Abstract
Information processing biases are hallmark features of major depressive disorder (MDD). Depressed individuals display biased memory and attention for negative material. Given that memory is highly dependent on attention for initial encoding, understanding the interplay of these processes may provide important insight into mechanisms that produce memory biases in depression. In particular, attentional control—the ability to selectively attend to task-relevant information by both inhibiting the processing of irrelevant information and disengaging attention from irrelevant material—may be one area of impairment in MDD. In the current study, clinically depressed (MDD: n = 15) and never depressed (non- MDD: n = 22) participants’ line of visual gaze was assessed while participants viewed positive and negative word pairs. For each word pair, participants were instructed to attend to one word (target) and ignore one word (distracter). Free recall of study stimuli was then assessed. Depressed individuals displayed greater recall of negatively valenced target words following the task. Although there were no group differences in attentional control in the context of negative words, attention to negative targets mediated the relationship between depression status and recall of negative words. Results suggest a stronger link between attention and memory for negative material in MDD.
Keywords: depression, information processing, attention, memory, eye-tracking
Cognitive theories of depression emphasize the role of cognitive processing biases in the development of the disorder (e.g., Teasdale, 1988). Accordingly, biased processing of negative information has been identified as a prominent feature of major depression (MDD) and been implicated in its maintenance and recurrence (e.g., Gotlib & Joormann, 2010). In particular, negative biases in attention, interpretation, and memory distinguish cognitive processing in depression from nondepressed individuals (Peckham, McHugh, & Otto, 2010).
Of these processing biases, memory biases may be the most robust (Gotlib & Joormann, 2010; Matt, Vázquez, & Campbell, 1992). MDD individuals recall more negative than positive words, while non-depressed individuals display enhanced recall of positive material (e.g., Matt et al., 1992). Depressed individuals also falsely recall semantically related negative words more frequently than nondepressed individuals (Joormann, Teachman, & Gotlib, 2009).
Given that cognitive biases are believed to maintain a depressive episode (e.g., Teasdale, 1988), understanding the mechanisms associated with these memory biases is important. Attentional control—the ability to attend to task-relevant information by inhibiting the processing of irrelevant information (Koster, De Lissnyder, Derakshan, & De Raedt, 2011)—may be a critical process operating across biases in MDD. In fact, recent theoretical accounts of cognitive mechanisms speculate that difficulties with general cognitive control may underlie both attention and memory biases (e.g., Joormann & Siemer, 2011). Attentional control may be a particularly important process involved in memory biases. Attentional control differs from attentional bias in that biases are captured in automatic processing of attention, whereas control reflects the later-stages of attention which rely heavily on executive functions such as disengagement and inhibition (De Raedt & Koster, 2010). In the only study of attentional control utilizing eye-tracking, depressed individuals displayed poor attentional control for emotional information using an antisaccade task (Derakshan, Salt, & Koster, 2009). Therefore, utilizing eye-tracking to measure depressed individuals’ attentional control may be a novel approach for examining both attentional biases and attentional control.
These memory and attention processes in MDD have been well supported empirically in isolation, yet less is known about their interplay (e.g., Everaert, Koster, & Derakshan, 2012). Recent theories have suggested that the impact of cognitive processing biases on specific disorders is best observed when they are combined or integrated (e.g., combined cognitive bias hypothesis; Everaert et al., 2012). In this context, simultaneous assessment of attention and memory may clarify whether differences observed between MDD and non-MDD individuals are because of differential encoding processes (i.e., those that aid in getting information into memory, such as attention) or retrieval processes (i.e., pulling information back out).
