Significance
Most disease-causing mutations in genes encoding enzymes impair the folding or catalytic function of the affected protein. Here we report that two missense mutations in the N-terminal cytoplasmic tail of a Golgi-localized enzyme result in the failure of the enzyme to be retained in the Golgi, with subsequent degradation in lysosomes/release from the cells. This mislocalization of catalytically active phosphotransferase represents the basis of the lysosomal storage disorder present in patients with these mutations. In addition, these findings provide a clue to the signals that retain this enzyme, and perhaps other Golgi-resident enzymes, in the Golgi complex.
Abstract
The lysosomal storage disorder mucolipidosis III αβ is caused by mutations in the αβ subunits of UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase (phosphotransferase). This Golgi-localized enzyme mediates the first step in the synthesis of the mannose 6-phosphate recognition marker on lysosomal acid hydrolases, and loss of function results in impaired lysosomal targeting of these acid hydrolases and decreased lysosomal degradation. Here we show that two patient missense mutations, Lys4Gln and Ser15Tyr, in the N-terminal cytoplasmic tail of the α subunit of phosphotransferase impair retention of the catalytically active enzyme in the Golgi complex. This results in mistargeting of the mutant phosphotransferases to lysosomes, where they are degraded, or to the cell surface and release into the medium. The finding that mislocalization of active phosphotransferase is the basis for mucolipidosis III αβ in a subset of patients shows the importance of single residues in the cytoplasmic tail of a Golgi-resident protein for localization to this compartment.
Lysosomes are vital for cells to degrade intracellular and endocytosed material. For this reason, defective lysosome biogenesis has detrimental effects on cellular function and is associated with a variety of diseases. Mucolipidosis (ML) III αβ is a lysosomal storage disorder caused by the impaired targeting of newly synthesized acid hydrolases to lysosomes. This results in severely decreased lysosomal degradative capacity and the accumulation of storage material in enlarged lysosomes in many tissues (1).
MLIII αβ results from mutations in the GNPTAB gene that encodes the α and β subunits of the enzyme UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase (phosphotransferase). Phosphotransferase catalyzes the first step in the generation of the mannose 6-phosphate (Man-6-P) recognition marker on newly synthesized acid hydrolases that directs them to the lysosome. It transfers GlcNAc-1-P to mannose residues on the hydrolases in the cis-Golgi complex. The GlcNAc is then excised by a second enzyme, termed uncovering enzyme, to generate the Man-6-P monoester, which serves as a high-affinity ligand for the Man-6-P receptors. These receptors transport the acid hydrolases from the trans-Golgi network to the endo-lysosomal system, where they function. Phosphotransferase is a heterohexameric enzyme composed of two α, β, and γ subunits (2). The α and β subunits are synthesized as a catalytically inactive αβ precursor of 1,256 amino acids. It is a type 3 transmembrane protein, with a 19-amino acid N-terminal tail (Fig. 1A) and a 21-amino acid C-terminal tail, both of which are cytoplasmically oriented. The αβ subunits undergo a proteolytic cleavage between amino acid Lys928 and Asp929 in the Golgi complex by the site 1 protease to generate the mature, catalytically active α and β subunits (3, 4). These subunits also have the ability to recognize the lysosomal acid hydrolases as specific substrates (5). The γ subunit, encoded by the GNPTG gene, is a soluble glycoprotein of 305 amino acids that enhances the phosphorylation of certain lysosomal enzyme substrates (5).
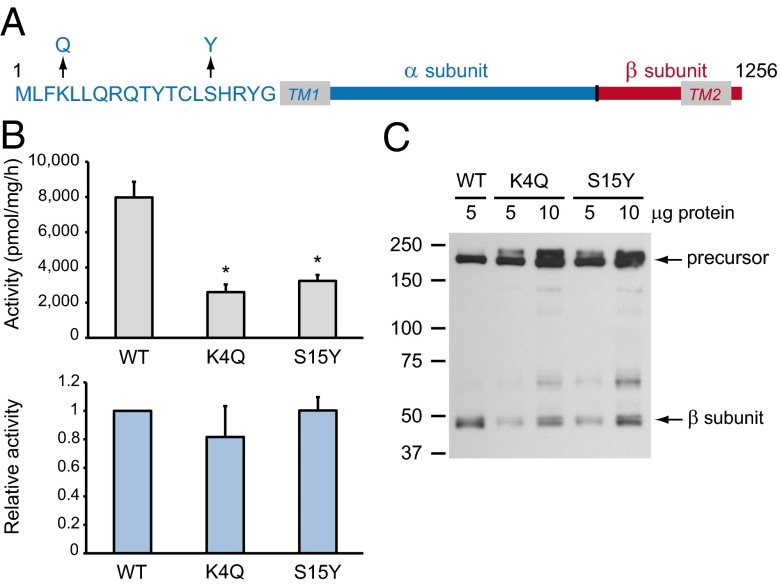
Fig. 1.
