Significance
Telomerase expression is essential for the long-term proliferation of most cancer cells. In cancer cells lacking telomerase, an alternative lengthening of telomeres (ALT) pathway is activated to maintain telomere length through telomere recombination. Using yeast as a model system, we found that the noncoding telomeric repeat-containing RNA (TERRA) plays a major role in recombination-mediated maintenance of telomere in telomerase-deficient cells. Increased levels of telomere-associated TERRA result in the accumulation of telomeric DNA–RNA hybrids and induce telomere recombination. Because the structure and function of TERRA are conserved among eukaryotes, our findings suggest that the accumulation of telomere-associated TERRA might also have a role in modulating the occurrence of ALT in cancer cells.
Abstract
In human somatic cells or yeast cells lacking telomerase, telomeres are shortened upon each cell division. This gradual shortening of telomeres eventually leads to senescence. However, a small population of telomerase-deficient cells can survive by bypassing senescence through the activation of alternative recombination pathways to maintain their telomeres. Although genes involved in telomere recombination have been identified, mechanisms that trigger telomere recombination are less known. The THO (suppressor of the transcriptional defects of Hpr1 mutants by overexpression) complex is involved in transcription elongation and mRNA export. Here we demonstrate that mutations in THO complex components can stimulate early senescence and type II telomere recombination in cells lacking telomerase. The accumulation of telomere-associated noncoding telomere repeat-containing RNA (TERRA) is required for the observed telomere effects in THO complex mutants; reduced transcriptional efficiency, or overexpression of RNase H or C1–3A RNA can severely impair the type II telomere recombination. The results highlight a unique function for telomere-associated TERRA, in the formation of type II survivors. Moreover, because TERRA is a long noncoding RNA, these results reveal a function for long noncoding RNA in regulating recombination.
In Saccharomyces cerevisiae, the telomere length is typically maintained by the constitutive expression of telomerase. However, in cells lacking telomerase, a gradual loss of telomere length during rounds of cell division eventually leads to the uncapping of telomeres and induces a checkpoint-mediated cell-cycle arrest termed senescence (1, 2). Although most cells die in the absence of telomerase, rare survivors do escape senescence by maintaining their telomeres through a radiation sensitive 52 (RAD52)-dependent recombination mechanism (3–5). At least two different types of telomeric recombination events contribute to telomere elongation in the absence of telomerase, generating two types of survivor cells that can be distinguished by different growth rates and distinct telomere patterns on Southern blots (3, 6–8). Type I survivors arise through gene-conversion events that occur in the Y′ sequence elements present in the subtelomeric region of most chromosomes; these cells have short terminal telomere repeats and grow slowly. In contrast, type II survivors have long, heterogeneous tracts of telomeric repeats, similar to the survivors identified in telomerase-deficient Kluyveromyces lactis and mammalian alternative lengthening of telomeres (ALT) cells (5, 9). The generation of both types of survivors and the stability of their telomeres depend on the function of RAD52 (3–5). However, two genetically distinct recombination pathways, defined by RAD50 and RAD51, govern the RAD52-dependent generation of specific types of survivors (6, 10). The type I survivors are absent in the rad51Δ strain, whereas type II survivors cannot arise in the rad50Δ strain (10). Neither type of survivor can be found in the rad51Δ rad50Δ double mutant (6). Despite the identification of these important genes that are involved in telomere recombination, mechanisms triggering telomere recombination have not been fully elucidated.
Telomeres are transcribed from subtelomeric regions into large noncoding telomeric repeat-containing RNA (TERRA) by RNA polymerase II. TERRA has been implicated in several telomere-related and non-telomere-related functions (11–15). For example, TERRA binds to telomeres and participates in regulating telomerase and the organization and maintenance of telomeric chromatin throughout development and cellular differentiation (11–13, 16). A study using an inducible telomere transcription system to investigate the effect of TERRA expression on telomere function (17) showed that telomeric transcription causes telomere shortening in a DNA replication-dependent manner; moreover, in the absence of both telomerase and telomere recombination, overexpression of TERRA at a single telomere is sufficient to induce early-onset senescence. Although the cellular localization of TERRA has not been demonstrated in yeast, RNA fluorescence in situ hybridization studies have showed that at least a fraction of TERRA molecules in human and mouse cells are stably associated with telomeric heterochromatin (11, 13). This telomeric association of TERRA may be achieved through interacting with telomeric proteins or, alternatively, through the direct formation of telomeric DNA–RNA hybrids.
THO (a suppressor of the transcriptional defects of Hpr1 mutants by overexpression) is a conserved eukaryotic complex involved in transcription elongation and mRNA export (18, 19). In S. cerevisiae, the THO complex comprises four proteins: Hpr1, Tho2, Mft1, and Thp2 (18). In mutants with a defective THO complex, stalled transcription and transcriptional DNA–RNA hybrids stimulate transcription-associated recombination, causing genome instability and chromosome fragility (20–23). Here, we used mutants of THO complex components to test the role of TERRA in telomere recombination. We found that premature senescence and formation of RAD50-dependent type II survivors were induced in THO mutants that were also lacking functional telomerase. The observed premature senescence and telomere recombination was transcription-dependent and required the accumulation of TERRA in telomeric DNA–RNA hybrids. Our results have revealed a mechanism in which the accumulation of telomere-associated TERRA in THO mutants induces recombination in the absence of telomerase. More significantly, we found that telomere-associated TERRA plays a role in type II telomere recombination to facilitate the bypass of senescence in cells lacking telomerase.
Results
Mutations in the THO Complex Components Induce Early Senescence and Type II Survivor Formation in the Absence of Telomerase.
