51. doi: 10.1681/ASN.2009121306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Michalek C, Zhang W, Zhu M, Xu X, Mende U. Regulation of cardiomyocyte signaling by RGS proteins: differential selectivity towards G proteins and susceptibility to regulation. Journal of Molecular and Cellular Cardiology. 2006;41:51–61. doi: 10.1016/j.yjmcc.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Hendriks-Balk MC, Peters SL, Michel MC, Alewijnse AE. Regulation of G protein-coupled receptor signalling: focus on the cardiovascular system and regulator of G protein signalling proteins. European Journal of Pharmacology. 2008;585:278–91. doi: 10.1016/j.ejphar.2008.02.088. [DOI] [PubMed] [Google Scholar]
- Heo K, Ha SH, Chae YC, Lee S, Oh YS, Kim YH, et al. RGS2 promotes formation of neurites by stimulating microtubule polymerization. Cellular Signalling. 2006;18:2182–92. doi: 10.1016/j.cellsig.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Hepler JR, Berman DM, Gilman AG, Kozasa T. RGS4 and GAIP are GTPase-activating proteins for Gqα and block activation of phospholipase Cβ by γ-thio-GTP-Gqα. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:428–32. doi: 10.1073/pnas.94.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heximer SP, Watson N, Linder ME, Blumer KJ, Hepler JR. RGS2/G0S8 is a selective inhibitor of Gqα function. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:14389–93. doi: 10.1073/pnas.94.26.14389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heximer SP, Lim H, Bernard JL, Blumer KJ. Mechanisms governing subcellular localization and function of human RGS2. Journal of Biological Chemistry. 2001;276:14195–203. doi: 10.1074/jbc.M009942200. [DOI] [PubMed] [Google Scholar]
- Heximer SP, Knutsen RH, Sun X, Kaltenbronn KM, Rhee MH, Peng N, et al. Hypertension and prolonged vasoconstrictor signaling in RGS2-deficient mice. Journal of Clinical Investigation. 2003;111:445–52. doi: 10.1172/JCI15598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden NS, Bell MJ, Rider CF, King EM, Gaunt DD, Leigh R, et al. β2-adrenoceptor agonist-induced RGS2 expression is a genomic mechanism of bronchoprotection that is enhanced by gluco-corticoids. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:19713–8. doi: 10.1073/pnas.1110226108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinger S, Hepler JR. Cellular regulation of RGS proteins: modulators and integrators of G protein signaling. Pharmacological Reviews. 2002;54:527–59. doi: 10.1124/pr.54.3.527. [DOI] [PubMed] [Google Scholar]
- Ingi T, Krumins AM, Chidiac P, Brothers GM, Chung S, Snow BE, et al. Dynamic regulation of RGS2 suggests a novel mechanism in G-protein signaling and neuronal plasticity. The Journal of Neuroscience. 1998;18:7178–88. doi: 10.1523/JNEUROSCI.18-18-07178.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KP, Covault J. Human miR-1271 is a miR-96 paralog with distinct non-conserved brain expression pattern. Nucleic Acids Research. 2011;39:701–11. doi: 10.1093/nar/gkq798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Tuomi JM, Chidiac P. Role of cholinergic innervation and RGS2 in atrial arrhythmia. Frontiers in Physiology. 2012;3:239. doi: 10.3389/fphys.2012.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kach J, Sethakorn N, Dulin NO. A finer tuning of G-protein signaling through regulated control of RGS proteins. American Journal of Physiology Heart and Circulatory Physiology. 2012;303:H19–35. doi: 10.1152/ajpheart.00764.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaiber M, Kruse M, Volker K, Schroter J, Feil R, Freichel M, et al. Novel insights into the mechanisms mediating the local antihypertrophic effects of cardiac atrial natriuretic peptide: role of cGMP-dependent protein kinase and RGS2. Basic Research in Cardiology. 2010;105:583–95. doi: 10.1007/s00395-010-0098-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koitabashi N, Kass DA. Reverse remodeling in heart failure -mechanisms and therapeutic opportunities. Nature Reviews Cardiology. 2012;9:147–57. doi: 10.1038/nrcardio.2011.172. [DOI] [PubMed] [Google Scholar]
