The safety of batteries is determined by numerous factors with complicated interplays, but is typically always inversely proportional to their energy/power densities. Past attempts to make such an energy storage device both energetic (as measured in Wh/kg) and powerful (as measured in W/kg) involve tightly packing a highly reactive oxidant (cathode material), reductant (anode material), and potential fuel (electrolyte) into a tiny enclosed space. The electrochemistry within these devices only proceeds along the designed pathway in the presence of protective interphases, known as the solid-electrolyte interphase (SEI), and generated from ad hoc decomposition chemistry of electrolyte components. Any deviation from this metastability would lead to a catastrophic “runaway” (i.e., the direct chemical reactions between the oxidants, reductants, and fuels). Following the emergence of the lithium ion battery (LIB) in the early 1990s and its rapid domination of the rechargeable battery market, increasingly higher expectations for energy/power densities were placed on this young battery chemistry by both cell and original equipment manufacturers, often at the expense of the above fragile metastability. In the past decade LIB safety incidents frequently appeared in headline news, such as laptop or cell phone fires, the fatal electric vehicle accidents, and the grounding of the Boeing 787 Dreamliner Fleet. At the center of these incidents stood the nonaqueous electrolytes, which consist of volatile and inflammable organic carbonate esters and toxic lithium salts. Wong et al. (1) have made a significant step toward eliminating this key hazard in the LIB safety by replacing those inflammable solvents with perfluoropolyethers (PFPE), and proved in concept that the electrolyte can support the lithium ion chemistry.
There are varieties of aprotic solvents that can dissolve lithium salts, but few of them qualify as electrolyte solvents because a series of stringent requirements must be met (ion conductivity, electrochemical stability or metastability on both electrodes, wettability with the separator, chemical and electrochemical inertness with other cell parts, and so forth) (2, 3). Extending their previous success of blending fluorinated (PFPEs) and nonfluorinated polyethers, Wong et al. (1) identified such a class of solvent based on functionalized nonflammable PFPEs that satisfies most of the electrolyte prerequisites. The solvents of varying molecular weight can dissolve lithium bis(trifloromethane)sulfonamide (LiTFSI), a benign alternative to the toxic lithium salt used in the current lithium ion industry. Unlike carbonate-based solvents, such as dimethyl carbonate, the highly fluorinated solvents are intrinsically nonflammable (Fig. 1). A surprising advantage of PFPE-based electrolytes is the ultrahigh transfer number of Li+, which has been much lower than 0.5 in state-of-the-art Li ion electrolytes because of the strong coordination of Li+ by the nucleophilic carbonates (Fig. 2A). Close to unity t+ was found in Wong et al.’s electrolytes instead, indicating that the salt dissolution must be accomplished mainly through anion-solvation, thus freeing Li+ as the conducting species (Fig. 2B). As pointed out by Wong et al., this unprecedented high Li+ mobility was the result of the significantly reduced nucleophilicity of carbonyl groups by the fluorine-presence in the molecules. Placed in a Li ion device, a higher Li+-transference number can reduce the polarization resistance, compensating in part the lower ion conductivity (2.2 × 10−5 S/cm). The above electrolytes were demonstrated with LiNi1/3Co1/3Mn1/3O2-based cathode chemistry with stable cycling performance, suggesting that the nonflammable electrolyte system could enable Li ion devices with enhanced safety.
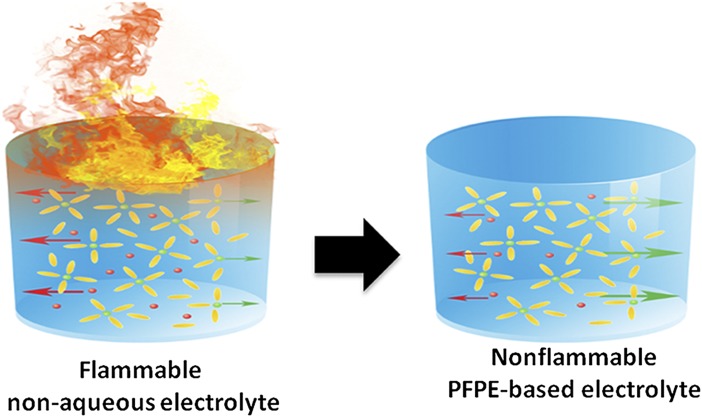
Fig. 1.
Schematic comparison between carbonate-based and PFPE-based electrolytes. The former, used in the state-of-art Li-ion batteries, are intrinsically flammable and characterized by low Li+ transference number. The latter, developed by Wong et al. (1), is nonflammable and promotes Li+ transport.