To date, five studies have attempted to link attention with memory in depression. In stably dysphoric individuals, a spatial cueing task revealed that greater negative attentional biases at later stages of processing predicted recall of negative words (Koster, De Raedt, Leyman, & De Lissnyder, 2010). In eye-tracking studies, greater attentional breadth for angry facial stimuli predicated better recognition of similar facial expressions (Wells, Beevers, Robison, & Ellis, 2010), while the lack of a positive attentional bias resulted in poorer recognition of positive word stimuli in dysphoric compared to non-dysphoric individuals (Ellis, Beevers, & Wells, 2011). Using an attention training paradigm, individuals with higher depression symptoms displayed less recall of negative words when trained away from this material than those not trained (Blaut, Paulewicz, Szastok, Prochwicz, & Koster, 2013). The only study with a clinical sample found negative biases for faces during a dot-probe task and greater recall of negative words in MDD, but no associations between memory and attention (Gotlib, Krasnoperova, Yue, & Joormann, 2004). Together, this evidence suggests that attentional processing affects recall/recognition in stably dysphoric individuals. These relationships have yet to be substantiated with clinically depressed individuals. Further, most of these studies examined the role of attentional bias, rather than attentional control, limiting the interpretation of findings to the role of preferential processing of negative material on memory. As such, the effect of attentional control (e.g., ability to sustain attention on a target while ignoring irrelevant information) on memory in MDD remains unknown.
The current, exploratory study aimed to determine whether MDD and non-MDD individuals differed in processing negative material. Specifically, we examined the role of attentional control through an eye-tracking paradigm where participants were required to view one emotionally valenced target word while ignoring a distracter word. We hypothesized that MDD individuals would display greater attention for negative words across both targets and distracters, compared to non-MDD. That is, they would display less attentional control (i.e. inability to sustain attention on targets) when distracters were negative, while displaying greater sustained attention for negative targets. Second, we expected the MDD group to display greater recall of negative words in a free-recall task compared to the non-MDD group. Finally, we hypothesized that attention for negative words would mediate the association between MDD status and recall.
Method
Participants
Thirty-seven participants with current major depressive disorder (MDD; n = 15) or no history of MDD (non-MDD; n = 22) were recruited from a large university and surrounding area. Interested participants completed the Beck Depression Inventory short form, and participants who scored 3 or less or a 7 or above completed a brief diagnostic interview. Inclusion criteria for the MDD group were 1) a diagnosis of current MDD and 2) no history of (hypo)mania. The non-MDD group had no history of MDD or (hypo)mania. The rights of human participants were protected and procedures were approved by the University’s institutional review board.
Measures
Beck Depression Inventory-II (BDI-II)
The BDI-II (Beck, Steer, & Brown, 1996) is a widely-used, 21-item, self-report measure that assesses depression symptoms. The BDI-II has demonstrated adequate to good test-retest reliability and construct validity (Dozois, Dobson, & Ahnberg, 1998).
Structured Clinical Interview for DSM-IV Diagnoses (SCID)
The mood disorders module from the Structured Clinical Interview for the DSM-IV Diagnoses – Patient Version (First & Gibbon, 2004) was used to determine a diagnosis of a major depressive disorder (MDD) or no history of depression (non- MDD). The diagnostician had 30 hours of training that involved watching videos, role-playing, and rating previously recorded interviews. Approximately half (53%) of the interviews were selected for reliability analyses and rated by an independent rater for presence or absence of a major depressive episode. Reliability was good (κ= .89).
Demographics
Participants completed a form that included age, gender, and ethnicity. Participants were representative of a college student sample. Importantly, there were no statistically significant differences on any of the demographic characteristics between the MDD (n = 15; Age: M=19.73, SD=1.67; 53.3% female) and non-MDD (n = 22; Age: M=19.67, SD=1.24; 50% female) groups (gender: χ2=.01, p= .92; age: F (1, 36) = .07, p=.79; race: F (1, 36)= .09, p=.77).
