Abstract
Connective tissue growth factor (CTGF/CCN2) is involved in extracellular matrix production, tumor cell proliferation, adhesion, migration and metastasis. Recent studies have shown that CTGF expression is elevated in precursor-B acute lymphoblastic leukemia (ALL) and that increased expression of CTGF is associated with inferior outcome in B-ALL. In this study, we characterized the functional role and downstream signaling pathways of CTGF in ALL cells. First, we utilized lentiviral shRNA to knock-down CTGF in RS4;11 and REH ALL cells expressing high levels of CTGF mRNA. Silencing of CTGF resulted in significant suppression of leukemia cell growth compared to control vector, which was associated with AKT/mTOR inactivation and increased levels of cyclin-dependent kinase inhibitor p27. CTGF knockdown sensitized ALL cells to vincristine and methotrexate. Treatment with an anti-CTGF monoclonal antibody, FG-3019, significantly prolonged survival of mice injected with primary xenograft B-ALL cells when co-treated with conventional chemotherapy (vincristine, L-asparaginase and dexamethasone). Data suggest that CTGF represents a targetable molecular aberration in B-ALL, and blocking CTGF signaling in conjunction with administration of chemotherapy may represent a novel therapeutic approach for ALL patients.
Keywords: ALL, CTGF, apoptosis
Introduction
Connective tissue growth factor (CTGF or CCN2) is an extracellular matrix-associated molecule and a member of the CCN family, which includes cysteine-rich protein 61 (Cyr61 or CCN1), CTGF/CCN2, nephroblastoma overexpressed protein (Nov or CCN3), Wnt-inducible secreted protein-1 (WISP-1 or CCN4), WISP-2 (CCN5) and WISP-3 (CCN6). The CCN family members possess an NH2-terminal signal peptide indicative of secreted proteins. CTGF is reported to interact with various proteins including integrins, bone morphogenetic proteins, transforming growth factor (TGF)-β, aggrecan, matrix metalloproteinases, fibronectin, perlecan, vascular endothelial growth factor (VEGF) and low-density lipoprotein receptor-related proteins [1-3]. CTGF is involved in extracellular matrix production, cell proliferation, cell survival, adhesion, migration, and metastasis [1-3]. None of the in vivo activities of CTGF have been unequivocally attributed to specific interactions, suggesting that CTGF might mediate its effects through multiple mechanisms.
CTGF over-expression has been associated with tumor progression and/or poor prognosis of solid cancers including breast cancer, glioblastoma and esophageal cancer [4-8]. In pancreatic cancer, CTGF is a critical regulator of tumor growth, and CTGF-specific antibody attenuates tumor growth and metastases in vivo [7, 8]. On the other hand, increased CTGF expression has been correlated with improved prognosis in chondrosarcoma patients and in patients with lung cancer [9, 10]. CTGF has been reported to confer anti-apoptotic properties and chemoresistance in cancer [11-14]. In hematological malignancies, elevated CTGF expression has been frequently and exclusively detected in precursor-B acute lymphoblastic leukemia (ALL) [15-18]. CTGF is poorly expressed in normal peripheral blood and hematopoietic bone marrow cells, AML or T-lineage ALL, while 70–80% of precursor-B ALL samples over-express CTGF [15-18]. High expression of CTGF has been associated with poor outcome in precursor-B ALL patients [18, 19]. It is thought that CTGF functions in a cell-type-specific, context-dependent manner and is a potential therapeutic target for some malignancies including precursor-B ALL. However, up until now, a direct role for CTGF in tumor suppression or progression has not been investigated in leukemias or with therapeutic agents with the capacity to inhibit CTGF function in vivo.
In this study, we characterized expression and function of CTGF in ALL cells and investigated the anti-leukemia efficacy of the human anti-CTGF monoclonal antibody FG-3019 (FibroGen, San Francisco, CA).
Methods
Reagents
CTGF monoclonal antibody
FG-3019 is a human IgG1κ monoclonal antibody recognizing domain 2 of human and rodent CTGF, provided by FibroGen (San Francisco, CA). In the indicated experiments, whole molecular human IgG, purified from serum (Jackson ImmunoResearch), was used as the control.
Cell lines, primary samples and cultures
RS4;11, REH, Raji and Jurkat cell lines were purchased from the American Type Culture Collection. NALM-6 was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkulturen. Cell lines were maintained in RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS). RS4;11, REH and NALM-6 are derived from precursor-B ALL patients, the mature B-cell Raji from a Burkitt lymphoma patient and Jurkat from a T-ALL patient. Human primary pre-B-ALL ALL xenografts (ALL-2 and ALL-10) were generated from pediatric ALL xenografts propagated in mice [20]. The clinical information has been also described [20]. Normal bone marrows were obtained after informed consent in accordance with institutional guidelines set forth by M. D. Anderson Cancer Center and the Declaration of Helsinki. Mononuclear cells were purified by Ficoll-Hypaque (Sigma Chemical Co., St. Louis, MO) density-gradient centrifugation, and non-adherent cells were resuspended in RPMI 1640 medium supplemented with 10% FBS at a density of 5 × 105 cell/mL. Cells were counted with a Vi-Cell Counter (Beckman Coulter, Brea, CA).