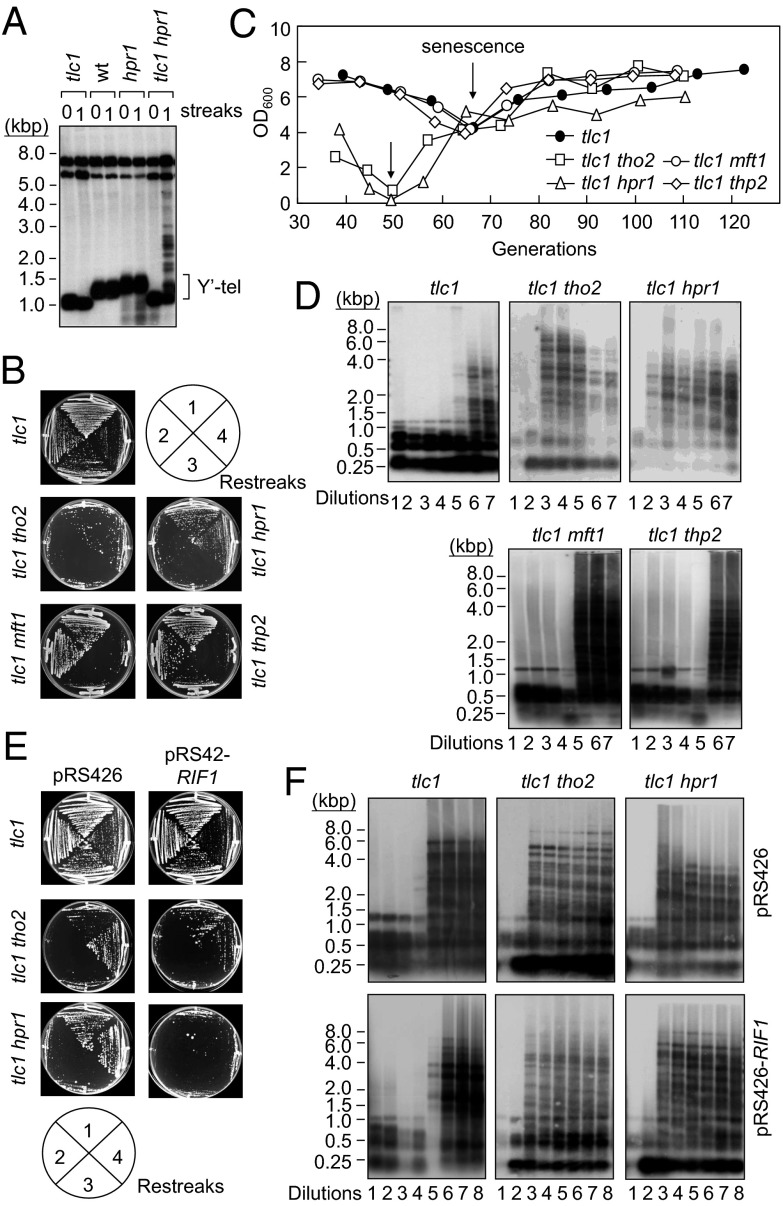
Our previous work demonstrated that telomeres are lengthened by ∼50–100 bp in tho2Δ or hpr1Δ strains but remain unaffected in mft1Δ or thp2Δ cells; moreover, mutations in these THO complex components affect telomere length by reducing the expression of a telomere-associated protein, Rif1 (24). To determine whether telomere lengthening in hpr1 and tho2 cells requires telomerase activity, the telomerase RNA component-encoding gene TLC1 was deleted in hpr1 and tho2 cells. Lacking functional telomerase, tlc1 cells exhibited progressive shortening of telomeres (Fig. 1A), whereas the lengths of Y′-bearing telomeres in hpr1 cells appeared ∼75 bp longer than those in wild-type cells, as reported (24). The telomere lengths in tlc1 hpr1 cells were similar to those in tlc1 cells, indicating that the increase of telomere lengths observed in hpr1 and tho2 cells required functional telomerase. Interestingly, the type II survivor pattern was frequently observed in tlc1 hpr1 and tlc1 tho2 double-mutant cells after restreaking, but not in wild-type or single-mutant cells (Fig. 1A and Fig. S1). The results suggest that the THO complex might somehow affect telomere recombination.
Fig. 1.
Loss of THO complex components HPR1 and THO2 induces early senescence and the formation of type II survivors in telomerase-deficient cells. (A) Early detection of type II survivors in tlc1 hpr1 cells. Colonies from the original spores (0th streak) and the restreaked cells were subsequently grown on YEPD medium at 30 °C. Genomic DNA from these cultures was collected, digested by using XhoI, and subjected to Southern blotting analysis by using a random-primed Y′ probe. (B) Premature senescence in tlc1 tho2 and tlc1 hpr1 cells. As described above, the colonies from fresh-dissected tetrads were continuously streaked on YEPD plates at 30 °C four times (first to fourth restreaks). Photographs of these cells with one to four restreaks on YEPD plates are represented. (C) Colonies from fresh-dissected tetrads were inoculated directly into liquid YEPD medium at a concentration of 3 × 105 cells per milliliter and cultured at 25 °C for 2 d. The cell growth was measured by using a spectrophotometer. The cultures were diluted to a concentration of 3 × 105 cells per milliliter and cultured at 25 °C for another 2 d. These procedures were repeated 8–10 times. (D) Early type II survivor formation in tlc1 tho2 and tlc1 hpr1 cells. Colonies from fresh-dissected tetrads were inoculated directly into liquid YEPD medium and cultured at 30 °C until the cells reached the stationary phase. The cultures were then diluted to 1:10,000 into fresh medium and cultured at 30 °C. The procedures were repeated seven to eight times. The genomic DNA was extracted from these cultures and digested by using a combination of AluI, HaeIII, HinfI, and MspI. Southern blotting analyses were conducted by using a C1–3A probe. (E) RIF1 overexpression does not restore the premature senescence of tlc1 tho2 and tlc1 hpr1 cells. The indicated yeast cells harboring pRS426 or pRS426-RIF1 were continuously streaked onto YC plates and cultured at 30 °C. (F) RIF1 overexpression does not restore the early formation of type II telomere recombination in tlc1 tho2 and tlc1 hpr1 cells. DNA from the indicated strains was collected and digested with a combination of AluI, HaeIII, HinfI, and MspI and blotted with a C1–3A probe.
To determine the role of the THO complex in senescence and cell survival in the absence of telomerase, we examined the growth of tlc1 cells with THO mutations. Cells were harvested directly from freshly dissected tlc1 spores and assayed by successive restreaking of grown colonies onto fresh plates four times. The tlc1 cells typically lost viability between their 60th and 80th generations after sporulation (on the second or third restreak), and the survivors appeared on the fourth restreak (Fig. 1B). The deletion of THO2 or HPR1 in tlc1 cells caused slow growth immediately after sporulation, and the cells showed severe growth defects on the second and third restreaks. Survivors that bypassed senescence first appeared after the third restreak and grew on the fourth restreak. The deletion of mft1 or thp2 did not affect the senescent phenotype of tlc1 cells (Fig. 1B). The effects of tho2 and hpr1 mutations on the viability of tlc1 cells were further measured in liquid culture assays (8). The tlc1 cells showed a decline in the growth rate, and survivors were generated after ∼70 generations (Fig. 1C). In contrast, the tlc1 tho2 and the tlc1 hpr1 mutants showed an early decline in growth, similar to that observed in the plate assays (Fig. 1B). Survivors from tlc1 tho2 and tlc1 hpr1 strains were generated after ∼50 generations. The deletion of MFT1 or THP2 did not affect the growth rate and survivor formation of tlc1 cells. These results indicate that both THO2 and HPR1 play a role in modulating the generation of survivors in the absence of telomerase.