- Lohse MJ, Calebiro D. Cell biology: receptor signals come in waves. Nature. 2013;495:457–8. doi: 10.1038/nature12086. [DOI] [PubMed] [Google Scholar]
- Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: fundamental concepts and new players. Nature Reviews Molecular Cell Biology. 2013;14:38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muinos-Gimeno M, Espinosa-Parrilla Y, Guidi M, Kagerbauer B, Sipila T, Maron E, et al. Human microRNAs miR-22, miR-138-2, miR-148a, and miR-488 are associated with panic disorder and regulate several anxiety candidate genes and related pathways. Biological Psychiatry. 2011;69:526–33. doi: 10.1016/j.biopsych.2010.10.010. [DOI] [PubMed] [Google Scholar]
- Nance MR, Kreutz B, Tesmer VM, Sterne-Marr R, Kozasa T, Tesmer JJ. Structural and functional analysis of the regulator of G protein signaling 2-Gαq complex. Structure. 2013;21:438–48. doi: 10.1016/j.str.2012.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattel S. G-protein signaling and arrhythmogenic atrial remodeling: relevance to novel therapeutic targets in atrial fibrillation. Heart Rhythm. 2009;6:85–6. doi: 10.1016/j.hrthm.2008.11.028. [DOI] [PubMed] [Google Scholar]
- Neitzel KL, Hepler JR. Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling. Seminars in Cell and Developmental Biology. 2006;17:383–9. doi: 10.1016/j.semcdb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Nguyen CH, Ming H, Zhao P, Hugendubler L, Gros R, Kimball SR, et al. Translational control by RGS2. Journal of Cell Biology. 2009;186:755–65. doi: 10.1083/jcb.200811058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni J, Qu L, Yang H, Wang M, Huang Y. Palmitoylation and its effect on the GTPase-activating activity and conformation of RGS2. The International Journal of Biochemistry and Cell Biology. 2006;38:2209–18. doi: 10.1016/j.biocel.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P, Sun X, Drenan RM, Steinberg TH, Blumer KJ. Regulation of RGS2 and second messenger signaling in vascular smooth muscle cells by cGMP-dependent protein kinase. Journal of Biological Chemistry. 2007;282:31656–65. doi: 10.1074/jbc.M706360200. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P, Sabharwal R, Kaltenbronn KM, Rhee MH, Chapleau MW, Dietrich HH, et al. Regulator of G protein signaling 2 deficiency causes endothelial dysfunction and impaired endothelium-derived hyperpolarizing factor-mediated relaxation by dysregulating Gi/o signaling. Journal of Biological Chemistry. 2012;287:12541–9. doi: 10.1074/jbc.M111.332130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park-Windhol C, Zhang P, Zhu M, Su J, Chaves, Maldonado AE, et al. Gq/11-mediated signaling and hypertrophy in mice with cardiac-specific transgenic expression of regulator of G-protein signaling 2. PLoS One. 2012;7:e40048. doi: 10.1371/journal.pone.0040048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin M, Huang H, Wang T, Hu H, Liu Y, Gu Y, et al. Atrial tachyarrhythmia in Rgs5-null mice. PLoS One. 2013;7:e46856. doi: 10.1371/journal.pone.0046856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy AA, Nunn C, Ming H, Zou MX, Penninger J, Kirshenbaum LA, et al. Up-regulation of endogenous RGS2 mediates cross-desensitization between Gs and Gq signaling in osteoblasts. Journal of Biological Chemistry. 2006;281:32684–93. doi: 10.1074/jbc.M604416200. [DOI] [PubMed] [Google Scholar]