Phosphotransferase mutations K4Q and S15Y result in reduced levels of the mature β subunit but do not affect the catalytic activity of the residual protein. (A) Schematic representation of the α (blue) and β (red) subunits of human phosphotransferase, showing the positions of the K4Q and S15Y mutations in the N-terminal tail. TM, transmembrane domain. (B) Phosphotransferase activity toward α-MM was measured in HEK293 cell lysates overexpressing the WT or mutant phosphotransferase αβ subunits (Upper). The relative activity (Lower) shows the activity corrected for the level of mature β subunit relative to WT. Data are average values of 3 independent experiments, error bars represent SDs. *P < 0.01 (Student t test). (C) HEK293 cell lysates expressing WT, K4Q, or S15Y phosphotransferase αβ subunits with a C-terminal V5-tag were subjected to SDS/PAGE and anti-V5 immunoblotting 48 h after transfection (representative blot of 3 independent experiments). The ratio of mature β subunit to the precursor is reduced in the case of the mutants compared with WT.
A number of missense mutations have been detected in individuals with MLIII αβ. In this study, the effect of two missense mutations in the N-terminal cytoplasmic tail of the α subunit of phosphotransferase, Lys4Gln (K4Q) and Ser15Tyr (S15Y) (6–8), was investigated (Fig. 1A). These mutations did not directly affect the catalytic function of the enzyme. Instead, they resulted in the failure of the mutant proteins to be retained in the Golgi complex, leading to enhanced degradation in lysosomes or release into the culture medium. As a result, the activity of these mutants in cells was significantly decreased. Thus, mislocalization of phosphotransferase was shown to be the underlying cause of the MLIII αβ phenotype in patients with K4Q and S15Y mutations. The finding that these two residues are critical for localization of phosphotransferase to the cis-Golgi complex may allow for a better understanding of the mechanism that localizes Golgi-resident enzymes to this compartment.
Results
The K4Q and S15Y Mutants Have a Normal Relative Activity.
The αβ precursors of wild-type (WT), K4Q, or S15Y phosphotransferase with a C-terminal V5-tag were expressed in HEK293 cells, and the catalytic activity was measured 48 h after transfection in an in vitro assay, using the simple sugar α-methyl d-mannoside (α-MM) as a substrate. Extracts of cells expressing the mutants exhibited decreased activity, with K4Q having 32 ± 2.7% and S15Y 41 ± 3.2% of WT activity (Fig. 1B, Upper). Analysis by SDS/PAGE and immunoblotting showed normal expression of the inactive mutant αβ precursors (∼190-kDa bands; Fig. 1C) but decreased levels of the mature β subunits (∼45 kDa; 41 ± 8.5% and 41 ± 4.6% of WT for K4Q and S15Y, respectively). Note that the α subunit is not detected, as the V5-tag is fused to the β subunit. This decrease in β subunit level was similar to the reduction in the total catalytic activity, thus showing a normal relative activity (Fig. 1B, Lower). Therefore, these mutations affect the level of the β subunits, but not the catalytic activity of the residual enzyme.
K4Q and S15Y αβ Phosphotransferase Mislocalize in Punctae.