Western blot analysis
Equal amounts of protein lysate were separated by SDS-PAGE. Proteins were transferred to nitrocellulose membrane, immunoblotted with primary antibodies followed by secondary antibodies (LI-COR Biosciences, Lincoln, NE), and detected by the Odyssey imaging system (LI-COR Biosciences). The following antibodies were used: goat polyclonal anti-CTGF (Santa Cruz Biotechnology, Santa Cruz, CA); rabbit polyclonal anti-AKT (Cell Signaling Technologies Beverly, MA); rabbit monoclonal anti-phospho-AKT (Ser473) (Cell Signaling Technologies); rabbit monoclonal anti-phospho-S6 Ribosomal Protein (S6RP) (Cell Signaling Technologies); rabbit polyclonal anti-phospho-S6RP (Ser240/244); rabbit polyclonal anti-4EBP1 (Cell Signaling Technologies); rabbit monoclonal anti-phospho-4EBP1 (Thr37/46) (Cell Signaling Technologies); mouse monoclonal anti-p27 (BD Biosciences, San Jose, CA); goat polyclonal anti-cIAP1 (R & D Systems, Minneapolis, MN), rabbit polyclonal anti-BCL-XL (BD Biosciences), rabbit polyclonal anti-BIM (Millipore, Billerica, MA), and mouse monoclonal anti-GAPDH (Millipore).
CTGF knockdown by lentiviral transduction
RS4;11 and REH cells were transduced with lentiviruses encoding either CTGF-specific shRNA (RHS3979-9629133; Open Biosystems, Lafayette, CO) or empty vector (RHS4080). Lentiviral infections were carried out according to the standard procedures for silencing experiments. In brief, 293T cells were co-transfected with viral packaging vectors pMD2.G and psPAX2 (Addgene, Cambridge MA), along with a lentiviral construct expressing either a specific CTGF-shRNA or the empty vector as control, using JetPrime transfection reagent (Polyplus-transfection, New York, NY) according to the manufacturer’s protocol. The transfection medium was replaced after 12 hours with fresh DMEM/10% FBS, and 48 hours later the viral supernatants were collected and used for infections. ALL cells were then transduced with a viral supernatant derived from empty lentiviral vector or the CTGF-shRNA–expressing vector. After incubation for 48 hours, the viral supernatant was replaced with complete cell culture medium containing 2 μg/ml puromycin (Invitrogen, Carlsbad, CA) for selection. ALL cells were cultured for 5 days in selection medium, which was then replaced with complete cell culture medium and used in the experiments.
Quantitative real-time PCR
cDNA were obtained by reverse transcription of 1 μg of DNase-treated total RNA from each sample using random hexamer priming in 20 μl reactions. The mRNA expression levels of CTGF and ABL1 were quantified using TaqMan gene expression assays (CTGF: Hs01026927_g1, ABL1: Hs01104728_m1, Applied Biosystems, Foster City, CA; H) on a 7900HT Fast Real-Time PCR System.
Cell cycle analysis
Cells were fixed in 70% ice-cold ethanol and stained with 25 μg/ml propidium iodide solution (Sigma Chemical). The DNA content was determined using a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, San Jose, CA). Cell cycle distribution was analyzed using ModFit LT software (Verity Software House, Topsham, ME).
Apoptosis assay
Leukemia cells were treated with different chemotherapy drugs for 48 hours. The apoptotic leukemia cells were detected by Annexin V flow cytometry. Briefly, cells were washed twice with binding buffer (10 mM HEPES, 140 mM NaCl, and 5 mM CaCl2 at pH 7.4; Sigma Chemical) and incubated with a 1:50 solution of FITC-conjugated Annexin V (Roche Diagnostic, Indianapolis, IN) for 15 minutes at room temperature. Stained cells were analyzed by flow cytometry and membrane integrity was simultaneously assessed by PI exclusion.
Animal experiments
All animal work was carried out in accordance with a protocol approved by the institutional animal care and use committee of MD Anderson Cancer Center. NOD/SCID mice (ALL-2) or NSG mice (ALL-10) were inoculated with 3.5 ×106 human primary pre-B-ALL ALL xenografts (ALL-2 and ALL-10) [20]. Treatment commenced when the percentage of human CD45+ cells in peripheral blood exceeded the median value of >1% for the complete cohort. In the experiment using ALL-2 cells, FG-3019 and control human IgG (10 mg/kg, twice weekly, i.p) were combined with conventional chemotherapy (VXL, vincristine, once a week, i.p., 0.15 mg/kg; L-asparaginase, 5 times a week, i.p., 1000 U/kg; dexamethasone, 5 times a week, i.p., 5 mg/kg) for 5 weeks total beginning at day 84 after leukemia cell injection (10 mice/group). FG-3019 and human IgG doses were increased to 20 mg/kg (twice weekly, i.p.) from week 6 – week 11 in the groups that also received VXL from week 1-5. For ALL-10 cells, FG-3019 and control human IgG (30 mg/kg, twice weekly, i.p) were combined with VXL as above for 3 weeks total beginning at day 3 following leukemia cell injection (8 mice/group). Mice were sacrificed if they became morbid or had a weight loss of 20% or greater.