- Salim S, Sinnarajah S, Kehrl JH, Dessauer CW. Identification of RGS2 and type V adenylyl cyclase interaction sites. Journal of Biological Chemistry. 2003;278:15842–9. doi: 10.1074/jbc.M210663200. [DOI] [PubMed] [Google Scholar]
- Schoeber JP, Topala CN, Wang X, Diepens RJ, Lambers TT, Hoenderop JG, et al. RGS2 inhibits the epithelial Ca2+ channel TRPV6. Journal of Biological Chemistry. 2006;281:29669–74. doi: 10.1074/jbc.M606233200. [DOI] [PubMed] [Google Scholar]
- Sethakorn N, Yau DM, Dulin NO. Non-canonical functions of RGS proteins. Cellular Signalling. 2010;22:1274–81. doi: 10.1016/j.cellsig.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukla AK, Xiao K, Lefkowitz RJ. Emerging paradigms of β-arrestin-dependent seven transmembrane receptor signaling. Trends in Biochemical Sciences. 2011;36:457–69. doi: 10.1016/j.tibs.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinnarajah S, Dessauer CW, Srikumar D, Chen J, Yuen J, Yilma S, et al. RGS2 regulates signal transduction in olfactory neurons by attenuating activation of adenylyl cyclase III. Nature. 2001;409:1051–5. doi: 10.1038/35059104. [DOI] [PubMed] [Google Scholar]
- Sjögren B, Blazer LL, Neubig RR. Regulators of G protein signaling proteins as targets for drug discovery. Progress in Molecular Biology and Translational Science. 2010;91:81–119. doi: 10.1016/S1877-1173(10)91004-1. [DOI] [PubMed] [Google Scholar]
- Sjögren B, Neubig RR. Thinking outside of the “RGS box”: new approaches to therapeutic targeting of regulators of G protein signaling. Molecular Pharmacology. 2010;78:550–7. doi: 10.1124/mol.110.065219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjögren B, Parra S, Heath LJ, Atkins KB, Xie ZJ, Neubig RR. Cardiotonic steroids stabilize regulator of G protein signaling 2 protein levels. Molecular Pharmacology. 2012;82:500–9. doi: 10.1124/mol.112.079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart A, Huang J, Fisher RA. RGS proteins in heart: brakes on the vagus. Frontiers in Physiology. 2012;3:95. doi: 10.3389/fphys.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BM, Harrison-Lavoie KJ, Marshansky V, Lin HY, Kehrl JH, Ausiello DA, et al. RGS4 and RGS2 bind coatomer and inhibit COPI association with Golgi membranes and intracellular transport. Molecular Biology of the Cell. 2000;11:3155–68. doi: 10.1091/mbc.11.9.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Kaltenbronn KM, Steinberg TH, Blumer KJ. RGS2 is a mediator of nitric oxide action on blood pressure and vasoconstrictor signaling. Molecular Pharmacology. 2005;67:631–9. doi: 10.1124/mol.104.007724. [DOI] [PubMed] [Google Scholar]
- Tadevosyan A, Vaniotis G, Allen BG, Hebert TE, Nattel S. G protein-coupled receptor signalling in the cardiac nuclear membrane: evidence and possible roles in physiological and pathophysiological function. The Journal of Physiology. 2012;590:1313–30. doi: 10.1113/jphysiol.2011.222794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takimoto E, Koitabashi N, Hsu S, Ketner EA, Zhang M, Nagayama T, et al. Regulator of G protein signaling 2 mediates cardiac compensation to pressure overload and antihypertrophic effects of PDE5 inhibition in mice. Journal of Clinical Investigation. 2009;119:408–20. doi: 10.1172/JCI35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Wang G, Lu P, Karas RH, Aronovitz M, Heximer SP, et al. Regulator of G-protein signaling-2 mediates vascular smooth muscle relaxation and blood pressure. Nature Medicine. 2003;9:1506–12. doi: 10.1038/nm958. [DOI] [PubMed] [Google Scholar]