A telomere Southern analysis was applied to evaluate the recombination of telomeres in the survivors obtained from liquid cultures after successive dilutions (7, 10). In tlc1 cells, the type I survivor pattern appeared first between the fourth and fifth dilutions, and subsequently the type II pattern was observed between the fifth and sixth dilutions (Fig. 1D) (10). In tlc1 tho2 and tlc1 hpr1 double mutants, the type-I to type-II conversion of recombination pattern occurred between the first and second dilutions. The deletion of MFT1 or THP2 did not affect the telomeric DNA pattern of tlc1 cells. Together, these results indicate that the loss of HPR1 or THO2 induces early senescence and the formation of type II survivors in telomerase-deficient cells.
A recent report demonstrated that the rif1 mutant has higher TERRA levels than those in wild-type cells (25). We therefore investigated whether the decreased Rif1 expression in THO mutants (24) underlies the early senescence and formation of type II survivors observed in tlc1 tho2 and tlc1 hpr1 cells. The results showed that, although the telomere lengthening observed in hpr1 and tho2 cells was suppressed by restoring Rif1 expression to the wild-type levels (24), the early senescence and formation of type II survivors in tlc1 tho2 and tlc1 hpr1 cells were not affected by plasmid-driven Rif1 expression (Fig. 1 E and F). These observations suggest that mutations in THO complex components may affect telomere functions through at least two different mechanisms: reducing Rif1 levels or causing accumulation of TERRA.
To further evaluate the effect of THO complex mutations on survivor formation, we analyzed the genomic DNA from independent survivors of tlc1 strains with or without THO mutations. We found that the deletion of THO2 or HPR1 did not affect the efficiency of survivor formation in the tlc1 background (Table S1). Southern analysis also revealed that the generation of either type of recombined telomere was not affected by the deletion of THO2 or HPR1 (Fig. S2). The results also showed that deleting THO2 in tlc1 cells did not cause obvious alteration of telomere-shortening rates (Fig. S3), indicating that the observed acceleration of senescence in tlc1 tho2 cells was not due to the increase of the overall telomere erosion rate.
Transcription Is Required for Induction of Early Senescence and Type II Survivors in Telomerase-Deficient tho2 or hpr1 Cells.
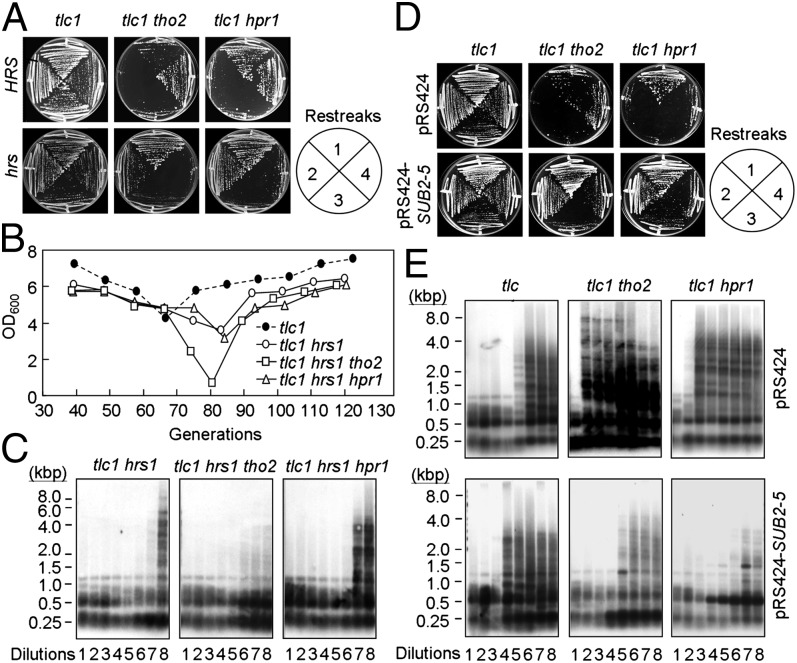
Because the THO complex functions in transcription elongation, it is possible that the observed premature senescence and type II survivor formation in tho2 and hpr1 cells might be related to their transcriptional defects. Hrs1, a component of the mediator complex of RNAPII conserved from yeast to humans, is involved in the regulation of transcription initiation (26, 27). Mutations in HRS1 can suppress the hyperrecombination phenotype of hpr1 cells (28). To determine whether hrs1 mutations can also suppress the senescence and telomere recombination phenotypes of tlc1 tho2 and tlc1 hpr1 mutants, hrs1 was introduced into these mutants, and the resulting strains were analyzed for growth and survivor formation. As shown in Fig. 2 A and B, the introduction of hrs1 suppressed the early onset of senescence in telomerase-deficient THO mutants. Remarkably, telomere Southern analysis showed that the type II survivor pattern was only detected in genomic DNA isolated from late dilutions of tlc1 hrs1 cultures in successive dilution experiments, and the type II recombination pattern also was not observed in early dilutions of tlc1 tho2 or tlc1 hpr1 double-mutant cells deleted of HRS1 (Fig. 2C). These findings suggest that HRS1 is required for the early induction of type II survivor formation in telomerase-deficient THO complex mutants.
Fig. 2.
Transcription is required for mutations of THO complex components to induce early senescence and formation of type II survivors in the absence of telomerase. (A) hrs1 restores the premature senescence of tlc1 tho2 and tlc1 hpr1 cells. Freshly dissected colonies with the indicated mutations were continuously streaked onto YC plates. (B) The senescence rates were measured in liquid culture by serially passaging spore products of the indicated genotypes. (C) hrs1 delayed type II telomere recombination in tlc1 cells. DNA from the indicated strains was collected, digested by using a combination of AluI, HaeIII, HinfI, and MspI, and blotted with a C1–3A probe. (D) sub2-5 overexpression restores the premature senescence of tlc1 tho2 and tlc1 hpr1 cells. The indicated yeast cells harboring pRS425 or pRS425–SUB2-5 were continuously streaked onto YC plates at 30 °C. (E) sub2-5 overexpression restored the early formation of type II telomere recombination in tlc1 tho2 and tlc1 hpr1 cells. DNA from the indicated strains was then analyzed by Southern blotting using a C1–3A probe.