Statistical analysis
Statistical analysis was performed using the 2-tailed Student’s t test or Mann-Whitney test if appropriate. Survival curves were created by the Kaplan-Meier method and compared by the log-lank test. Results were considered statistically significant at p-values < .05. Unless otherwise indicated, average values were expressed as mean ± SD.
Results
CTGF is highly expressed in precursor-B ALL cells
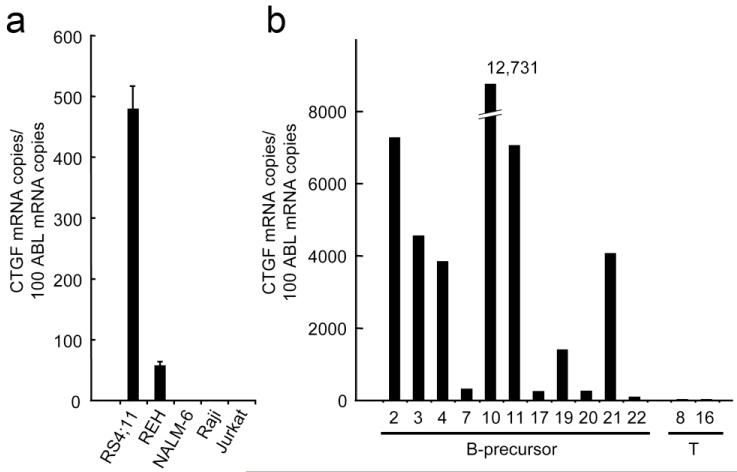
We first investigated CTGF mRNA expression in ALL cell lines. RS4;11 and REH expressed CTGF mRNA (479.3 ± 37.2 and 57.3 ± 5.9 per 100 copies of ABL1, respectively) (Fig. 1a). CTGF expression was not detected in NALM-6, Raji or Jurkat cells. In 11 primary precursor-B ALL patient samples (8 xenograft B-ALL cells [20] and 3 primary samples), CTGF mRNA levels varied from 68.7 to 12731 per 100 copies of ABL1 (Fig. 1b). The median mRNA expression in patient blasts (3823) was 8 times higher than CTGF mRNA levels in RS4;11 cells, suggesting an active involvement of CTGF in primary precursor-B ALL blasts.
Figure 1. CTGF is highly expressed in precursor-B ALL cells.
(A, B) Real-time PCR measurement of steady state expression of CTGF in ALL cell lines (A) and patient precursor-B ALL (B precursor) and T-ALL (T) samples (B). The abundance of mRNA was normalized to that of ABL1. Results are expressed as mean ± SD of triplicate measurements in cell lines and mean of duplicate measurements in patient samples. Precursor-B ALL cells expressed high levels of CTGF mRNA.
CTGF knockdown exhibits anti-leukemia effects in precursor-B ALL
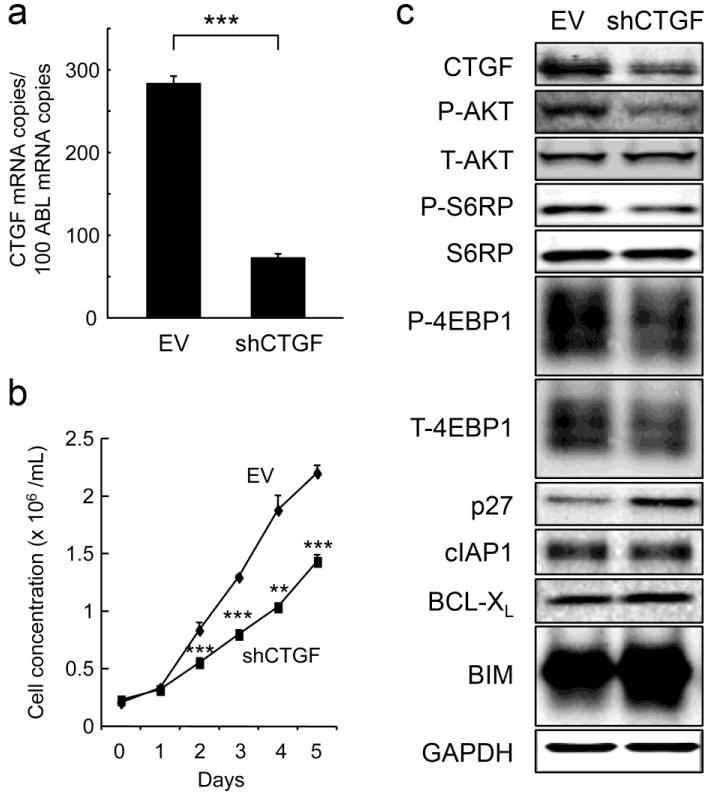
To investigate the biological consequences of CTGF expression in ALL cells, RS4;11 and REH cells were infected with lentivirus encoding either empty vector (RS4;11-EV) or CTGF-specific shRNA (RS4;11-shCTGF). CTGF-specific shRNA led to reduced basal CTGF mRNA expression by 65% in RS4;11 cells (Fig. 2a) and by 55% in REH cells (data not shown). We observed retarded growth of CTGF knockdown cells as compared with control cells (Fig. 2b, Supplementary Fig. S1). CTGF knockdown caused significant inhibition of the G1/S transition, with accumulation of cells in the G1 phase (Supplemental Fig. S1). It has been reported that CTGF stimulates AKT-mediated reduction of p27, which is a key regulator of G1/S transition [21, 22]. Consistent with previous reports, CTGF knockdown resulted in decreased levels of phospho-AKT, downstream targets of mTOR phospho-S6RP and phospho-4EBP1 and increased levels of p27 (Fig. 2c), which could cause G1 cell cycle arrest. Levels of anti-apoptotic proteins cIAP1 and BCL-XL did not change. CTGF knockdown led to increased levels of the pro-apoptotic BCL-2 family protein BIM.