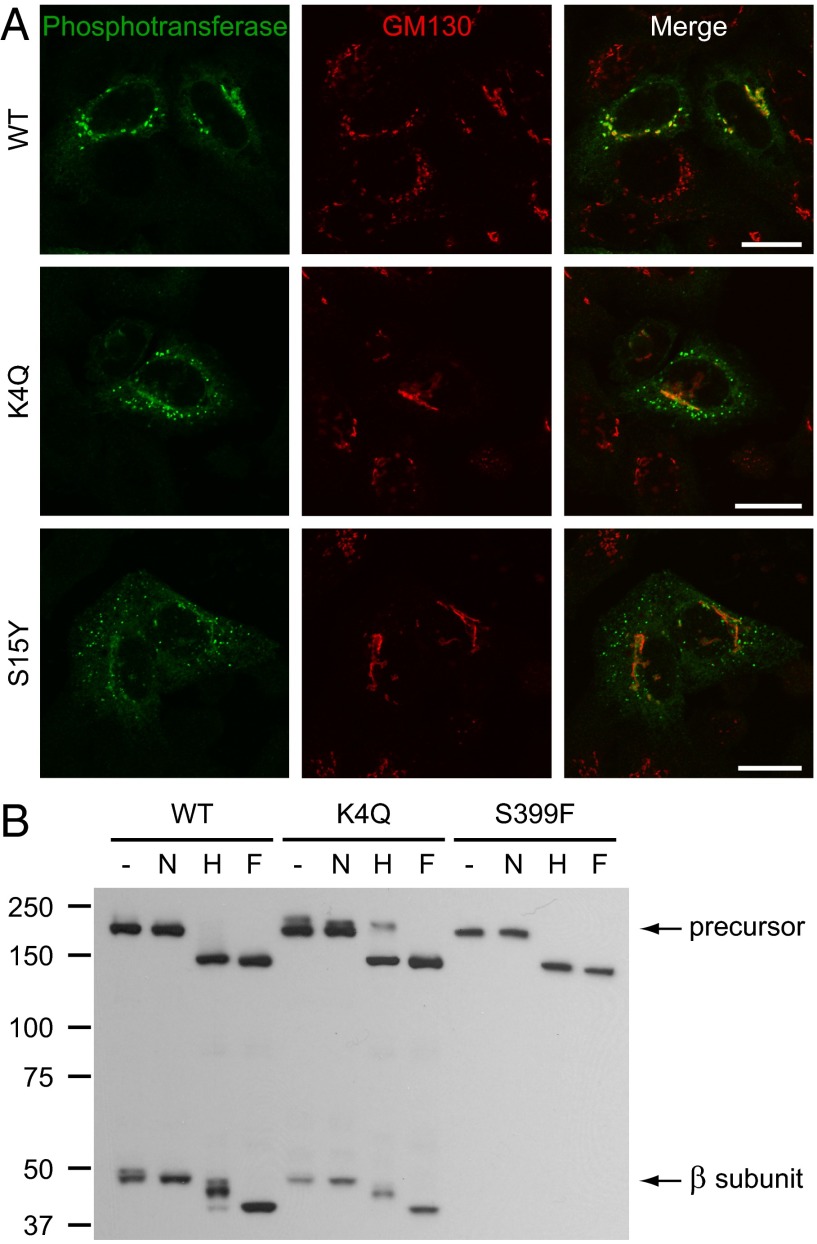
The αβ precursor of phosphotransferase is cleaved into active α and β subunits in the Golgi complex. Therefore, a block in exit from the endoplasmic reticulum (ER) could cause the reduced β subunit levels that are observed with the K4Q and S15Y mutants by Western blotting. To examine this possibility, the subcellular localization of the WT enzyme and mutants was compared by confocal immunofluorescence microscopy. The localization of WT αβ phosphotransferase was dependent on the level of overexpression. In HeLa cells with the highest levels of overexpression, phosphotransferase was detected in the Golgi complex, as well as in the ER and on the plasma membrane. In low and moderate overexpressors, phosphotransferase was confined to the Golgi complex, where it colocalized with the cis-Golgi marker GM130 (Fig. 2A). The K4Q and S15Y mutants had a markedly altered localization compared with WT. Low levels were found in the Golgi complex and in the ER, but the majority was present in punctae throughout the cytoplasm (Fig. 2A). The punctae did not represent ER or ER exit sites, as no significant colocalization was observed with the markers calnexin or Sec31A, respectively (Fig. S1 A and B). This is in contrast to the findings with another MLIII αβ mutant, Ser399Phe (S399F) (9), which gave a predominant ER staining by immunofluorescence (Fig. S1A) and an undetectable β subunit by Western blotting (Fig. 2B) (10), indicative of misfolding in the ER. Thus, reduced ER exit is unlikely to be the cause of the low β subunit levels of the K4Q and S15Y mutants.
Fig. 2.
