Abstract
Influenza vaccines with broad cross-protection are urgently needed. The highly conserved ectodomain of the influenza matrix protein 2 (M2e) can be a promising candidate if its low immunogenicity was overcome. In this study, we generated protein nanoclusters self-assembled from conformation-stabilized M2e tetramers (tM2e) to improve its immunogenicity. The resulting nanoclusters showed an average hydrodynamic diameter of 268 nm. Vaccination with the nanoclusters by an intranasal route elicited high levels of serum antigen-specific IgG in mice (approximately 100-fold higher than that obtained with soluble tM2e), as well as antigen-specific T cell and mucosal antibody responses. The immunity conferred complete protection against lethal challenge with homo- as well as heterosubtypic viruses. These results demonstrate that nanoclusters assembled from conformation-stabilized M2e are promising as a potential universal influenza A vaccine. Self-assembly into nanoclusters represents a novel approach for increasing the immunogenicity of vaccine antigens.
Keywords: Cross protection, Matrix protein 2 (M2), Nanocluster
Background
Influenza virus is one of the most important respiratory pathogens with significant medical and economic burdens1. Although infection is preventable by vaccination2, current influenza vaccines only induce efficient protection against similar viral strains and are inadequate against possible pandemic strains. Conserved influenza epitopes are ideal components of an improved vaccine with broad cross protection.
M2 is an integral transmembrane protein with a conserved ectodomain (M2e) 3. Because it is highly conserved among influenza A viruses, M2e is considered a promising target for inducing cross protection against different influenza A virus subtypes 4. M2e-specific antibodies can reduce viral plaque size, and passive immunization with these antibodies reduce virus titers in the lungs of mice infected with influenza A viruses5, 6. However, M2e-specific antibodies are rarely detected after natural influenza virus infection or seasonal vaccination7, 8. Various platforms have been used to overcome the low immunogenicity of M2e, including fusing the protein with carrier molecules, using multiple antigenic peptides, or delivering protein in vectored live vaccines9–11. However, in most studies M2e was not presented in its native tetrameric form.
Nanoparticles are a promising vaccine delivery system, and a variety of carrier materials, including polymers, liposomes, and virus-like particles, have been proposed12–14. Nanoparticles, in addition to controlling release and protecting vaccine antigens, also exhibit adjuvant effects and stimulate antigen-presenting cells (APCs) upon binding and/or internalization15, 16. However, in many cases the amount of antigen loaded into the nanoparticle is low, and the process by which the particle is made can damage or unfold the antigen15, 17. As an alternative, we have designed vaccine nanoclusters that are assembled directly from protein antigens with no encapsulating agent to maximize protein loading and use gentle fabrication conditions. Here, we generated nanoclusters from M2e stabilized with a tetramerization motif to investigate as a potential influenza vaccine. Our results demonstrate that self-assembly of antigens into nanoclusters presents a very promising approach to increase vaccine immunogenicity.
Methods
Peptides, CpG-ODN, Cell lines and viruses
The M2e peptides were synthesized at GenScript (Piscataway, NJ, USA) as shown in Table 1. The purity of the peptide was above 95%. CpG-ODN 1826 (5′-TCCATGACGTTCCTGACGTT-3′) was purchased from InvivoGen (CA, USA), and stored at −20°C before use. Spodoptera frugiperda Sf9 (Sf9, ATCC, CRL-1711) and Madin-Darby canine kidney (MDCK) cells were obtained from Dr. A. Pekosz18. Mouse-adapted influenza viruses Phi/82 and CA/09 were prepared as lung homogenates from intranasally infected mice. The viruses were titrated by infection of mice with serial dilutions, and the LD50 (50% lethal dose) was calculated by the method of Reed and Muench19.
Table 1.
M2e amino acid of influenza A virus
| Viral strains | Subtype | M2e amino acid sequence |
|---|---|---|
| M2e in nanoclusters | N/A* | MSLLTEVETP IRNEWGCRCN D |
| A/Philippines/2/82 | H3N2 | MSLLTEVETP IRNEWGCRCN D |
| A/Puerto Rico/8/34 | H1N1 | MSLLTEVETP IRNEWGCRCN G |
| A/California/04/09 | H1N1 | MSLLTEVETPT RSEWECRCS D |
| A/Vietnam/1203/04 | H5N1 | MSLLTEVETPT RNEWECRCS D |
M2e consensus of human influenza A viruses
Purification and characterization of recombinant tM2e
A GCN4 sequence-stabilized tetrameric M2e (tM2e) construct was generated as described by introducing a foreign tetramerization motif GCN4 (tGCN4), a modified form of the leucine-zipper region of a yeast transcription factor20 with a signal peptide encoding sequence from the honeybee melittin protein in frame to facilitate protein expression in insect cells 21. The full-length tM2e encoding gene was subcloned into a transfer vector pFastBac-1 (Invitrogen, Grand Island, NY). Recombinant baculovirus (rBV) expressing tM2e was generated using the Bac-to-Bac protein expression kit (Invitrogen, Grand Island, NY) according to the manufacturer’s instructions. To purify recombinant tM2e, Sf9 cells were infected with the above rBVs at a MOI of 1 and incubated for 48 hours. Supernatants were collected and clarified by a brief centrifugation. Recombinant tM2e was purified from the supernatants using nickel-agarose (Qiagen, Valencia, CA) affinity chromatography. tM2e purity was confirmed by SDS-PAGE followed by Western blot. After dialysis against phosphate buffered saline (PBS, pH7.2), purified tM2e was stored at −80°C. The oligomeric status of purified tM2e was determined using the water soluble BS3 crosslinker (Pierce-Rockford, IL). Briefly, 1 μg of tM2e was incubated at room temperature in the presence of BS3 at different concentrations (final concentrations: 0, 1, 2, 4 and 8 mM, respectively) for 30 minutes. The crosslinking reaction was stopped by the addition of 1M Tris-HCl pH 8.0 to a final concentration of 50 mM. After crosslinking, proteins were separated on a 5–15% SDS-PAGE under reducing conditions (1% mercaptoethanol) and followed by Western blot using anti-M2e antibody (14C2).