Figure 2. CTGF knockdown inhibits ALL cell proliferation in vitro.
(A, B) CTGF expression levels (A) and growth curves (B) of RS4;11 cells expressing either empty vector (EV) or CTGF shRNA (shCTGF). The abundance of mRNA was normalized to that of ABL1. Cell proliferation was analyzed by counting absolute cell numbers with a Vi-Cell XR cell counter. Results are expressed as mean ± SD of triplicate measurements. Statistical significances are denoted as follows: **P < 0.01, ***P < 0.001. (C) CTGF knockdown led to inactivated AKT/mTOR components and increased levels of p27. Protein expression was determined by Western blotting.
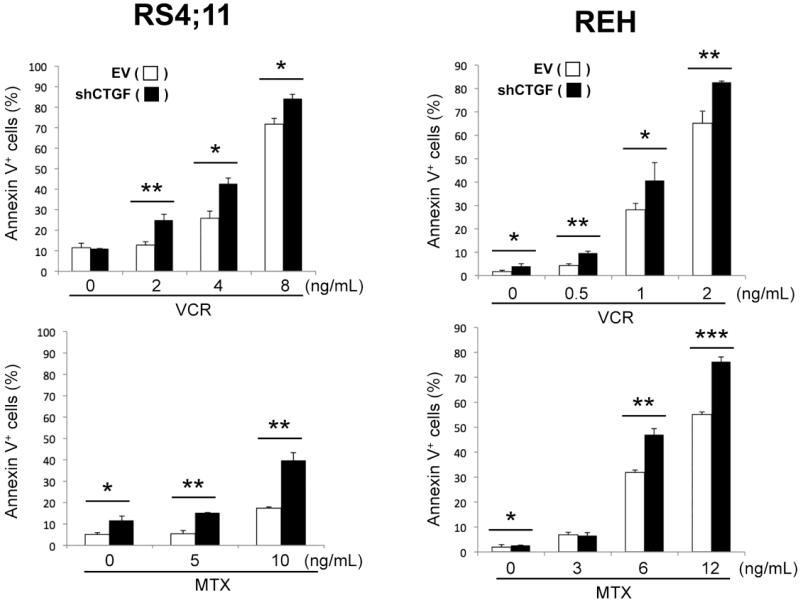
To investigate if modulation of CTGF expression can sensitize ALL cells to conventional chemotherapeutic agents, RS4;11-EV/-shCTGF and REH-EV/-shCTGF cells were treated for 48 hours with vincristine (VCR) and methotrexate (MTX) in vitro. CTGF knockdown was associated with modest phosphatidylserine externalization in both cells (Fig. 3). On the contrary, shCTGF-expressing cells were more susceptible to VCR and MTX, implying that reduced CTGF expression may sensitize ALL cells to these agents.
Figure 3. CTGF knockdown enhances chemotherapy-induced apoptosis.
RS4;11 and REH cells expressing either empty vector (EV) or CTGF shRNA (shCTGF) cells were treated for 48 hours with the indicated concentrations of vincristine (VCR) and methotrexate (MTX), and Annexin V-positive fractions were measured. Results are expressed as mean ± SD of triplicate measurements. Statistically significant differences are denoted as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
Anti-CTGF monoclonal antibody attenuates tumor growth of precursor-B ALL in vivo
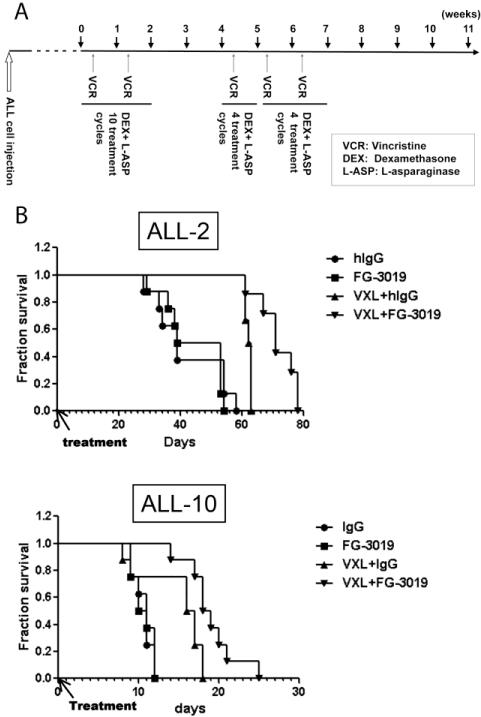
We next investigated the anti-leukemic efficacy of the monoclonal anti-CTGF antibody FG-3019 in ALL models. FG-3019 failed to affect cell growth in liquid cultures or chemosensitivity of ALL cells cultured in vitro, consistent with previously reported findings in pancreatic cancer cell models [23]. Since CTGF most likely plays a role in the interaction of ALL cells with cells of the bone marrow microenvironment, we examined efficacy of FG-3019 in the in vivo ALL model. The anti-leukemic effects of FG-3019 in combination with an induction-type regimen consisting of vincristine, L-asparaginase and dexamethasone (VXL) [24] were evaluated in mice injected with cells from two pediatric ALL patients propagated in vivo (ALL-2 and ALL-10) [20]. ALL-2 and ALL-10 expressed high levels of CTGF mRNA at 7,248 and 12,731 per 100 copies of ABL1, respectively (Fig. 1B). While ALL-10 was far more aggressive than ALL-2, FG-3019 alone did not affect leukemia progression in either of these primary xenograft models (ALL-2: p = 0.61; ALL-10: p = 0.84) (Fig. 4). VXL treatment significantly extended mouse survival compared to IgG control in ALL-2 (p = 0.023) but not in ALL-10 (p = 0.32). The combination of FG-3019 with VXL significantly prolonged mouse survival compared with IgG with VXL in both cases (ALL-2, p = 0.027; ALL-10, p = 0.033).