K4Q and S15Y αβ phosphotransferase mislocalize in punctae that represent post-ER compartments. (A) Immunofluorescence staining for phosphotransferase (anti-α subunit) and the cis-Golgi marker GM130 in HeLa cells 16 h after transfection with WT, K4Q, or S15Y αβ phosphotransferase. Although the WT enzyme localizes to the Golgi complex, the mutants localize in punctae with only low levels in the Golgi. (Scale bars, 20 μm.) (B) Western blots (anti-V5 antibody) of transfected HEK293 cell lysates treated with neuraminidase (N), Endo Hf (H), or PNGase F (F) (3 h at 37 °C) or untreated (−) show partial digestion of the β subunits (WT and K4Q) by Endo Hf. Although the WT αβ precursor almost completely shifts to ∼150 kDa after digestion by Endo Hf, the K4Q precursor is partially insensitive to Endo Hf (∼210-kDa band) and shows a clear shift after neuraminidase treatment, indicating the presence of complex-type N-linked glycans.
K4Q αβ Phosphotransferase Contains Increased Complex-Type N-Linked Glycans.
Further evidence that the punctate staining pattern displayed by the two cytoplasmic tail mutants represents post-ER compartments was obtained by analyzing the nature of the sugar modifications of WT, K4Q, and S399F αβ phosphotransferase. The αβ precursor contains multiple N-linked glycosylation sites that receive high mannose-type glycans in the ER that can be further modified to complex-type units if the enzyme reaches the medial/trans-Golgi complex. Treatment with endoglycosidase Hf (Endo Hf) and N-glycosidase F (PNGase F) allows identification of the types of N-linked glycans, as Endo Hf only digests high mannose-type units, whereas PNGase F cleaves both types of glycans. In addition, neuraminidase treatment was used to identify glycans with sialic acids that are added to N- and O-linked oligosaccharides in the trans-Golgi complex. In the case of the S399F precursor, treatment with Endo Hf or PNGase F had the same effect on its migration (shift to ∼150 kDa; Fig. 2B), showing that all of the N-linked glycans are of the high mannose-type, which is consistent with its ER localization. The WT precursor contained a very low amount of complex-type glycans, as treatment with neuraminidase caused a slight shift in its migration and Endo Hf a near complete shift to 150 kDa (Fig. 2B). This suggests that at least a small portion of the uncleaved precursor is present in the Golgi complex, indicating that cleavage by the site 1 protease in that compartment does not occur immediately on arrival of the precursor in that organelle. In contrast, the β subunit was found to contain a mixture of high mannose- and complex-type units, as indicated by the shift in gel migration after neuraminidase treatment and the partial digestion by Endo Hf, with just a small portion containing only high mannose units. These results suggest that most of the mature Golgi-localized phosphotransferase and a small portion of the αβ precursor reach the medial/trans cisternae, where they then undergo retrograde transport to maintain their concentration in the cis-Golgi.
Although the K4Q β subunit behaved in a manner similar to WT, Endo Hf treatment of the precursor revealed a discrete band at ∼210 kDa in addition to the 150-kDa, completely digested band (Fig. 2B). Neuraminidase digestion of this precursor also resulted in a significant downward shift of the slowest migrating band. This shows that a significant portion of the mutant precursor contains complex-type N-linked glycans, consistent with some of the mutant precursor reaching the medial/trans cisternae before being cleaved by the site 1 protease.
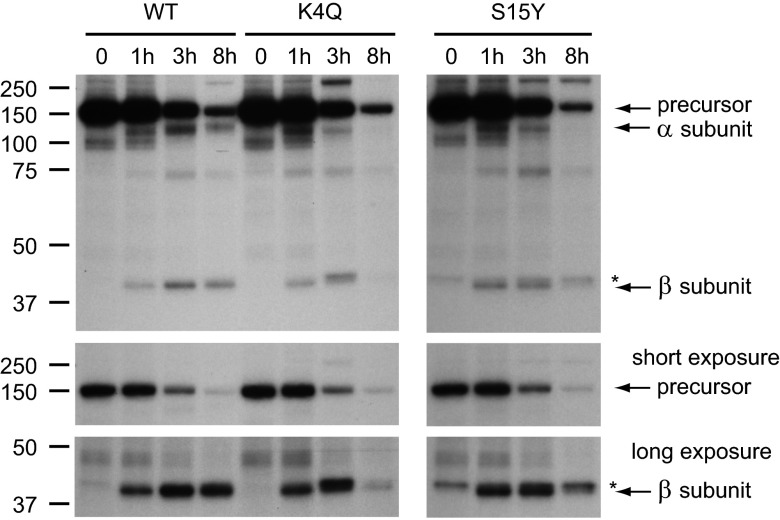
The Half-life of the K4Q and S15Y Mutants Is Decreased.