Eye Tracking Paradigm
Word Selection
The task presented word pairs selected from the Affective Norms for English Words (ANEW; (Bradley & Lang, 1999)—32 positively valenced and 32 negatively valenced (Valence rating: F(1, 63)=1121.00, p < .00). The words selected were adjectives or nouns that a person could use to describe him/herself (e.g., loyal, failure, pretty, ugly). These words did not differ in arousal rating (F(1, 63)= .01, p= .93) or frequency in the English language (F(1, 63)= .13, p= .72). The words were further divided into the following categories: Positive Targets (PT), Positive Distracters (PD), Negative Targets (NT) and Negative Distracters (ND). These were also examined for differences in valence and arousal. As expected, when the groups were compared on valence, significant differences emerged between the positive and negative words (F(3, 63)= 368.33, p<.00), but no group differences were observed between groups on arousal (F(3, 63)= .60, p= .62). Words pairs were matched for letter-length.
Eye-tracking task
A target word, displayed in red font, and opposite-valenced distracter word, displayed in white font, of equal length were presented one pair at a time over 32 trials. Participants were instructed to look only at the word in red (target), while ignoring the word in white (distracter) and to indicate whether the word in red described them by pressing a “yes” or “no” key on a response box. Words were presented in the middle of the screen with one word above the other.
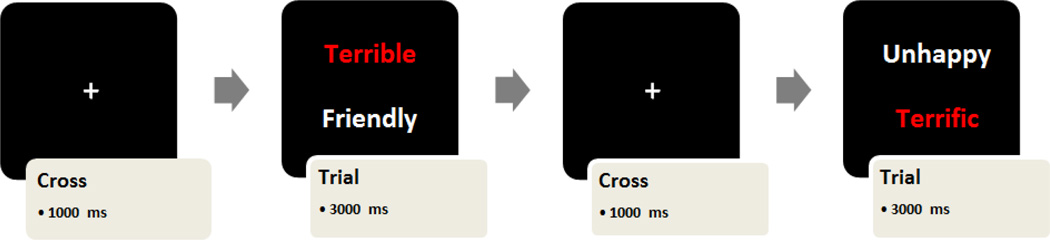
Each trial began with a 1000 ms centrally presented fixation cross. The experimenter manually advanced each trial when subjects’ line of gaze was stable within 1° of visual angle around fixation cross. This was followed by presentation of stimuli for 3 seconds. Stimuli location (i.e., top or bottom), target valence, and presentation order of word pairs were randomly determined for each participant by stimulus presentation software. However, because word pairs were matched on letter length, pairing of words was held constant across participants (Figure 1). In order to minimize demand effects, instructions for the task emphasized measurement of pupil dilation. Five individuals (1 MDD, 4 non-MDD) were not included in the final analyses due to difficulty with eye-tracking (e.g., falling asleep, poor quality of data for the majority of trials) resulting in an n of 32 for eye tracking analyses. Importantly, these excluded cases did not differ from their group in age, race or gender.
Figure 1.
This figure represents two independent trials where the participant was asked to rate whether the word in red was self-descriptive (yes/no), while ignoring the word in white.
Each word stimulus in a pair (plus an additional one degree of visual angle around each word) was designated as an area of interest (AOI) to determine the measure of attention. In this paradigm, we operationalized attentional control as the ability to attend to target words in the presence of competing distracter words. This ability was indexed by percentage of fixations directed towards target versus distracter words and will be further referred to as fixation bias. Further, positive and negative target/distracter words were used, allowing us to examine the influence of valence on attentional control.
Eye Tracking System
Line of visual gaze was assessed using a remote optics eye tracking system model R6 from Applied Science Laboratories (Bedford, MA). Location of gaze was sampled every 16.7 ms (60Hz). Eye movements that were stable for more than 100ms within a 1° of visual angle were classified as a fixation. Areas of interest (AOIs) reflected word location in each of two quadrants on the screen. E-prime software presented stimuli and automated recording of eye location with the eye tracking system. Participants’ eye location was 70 cm from monitor. Height of word stimuli was 0.6 cm (0.5° visual angle) and length of word stimuli ranged from 1.75 to 4.5 cm (1.4° to 3.7° visual angle). Distance between words was approximately 2.9 cm (2.4° visual angle) when measured from the center.