Nanocluster fabrication
The nanoclusters were formed by desolvation22. Ethanol was dripped into a solution of tM2e at a rate of 1mL/min under constant stirring. The solution contained 1.7–2.8 mg/mL protein with or without 1.7–2.8 mg/mL of CpG ODN (InvivoGen, San Diego, CA) in PBS and was desolvated with a 4:1 volume ratio of ethanol to protein solution. To stabilize the nanoclusters, an amine crosslinking reaction was performed using 0.4 mM glutaraldehyde for 20 minutes under stirring and an additional 20 minutes with sonication. Linkages occurred among ε-amino groups of the five lysine residues, all located in the tGCN4 region of tM2e, as well as the terminal amine20. The nanoclusters were collected by centrifugation, resuspended in sterile PBS, and stored at 4°C. Protein concentration in the supernatant following centrifugation was measured by micro-volume UV-Vis spectrometry to determine the total protein content in nanoclusters. CpG ODN in the supernatant was quantified with OliGreen fluorescence (Molecular Probes, Eugene, OR). Dynamic light scattering (DLS) was performed in PBS with a Malvern Zetasizer Nano ZS to assess nanocluster size distributions. Nanoclusters were resuspended in water, dried, and sputter coated with gold prior to visualization with a Zeiss Ultra60 FE scanning electron microscope.
Immunization and challenge
Groups of 18 mice were intranasally (i.n.) immunized with 10 μg of tM2e protein alone (G1), 10 μg tM2e nanoclusters (G2), or 10 μg tM2e + CpG ODN nanoclusters (G3) at day 0, day 28 and day 56, respectively. Sera were collected at 2 weeks after each immunization. Vaccinated mice were lightly anesthetized by inhalation of isoflurane and challenged i.n. with 5 LD50 of mouse-adapted A/Philippines/82 (Phi/82) or A/California/04/09 (CA/09) viruses, respectively, 4 weeks after the final immunization. Body weight loss and survival rates were monitored daily for 14 days post infection (p.i.). Weight loss of ≥25% was used as the endpoint at which mice were euthanized according to IACUC guidelines.
Sample collection
Blood samples were collected at one week before immunization for pre-immune sera and 2 weeks after each immunization for immune sera by retro-orbital plexus puncture. Sera were collected by a brief spin (5000 rpm for 5 min) after clotting (about 2 hours at room temperature). Mucosal samples were collected before challenge and 4 days after challenge. Nasal washes were collected by lavaging mouse nostrils repetitively with 1ml PBS containing 0.05% Tween 20 (PBST). For lung washes, individual mouse lungs were lavaged repetitively with 1 ml PBST. After a brief centrifugation (8000 rpm) for 10 min, supernatants were filtered through a 0.22 μm filter and stored at −80°C for further assays. Lymphocytes from lung and spleen samples were collected from mice euthanized 4 weeks after the final boost and used for ELISPOT as described23.
Antibody ELISA and cytokine ELISpot
M2e specific antibody (Ab) titers in immune sera were determined by ELISA using synthesized M2e peptides (1 μg/ml) as coating antigens. Antibodies recognizing native M2 protein were determined by cell surface ELISA as described 24 (Supplementary Information, SI: Methods). Interferon gamma (INF-γ) and interleukin 4 (IL-4) secretions from immunized mouse splenocytes or lung cells were evaluated using ELISpot kits (eBioscience, San Diego, CA) according to the manufacturer’s instructions (SI: Methods).
Lung viral titers
The lungs were collected at day 4 post-infection (p.i.), and ground using cell strainers (BD Falcon, Franklin Lakes, NJ). Lung homogenates were centrifuged at 1000 RPM for 10 min to remove tissue debris. MDCK cell-based plaque assay was used for lung virus titration as described elsewhere25.
Passive immunization of mice
Naive mice were injected intraperitoneally with pooled immune sera (200 μl per mouse) from mice receiving tM2e nanoclusters or mice receiving tM2e + CpG ODN nanoclusters. After 24 h, the recipient mice were i.n. challenged with 5LD50 of Phi/82 viruses. Mouse body weight loss and survival rates were monitored daily for 14 days.
Statistical analysis
Comparisons among vaccinated groups were performed using a one-way ANOVA followed by Bonferroni’s multiple comparison post-test. Comparison of survival rate was performed using the Log-rank (Mantel-Cox) test. The analyses were done by using GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego, CA). P values of less than 0.05 (p<0.05) were considered to be statistically significant.
Results
Characterization of tM2e and nanoclusters
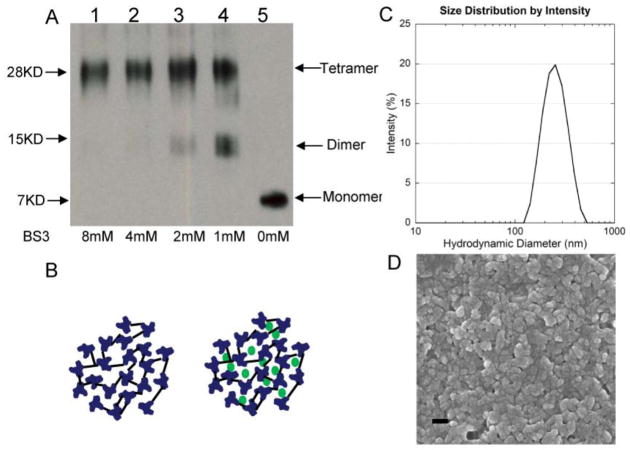
To confirm the tetrameric structure of tM2e after expression and purification, we used the chemical cross-linker Bis[sulfosuccinimidyl] suberate(BS3) to fix its polymeric state. Following cross-linking, SDS-PAGE revealed a major band with a molecular mass of 28 kDa representing the M2e tetramer and a band with a molecular mass of 14 kDa representing dimers (Fig 1A, lanes 1–4). We observed only one band with a molecular mass of 7 kDa representing the M2e monomer without the addition of BS3 (Fig 1A, lane 5). These results demonstrate that the tGCN4-stabilized tM2e was expressed in a soluble tetrameric form20.
Figure 1.