A high-copy suppressor screen has revealed that SUB2 can suppress the transcriptional defect of hpr1 cells (29–31). SUB2 encodes a DEAD-box RNA helicase involved in spliceosome assembly and mRNA export (32–34). Sub2 binds to Yra1 and the THO complex to form a TREX (TRanscription/EXport) complex that couples transcription elongation and mRNA export (19, 34, 35). To determine whether overexpressing SUB2 also has an effect on early senescence and the formation of type II survivors, we analyzed tlc1 tho2 and tlc1 hpr1 cells with a plasmid carrying SUB2 or sub2-5. The sub2-5 allele expresses a mutant Sub2 protein with a Q308R mutation near helicase motif IV, which does not cause any detectable defects in the splicing activity or spliceosome assembly (36). However, the expression from the sub2-5 allele is higher than that from the SUB2 allele for unknown reasons (37). As shown, overexpressing SUB2 or sub2-5 suppressed the occurrence of early senescence in tlc1 tho2 or tlc1 hpr1 cells (Fig. 2D and Fig. S4). Telomere Southern analysis also showed that, similar to the effect of deleting HRS1, overexpressing SUB2 or sub2-5 suppressed type II telomere recombination and delayed type II survivor formation in both tlc1 tho2 and tlc1 hpr1 cells with a timing similar to that in the tlc1 cells (Fig. 2E and Fig. S4). Together, these results indicate that defective transcription underlies the premature senescence and the early formation of type II survivors in tlc1 cells with THO complex mutations.
Accumulation of Telomere-Associated TERRA in THO Mutants.
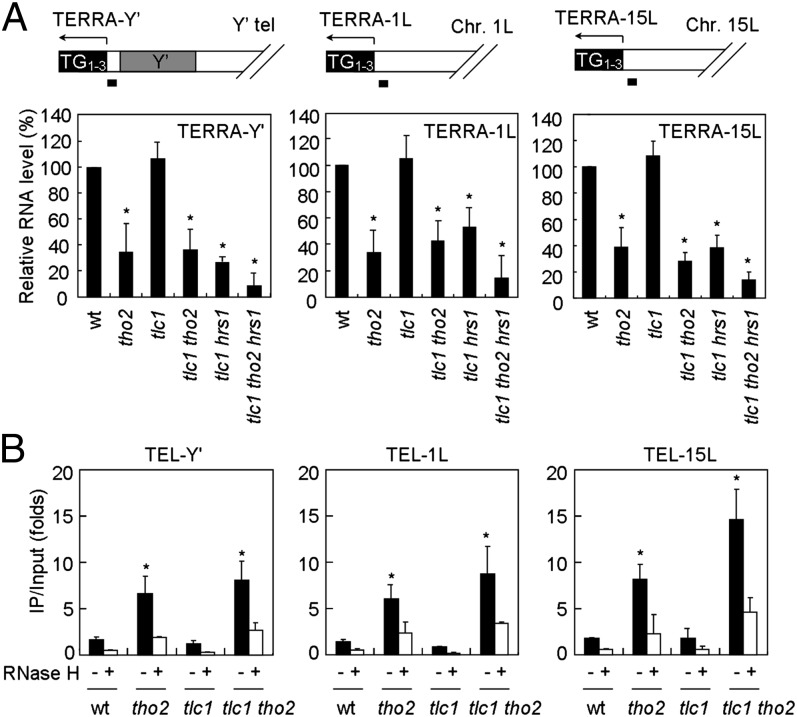
To further investigate the influence of transcriptional defects on senescence and telomere recombination in the THO complex mutants, we analyzed the status of telomere transcript TERRA. A quantitative real-time RT-PCR assay was adopted to detect the presence of TERRA in yeast cells (12, 25). TERRA of the Y′ elements (TERRA-Y′) and the left arms of chromosomes 1 (TERRA-1L) and 15 (TERRA-15L) were analyzed. Using oligodT (polyT18) as the primer for reverse transcription, we found that cells with tho2 mutations expressed the three analyzed TERRA species at levels ∼30–40% of those in wild-type cells (Fig. 3A). Wild-type-like TERRA levels were observed in the early passages of tlc1 cells, suggesting that telomerase does not affect the expression of TERRA. Interestingly, the introduction of tho2 or hrs1 mutations in tlc1 cells caused a reduction in the levels of all three tested TERRA species, indicating that the transcriptional status of cells can affect the overall TERRA production. We also examined TERRA levels in tlc1, tlc1 tho2, and tlc1 hpr1 cells using RT-PCR primed with C1–3A oligonucleotides and observed a reduction in the TERRA levels in THO complex mutants (Fig. S5).
Fig. 3.
Accumulation of DNA-associated TERRA in THO mutants. All strains were collected after 30 doublings. (A) TERRA level is decreased in THO mutants. Total RNA from the indicated strains was extracted by using acid phenol. After reverse transcription primed by poly-T18, quantitative PCR analysis was conducted by using primer pairs specific for telomere 1L, 15L, and Y′-bearing telomeres. The resulting CT values were normalized to ACT1 RNA levels and presented as relative RNA levels by using the wild-type value as 1. The error bars represent the SDs from four independent experiments. *P < 0.05. (B) Genomic DNA extracted from the indicated strains was sonicated and immunoprecipitated by using an S9.6 antibody. The levels of S9.6-associated TERRA were quantified as above. For RNase H treatment, the sonicated genomic DNA was incubated with 10 units of RNase H at 37 °C for 1 h. *P < 0.05.
Because the stalled R loop is a common intermediate in THO mutants (20, 21), the level of TERRA in the form of a DNA–RNA hybrid was further analyzed in various yeast strains. We carried out direct immunoprecipitation (IP) analysis using the S9.6 antibody on DNA samples prepared by a neutral phenol extraction method; this extraction method preserves the DNA-associated RNA. Compared with wild-type cells, tho2, tlc1 tho2, hpr1, and tlc1 hpr1 mutant strains displayed increased amounts of Y′, 1L, and 15L telomeres (Fig. 3B and Fig. S6). Significantly, the PCR-amplified signals were sensitive to RNase H treatment in vitro, confirming that these PCR products were indeed from DNA–RNA hybrids. Thus, although the overall TERRA levels ware reduced, the amounts of telomere-associated TERRA were increased in tho2 or hpr1 mutant strains.
TERRA Induces Type II Survivor Formation.