Figure 4. FG-3019 significantly prolongs overall survival in patient-derived human ALL xenograft models when combined with conventional chemotherapy.
(A) Schematic representation of the in vivo xenograft experiment and chemotherapy/FG-3019 administration. (B) Overall survival of control (hIgG-treated) and FG-3019-treated mice with or without conventional chemotherapy (vincristine, L-asparaginase and dexamethasone, VXL) in the ALL-2 and ALL-10 xenograft model. X axis, days post initiation of treatment (day 84 for ALL-2, day 3 for ALL-10).
Discussion
Recent studies have reported increased expression of CTGF in precursor-B ALL, which was significantly associated with inferior outcome [18, 19]. In this study, we characterized mRNA expression and function of CTGF in ALL cell lines and in samples from primary ALL patients. In accordance with previous reports, precursor-B ALL cell lines and patient samples expressed CTGF mRNA. CTGF mRNA expression was detected in 2 of 3 precursor-B ALL cell lines (positive for RS4;11 and REH and negative for NALM-6) and in all 11 primary precursor-B ALL patient samples, raising the possibility that CTGF is actively involved in this specific subtype of ALL. Notably, the majority of primary ALL cells express higher CTGF levels than cell lines, and the expression in the xenograft cells passaged in vivo (ALL-2 and ALL-10) appear to be more closely aligned with the primary samples than with cell lines. This suggests that CTGF expression may be important in the context of the bone marrow microenvironment where the cells are propagated.
The mechanisms of CTGF over-expression in B precursor ALL remain unknown. The CTGF gene is located on Chromosome 6q23, and trisomy 6 or 6q23 abnormalities are rare in ALL (Atlas of Genetics and Cytogenetics in Oncology and Haematology. URL http://AtlasGeneticsOncology.org). CTGF gene mutations or amplification have not been reported. Alternatively, CTGF mRNA could be upregulated downstream of specific microRNA overexpression [25, 26]. As such, CTGF is a known predicted target of miR-18a, miR-133, miR-30, miR-17-92 microRNA cluster, from which miR-17-92 cluster and miR-30e-5p were found to be upregulated in ALL. Further, CTGF is a well-known immediate early gene that is potently induced by a variety of stimuli that normally regulate extracellular matrix deposition, tissue remodeling, and neovascularization, including platelet-derived growth factor, TGF-β, basic fibroblast growth factor and VEGF; however, the role of these factors in the biology of human ALL has not been defined. Interestingly, CTGF expression can be induced by hypoxia [27-29]. Hypoxia occurs along with bone marrow infiltration by precursor-B ALL cells [30]. Hypoxia has been reported to induce CTGF via the HIF-1 pathway [27, 28]. Hypoxia has also been reported to increase CTGF expression by 3′-untranslated region-mediated increases in CTGF mRNA stability [29]. Thus, we propose the hypothesis that precursor-B ALL cells generate a hypoxic microenvironment and thereby induce CTGF to support their survival advantage and proliferation.
We found that CTGF knockdown in precursor-B ALL cells leads to reduced cell proliferation with G1 arrest. Although identification of downstream mediators of CTGF that promote cell survival and proliferation requires further investigation, our data point to the relevance of AKT/mTOR activation, affecting protein expression of cell cycle regulator p27 and pro-apoptotic BH3 protein BIM. Possibly as a result of blockade of these pro-survival pathways, CTGF knockdown sensitized precursor-B ALL cells to chemotherapeutic agents VCR and MTX. These findings suggest that CTGF is critically involved in ALL progression and is a potential therapeutic target for ALL.