Memory Task
Following the task, participants were asked to generate as many words, regardless of font color, that they could recall from the words shown. Participants were not told that they would be required to recall any words prior to task completion. Our primary assessment of recall accuracy was total number of words recalled within each category (PT, PD, NT, and ND).
Procedure
Following consent, participants were administered the Mood Disorders module of the SCID. After completion of a demographics questionnaire, participants were seated in an adjustable chair and participants’ eyes were aligned with the middle of the 17-inch monitor on which stimuli were presented. A 9-point calibration procedure was repeated until the experimenter confirmed that the eye tracker was recording line of visual gaze within 1° of visual angle for each calibration point. Following calibration, task instructions were presented via the computer screen. From an adjacent room, the experimenter monitored stimulus presentation and eye tracking.
Immediately after the 32 eye tracking trials had been completed, participants were given paper and pencil and asked to recall any words (targets or distracters) they had been shown during the task. Participants were debriefed and thanked for their time.
Results
Behavioral Data
Group differences in reaction time were examined with univariate analysis of variance (ANOVA). For reaction time in responding to both negative and positive words, no group differences emerged (negative: F (1, 35)= .01, p= .93, partial η2=.00; positive: F (1, 35)= .39, p= .54, partial η2=.01). That is, MDD individuals were neither faster nor slower at determining whether words described them or not, compared to non-MDD. There were no differences in the length of constrained viewing that occurred following response between the two groups.
Fixation Bias
A 2 (MDD group: MDD, non-MDD)×2 (Word Type: Target, Distracter)×2 (Valence: Positive, Negative) repeated measures ANOVA examined whether MDD group was associated with fixation bias for particular stimuli. The three-way MDD by valence by word type interaction (F (1, 31)= .38, p= .54, partial η2=.01), the MDD by valence interaction, (F (1, 31)= 1.83, p= .19, partial η2=.06) and the valence by word type interaction (F (1, 31)= .22, p= .64, partial η2=.01) were non-significant. However, there was a word type by MDD interaction (F (1, 31)= 8.03, p= .01, partial η2=.21). There was also a significant effect of MDD group, (F (1, 31)= 6.37, p= .01, partial η2=.18) which indicated that MDD individuals had a higher fixation percentage than non-MDD. As expected, the main effect for word type was also significant (F (1, 31)= 223.07, p= .00, partial η2=.88), with greater fixation bias on target words than distracter words. However, the main effect of word valence was non-significant (F (1, 31) = .62, p= .44, partial η2=.02) indicating that, across groups, valence did not affect number of fixations.
To further investigate the MDD×word type interaction, mean for target words and distracter words were examined between MDD groups. MDD individuals fixated more than non-MDD on target words (F (1, 31) = 4.17, p= .05), whereas non-MDD individuals displayed a trend to fixate more on distracter words (F (1, 31) = 3.25, p= .08).
Free Recall
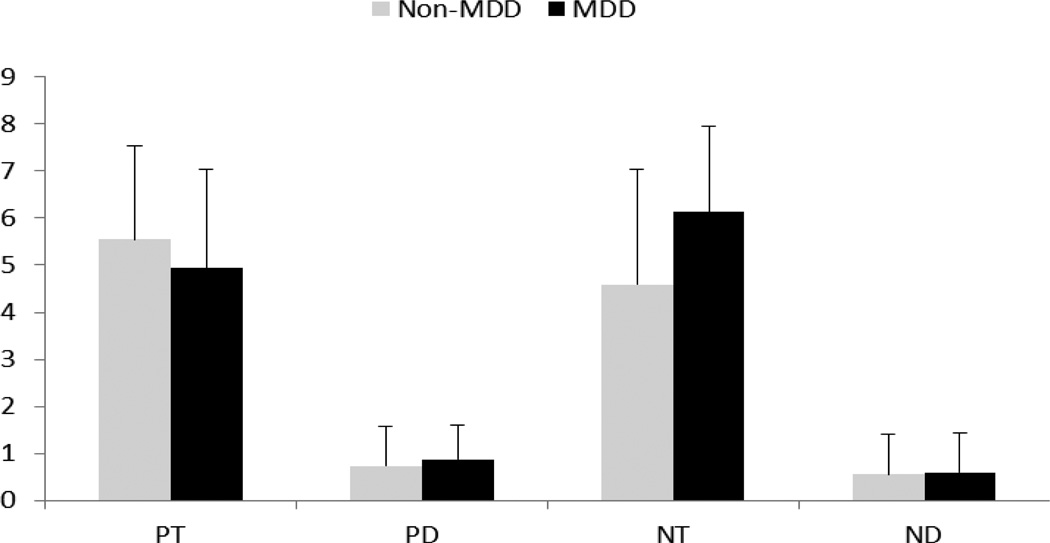