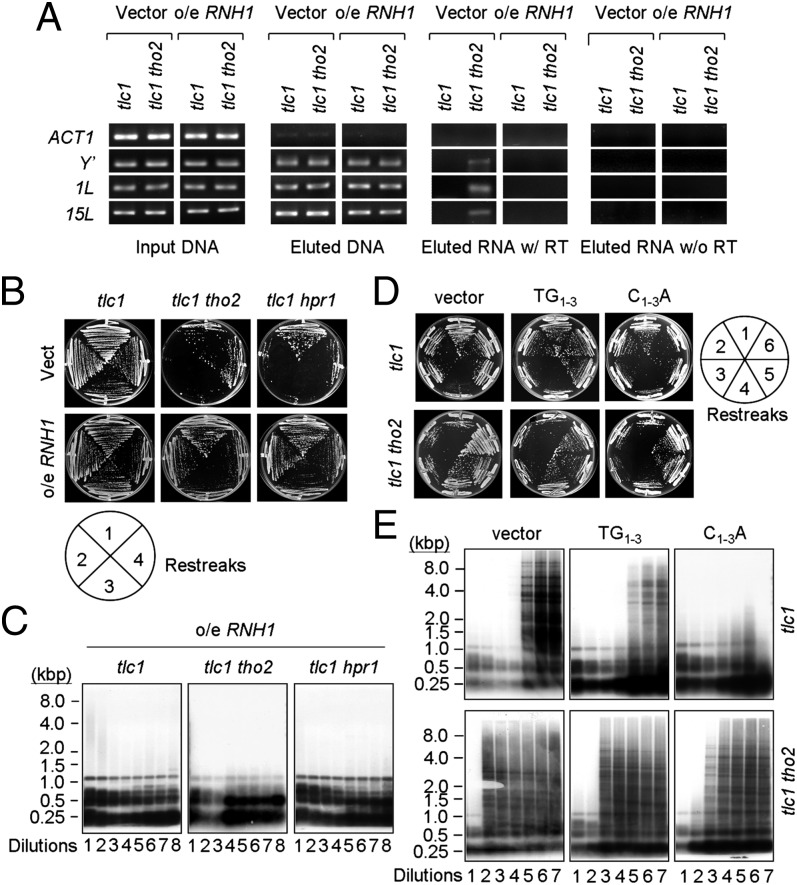
We next used chromatin IP (ChIP) to directly analyze the telomere-associated TERRA using an anti-Rap1 antibody. Rap1 is a telomere binding protein that binds to double-stranded telomeric DNA (38). The results showed that TERRA of Y′, 1L, and 15L was only detected in Rap1 immunoprecipitates from tlc1 tho2 cells, but it was not detectable in samples from tlc1 tho2 cells overexpressing RNase H1 (Fig. 4A), suggesting that TERRA was accumulated in the form of a DNA–RNA hybrid on the telomeres of tlc1 tho2 cells. Results from restreaking assays showed that overexpressing RNase H1 readily suppressed the early senescence of both tlc1 tho2 and tlc1 hpr1 cells (Fig. 4B). Remarkably, telomere Southern analysis showed that the formation of type II survivors in tlc1, tlc1 tho2, or tlc1 hpr1 cells was blocked through RNase H1 overexpression (Fig. 4C). Overexpressing RNH35, which encodes RNase H2, also affected the formation of type II survivors (Fig. S7). We also found that overexpressing RNase H1 did not substantially affect the temperature sensitivity or telomere length of the tho2 or hpr1 cells (Fig. S8); this observation excluded the possibility that the elimination of type II survivors by overexpressing RNase H1 was a consequence of affecting the general transcriptional defects or telomere length in THO mutants. Interestingly, none of the yeast tlc1 rnh1, tlc1 rnh35, or tlc1 rnh1 rnh35 strains affected type II survivor formation (Fig. S9).
Fig. 4.
Accumulation of telomere-associated TERRA induces premature senescence and telomere recombination. (A) Overexpressing RNase H1 reduces the level of telomere-associated TERRA in tlc1 tho2 cells. Freshly dissected tlc1 and tlc1 tho2 cells harboring the plasmid pGAL1–RNH1 were used in ChIP analyses with antibodies against Rap1. PCR analyses were used to determine the levels of Rap1-associated TERRA. (B) Overexpressing RNase H1 rescues premature senescence in tlc1 tho2 and tlc1 hpr1 cells. Freshly dissected colonies with the indicated mutations were continuously streaked onto YC plates. (C) RNH1 overexpression delays type II telomere recombination in tlc1 cells. DNA from the indicated strains was then analyzed by Southern blotting using a C1–3A probe. (D) Overexpressing C1–3A transcripts delays survivor formation in tlc1 cells. Freshly dissected tlc1 cells carrying plasmid pRS426-Gal (vector), pRS426-Gal-TG1–3 (TG1–3), or pRS426-Gal-C1–3A (C1–3A) were continuously streaked at 30 °C. These plates were photographed after incubation at 30 °C for 3–4 d. (E) Excess amounts of C1–3A transcript delays type II recombination in tlc1 cells. DNA from the indicated strains was then analyzed by Southern blotting using a C1–3A probe.
If the telomeric DNA–RNA hybrid formed by TERRA is required for type II survivor formation, it is likely that expressing excessive amounts of C1–3A-containing RNA might compete for binding to TERRA, and hence affect type II survivor formation. We tested the above possibility by using plasmids expressing 270 bp of TG1–3 or C1–3A sequences under the control of a GAL1 promoter. As shown in Fig. 4D, overexpressing TG1–3 or C1–3A did not affect the senescence rate of tlc1 cells. Telomere Southern analysis showed that overexpressing C1–3A markedly suppressed the formation of type II survivors (Fig. 4E). Because the formation of type II survivors was not affected by overexpressing TG1–3 RNA, it is likely that TERRA in trans might not affect telomere recombination. Together, our results indicate that the DNA–RNA hybrid formed by TERRA is required for the formation of type II survivors.
Induction of the DNA Damage Response by Accumulation of TERRA on Telomeres.
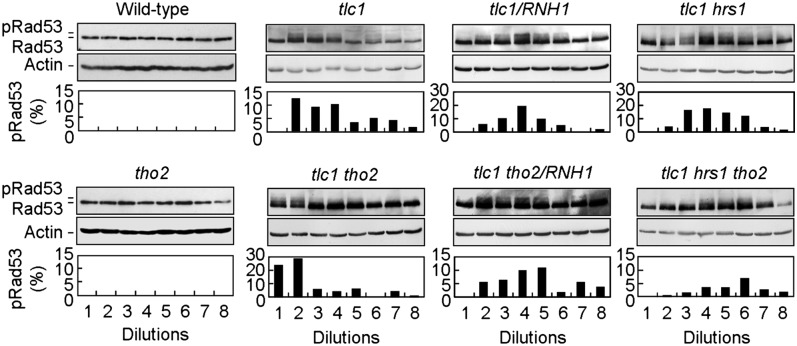
DNA–RNA hybrids are a common intermediate in different forms of unscheduled DNA damage (22). It is possible that telomeric DNA–RNA hybrids formed by telomere-associated TERRA facilitate the induction of the DNA damage response to initiate telomere recombination. To test this hypothesis, we monitored the DNA damage response during the process of senescence and survivor formation in telomerase-deficient and/or THO-defective cells by examining the phosphorylation of Rad53. Rad53 phosphorylation is required for cell-cycle arrest in response to DNA damage. As shown in Fig. 5, phosphorylated Rad53 was detected in the second to the fourth dilutions of tlc1 cell cultures in the successive dilution assay, which was equivalent to ∼30–60 generations after losing telomerase. In contrast, Rad53 phosphorylation was not detectable in either wild-type or tho2 cells. Notably, the generations in which the DNA damage response was detectable occurred immediately before the formation of survivors (compared with Fig. 1D). After the survivors were formed, the DNA damage response subsided. In tlc1 tho2 cells, Rad53 phosphorylation was readily detected as early as the first dilution. RNase H overexpression or the introduction of hrs1 mutation in both tlc1 and tlc1 tho2 cells delayed the occurrence of Rad53 phosphorylation to later dilutions. These results suggest a temporal correlation among telomere erosion, senescence, type II survivor formation, DNA damage response induction, and the accumulation of TERRA in telomeres.