Generation and characterization of tM2e and nanoclusters. A, tM2e characterization. Tetrameric M2e was purified as described in SI Materials and Methods. 1μg of tM2e was cross-linked with BS3 at concentrations of 8, 4, 2, 1 and 0 mM, respectively. Cross-linked tM2e samples were applied to SDS-PAGE and Western blot probed using mouse monoclonal antibody 14C2 (Abcam Inc., Cambridge, MA). B, tM2e nanocluster schematics. Nanoclusters were prepared as described in Materials and Methods. Left panel, tM2e nanocluster. Right panel, tM2e nanocluster with CpG ODN trapped. C, Nanocluster homogeneity analysis. The distribution of nanocluster hydrodynamic diameters was measured in PBS by dynamic light scattering (DLS) and plotted as function of the intensity of the signal for a given size as a percentage of the total signal intensity. D, Nanocluster morphology. Dried nanoclusters were imaged by scanning electron microscopy, scale bar 200 nm.
Nanoclusters were formed by a modified desolvation process 12 and stabilized by crosslinking. Tetrameric M2e nanoclusters contained only tM2e, while tM2e + CpG ODN nanoclusters were made by the co-desolvation of CpG ODN with tM2e in an equal mass ratio (Fig 1B). Production efficiency was high, with 87% of tM2e and 30% of CpG ODN incorporated into nanoclusters. CpG ODN retention in particles was found to be ≥99.6% after storage in PBS at 4°C for 2 weeks. DLS of the nanoclusters indicated a single, reproducible population distribution with an average hydrodynamic diameter of 227 nm and an average polydispersity of 0.42 (Fig 1C). This polydispersity corresponds to a distribution of 227 ± 147 nm, and variations in diameter among batches and after one month storage in PBS at 4°C were statistically insignificant. Scanning electron microscopy of dried nanoclusters showed smaller particulates that were relatively spherical, indicating the particles contained significant amounts of water in their normal, hydrated state (Fig 1D).
Tetrameric M2e nanoclusters induced strong humoral responses and broadly cross-reactive M2e-specific antibodies
To determine if i.n. immunization with tM2e nanoclusters can induce enhanced humoral responses, immune sera were evaluated for antigen-specific IgG titers using ELISA with M2e peptides as coating antigens, or cell surface ELISA using M2-expressing MDCK cells. As shown in Fig 2A, M2e-specific IgG titers in sera of mice receiving tM2e nanoclusters or tM2e + CpG ODN nanoclusters were approximately 100-foldhigher than that of the mouse group immunized with only soluble tM2e (p< 0.001) (Fig 2A). The levels of M2e-specific IgG1, IgG2a and IgG2b were also significantly higher than those of mice receiving only tM2e (SI Fig S1). Both tM2e and tM2e + CpG ODN nanocluster groups showed similar levels of IgG1 and IgG2a, which indicated that the nanoparticles elicit a balanced Th1/Th2 immune response 26. CpG ODN did not show a significant adjuvant effect on the magnitude of M2e-specific Ab responses when integrated into nanoclusters (Fig 2), indicating that nanoclusters themselves are strong adjuvants which may mask the effect of other adjuvants. Immune sera also showed similar binding reactivity to native M2 expressed on MDCK cell surfaces, suggesting that antibodies induced by tM2e nanoclusters can recognize conformational epitopes on native M2.
Figure 2.
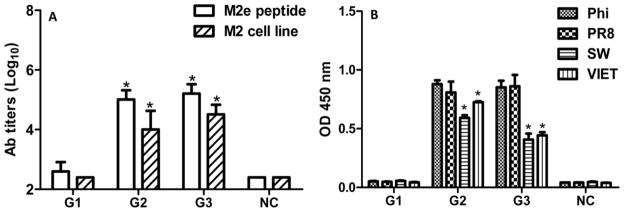
Serum M2e-specific IgG antibodies and cross-reactivity with variant M2e peptides. Serum antibodies specific for M2e peptides and MDCK expressed M2 were determined by ELISA. The levels of serum M2e-specific IgG antibody titers were measured by antibody endpoint titer, whereas the levels of cross-reactivity with variant M2e peptide were measured by OD450 value (1:5,000 dilution). The reciprocal of the highest dilution of sera that gave an OD450 of twice that of the naïve group at the lowest dilution was designated as the Ab endpoint titer. Representative data are the mean±S.D. of six mice in each group. G1: Soluble tM2e (10 μg); G2: tM2e nanoclusters (10 μg); G3: tM2e + CpG ODN nanoclusters (10 μg each of tM2e and CpG ODN). NC: naïve mice control A, Serum IgG to M2e peptide or M2e expressed on surface of MDCK cell; B, Cross-reactivity of M2e antibodies to variant M2e-sequence peptides from A/Philippines/2/82 (Phi/82), A/Puerto Rico/8/34 (PR/8), A/California/04/09 (CA/09) and A/Vietnam/1203/04 (VIET/04). * p<0.05: compared to antibody titers of G1 (A) or to the binding avidity with M2e peptide of Phi/82 or PR8 (B).
Although M2e is highly conserved in influenza A viruses, some amino acid substitutions occur in different strains and subtypes. To evaluate the cross-reactivity of M2e-specific Abs induced by tM2e nanoclusters, sera from mice immunized i.n. with tM2e nanoclusters were assayed using ELISA with variant M2e peptide as coating antigens. As shown in Table 1, the M2e sequence in tM2e nanoclusters is the consensus as in Phi/82 H3N2 virus, whereas there are 1, 4 and 3 amino acid differences respectively in M2e of PR/8, CA/09 and VIET/04 viruses. As demonstrated in Fig 2B, M2e Abs bound strongly to the M2e peptides of Phi/82 and PR/8. The levels of Ab binding were significantly lower to CA/09 or VIET/04 M2e peptides (p<0.05). Levels of IgG subtype binding to various M2e peptides were also compared (SI Fig S2). M2e-specific IgG subclass antibodies reacted well with the M2e domain of Phi/82 and PR/8, but at a significantly lower level with that of CA/09 and VIET/04 (p<0.05) for IgG1, IgG2a and IgG2b. These results demonstrate that the assembly of the tM2e antigen into nanoclusters can significantly enhance the immunogenicity of tM2e, elicit balanced Th cell responses and induce M2e-specific antibodies with some cross reactivity to variant M2e sequences.