In addition to autocrine effects of CTGF on ALL cell signaling, CTGF is known to directly bind to several integrin extracellular matrix receptors and regulate cell adhesion and migration of several cell types [31]. Integrin αIIbβ3a (CD41/CD61), a CTGF binding partner, has recently been identified on a subpopulation of primitive HSC, both human and murine [32, 33], and on most hematopoietic progenitor cells derived in vitro from murine embryonic stem cells [34]. In our cell line studies we failed to detect significant effects of FG-3019 on CXCL12-induced migration or adhesion of ALL cells to fibronectin in vitro, however this may reflect our inability to recreate an appropriate microenvironment that mimics the conditions of the in vivo setting. Notably, CTGF was recently identified as the top upregulated gene in a large cohort of primary B-ALL samples by microarray analysis, and an in silico functional analysis suggested the linkage to pathways regulating leukemia-microenvironment interactions [16].
To determine potential therapeutic efficacy of CTGF blockade in the in vivo leukemia models, we investigated the effects of the human anti-CTGF monoclonal antibody FG-3019 in the mouse model of precursor-B ALL, using patient-derived leukemia cells. A mouse survival study showed beneficial results of conventional chemotherapy when combined with FG-3019 in primary human ALL xenografts. FG-3019 has been evaluated in several phase 1 clinical studies in patients with diabetic kidney diseases [35], idiopathic lung fibrosis and pancreatic cancer and is currently in phase 2 testing in idiopathic lung fibrosis (NCT01262001) and chronic hepatitis B infection-related liver fibrosis (NCT01217632). The phase 1 study of FG-3019 in combination with Gemcitabine and Erlotinib in patients with advanced pancreatic cancer (NCT01181245) has completed patient enrollment, and results of interim analysis demonstrated impressive PET responses at the highest dose in some patients [36]. It is noteworthy, that at least in pancreatic cancer, CTGF is expressed not only in the cancer cells, but also in the tumor stroma and is most abundant in pancreatic stellate cells within the stroma [37]. CTGF acts as both an autocrine and paracrine factor but as an ECM-associated factor, may exert it’s effects locally. It was therefore proposed that the efficacy of FG-3019 in this tumor type could be partly attributable to the interference with tumor-associated stroma formation and function, including pancreatic stellate cell activation, thereby attenuating collagen I deposition, suppressing further stromal interactions with the tumor, and decreasing stroma-derived tumorigenic factors, such as VEGF-A. Although the effects of CTGF blockade on bone marrow microenvironment remains to be further investigated, CTGF was shown to be upregulated in leukemia inhibitory factor-stimulated stroma cultures, which in turn augmented their ability to support hematopoiesis [38]. Our data indeed demonstrate high expression of CTGF in bone marrow-derived mesenchymal stem cells [39], indicating a potential role of CTGF in leukemia-stroma interactions. Hence, CTGF may represent a unique target functionally important for both, leukemia cell survival and microenvironment-mediated chemoresistance. In summary, our data demonstrate that autocrine CTGF is highly expressed in pre B-ALL cell lines and primary ALL samples and represents a potential druggable target for therapies blocking CTGF signaling.
Supplementary Material
Acknowledgments
Grant Support: Supported in part by grants from National Institutes of Health Lymphoma SPORE (CA136411), P01 “The Therapy of AML” (CA55164), Leukemia SPORE (CA100632), Cancer Center Support Grant (CA16672), the Paul and Mary Haas Chair in Genetics (M. Andreeff) and by 1R01CA155056-01, CDP-01, Leukemia and Lymphoma Society and DRP, Leukemia Spore 5 P50 CA100632-08 (to M. Konopleva).
Footnotes
STATEMENT OF AUTHORSHIP
H.L., V.L.B., B.K., Y.S., Y.C., designed and performed the research and analyzed the data; K.K. analyzed the data and wrote the manuscript; S.S. provided anti-CTGF monoclonal antibody, contributed to discussion and edited the paper; D.A.T. and H.K. provided primary patients’ samples, analyzed the data and contributed to discussion; R.B.L. established human primary pre-B-ALL ALL xenografts and analyzed the data; M.A. designed the research and analyzed the data; M.K. initiated, supervised the research, analyzed the data and wrote and edited the paper.
Disclosure of Potential Conflicts of Interest: S.S. is an employee of FibroGen, a biopharmaceutical company that develops anti-CTGF monoclonal antibody.