Fig. 5.
Induction of the DNA damage response and recruitment of recombination protein onto telomeres in THO mutants lacking telomerase. Colonies from fresh-dissected tetrads were inoculated directly into liquid YEPD medium and cultured at 30 °C until the stationary phase. The cultures were diluted and cultured at 30 °C. The procedures were repeated seven to eight times. Total cell extracts were prepared form these cells and immunoblotted with antibodies against Rad53 (Upper) or actin (Lower). Quantifications for the percentage of phosphorylated Rad53 over total Rad53 were plotted.
Discussion
TERRA transcripts are versatile molecules that participate in multiple cellular functions. TERRA can base-pair with the RNA template of telomerase RNA and bind to the telomerase reverse transcriptase (TERT) polypeptide (39); these interactions might be responsible for the TERRA-mediated inhibition of telomerase activity. TERRA is believed to be involved in regulating telomeric chromatin organization and maintenance throughout development and cellular differentiation (11–13, 15–17, 40). TERRA has also been implicated in the recruitment of the origin recognition complex to telomeres (41) and can collaborate with hnRNPA1 and POT1 to remove RPA from telomeric DNA tail after DNA replication (23). Here, our results reveal a unique function of TERRA in triggering early senescence and telomere recombination. In THO complex mutants, the general reduction in the efficiency of transcription elongation causes the stalling of transcriptional elongation complex (20, 42). The stalled transcription elongation complex does not stimulate recombination in cells with active telomerase. However, in cells that lack telomerase, the transcribed TERRA might invade duplex telomeric DNA to form a DNA–RNA hybrid and thereby induce recombination. Significantly, although our initial observation of TERRA-induced telomere recombination was made in THO mutants, we also provide strong evidence showing that the accumulation of TERRA itself is sufficient to induce recombination. The strong correlation of TERRA expression and telomere association with suppression of very early senescence suggest that telomere-associated TERRA has a role in inducing type II recombination in cells lacking telomerase.
TERRA also represents a type of long noncoding cellular RNA. Long noncoding RNAs larger than ∼200 nucleotides are produced in eukaryotes (43). These long noncoding RNAs include antisense, intronic, and intergenic transcripts, as well as pseudogenes and retrotransposons. Long noncoding RNAs are expressed in a developmentally regulated manner and are known to play roles in higher-order chromosomal dynamics, subcellular structural organization, and telomere biology. Our finding that TERRA induces telomere recombination provides a previously unidentified example of long noncoding RNA functioning in recombination. Mechanistically, long noncoding RNAs mediate locus- and allele-specific functions through tethering to specific sites of chromosomes. For example, long noncoding RNAs are tethered to the site of transcription through the RNA polymerase ternary complex, thereby enabling functions as locus- or allele-specific tags. Long noncoding RNAs could also recruit protein factors to the transcription sites to exert their functions. It is likely that the stalled RNA polymerase ternary complex in cells with mutations of the THO complex components could prolong tethering and facilitate the recruitment of recombination factors onto telomeres for recombination. This notion is supported by the observation that Rad53-mediated DNA damage response was activated in cells with accumulation of telomere-associated TERRA. Thus, TERRA might adopt the general mechanism used by long noncoding RNAs to facilitate their various functions in cells.
Telomerase expression is pivotal for maintaining long-term proliferation of cancer cells. Telomerase has long been recognized as an important target for anticancer therapies (44). The inhibition of telomerase activity in cancer cells should cause telomere shortening and eventually lead to senescence. However, because telomeres in most cancer cells are maintained in a long length, it could take a long time for telomerase inhibition therapy to take effect in inducing the senescence response; therefore, sustained inhibition of telomerase activity is required for the best anticancer therapeutic effects. This treatment scenario is expected to affect other active proliferating cells in the body and may cause more side effects. Our finding that tampering THO complex function in yeast cells lacking telomerase activity can induce premature senescence provides a unique strategy in anticancer treatment. Because the THO complex is structurally and functionally conserved in eukaryotic cells, it is possible that the inhibition of the THO complex might also induce early senescence in cancer cells treated with telomerase inhibitors. Thus, the combination of both the THO complex and telomerase inhibitors might shorten the time between drug administration and clinical response. In addition, because the structure and function of TERRA are also conserved among eukaryotes, one could envision that increasing the accumulation of telomere-associated TERRA could enhance early senescence in cancer cells. Thus, agents that pause or stall the RNA polymerase elongation complex are also candidates for adjuvant therapeutics for telomerase inhibitors in anticancer therapy. Telomeres of the type II recombination survivors are similar to those of mammalian ALT cells. Our findings also implicate an evolutionarily conserved mechanism of generating survivors in cells without telomerase activity.
Materials and Methods
Senescence Rate Determination.
The senescence rate was measured in the liquid culture through serial passages of spore products. Colonies grown from the dissected tetrads were inoculated directly into liquid yeast complete (YC) medium and cultured for 2 d at 25 °C. The cultures were diluted to 3 × 105 cells per milliliter in fresh YC medium and cultured for additional 2 d. The procedure was repeated 8–10 times. A spectrophotometer was used to measure the growth rate. The growth rates were converted to population doublings. By estimation, it takes ∼20 population doublings for a spore to grow into a colony.
ChIP Analysis.
The ChIP analysis was conducted according to a described procedure (45). The Rap1p (SC-6662) antibody was purchased from Santa Cruz Biotechnology. PCR amplifications were carried out to detect the presence of TERRA-Y′, -1L, -15L, and ACT1. In the RNA–ChIP assays, a similar protocol was applied as in the regular ChIP assays, except that RNase inhibitors (Fermentas no. EO0381) were added to the chromatin lysates during the IP procedure. The precipitated RNA was also treated with DNase I to digest genomic DNA. Reverse transcription was conducted by using the same RT-PCR procedure with poly-T18 as primer.
DNA–RNA Hybrid IP.