Tetrameric M2e nanoclusters induce robust mucosal antibody responses
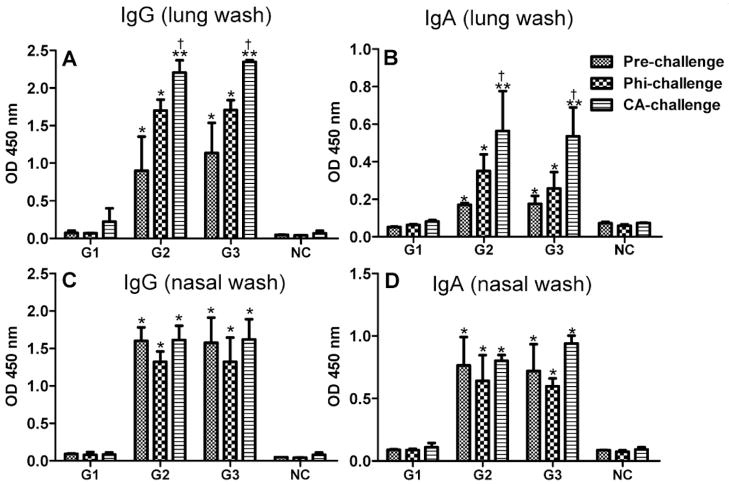
A strong mucosal antibody response is associated with the prevention or mediation of viral entry in the upper respiratory tract of mice27. To evaluate whether i.n. immunization with tM2e nanoclusters can induce mucosal Ab responses, we measured M2e-specific IgA and IgG Abs in lung and nasal washes by ELISA in mice pre-challenge and 4 days after challenge (Fig 3). As shown in Fig 3A and 3B, significantly higher levels of IgG and IgA were observed in lung washes of pre-challenged mice of tM2e or tM2e + CpG ODN nanocluster groups compared to that of mice which received only soluble tM2e (p<0.05). After challenges with Phi/82 or CA/09 virus, the levels of lung wash IgG and IgA increased. In CA/09 challenged mice a significantly higher level of Ab response was observed compared to pre-challenge in the nanoparticle-immunized groups (p<0.05). Similarly, significantly higher levels of IgG and IgA were observed in nasal washes of pre-challenged mice immunized with tM2e or tM2e + CpG ODN nanoclusters (p<0.05) and there was no significant change between pre-challenge and post-challenge nasal washes (Figs 3C and 3D). These data demonstrate that tM2e nanoclusters stimulate M2e-specific mucosal Ab in mice.
Figure 3.
Mucosal M2e-specific IgG and IgA antibody levels. Lung and nasal washes were collected from individual mice before and at day 4 post challenge with Phi/82 (H3N2) or CA/09 (H1N1) viruses. IgA and IgG antibodies binding to M2e peptide of Phi/82 were determined in 5-fold diluted lung washes or undiluted nasal washes by ELISA. Groups of mice were the same as denoted in Figure 1. A, IgG in lung washes. B, IgA in lung washes. C, IgG in nasal washes. D, IgA in nasal washes. * p<0.05 or ** p<0.01: compared to G1; † p<0.05: CA/09-challenge versus pre-challenge in G2 or G3.
Tetrameric M2e nanoclusters activate M2e-specific T cell responses
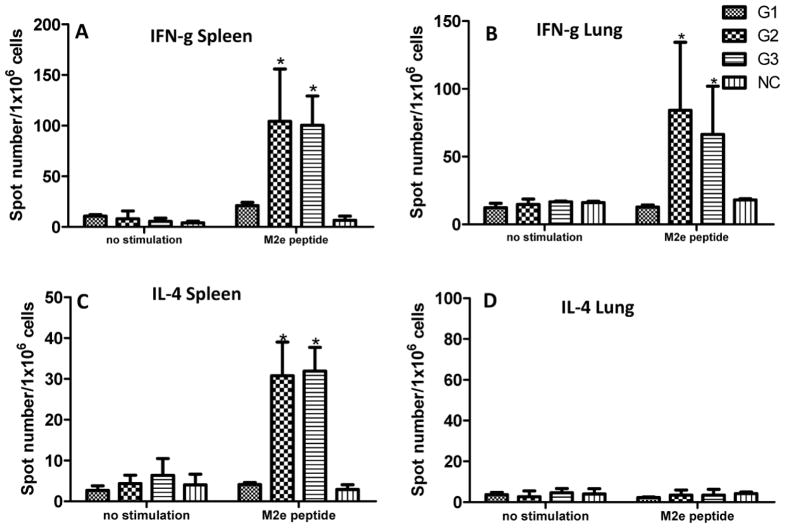
T cell responses are important for the generation and regulation of an effective immune response and are known to contribute to broad cross protective immunity28, 29. IFN-γ and IL-4 secreting cells in both spleens and lungs of immunized mice were evaluated by cytokine ELISpot25. As shown in Fig 4, mice that received either tM2e or tM2e+CpG nanoclusters showed significantly higher IFN-γ secreting cell populations in the spleen and lungs after stimulation with M2e peptide compared to mice immunized with soluble tM2e (Figs 4A and 4B, p<0.05). A higher frequency of IL-4 secreting cells was also detected in the spleens of immunized mice in either tM2e or tM2e+CpG ODN nanocluster-immunized groups compared to that of mice vaccinated with soluble tM2e (Fig 4C, p<0.05). However, no IL-4 secreting cells were detected in the lungs of mice from any group. It has been reported that vaccination by i.n. route with Macobacterium tuberculosis vaccine candidates in BALB/c mice induced more antigen-specific IL-4 secreting cells in the lung30. However, low IL-4 secreting population was observed from immunized mouse lungs with M2e nanocluster immunization in this study. A possible reason for the difference observed may be resulted from different antigen delivering platform. The exact mechanism is still need to be studied in the future. The tM2e group showed only background levels of cytokine secreting cells after M2e peptide stimulation. These results provide evidence that i.n. immunization with M2e nanoclusters induces enhanced M2e specific T cell immune responses.
Figure 4.
M2e-specific cellular immune responses. Spleens and lungs of mice were collected after immunization with soluble tM2e, tM2e nanoclusters or tM2e + CpG ODN nanoclusters, respectively. Cells from homogenates of spleens (A, C) and lungs (B, D) were stimulated with M2e peptides for 40 hours; then IFN-g or IL-4 cytokine-secreting cell clones were determined by ELISPOT assay. Groups of mice were the same as denoted in Figure 1. * p<0.05: compared to G1.
Tetrameric M2e nanoclusters protect mice from lethal virus challenge
To evaluate the protective efficacy of tM2e nanoclusters, mice were infected with 5LD50 of Phi/82 H3N2 virus or CA/09 H1N1 virus 4 weeks after the final boost. Mouse body weight loss and survival were monitored for 14 days. Day 4 post-infection was chosen to evaluate lung virus titers because naive mice were shown previously to have substantial titers of virus in lungs at this time point 31.