A 2 (MDD group: MDD, non-MDD)×2 (Word Type: Target, Distracter)×2 (Valence: Positive, Negative) repeated measures ANOVA revealed an MDD group×word type×valence interaction (F (1, 35)= 9.02, p= .01, partial η2=.21) (Figure 2). The valence by MDD group interaction (F (1, 35)= 5.76, p= .02, partial η2=.14) suggested greater recall of negative words among MDD and greater recall of positive words by non-MDD. MDD group by word type interaction was non-significant (F (1, 35)= .34, p= .56, partial η2=.01). Finally, there was an effect of word type (F (1, 35)= 216.21, p< .00, partial η2=.86), where participants recalled more target words than distracter words. There were no main effects of MDD status (F (1, 35)= .68, p= .42, partial η2=.02) or valence (F (1, 35)= .06, p= .82, partial η2<.01).
Figure 2.
Means and standard deviations for number of words recalled within each word type and valence across depression groups. PT = positively valenced target words. PD = positively valenced distracter words. NT = negatively valenced target words. ND = negatively valenced distracter words.
To further investigate the three-way interaction, the effects of valence and word type were examined within each MDD group. Within MDD, target words were recalled more than non-targets (F (1, 14)= 92.34, p< .01, partial η2=.87), but there was no main effect of valence, F (1, 14)= 2.10, p= .17, partial η2=.13. Additionally, a word type by valence interaction was revealed (F (1, 14)= 5.40, p= .04, partial η2=.28) whereby MDD individuals recalled more negative target words than other word types.
Within non-MDD, better recall was demonstrated for target versus distracter words (F (1, 21)= 128.44, p< .01, partial η2=.86). In addition, there was a trend toward a valence effect, F (1, 21)= 4.07, p= .06, partial η2=.16, with better recall of positive over negative words. The word type by valence interaction was non-significant, (F (1, 21)= 3.07, p= .09, partial η2=.13). These data suggest that non- MDD participants showed a propensity toward better recall of positively valenced words than negatively valenced.
Mediation analyses
Path analysis was used to test relations between MDD status, attention for target and distracter words, and recall of word stimuli. Specifically, we examined whether attention for word stimuli (indexed by fixations for positive and negative stimuli) mediated the relationship between MDD status and recall of word stimuli. The first model examined attention for negative targets as putative mediators of the relationship between MDD status and recall of negative target stimuli. The second model examined attention for negative distracters as a putative mediator. We did not examine attention for positive targets and distracters as mediators because they were strongly correlated with their opposite counterparts (negative distracters and targets, respectively).
Several indices are often used to determine quality of path model fit. Among the most commonly used are: χ2, Comparative Fit Index (CFI), and Root Mean Squared Error of Approximation (RMSEA). Model fit that includes CFI ≥ .90 and RMSEA ≤ .10 is generally acceptable (Kline, 1998). These criteria were used in the present study.
The initial path model posited direct effects between 1) MDD status and attention for negative targets and 2) attention for negative targets and recall of negative targets. This model provided good fit to the data, χ2 (df = 1) = 0.79, p = .37, RMSEA = .00, CFI = 1.0. MDD status predicted greater attention for negative targets (β = .49, p < .001), which in turn, predicted greater recall of negative targets (β = .32, p = .04). The indirect effect from MDD status to recall of negative targets via attention for negative targets approached statistical significance (β = .72, p = .09). This marginally significant indirect effect suggests partial mediation.