The colonies from freshly dissected tetrads were inoculated directly into 10 mL of liquid yeast extract peptone dextrose (YEPD) medium and cultured to 3 × 108 cells. The cells were resuspended in DNA extraction buffer [2% (vol/vol) Triton X-100, 1% SDS, 200 mM NaCl, 10 mM EDTA, 10 mM Tris⋅HCl, pH 8.0, and 25 units of RNase inhibitor in 500 μL of buffer] and lysed by vortexing with glass beads using the FastPrep-24 system (MP Biomedicals). The extracted DNA was fragmented into ∼500 bp by using a cup horn sonicator (Qsonica Sonicator 4000), followed by treatment with 200 μg of proteinase K at 42 °C for 1 h to eliminate the proteins. DNA was extracted by using phenol/chloroform (pH 8.0) and precipitated by using ethanol. For IP experiments, DNA at the concentration of 0.25 μg/μL in FA1 buffer (0.1% SDS, 1% Triton X-100, 0.1% Na deoxycholate, 275 mM NaCl, 10 mM Hepes, pH 7.5) was incubated with 5 μg of S9.6 antibody and precipitated upon the addition of protein G Sepharose (SIGMA-Aldrich). The levels of DNA–RNA hybrids were determined by using real-time quantitative PCR. The IP efficiency was represented as folds of immunoprecipitates over input using ACT1 as internal control: (TEL/ACT1)IP /(TEL/ACT1)input. For the RNase H sensitivity assays, the sonicated genomic DNA was incubated with 10 units of RNase H at 37 °C for 1 h and inactivated at 65 °C for 20 min before IP with S9.6 antibody.
Note.
While this work was under review for publication, two papers also published similar conclusions (46, 47).
Supplementary Material
Acknowledgments
We thank Drs. S.-C. Teng, V. A. Zakian, D. Shore, E. Lahue, R. Crouch, and D. E. Gottschling for providing yeast strains and reagents, and Dr. Mei-Yu Chen for critical reading of the manuscript. This research was supported by National Health Research Institute Grant NHRI-EX100-10050SI, National Science Council Grants 99-3112-B-010-001 and 100-2311-B-010-001, and the Ministry of Education.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1307415111/-/DCSupplemental.
References
- 1.Singer MS, Gottschling DE. TLC1: Template RNA component of Saccharomyces cerevisiae telomerase. Science. 1994;266(5184):404–409. doi: 10.1126/science.7545955. [DOI] [PubMed] [Google Scholar]
- 2.Lundblad V, Szostak JW. A mutant with a defect in telomere elongation leads to senescence in yeast. Cell. 1989;57(4):633–643. doi: 10.1016/0092-8674(89)90132-3. [DOI] [PubMed] [Google Scholar]
- 3.Lundblad V, Blackburn EH. An alternative pathway for yeast telomere maintenance rescues est1- senescence. Cell. 1993;73(2):347–360. doi: 10.1016/0092-8674(93)90234-h. [DOI] [PubMed] [Google Scholar]
- 4.Lendvay TS, Morris DK, Sah J, Balasubramanian B, Lundblad V. Senescence mutants of Saccharomyces cerevisiae with a defect in telomere replication identify three additional EST genes. Genetics. 1996;144(4):1399–1412. doi: 10.1093/genetics/144.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McEachern MJ, Blackburn EH. Cap-prevented recombination between terminal telomeric repeat arrays (telomere CPR) maintains telomeres in Kluyveromyces lactis lacking telomerase. Genes Dev. 1996;10(14):1822–1834. doi: 10.1101/gad.10.14.1822. [DOI] [PubMed] [Google Scholar]
- 6.Le S, Moore JK, Haber JE, Greider CW. RAD50 and RAD51 define two pathways that collaborate to maintain telomeres in the absence of telomerase. Genetics. 1999;152(1):143–152. doi: 10.1093/genetics/152.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teng S-C, Zakian VA. Telomere-telomere recombination is an efficient bypass pathway for telomere maintenance in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19(12):8083–8093. doi: 10.1128/mcb.19.12.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Q, Ijpma A, Greider CW. Two survivor pathways that allow growth in the absence of telomerase are generated by distinct telomere recombination events. Mol Cell Biol. 2001;21(5):1819–1827. doi: 10.1128/MCB.21.5.1819-1827.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cesare AJ, Reddel RR. Alternative lengthening of telomeres: Models, mechanisms and implications. Nat Rev Genet. 2010;11(5):319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 10.Teng S-C, Chang J, McCowan B, Zakian VA. Telomerase-independent lengthening of yeast telomeres occurs by an abrupt Rad50p-dependent, Rif-inhibited recombinational process. Mol Cell. 2000;6(4):947–952. doi: 10.1016/s1097-2765(05)00094-8. [DOI] [PubMed] [Google Scholar]
- 11.Azzalin CM, Reichenbach P, Khoriauli L, Giulotto E, Lingner J. Telomeric repeat containing RNA and RNA surveillance factors at mammalian chromosome ends. Science. 2007;318(5851):798–801. doi: 10.1126/science.1147182. [DOI] [PubMed] [Google Scholar]
- 12.Luke B, et al. The Rat1p 5′ to 3′ exonuclease degrades telomeric repeat-containing RNA and promotes telomere elongation in Saccharomyces cerevisiae. Mol Cell. 2008;32(4):465–477. doi: 10.1016/j.molcel.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Schoeftner S, Blasco MA. Developmentally regulated transcription of mammalian telomeres by DNA-dependent RNA polymerase II. Nat Cell Biol. 2008;10(2):228–236. doi: 10.1038/ncb1685. [DOI] [PubMed] [Google Scholar]
- 14.Bah A, Wischnewski H, Shchepachev V, Azzalin CM. The telomeric transcriptome of Schizosaccharomyces pombe. Nucleic Acids Res. 2012;40(7):2995–3005. doi: 10.1093/nar/gkr1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeiffer V, Lingner J. TERRA promotes telomere shortening through exonuclease 1-mediated resection of chromosome ends. PLoS Genet. 2012;8(6):e1002747. doi: 10.1371/journal.pgen.1002747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng Z, Norseen J, Wiedmer A, Riethman H, Lieberman PM. TERRA RNA binding to TRF2 facilitates heterochromatin formation and ORC recruitment at telomeres. Mol Cell. 2009;35(4):403–413. doi: 10.1016/j.molcel.2009.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maicher A, Kastner L, Dees M, Luke B. Deregulated telomere transcription causes replication-dependent telomere shortening and promotes cellular senescence. Nucleic Acids Res. 2012;40(14):6649–6659. doi: 10.1093/nar/gks358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chávez S, et al. A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae. EMBO J. 2000;19(21):5824–5834. doi: 10.1093/emboj/19.21.5824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Strässer K, et al. TREX is a conserved complex coupling transcription with messenger RNA export. Nature. 2002;417(6886):304–308. doi: 10.1038/nature746. [DOI] [PubMed] [Google Scholar]