As shown in Fig 5, all mice immunized with tM2e alone or naive groups died by 7 to 8 days p.i. (Fig 5C) with high titers of viruses in the lungs (Fig 5A) and over 25% body weight loss (Fig 5B). Mice receiving tM2e alone did not show substantial virus reduction in the lungs compared with control mice (p>0.05). In contrast, mice immunized with tM2e or tM2e+CpG ODN nanoclusters had over 20-fold lower virus titers in their lungs after Phil/82 challenge and exhibited significantly reduced morbidity as demonstrated by a low weight loss ≤10% compared with mice in the tM2e group (p<0.05). All nanocluster-immunized mice survived the lethal challenge with homologous Phi/82 H3N2 virus. These results indicate that i.n. immunization of mice with M2e nanocluster vaccines significantly reduces virus titers in the lungs, and completely protects mice against severe disease and death from lethal infection with the homologous virus.
Figure 5.
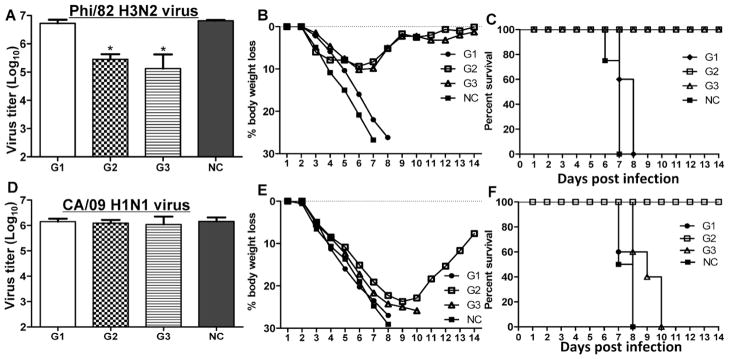
Protective efficacy against infection by Phi/82 or CA/09 strains. Immunized mice were challenged with a lethal dose (5LD50) of Phi/82 or CA/09 virus in a volume of 30 μl PBS by i.n. instillation. Mouse survival rate and body weight changes were monitored daily for 14 days. Mouse lung virus titers at day 4 post challenge were determined by standard plaque assays. Bars represent mean virus titers (Log10 pfu/ml) ±standard errors from three independent assays. Groups of mice were the same as denoted in Fig 1. A, lung virus titers, B, body weight changes and C, survival rate post Phi/82 virus infection; D, lung virus titers, E, body weight changes, and F, survival rate post CA/09 infection. * p<0.05: compared to G1 or NC group.
To further evaluate whether tM2e nanoclusters elicit immunity conferring broad protection, immunized mice were subjected to a heterologous virus challenge. As shown in Fig. 5, although mice immunized with tM2e nanoclusters exhibited severe body weight loss after lethal viral exposure with a heterosubtypic CA/09 H1N1 virus, they survived the challenge and began to regain body weight after day 9 post-challenge (Figs 5E and 5F). Mice in the other groups including the group of tM2e nanoclusters plus CpG ODN reached their end-point in 7 to 10 days p.i. However mice in G2 did not show significantly lower lung virus titers compared with the other groups after challenge with CA/09 H1N1 virus (Fig 5D). These results demonstrate that i.n. immunization of mice with tM2e nanoclusters survive after homologous or heterosubtypic virus challenges.
Protective effect of immune sera from tM2e nanocluster-immunized mice
To determine if M2e-specific antibodies can protect mice from lethal influenza infection, we administered immune sera from mice immunized with tM2e or tM2e+CpG ODN nanoclusters i.p. to two groups of naive mice. After 24 h, mice were challenged i.n. with 5LD50 of Phi/82 H3N2 virus. Body weight loss and survival were monitored daily for 14 days p.i. Although mice initially showed substantial body weight loss, the two groups receiving immune sera exhibited >70% survival from a lethal dose infection by Phi/82 H3N2 virus (Fig 6). In contrast, all mice in a control group died 6 to 8 days p.i. These data provide evidence that M2e-specific antibodies contribute at least partially to the protection observed above.
Figure 6.
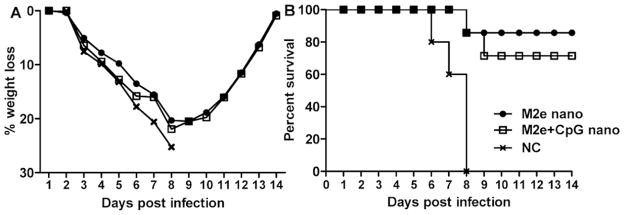
Protection by passive transfer of immune sera. Immune sera from M2e nanocluster vaccine-immunized mice was pooled. A total of 200 μl of pooled sera were transferred to naive recipient mice via intraperitoneal (i.p.) injection. Twenty-four hours post-transfer, mice were infected with 5LD50 of Phi/82 virus as described in Fig 5. A, mouse body weight loss and B, survival rate were monitored for 14 days.
Discussion
Our results demonstrate that intranasal vaccination with tM2e nanoclusters induces high levels of humoral and mucosal M2e-specific antibody and T cell responses. M2e is considered to be a promising target for inducing cross-protection against different influenza A virus subtypes because it is highly conserved among influenza A viruses 4. However, the immunogenicity of M2e is very low due in part to its low incorporation into influenza virus particles and steric blocking by HA and NA on the surface of the virus. In this study, nanoclusters were directly assembled from tM2e in the absence of HA and NA using an extremely low level of cross-linker. Thus tM2e could be delivered to immune cells at a high epitope density to overcome the limitations of M2e presentation during viral infection or vaccination. Since no encapsulation materials are used, the nanoclusters contain much higher level of protein antigen compared to particles that contain polymeric or liposomal materials. Antigen is present not only within the nanoclusters, but also on the nanocluster surface, mimicking natural presentation. As antibodies specific to conformational epitopes presented in quaternary structures may be more effective at binding M28, a tetrameric conformation-stabilized recombinant M2e was predicted to be more immunogenic than other M2e forms. The tetrameric M2e that we produced was stabilized by addition of tGCN4, and was expressed efficiently in a recombinant baculovirus protein expression system in insect cells. Our data demonstrate that i.n. immunization with tM2e nanoclusters dramatically enhanced the antibody responses to M2e compared to those observed with soluble tM2e, and these Abs were shown to recognize native M2 on cell surfaces.