We then ran the same model testing attention for negative distracters as the mediator, as attention for negative distracters could interfere with recall of negative targets. This model provided adequate fit to the data, χ2 (df = 1) = 1.29, p = .26, RMSEA = .10, CFI = 0.95. Evidence of mediation for attention towards distractors was marginal, as the effect of MDD status on attention for negative distracters approached statistical significance (β = −.28, p = .07); that is, the MDD group was less likely to attend to negative distracters than the non-MDD group. Nevertheless, attention for negative distracters was inversely associated with recall of negative targets (β = −.39, p = .009). In summary, path analyses suggest a stronger link between attention for negative stimuli and memory in MDD than among healthy control participants.
Discussion
Biased cognition has been robustly supported in the literature as a central component of depression, yet few studies have integrated cognitive processes to examine their associations within a single study. Even fewer have attempted to examine these associations in a clinically depressed sample (e.g., Gotlib et al., 2004). One of the primary aims of the current study was to explore the association between attentional control and memory in MDD.
The first goal of the current study was to use eye-tracking methods to demonstrate attentional control deficits in MDD. Although we expected MDD individuals to show more difficulty ignoring (i.e., not attending to) negative distracters, this was not supported. Surprisingly, results indicated that depressed individuals were better able to direct their attention towards target words, regardless of valence, than non-depressed individuals. This suggests that under conditions when passive attentional control is required, (i.e., circumstances where individuals are not required to shift attention but instead sustain attention on a target), MDD individuals are able to do fairly well. Although there were no effects of valence, this may suggest that MDD individuals have difficulties with appropriate shifting of attention. This is consistent with studies where depressed individuals have difficulty with more active forms of attentional control, such as shifting their attention away from negative stimuli (Koster, De Raedt, Goeleven, Franck, & Crombez, 2005). Along these lines, in passive viewing tasks (with no control aspect), MDD individuals exhibit sustained attention, particularly for negative stimuli (e.g., Kellough, Beevers, Ellis, & Wells, 2008). Future studies should compare passive and active forms of cognitive control within the same depressed sample and further establish which forms of attentional control are disrupted in depression and which aspects are enhanced.
It is unclear why MDD individuals displayed greater attention for targets while non-MDD displayed more attention to distracters. It seems unlikely that MDD individuals are merely “better” at following task instructions (e.g., ignore the word in white); more likely, however, is that the gaze pattern observed in healthy individuals represents an adaptive attentional process. Appropriate flexibility in response to changing environmental stimuli is a critical component of a number of emotional processes (e.g., emotion reactivity,(Rottenberg, Gross, & Gotlib, 2005). The current findings suggest that while MDD individuals appeared to become “stuck” in their attention for target words, healthy individuals displayed modulation of attention. These results may suggest that flexible attention is an adaptive process in psychological healthy individuals.
The second goal of the study was to examine the link between attentional processes and memory in MDD. Findings indicated that MDD and non-MDD groups did not differ in attentional control over negative information, yet only among MDD individuals, was attention significantly related to recall. These findings lend support to the robust nature of negative recall biases in depression (e.g Matt et al., 1992), while also providing evidence of the role of attention in these biases. They further highlight negative valence specificity in the linkage between attention and memory in MDD, which was not observed in dysphoric individuals (Ellis et al., 2011). These findings are also the first to document a stronger association between mood congruent attention and memory in MDD than in healthy individuals.
Understanding why the strength of association between these processes was stronger in MDD may be explained by the associative network model of memory, which suggests that subconscious processing of negative material relies on implicit associations with prior memories formed with repeated experience (Smith & DeCoster, 2000). It may be that associative processing biases facilitate encoding, thus enhancing later recall through easier access of highly elaborated negative material. In line with this idea, depressed individuals display difficulties inhibiting negative information from entering working memory, (e.g., Goeleven, De Raedt, Baert, & Koster, 2006; Joormann & Gotlib, 2010) and also show impairments with removing irrelevant information from working memory (Joormann & Gotlib, 2008). Together, these suggests that cognitive control more generally, not necessarily at the attentional level, may contribute to memory biases in MDD (see (Joormann & Siemer, 2011) and suggest that, despite similar attentional processing, MDD individuals may have continued to process (i.e., not able to disengage from) this negative information.