- 20.Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Mol Cell. 2003;12(3):711–721. doi: 10.1016/j.molcel.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 21.Gómez-González B, Felipe-Abrio I, Aguilera A. The S-phase checkpoint is required to respond to R-loops accumulated in THO mutants. Mol Cell Biol. 2009;29(19):5203–5213. doi: 10.1128/MCB.00402-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aguilera A, García-Muse T. R loops: From transcription byproducts to threats to genome stability. Mol Cell. 2012;46(2):115–124. doi: 10.1016/j.molcel.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Stirling PC, et al. R-loop-mediated genome instability in mRNA cleavage and polyadenylation mutants. Genes Dev. 2012;26(2):163–175. doi: 10.1101/gad.179721.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu T-Y, Wang C-Y, Lin J-J. Depleting components of the THO complex causes increased telomere length by reducing the expression of the telomere-associated protein Rif1p. PLoS ONE. 2012;7(3):e33498. doi: 10.1371/journal.pone.0033498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iglesias N, et al. Subtelomeric repetitive elements determine TERRA regulation by Rap1/Rif and Rap1/Sir complexes in yeast. EMBO Rep. 2011;12(6):587–593. doi: 10.1038/embor.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boube M, Joulia L, Cribbs DL, Bourbon HM. Evidence for a mediator of RNA polymerase II transcriptional regulation conserved from yeast to man. Cell. 2002;110(2):143–151. doi: 10.1016/s0092-8674(02)00830-9. [DOI] [PubMed] [Google Scholar]
- 27.Reeves WM, Hahn S. Targets of the Gal4 transcription activator in functional transcription complexes. Mol Cell Biol. 2005;25(20):9092–9102. doi: 10.1128/MCB.25.20.9092-9102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santos-Rosa H, Aguilera A. Isolation and genetic analysis of extragenic suppressors of the hyper-deletion phenotype of the Saccharomyces cerevisiae hpr1 delta mutation. Genetics. 1995;139(1):57–66. doi: 10.1093/genetics/139.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fan H-Y, Merker RJ, Klein HL. High-copy-number expression of Sub2p, a member of the RNA helicase superfamily, suppresses hpr1-mediated genomic instability. Mol Cell Biol. 2001;21(16):5459–5470. doi: 10.1128/MCB.21.16.5459-5470.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jimeno S, Rondón AG, Luna R, Aguilera A. The yeast THO complex and mRNA export factors link RNA metabolism with transcription and genome instability. EMBO J. 2002;21(13):3526–3535. doi: 10.1093/emboj/cdf335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.West RW, Jr, Milgrom E. DEAD-box RNA helicase Sub2 is required for expression of lacZ fusions in Saccharomyces cerevisiae and is a dosage-dependent suppressor of RLR1 (THO2) Gene. 2002;288(1-2):19–27. doi: 10.1016/s0378-1119(02)00482-1. [DOI] [PubMed] [Google Scholar]
- 32.Libri D, Graziani N, Saguez C, Boulay J. Multiple roles for the yeast SUB2/yUAP56 gene in splicing. Genes Dev. 2001;15(1):36–41. doi: 10.1101/gad.852101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jensen TH, Boulay J, Rosbash M, Libri D. The DECD box putative ATPase Sub2p is an early mRNA export factor. Curr Biol. 2001;11(21):1711–1715. doi: 10.1016/s0960-9822(01)00529-2. [DOI] [PubMed] [Google Scholar]
- 34.Strässer K, Hurt E. Splicing factor Sub2p is required for nuclear mRNA export through its interaction with Yra1p. Nature. 2001;413(6856):648–652. doi: 10.1038/35098113. [DOI] [PubMed] [Google Scholar]
- 35.Zenklusen D, Vinciguerra P, Wyss JC, Stutz F. Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol Cell Biol. 2002;22(23):8241–8253. doi: 10.1128/MCB.22.23.8241-8253.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kistler AL, Guthrie C. Deletion of MUD2, the yeast homolog of U2AF65, can bypass the requirement for sub2, an essential spliceosomal ATPase. Genes Dev. 2001;15(1):42–49. doi: 10.1101/gad.851301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lahue E, Heckathorn J, Meyer Z, Smith J, Wolfe C. The Saccharomyces cerevisiae Sub2 protein suppresses heterochromatic silencing at telomeres and subtelomeric genes. Yeast. 2005;22(7):537–551. doi: 10.1002/yea.1231. [DOI] [PubMed] [Google Scholar]
- 38.Conrad MN, Wright JH, Wolf AJ, Zakian VA. RAP1 protein interacts with yeast telomeres in vivo: Overproduction alters telomere structure and decreases chromosome stability. Cell. 1990;63(4):739–750. doi: 10.1016/0092-8674(90)90140-a. [DOI] [PubMed] [Google Scholar]
- 39.Redon S, Reichenbach P, Lingner J. The non-coding RNA TERRA is a natural ligand and direct inhibitor of human telomerase. Nucleic Acids Res. 2010;38(17):5797–5806. doi: 10.1093/nar/gkq296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sandell LL, Gottschling DE, Zakian VA. Transcription of a yeast telomere alleviates telomere position effect without affecting chromosome stability. Proc Natl Acad Sci USA. 1994;91(25):12061–12065. doi: 10.1073/pnas.91.25.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Norseen J, et al. RNA-dependent recruitment of the origin recognition complex. EMBO J. 2008;27(22):3024–3035. doi: 10.1038/emboj.2008.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.González-Aguilera C, et al. The THP1-SAC3-SUS1-CDC31 complex works in transcription elongation-mRNA export preventing RNA-mediated genome instability. Mol Biol Cell. 2008;19(10):4310–4318. doi: 10.1091/mbc.E08-04-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: Insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 44.Harley CB. Telomerase and cancer therapeutics. Nat Rev Cancer. 2008;8(3):167–179. doi: 10.1038/nrc2275. [DOI] [PubMed] [Google Scholar]
- 45.Hsieh YC, et al. The U3 small nucleolar ribonucleoprotein component Imp4p is a telomeric DNA-binding protein. Biochem J. 2007;408(3):387–393. doi: 10.1042/BJ20070968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balk B, et al. Telomeric RNA-DNA hybrids affect telomere-length dynamics and senescence. Nat Struct Mol Biol. 2013;20(10):1199–1205. doi: 10.1038/nsmb.2662. [DOI] [PubMed] [Google Scholar]
- 47.Pfeiffer V, Crittin J, Grolimund L, Lingner J. The THO complex component Thp2 counteracts telomeric R-loops and telomere shortening. EMBO J. 2013;32(21):2861–2871. doi: 10.1038/emboj.2013.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.