Although anti-M2 antibodies may not directly neutralize the virus 32, influenza virions bound to M2 antibodies might be preferentially recognized and cleared by opsono-phagocytosis by macrophages 24. Fu et al. demonstrated that M2 monoclonal antibodies that preferentially bind to polymeric M2 were protective independent of natural killer cell mediated effector functions33. Another study showed that alveolar macrophages and Fc receptor-dependent elimination of influenza A virus-infected cells are essential for protection mediated by anti-M2e IgG34. Therefore, it is possible that the protective efficacy demonstrated in our study may be correlated with the level of circulating M2e-specific Abs. This is supported by the results of the passive transfer of immune serum in our study. Studies have been reported about the involvement of Ab-dependent cell-mediated cytotoxicity (ADCC) by natural killer (NK) and/or natural killer T (NKT) cells which suggested the involvement of B cell responses in the protection mechanism of M2e vaccine32. However, the mechanism of how M2e-specific Abs protects mice from lethal challenge is still unclear. As there was only about a 70~80% survival rate in the passive transfer study, other factors such as mucosal immunity and T cell responses may also contribute to the protection observed by nanocluster immunization.
Cell-mediated immunity plays an important role in many vaccinations 35. Although it is recognized that antibodies are the main effectors in M2e-induced immune responses, and so far only a single human CTL epitope has been identified for M2e specific memory CTL activity 36, M2e-specific cellular immune responses can be elicited and can contribute to protection against experimental infection in mice and ferrets 37. Our results demonstrate that tM2e nanovaccines induce strong M2e-specific CD4 helper T cell (Th) responses. Furthermore, these appear to be balanced Th1/Th2 responses based on the similarity of M2e-specific IgG1 and IgG2a levels observed post immunization.
The mucosal immune system is the first immunological barrier against pathogens that invade the body via the mucosal surface 38. M2e-based vaccines were found to induce mucosal immune responses when delivered by the i.n. route39. Several studies have demonstrated that IgA in upper respiratory tract secretions plays a major role in antiviral immunity40, 41. The local production of IgG is also an important component of the immune responses following traditional mucosal immunization or infection42. We found that high levels of IgA and IgG were induced in both nasal mucosal and lung surfaces after i.n. immunization with tM2e nanoclusters, and these mucosal antibodies may contribute to protective immunity.
Although M2e is relatively conserved in all influenza A viruses, variation of M2e sequences in different virus strains may limit the protective efficacy of M2e vaccines to virus infections. Nanoclusters assembled from tM2e conferred complete survival of mice with little body weight loss upon Phi/82 challenge, in which the M2e sequence is identical with that of the tM2e used for immunization. In contrast, although they survived, mice showed much more severe illness upon CA/09 challenge, as demonstrated by increased body weight loss and high lung virus titers. This may be a result of 4-aa substitutions in the M2e of CA/09, which represents an extreme in influenza A M2e sequence variation. In ELISA binding assays, even a 1-aa difference was found to impact the binding of M2e-specific Abs to synthetic peptides. Therefore, other approaches are needed to enhance the cross-protective effect of M2e vaccines, such as incorporating representative M2e sequences from multiple strains and subtypes.
An interesting finding is that CpG ODN trapped in nanoclusters did not show any adjuvant effect; and immunized mice were less protected compared to mice in the group of nanoclusters without CpG ODN upon challenge infection. A previous study also reported that with the addition of CpG ODN, the protective efficacy of influenza M2e peptide vaccine was weakened43. Although nanoclusters exert adjuvant effect themselves and may shield the role of the other adjuvants co-administered, it may not explain the reduced protection. Mechanisms of the adverse adjuvant effect observed should be studied in the future.
Although enhanced M2e-specific B and T cell responses were observed, mechanisms for how M2e vaccines conferring protection is still unclear. There were several conflict observations regarding to the possible mechanisms of protective immunity induced by M2e vaccines. Nevertheless, it is commonly accepted that M2e-specific Abs may be important players in providing such protection although a few studies claimed otherwise44–46. Increasing studies support the conclusion that M2e-specific antibodies do not neutralize viruses but mediate effector cell function such as antibody-dependent cell-mediated cytotoxicity/virus-inhibition, conferring viral protection 32, 47, 48, 34. To consolidate the conclusion, certain studies may need to be conducted in the future, for instance, to find out how antibodies functioning on portal surface where influenza virus enter the host and replicate itself.
Nanoclusters assembled from conformation-stabilized M2e are promising as a potential universal influenza A vaccine. The approach of self-assembly of vaccine antigens into nanoclusters represents a potent general strategy for increasing vaccine-induced immune responses. It is also readily amenable for use with mixtures of multiple antigens or with mixtures of antigens and adjuvants. The nanocluster formulations described here share the immunological advantages of particulate antigens with other nanoparticle vaccines. However they are unique in providing nanoparticles consisting entirely of the antigen of interest, or of antigen-adjuvant combinations.
Supplementary Material
Acknowledgments
We thank Won Min Park for SEM images and Erin-Joi Collins for her valuable assistance in the preparation of the manuscript.