There are several limitations to the current study. First, the study utilized a small, college-aged sample which reduces the generalizability of the findings to an older or less educated population. Despite age, however, the population was held to the same strict diagnostic criteria for a major depressive episode—which was further strengthened by the stability of mood evidenced by the two-step process used during recruitment. In addition, of the few studies that have examined the associations between cognitive processes in depression, even fewer have used a clinical sample (e.g., Gotlib et al., 2004). Thus, although exploratory in nature due to the sample size, the current study, contributes to this body of work.
Next, despite using a novel task to further examine attentional control in MDD, there are ways in which the task may have been improved. Since there were only two types of word stimuli, time spent viewing one type (e.g., target) would reduce the time spent viewing the other (e.g., distracter), potentially confounding overall effects. It could also be argued that the ease and simplicity of the current task was too passive and not engaging enough to capture the construct. It may be that a better task would be one where task-irrelevant information is presented while participants are actively engaged in another task and requiring active disengagement. This would manipulate the nature of the task, rather than the constraints on viewing (attend v. ignore) during the task. Future work should explore the effects of specifically manipulating viewing constraints versus task engagement on memory biases.
Despite these limitations, these results are the first to reveal a stronger link between attentional control and memory in clinical depression compared to healthy individuals. These results provide new insight into the interplay of cognitive biases in MDD and further highlight the importance of the development of interventions specifically designed to modify these biases (e.g., cognitive bias modification;(Wells & Beevers, 2010).
Footnotes
Mean time to first fixation for each stimuli type was also examined for MDD group differences using repeated measures anova. The valence by MDD group (F (1, 31)= 3.31, p= .08, partial η2=.10) and word type by MDD group (F (1, 31)= 3.29, p= .08, partial η2=.10) approached, but failed to meet significance. Further, the three-way, MDD group by valence by word time interaction was not significant (F (1, 31)= .22, p= .64, partial η2=.01). Thus, there were no effects of attention initiation between groups.
References
- 1.Beck AT, Steer RA, Brown GK. Manual for the BDI-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 2.Blaut A, Paulewicz B, Szastok M, Prochwicz K, Koster E. Are attentional bias and memory bias for negative words causally related? Journal of Behavior Therapy and Experimental Psychiatry. 2013;44(3):293–299. doi: 10.1016/j.jbtep.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 3.Bradley MM, Lang PJ. Fearfulness and affective evaluations of pictures. Motivation and Emotion. 1999;23(1):1–13. [Google Scholar]
- 4.De Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cognitive, Affective & Behavioral Neuroscience. 2010;10(1):50–70. doi: 10.3758/CABN.10.1.50. [DOI] [PubMed] [Google Scholar]
- 5.Derakshan N, Salt M, Koster EHW. Attentional control in dysphoria: An investigation using the antisaccade task. Biological Psychology. 2009;80(2):251–255. doi: 10.1016/j.biopsycho.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Dozois DJ, Dobson KS, Ahnberg JL. A psychometric evaluation of the Beck Depression Inventory-II. Psychological assessment. 1998;10(2):83–89. [Google Scholar]
- 7.Ellis AJ, Beevers CG, Wells TT. Attention allocation and incidental recognition of emotional information in dysphoria. Cognitive Therapy and Research. 2011;35(5):425–433. [Google Scholar]