References
- 1.Leask A, Abraham DJ. All in the CCN family: essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119(Pt 23):4803–10. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 2.Dhar A, Ray A. The CCN family proteins in carcinogenesis. Exp Oncol. 2010;32(1):2–9. [PubMed] [Google Scholar]
- 3.Hall-Glenn F, Lyons KM. Roles for CCN2 in normal physiological processes. Cell Mol Life Sci. 2011;68(19):3209–17. doi: 10.1007/s00018-011-0782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, et al. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 5.Xie D, Yin D, Wang HJ, Liu GT, Elashoff R, Black K, et al. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res. 2004;10(6):2072–81. doi: 10.1158/1078-0432.ccr-0659-03. [DOI] [PubMed] [Google Scholar]
- 6.Koliopanos A, Friess H, di Mola FF, Tang WH, Kubulus D, Brigstock D, et al. Connective tissue growth factor gene expression alters tumor progression in esophageal cancer. World J Surg. 2002;26(4):420–7. doi: 10.1007/s00268-001-0242-x. [DOI] [PubMed] [Google Scholar]
- 7.Bennewith KL, Huang X, Ham CM, Graves EE, Erler JT, Kambham N, et al. The role of tumor cell-derived connective tissue growth factor (CTGF/CCN2) in pancreatic tumor growth. Cancer Res. 2009;69(3):775–84. doi: 10.1158/0008-5472.CAN-08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther. 2006;5(5):1108–16. doi: 10.1158/1535-7163.MCT-05-0516. [DOI] [PubMed] [Google Scholar]
- 9.Shakunaga T, Ozaki T, Ohara N, Asaumi K, Doi T, Nishida K, et al. Expression of connective tissue growth factor in cartilaginous tumors. Cancer. 2000;89(7):1466–73. [PubMed] [Google Scholar]
- 10.Chen PP, Li WJ, Wang Y, Zhao S, Li DY, Feng LY, et al. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One. 2007;2(6):e534. doi: 10.1371/journal.pone.0000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai D, Ho KC, Hao Y, Yang X. Taxol resistance in breast cancer cells is mediated by the hippo pathway component TAZ and its downstream transcriptional targets Cyr61 and CTGF. Cancer Res. 2011;71(7):2728–38. doi: 10.1158/0008-5472.CAN-10-2711. [DOI] [PubMed] [Google Scholar]
- 12.Wang MY, Chen PS, Prakash E, Hsu HC, Huang HY, Lin MT, et al. Connective tissue growth factor confers drug resistance in breast cancer through concomitant up-regulation of Bcl-xL and cIAP1. Cancer Res. 2009;69(8):3482–91. doi: 10.1158/0008-5472.CAN-08-2524. [DOI] [PubMed] [Google Scholar]
- 13.Chen PS, Wang MY, Wu SN, Su JL, Hong CC, Chuang SE, et al. CTGF enhances the motility of breast cancer cells via an integrin-alphavbeta3-ERK1/2-dependent S100A4-upregulated pathway. J Cell Sci. 2007;120(Pt 12):2053–65. doi: 10.1242/jcs.03460. [DOI] [PubMed] [Google Scholar]
- 14.Yin D, Chen W, O’Kelly J, Lu D, Ham M, Doan NB, et al. Connective tissue growth factor associated with oncogenic activities and drug resistance in glioblastoma multiforme. Int J Cancer. 2010;127(10):2257–67. doi: 10.1002/ijc.25257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vorwerk P, Wex H, Hohmann B, Oh Y, Rosenfeld RG, Mittler U. CTGF (IGFBP-rP2) is specifically expressed in malignant lymphoblasts of patients with acute lymphoblastic leukaemia (ALL) Br J Cancer. 2000;83(6):756–60. doi: 10.1054/bjoc.2000.1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tesfai Y, Ford J, Carter KW, Firth MJ, O’Leary RA, Gottardo NG, et al. Interactions between acute lymphoblastic leukemia and bone marrow stromal cells influence response to therapy. Leuk Res. 2012;36(3):299–306. doi: 10.1016/j.leukres.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Boag JM, Beesley AH, Firth MJ, Freitas JR, Ford J, Brigstock DR, et al. High expression of connective tissue growth factor in pre-B acute lymphoblastic leukaemia. Br J Haematol. 2007;138(6):740–8. doi: 10.1111/j.1365-2141.2007.06739.x. [DOI] [PubMed] [Google Scholar]
- 18.Sala-Torra O, Gundacker HM, Stirewalt DL, Ladne PA, Pogosova-Agadjanyan EL, Slovak ML, et al. Connective tissue growth factor (CTGF) expression and outcome in adult patients with acute lymphoblastic leukemia. Blood. 2007;109(7):3080–3. doi: 10.1182/blood-2006-06-031096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang H, Chen IM, Wilson CS, Bedrick EJ, Harvey RC, Atlas SR, et al. Gene expression classifiers for relapse-free survival and minimal residual disease improve risk classification and outcome prediction in pediatric B-precursor acute lymphoblastic leukemia. Blood. 2010;115(7):1394–405. doi: 10.1182/blood-2009-05-218560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liem NL, Papa RA, Milross CG, Schmid MA, Tajbakhsh M, Choi S, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103(10):3905–14. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 21.Crean JK, Furlong F, Mitchell D, McArdle E, Godson C, Martin F. Connective tissue growth factor/CCN2 stimulates actin disassembly through Akt/protein kinase B-mediated phosphorylation and cytoplasmic translocation of p27 (Kip-1) FASEB J. 2006;20(10):1712–4. doi: 10.1096/fj.05-5010fje. [DOI] [PubMed] [Google Scholar]