Footnotes
Conflict of Interests
This study was supported by grants AI068003 (R.W.C) and AI101047 (B.Z.W) from the National Institutes of Health and startup funding from Georgia Institute of Technology (J.A.C.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. AH was supported by the Stamps Leadership Scholar Program. Chemical reagents and computer programs used in this study were purchased from manufacturers or commercial vendors. They had no role in the performance of this study. No conflict of interest was found for any of the coauthors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Thompson WW, Shay DK, Weintraub E, Brammer L, Bridges CB, Cox NJ, et al. Influenza-associated hospitalizations in the United States. Jama. 2004;292:1333–40. doi: 10.1001/jama.292.11.1333. [DOI] [PubMed] [Google Scholar]
- 2.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. Jama. 2003;289:179–86. doi: 10.1001/jama.289.2.179. [DOI] [PubMed] [Google Scholar]
- 3.Lamb RA, Zebedee SL, Richardson CD. Influenza virus M2 protein is an integral membrane protein expressed on the infected-cell surface. Cell. 1985;40:627–33. doi: 10.1016/0092-8674(85)90211-9. [DOI] [PubMed] [Google Scholar]
- 4.Schotsaert M, De Filette M, Fiers W, Saelens X. Universal M2 ectodomain-based influenza A vaccines: preclinical and clinical developments. Expert Rev Vaccines. 2009;8:499–508. doi: 10.1586/erv.09.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hughey PG, Roberts PC, Holsinger LJ, Zebedee SL, Lamb RA, Compans RW. Effects of antibody to the influenza A virus M2 protein on M2 surface expression and virus assembly. Virology. 1995;212:411–21. doi: 10.1006/viro.1995.1498. [DOI] [PubMed] [Google Scholar]
- 6.Treanor JJ, Tierney EL, Zebedee SL, Lamb RA, Murphy BR. Passively transferred monoclonal antibody to the M2 protein inhibits influenza A virus replication in mice. J Virol. 1990;64:1375–7. doi: 10.1128/jvi.64.3.1375-1377.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black RA, Rota PA, Gorodkova N, Klenk HD, Kendal AP. Antibody response to the M2 protein of influenza A virus expressed in insect cells. J Gen Virol. 1993;74(Pt 1):143–6. doi: 10.1099/0022-1317-74-1-143. [DOI] [PubMed] [Google Scholar]
- 8.Feng J, Zhang M, Mozdzanowska K, Zharikova D, Hoff H, Wunner W, et al. Influenza A virus infection engenders a poor antibody response against the ectodomain of matrix protein 2. Virol J. 2006;3:102. doi: 10.1186/1743-422X-3-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hikono H, Miyazaki A, Mase M, Inoue M, Hasegawa M, Saito T. Induction of a cross-reactive antibody response to influenza virus M2 antigen in pigs by using a Sendai virus vector. Vet Immunol Immunopathol. 2012;146:92–6. doi: 10.1016/j.vetimm.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 10.De Filette M, Ysenbaert T, Roose K, Schotsaert M, Roels S, Goossens E, et al. Antiserum against the conserved nine amino acid N-terminal peptide of influenza A virus matrix protein 2 is not immunoprotective. J Gen Virol. 2011;92:301–6. doi: 10.1099/vir.0.027086-0. [DOI] [PubMed] [Google Scholar]
- 11.De Filette M, Fiers W, Martens W, Birkett A, Ramne A, Lowenadler B, et al. Improved design and intranasal delivery of an M2e-based human influenza A vaccine. Vaccine. 2006;24:6597–601. doi: 10.1016/j.vaccine.2006.05.082. [DOI] [PubMed] [Google Scholar]
- 12.Peek LJ, Middaugh CR, Berkland C. Nanotechnology in vaccine delivery. Adv Drug Deliv Rev. 2008;60:915–28. doi: 10.1016/j.addr.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Champion CI, Kickhoefer VA, Liu G, Moniz RJ, Freed AS, Bergmann LL, et al. A vault nanoparticle vaccine induces protective mucosal immunity. PLoS ONE. 2009;4:e5409. doi: 10.1371/journal.pone.0005409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui Z, Han SJ, Vangasseri DP, Huang L. Immunostimulation mechanism of LPD nanoparticle as a vaccine carrier. Mol Pharm. 2005;2:22–8. doi: 10.1021/mp049907k. [DOI] [PubMed] [Google Scholar]
- 15.Cheng X, Liu R, He Y. A simple method for the preparation of monodisperse protein-loaded microspheres with high encapsulation efficiencies. Eur J Pharm Biopharm. 2010;76:336–41. doi: 10.1016/j.ejpb.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 16.Jin T, Zhu J, Wu F, Yuan W, Geng LL, Zhu H. Preparing polymer-based sustained-release systems without exposing proteins to water-oil or water-air interfaces and cross-linking reagents. J Control Release. 2008;128:50–9. doi: 10.1016/j.jconrel.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Park TG, Lu WQ, Crotts G. Importance of in vitro experimental conditions on protein release kinetics, stability and polymer degradation in protein encapsulated poly (d,l-lactic acid-co-glycolic acid) microspheres. J Control Release. 1995;33:211–222. [Google Scholar]
- 18.Grantham ML, Stewart SM, Lalime EN, Pekosz A. Tyrosines in the influenza A virus M2 protein cytoplasmic tail are critical for production of infectious virus particles. J Virol. 2010;84:8765–76. doi: 10.1128/JVI.00853-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reed MH, LJ A simple method of estimating fifty per cent end points. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 20.De Filette M, Martens W, Roose K, Deroo T, Vervalle F, Bentahir M, et al. An influenza A vaccine based on tetrameric ectodomain of matrix protein 2. J Biol Chem. 2008;283:11382–7. doi: 10.1074/jbc.M800650200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang BZ, Liu W, Kang SM, Alam M, Huang C, Ye L, et al. Incorporation of high levels of chimeric human immunodeficiency virus envelope glycoproteins into virus-like particles. J Virol. 2007;81:10869–78. doi: 10.1128/JVI.00542-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer K, Anhorn MG, Steinhauser I, Dreis S, Celebi D, Schrickel N, et al. Human serum albumin (HSA) nanoparticles: reproducibility of preparation process and kinetics of enzymatic degradation. Int J Pharm. 2008;347:109–17. doi: 10.1016/j.ijpharm.2007.06.028. [DOI] [PubMed] [Google Scholar]