- 8.Everaert J, Koster EHW, Derakshan N. The combined cognitive bias hypothesis in depression. Clinical Psychology Review. 2012;32(5):413–424. doi: 10.1016/j.cpr.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 9.First MB, Gibbon M. The Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) and the Structured Clinical Interview for DSM-IV Axis II Disorders (SCID-II) Hoboken, NJ, US: John Wiley & Sons Inc, Hoboken, NJ; 2004. [Google Scholar]
- 10.Goeleven E, De Raedt R, Baert S, Koster EHW. Deficient inhibition of emotional i nformation in depression. Journal of Affective Disorders. 2006;93(1–3):149–157. doi: 10.1016/j.jad.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 11.Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional Biases for Negative Interpersonal Stimuli in Clinical Depression. Journal of Abnormal Psychology. 2004;113(1):127–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- 13.Joormann J, Gotlib IH. Updating the contents of working memory in depression: Interference from irrelevant negative material. Journal of Abnormal Psychology. 2008;117(1):182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- 14.Joormann J, Gotlib IH. Emotion regulation in depression: Relation to cognitive inhibition. Cognition and Emotion. 2010;24(2):281–298. doi: 10.1080/02699930903407948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Joormann J, Siemer M. Affective Processing and Emotion Regulation in Dysphoria and Depression: Cognitive Biases and Deficits in Cognitive Control. Social & Personality Psychology Compass. 2011;5(1):13–28. [Google Scholar]
- 16.Joormann J, Teachman BA, Gotlib IH. Sadder and less accurate? False memory for negative material in depression. Journal of Abnormal Psychology. 2009;118(2):412–417. doi: 10.1037/a0015621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kellough JL, Beevers CG, Ellis AJ, Wells TT. Time course of selective attention in clinically depressed young adults: An eye tracking study. Behaviour Research and Therapy. 2008;46(11):1238–1243. doi: 10.1016/j.brat.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kline P. The new psychometrics: Science, psychology and measurement. Florence, KY, US: Taylor & Frances/Routledge, Florence, KY; 1998. [Google Scholar]
- 19.Koster EHW, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: The impaired disengagement hypothesis. Clinical Psychology Review. 2011;31(1):138–145. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Koster EHW, De Raedt R, Goeleven E, Franck E, Crombez G. Mood-Congruent Attentional Bias in Dysphoria: Maintained Attention to and Impaired Disengagement From Negative Information. Emotion. 2005;5(4):446–455. doi: 10.1037/1528-3542.5.4.446. [DOI] [PubMed] [Google Scholar]
- 21.Koster EHW, De Raedt R, Leyman L, De Lissnyder E. Mood-congruent attention and memory bias in dysphoria: Exploring the coherence among information-processing biases. Behaviour Research and Therapy. 2010;48(3):219–225. doi: 10.1016/j.brat.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Matt GE, Vázquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clinical Psychology Review. 1992;12(2):227–255. [Google Scholar]
- 23.Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depression and Anxiety. 2010;27(12):1135–1142. doi: 10.1002/da.20755. [DOI] [PubMed] [Google Scholar]
- 24.Rottenberg J, Gross JJ, Gotlib IH. Emotion Context Insensitivity in Major Depressive Disorder. Journal of Abnormal Psychology. 2005;114(4):627–639. doi: 10.1037/0021-843X.114.4.627. [DOI] [PubMed] [Google Scholar]
- 25.Smith ER, DeCoster J. Dual-process models in social and cognitive psychology: Conceptual integration and links to underlying memory systems. Personality and Social Psychology Review. 2000;4(2):108–131. [Google Scholar]
- 26.Teasdale JD. Cognitive vulnerability to persistent depression. Cognition and Emotion. 1988;2(3):247–274. [Google Scholar]
- 27.Wells TT, Beevers CG. Biased attention and dysphoria: Manipulating selective attention reduces subsequent depressive symptoms. Cognition and Emotion. 2010;24(4):719–728. [Google Scholar]
- 28.Wells TT, Beevers CG, Robison AE, Ellis AJ. Gaze behavior predicts memory bias for angry facial expressions in stable dysphoria. Emotion. 2010;10(6):894–902. doi: 10.1037/a0020022. [DOI] [PubMed] [Google Scholar]