- 22.Crawford LA, Guney MA, Oh YA, Deyoung RA, Valenzuela DM, Murphy AJ, et al. Connective tissue growth factor (CTGF) inactivation leads to defects in islet cell lineage allocation and beta-cell proliferation during embryogenesis. Mol Endocrinol. 2009;23(3):324–36. doi: 10.1210/me.2008-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dornhöfer N, Spong S, Bennewith K, Salim A, Klaus S, Kambham N, et al. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66(11):5816–27. doi: 10.1158/0008-5472.CAN-06-0081. [DOI] [PubMed] [Google Scholar]
- 24.Szymanska B, Wilczynska-Kalak U, Kang MH, Liem NL, Carol H, Boehm I, et al. Pharmacokinetic modeling of an induction regimen for in vivo combined testing of novel drugs against pediatric acute lymphoblastic leukemia xenografts. PLoS One. 2012;7(3):e33894. doi: 10.1371/journal.pone.0033894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanette DL, Rivadavia F, Molfetta GA, Barbuzano FG, Proto-Siqueira R, Silva WA, Jr, et al. miRNA expression profiles in chronic lymphocytic and acute lymphocytic leukemia. Braz J Med Biol Res. 2007;40(11):1435–40. doi: 10.1590/s0100-879x2007001100003. [DOI] [PubMed] [Google Scholar]
- 26.Schotte D, Chau JC, Sylvester G, Liu G, Chen C, van der Velden VH, et al. Identification of new microRNA genes and aberrant microRNA profiles in childhood acute lymphoblastic leukemia. Leukemia. 2009;23(2):313–22. doi: 10.1038/leu.2008.286. [DOI] [PubMed] [Google Scholar]
- 27.Hong KH, Yoo SA, Kang SS, Choi JJ, Kim WU, Cho CS. Hypoxia induces expression of connective tissue growth factor in scleroderma skin fibroblasts. Clin Exp Immunol. 2006;146(2):362–70. doi: 10.1111/j.1365-2249.2006.03199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recchia AG, De Francesco EM, Vivacqua A, Sisci D, Panno ML, Andò S, et al. The G protein-coupled receptor 30 is up-regulated by hypoxia-inducible factor-1alpha (HIF-1alpha) in breast cancer cells and cardiomyocytes. J Biol Chem. 2011;286(12):10773–82. doi: 10.1074/jbc.M110.172247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondo S, Kubota S, Mukudai Y, Moritani N, Nishida T, Matsushita H, et al. Hypoxic regulation of stability of connective tissue growth factor/CCN2 mRNA by 3′-untranslated region interacting with a cellular protein in human chondrosarcoma cells. Oncogene. 2006;25(7):1099–110. doi: 10.1038/sj.onc.1209129. [DOI] [PubMed] [Google Scholar]
- 30.Benito J, Shi Y, Szymanska B, Carol H, Boehm I, Lu H, et al. Pronounced hypoxia in models of murine and human leukemia: high efficacy of hypoxia-activated prodrug PR-104. PLoS One. 2011;6(8):e23108. doi: 10.1371/journal.pone.0023108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363(9402):62–4. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- 32.Debili N, Robin C, Schiavon V, Letestu R, Pflumio F, Mitjavila-Garcia MT, et al. Different expression of CD41 on human lymphoid and myeloid progenitors from adults and neonates. Blood. 2001;97(7):2023–30. doi: 10.1182/blood.v97.7.2023. [DOI] [PubMed] [Google Scholar]
- 33.Corbel C, Salaün J. AlphaIIb integrin expression during development of the murine hemopoietic system. Dev Biol. 2002;243(2):301–11. doi: 10.1006/dbio.2001.0553. [DOI] [PubMed] [Google Scholar]
- 34.Mitjavila-Garcia MT, Cailleret M, Godin I, Nogueira MM, Cohen-Solal K, Schiavon V, et al. Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development. 2002;129(8):2003–13. doi: 10.1242/dev.129.8.2003. [DOI] [PubMed] [Google Scholar]
- 35.Adler SG, Schwartz S, Williams ME, Arauz-Pacheco C, Bolton WK, Lee T, et al. Phase 1 study of anti-CTGF monoclonal antibody in patients with diabetes and microalbuminuria. Clin J Am Soc Nephrol. 2010;5(8):1420–8. doi: 10.2215/CJN.09321209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heestand GM, Pipas JM, Valone F, McMullen AD, Gadea P, Williams D, et al. A phase I trial of the monoclonal antibody FG-3019 to connective tissue growth factor (CTGF) in locally advanced or metastatic pancreatic cancer [Abstract] J Clin Oncol. 2011;29(Suppl. 4) Abstract 269. [Google Scholar]
- 37.Hartel M, Di Mola FF, Gardini A, Zimmermann A, Di Sebastiano P, Guweidhi A, et al. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg. 2004;28(8):818–25. doi: 10.1007/s00268-004-7147-4. [DOI] [PubMed] [Google Scholar]
- 38.Ueno H, Sakita-Ishikawa M, Morikawa Y, Nakano T, Kitamura T, Saito M. A stromal cell-derived membrane protein that supports hematopoietic stem cells. Nat Immunol. 2003;4(5):457–63. doi: 10.1038/ni916. [DOI] [PubMed] [Google Scholar]
- 39.Battula VL, Cabreira M, Wang Z, Ma W, Benito J, Ruvolo PP, et al. Connective tissue growth factor (CTGF) is essential for self renewal and proliferation of mesenchymal stromal cells (MSCs) and affects leukemia-stromal interactions [Abstract] Blood. 2010;116(21):1573. Abstract 3845. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.