- Tsingotjidou A, Nervina JM, Pham L, Bezouglaia O, Tetradis S. Parathyroid hormone induces RGS-2 expression by a cyclic adenosine 3′,5′-monophosphate-mediated pathway in primary neonatal murine osteoblasts. Bone. 2002;30:677–84. doi: 10.1016/s8756-3282(02)00698-1. [DOI] [PubMed] [Google Scholar]
- Tuomi JM, Chidiac P, Jones DL. Evidence for enhanced M3 muscarinic receptor function and sensitivity to atrial arrhythmia in the RGS2-deficient mouse. American Journal of Physiology Heart and Circulatory Physiology. 2010;298:H554–61. doi: 10.1152/ajpheart.00779.2009. [DOI] [PubMed] [Google Scholar]
- Van Rooij E, Marshall WS, Olson EN. Toward microRNA-based therapeutics for heart disease: the sense in antisense. Circulation Research. 2008;103:919–28. doi: 10.1161/CIRCRESAHA.108.183426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Violin JD, Soergel DG, Boerrigter G, Burnett, Lark MW. GPCR-biased ligands as novel heart failure therapeutics. Trends in Cardiovascular Medicine. 2013 doi: 10.1016/j.tcm.2013.01.002. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Wang X, Zeng W, Soyombo AA, Tang W, Ross EM, Barnes AP, et al. Spinophilin regulates Ca2+ signalling by binding the N-terminal domain of RGS2 and the third intracellular loop of G-protein-coupled receptors. Nature Cell Biology. 2005;7:405–11. doi: 10.1038/ncb1237. [DOI] [PubMed] [Google Scholar]
- Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiological Reviews. 2005;85:1159–204. doi: 10.1152/physrev.00003.2005. [DOI] [PubMed] [Google Scholar]
- Wolff DW, Xie Y, Deng C, Gatalica Z, Yang M, Wang B, et al. Epigenetic repression of regulator of G-protein signaling 2 promotes androgen-independent prostate cancer cell growth. International Journal of Cancer. 2012;130:1521–31. doi: 10.1002/ijc.26138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Liu D, Liu S, Calderon L, Zhao G, Turk J, et al. Identification of a cAMP-response element in the regulator of G-protein signaling-2 (RGS2) promoter as a key cis-regulatory element for RGS2 transcriptional regulation by angiotensin II in cultured vascular smooth muscles. Journal of Biological Chemistry. 2011;286:44646–58. doi: 10.1074/jbc.M111.265462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Kamide K, Kokubo Y, Takiuchi S, Tanaka C, Banno M, et al. Genetic variations of regulator of G-protein signaling 2 in hypertensive patients and in the general population. Journal of Hypertension. 2005;23:1497–505. doi: 10.1097/01.hjh.0000174606.41651.ae. [DOI] [PubMed] [Google Scholar]
- Zhang P, King M, Ayrapetov M, Mende U. RGS2 is a negative regulator of Gq/11-mediated signaling and cell proliferation in adult rat ventricular fibroblasts. The FASEB Journal. 2008;22(588):2. [Google Scholar]
- Zhang P, Mende U. Regulators of G-protein signaling in the heart and their potential as therapeutic targets. Circulation Research. 2011;109:320–33. doi: 10.1161/CIRCRESAHA.110.231423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Su J, King ME, Maldonado AE, Park C, Mende U. Regulator of G protein signaling 2 is a functionally important negative regulator of angiotensin II-induced cardiac fibroblast responses. American Journal of Physiology Heart and Circulatory Physiology. 2011;301:H147–56. doi: 10.1152/ajpheart.00026.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Anger T, Su J, Hao J, Xu X, Zhu M, et al. Selective loss of fine tuning of Gq/11 signaling by RGS2 protein exacerbates cardiomyocyte hypertrophy. Journal of Biological Chemistry. 2006;281:5811–20. doi: 10.1074/jbc.M507871200. [DOI] [PubMed] [Google Scholar]
- Zou MX, Roy AA, Zhao Q, Kirshenbaum LA, Karmazyn M, Chidiac P. RGS2 is upregulated by and attenuates the hypertrophic effect of α1-adrenergic activation in cultured ventricular myocytes. Cellular Signalling. 2006;18:1655–63. doi: 10.1016/j.cellsig.2006.01.012. [DOI] [PubMed] [Google Scholar]