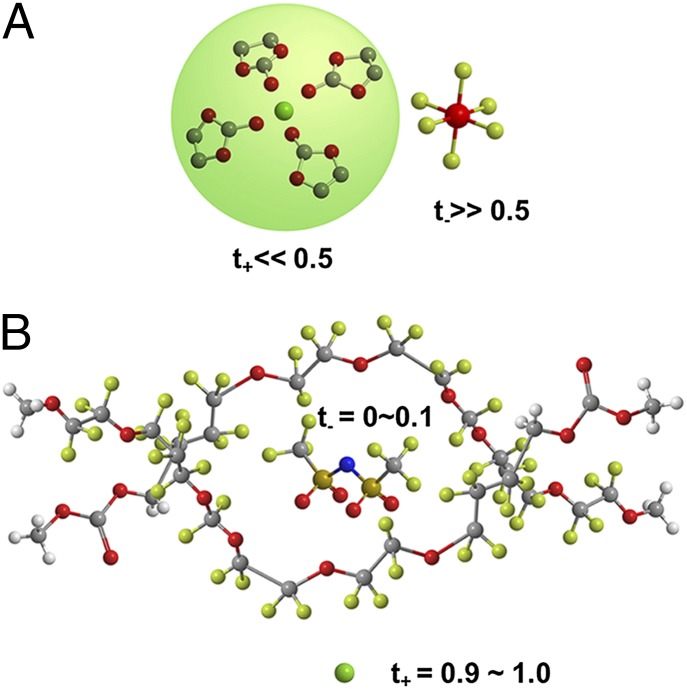
Fig. 2.
(A) In conventional nonaqueous electrolytes, Li+ is tightly solvated and slowed down in its migration by coordinating carbonate molecules, resulting in low t+. (B) In electrolytes based on highly fluorinated solvents, anion TFSI is solvated instead, thus freeing Li+ as the conducting species and resulting in almost unity t+.
Despite its fundamental significance, the electrolyte system proposed by Wong et al. (1) still needs major improvements to suit practical applications. Aside from cost concerns that always accompany fluorinated organic solvents, the ion conductivities (∼10−5 S/cm) of the system are far from the ideal 10−3 S/cm range suggested by Goodenough and Kim (4), which would impose limits on the cell chemistry kinetics and eventually the power density. This limitation is reflected in the maximum rate C/8 used in the article that is short of the C/3 goal set by the Department of Energy. The ability of these fluorinated solvents to form a protective SEI on graphitic carbon electrodes is yet
The work of Wong et al. represents an encouraging advance toward a safer battery.
to be proved, as the article only demonstrated cathode-half cells wherein metallic lithium was used as surrogate anode. This is a critical prerequisite for LIB electrolytes before graphite can be replaced by other more energetic candidates as anode materials. Most importantly, nonflammability does not always translate into higher tolerance against abuse in an electrochemical environment. According to Shigematsu et al. (5), the former concerns the combustion behavior in atmosphere and the latter the electrochemical reactivity in cell environments. This finding was further supported by Wang et al. (6), who demonstrated that even the intrinsically nonflammable ionic liquid can still lead to potential thermal runaway in the presence of charged electrode materials; therefore, although nonflammable electrolytes did help enhance thermal safety of devices in the event of cell rupture with external ignition sources, their impact on the overall LIB safety under extreme abusive conditions needs to be examined in large-format cells. The work of Wong et al. (1) represents an encouraging advance toward a safer battery regardless, and benefits perhaps not only Li ion batteries, but other “beyond Li ion” battery chemistry as well.
Footnotes
The authors declare no conflict of interest.
See companion article on page 3327.
References
- 1.Wong DHC, et al. Nonflammable perfluoropolyether-based electrolytes for lithium batteries. Proc Natl Acad Sci USA. 2013;111:3327–3331. doi: 10.1073/pnas.1314615111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem Rev. 2004;104(10):4303–4417. doi: 10.1021/cr030203g. [DOI] [PubMed] [Google Scholar]
- 3.Hu Z, et al. Optically transparent, amphiphilic networks based on blends of perfluoropolyethers and poly(ethylene glycol) J Am Chem Soc. 2008;130(43):14244–14252. doi: 10.1021/ja803991n. [DOI] [PubMed] [Google Scholar]
- 4.Goodenough JB, Kim Y. Challenges for rechargeable Li batteries. Chem Mater. 2010;22(3):587–603. [Google Scholar]
- 5.Shigematsu Y, Ue M, Yamaki J. Thermal behavior of charged graphite and LixCoO2 in electrolytes containing alkyl phosphate for lithium-ion cells batteries and energy storage. J Electrochem Soc. 2009;156(3):176–180. [Google Scholar]
- 6.Wang Y, et al. Accelerating rate calorimetry studies of the reactions between ionic liquids and charged lithium ion battery electrode materials. Electrochim Acta. 2007;52(22):6346–6352. [Google Scholar]