- 23.Wang BZ, Quan FS, Kang SM, Bozja J, Skountzou I, Compans RW. Incorporation of membrane-anchored flagellin into influenza virus-like particles enhances the breadth of immune responses. J Virol. 2008;82:11813–23. doi: 10.1128/JVI.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Song JM, Wang BZ, Park KM, Van Rooijen N, Quan FS, Kim MC, et al. Influenza virus-like particles containing M2 induce broadly cross protective immunity. PLoS ONE. 2011;6:e14538. doi: 10.1371/journal.pone.0014538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang BZ, Xu R, Quan FS, Kang SM, Wang L, Compans RW. Intranasal immunization with influenza VLPs incorporating membrane-anchored flagellin induces strong heterosubtypic protection. PLoS ONE. 2010;5:e13972. doi: 10.1371/journal.pone.0013972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–73. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 27.Ito R, Ozaki YA, Yoshikawa T, Hasegawa H, Sato Y, Suzuki Y, et al. Roles of anti-hemagglutinin IgA and IgG antibodies in different sites of the respiratory tract of vaccinated mice in preventing lethal influenza pneumonia. Vaccine. 2003;21:2362–71. doi: 10.1016/s0264-410x(03)00078-1. [DOI] [PubMed] [Google Scholar]
- 28.Kreijtz JH, Bodewes R, van Amerongen G, Kuiken T, Fouchier RA, Osterhaus AD, et al. Primary influenza A virus infection induces cross-protective immunity against a lethal infection with a heterosubtypic virus strain in mice. Vaccine. 2007;25:612–20. doi: 10.1016/j.vaccine.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 29.Seo SH, Webster RG. Cross-reactive, cell-mediated immunity and protection of chickens from lethal H5N1 influenza virus infection in Hong Kong poultry markets. J Virol. 2001;75:2516–25. doi: 10.1128/JVI.75.6.2516-2525.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sable SB, Cheruvu M, Nandakumar S, Sharma S, Bandyopadhyay K, Kellar KL, et al. Cellular immune responses to nine Mycobacterium tuberculosis vaccine candidates following intranasal vaccination. PLoS One. 2011;6:e22718. doi: 10.1371/journal.pone.0022718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X, Tumpey TM, Morken T, Zaki SR, Cox NJ, Katz JM. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–11. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jegerlehner A, Schmitz N, Storni T, Bachmann MF. Influenza A vaccine based on the extracellular domain of M2: weak protection mediated via antibody-dependent NK cell activity. J Immunol. 2004;172:5598–605. doi: 10.4049/jimmunol.172.9.5598. [DOI] [PubMed] [Google Scholar]
- 33.Fu TM, Freed DC, Horton MS, Fan J, Citron MP, Joyce JG, et al. Characterizations of four monoclonal antibodies against M2 protein ectodomain of influenza A virus. Virology. 2009;385:218–26. doi: 10.1016/j.virol.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 34.El Bakkouri K, Descamps F, De Filette M, Smet A, Festjens E, Birkett A, et al. Universal vaccine based on ectodomain of matrix protein 2 of influenza A: Fc receptors and alveolar macrophages mediate protection. J Immunol. 2011;186:1022–31. doi: 10.4049/jimmunol.0902147. [DOI] [PubMed] [Google Scholar]
- 35.Salerno-Goncalves R, Sztein MB. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 2006;14:536–42. doi: 10.1016/j.tim.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Gianfrani C, Oseroff C, Sidney J, Chesnut RW, Sette A. Human memory CTL response specific for influenza A virus is broad and multispecific. Hum Immunol. 2000;61:438–52. doi: 10.1016/s0198-8859(00)00105-1. [DOI] [PubMed] [Google Scholar]
- 37.Lalor PA, Webby RJ, Morrow J, Rusalov D, Kaslow DC, Rolland A, et al. Plasmid DNA-based vaccines protect mice and ferrets against lethal challenge with A/Vietnam/1203/04 (H5N1) influenza virus. J Infect Dis. 2008;197:1643–52. doi: 10.1086/588431. [DOI] [PubMed] [Google Scholar]
- 38.Mestecky J, McGhee JR. Immunoglobulin A (IgA): molecular and cellular interactions involved in IgA biosynthesis and immune response. Adv Immunol. 1987;40:153–245. doi: 10.1016/s0065-2776(08)60240-0. [DOI] [PubMed] [Google Scholar]
- 39.De Filette M, Ramne A, Birkett A, Lycke N, Lowenadler B, Min Jou W, et al. The universal influenza vaccine M2e-HBc administered intranasally in combination with the adjuvant CTA1-DD provides complete protection. Vaccine. 2006;24:544–51. doi: 10.1016/j.vaccine.2005.08.061. [DOI] [PubMed] [Google Scholar]
- 40.Asahi Y, Yoshikawa T, Watanabe I, Iwasaki T, Hasegawa H, Sato Y, et al. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J Immunol. 2002;168:2930–8. doi: 10.4049/jimmunol.168.6.2930. [DOI] [PubMed] [Google Scholar]
- 41.Renegar KB, Johnson CD, Dewitt RC, King BK, Li J, Fukatsu K, et al. Impairment of mucosal immunity by total parenteral nutrition: requirement for IgA in murine nasotracheal anti-influenza immunity. J Immunol. 2001;166:819–25. doi: 10.4049/jimmunol.166.2.819. [DOI] [PubMed] [Google Scholar]
- 42.Parr EL, Parr MB. Immunoglobulin G, plasma cells, and lymphocytes in the murine vagina after vaginal or parenteral immunization with attenuated herpes simplex virus type 2. J Virol. 1998;72:5137–45. doi: 10.1128/jvi.72.6.5137-5145.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu F, Yuan XY, Li J, Chen YH. The co-administration of CpG-ODN influenced protective activity of influenza M2e vaccine. Vaccine. 2009;27:4320–4. doi: 10.1016/j.vaccine.2009.04.075. [DOI] [PubMed] [Google Scholar]
- 44.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou WM, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–63. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 45.Bessa J, Schmitz N, Hinton HJ, Schwarz K, Jegerlehner A, Bachmann MF. Efficient induction of mucosal and systemic immune responses by virus-like particles administered intranasally: implications for vaccine design. Eur J Immunol. 2008;38:114–26. doi: 10.1002/eji.200636959. [DOI] [PubMed] [Google Scholar]
- 46.Mozdzanowska K, Zharikova D, Cudic M, Otvos L, Gerhard W. Roles of adjuvant and route of vaccination in antibody response and protection engendered by a synthetic matrix protein 2-based influenza A virus vaccine in the mouse. Virol J. 2007;4:118. doi: 10.1186/1743-422X-4-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mozdzanowska K, Furchner M, Zharikova D, Feng J, Gerhard W. Roles of CD4+ T-cell-independent and -dependent antibody responses in the control of influenza virus infection: evidence for noncognate CD4+ T-cell activities that enhance the therapeutic activity of antiviral antibodies. J Virol. 2005;79:5943–51. doi: 10.1128/JVI.79.10.5943-5951.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tompkins SM, Zhao ZS, Lo CY, Misplon JA, Liu T, Ye Z, et al. Matrix protein 2 vaccination and protection against influenza viruses, including subtype H5N1. Emerg Infect Dis. 2007;13:426–35. doi: 10.3201/eid1303.061125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.