We next performed pulse-chase labeling experiments to obtain a more quantitative measure of the trafficking of αβ phosphotransferase and to explore the possibility that the low levels of the β subunits of the K4Q and S15Y mutants might be a result of enhanced turnover of the mature enzyme. In the initial experiments, HEK293 cells were transfected with a plasmid encoding WT αβ precursor. After overnight transfection, the cells were labeled for 20 min with [35S]methionine/cysteine and either harvested or chased for 3 h. Phosphotransferase was then immunoprecipitated via its C-terminal V5-tag and analyzed by SDS/PAGE and autoradiography. After the pulse, a single band at ∼190 kDa appeared, which corresponded to the αβ precursor (Fig. S2A). The precursor contained only high mannose-type glycans, as shown by its complete sensitivity to Endo Hf (Fig. S2A), consistent with an ER localization. After the 3-h chase, a portion of the immunoprecipitate had become partially resistant to Endo Hf and shifted to ∼110 kDa after treatment with PNGase F. We reasoned that this band represented the α subunit, which likely contains more complex-type glycans and, therefore, despite its smaller polypeptide size, runs at a similar molecular weight to that of the precursor in the untreated lane. In addition, the β subunit was detected after the chase and contained a mixture of high mannose and complex-type N-linked glycans (Fig. S2A). When an antibody to the α subunit was used in the immunoprecipitation, the same ∼110-kDa band appeared after PNGase F treatment (Fig. S2B), together with the 150-kDa precursor and the β subunit, indicating that the cleaved α and β subunits are immunoprecipitated as a complex.
Pulse-chase experiments were then performed with the K4Q, S15Y, and S399F mutants, along with the WT control (Fig. 3; Fig. S3). Phosphotransferase was immunoprecipitated either at the end of the 20-min pulse or after 1, 3, or 8 h of chase. All samples were treated with PNGase F to allow the cleaved α subunit to be distinguished from the αβ precursor. In the case of WT phosphotransferase, the cleaved α (110 kDa) and β (40 kDa) subunits appeared after the 1-h chase and peaked after 3 h; at 8 h, substantial amounts were still detected (Fig. 3). Consistent with the appearance of the cleaved subunits, the level of αβ precursor decreased after the 1-h chase (see short exposure in Fig. 3). However, the loss of the precursor could not be fully accounted for by the formation of the cleaved subunits, suggesting that a substantial portion of the newly synthesized αβ precursor is degraded before it exits the ER. The various mutants showed a similar pattern.
Fig. 3.
The half-life of K4Q and S15Y αβ phosphotransferase is reduced compared with WT. Transfected HEK293 cells were labeled with TRAN 35S-LABEL methionine/cysteine for 20 min, and αβ phosphotransferase was immunoprecipitated with anti-V5 either immediately or after a 1-, 3-, or 8-h chase. All samples were treated with PNGase F (3 h at 37 °C). After the 8-h chase, the K4Q and S15Y mature β subunits are clearly decreased compared with WT. *Background band just above the 40-kDa β subunit. Note that a similar band is present after immunoprecipitation of the ΔCT and S399F mutants (Fig. S3). The autoradiographs are representative of 3–5 experiments.
The appearance of the cleaved α and β subunits of the K4Q and S15Y mutants followed the same course as seen with the WT, indicating that ER exit was not delayed. Importantly, the later chase times showed that the half-life of the mutants was significantly decreased, with the α and β subunits being almost completely gone by 8 h (see long-exposure β subunit in Fig. 3). In some cases, a band just above the 40-kDa β subunit was detected (indicated by an asterisk in Fig. 3 and Fig. S3). We concluded that this represents a background band, based on the observation that it was seen with the S399F mutant that makes only trace amounts of β (Fig. S3). Further, an αβ mutant that lacks most of the C-terminal cytoplasmic tail showed a clear separation of the background band from the truncated β subunit (ΔCT; Fig. S3). This mutant behaved very similarly to WT αβ phosphotransferase, which suggests that the lack of the C-terminal tail does not substantially impair the exit of the precursor from the ER or the stability of the enzyme in the Golgi. In agreement with this, the ΔCT mutant colocalized with GM130 and had normal catalytic activity.
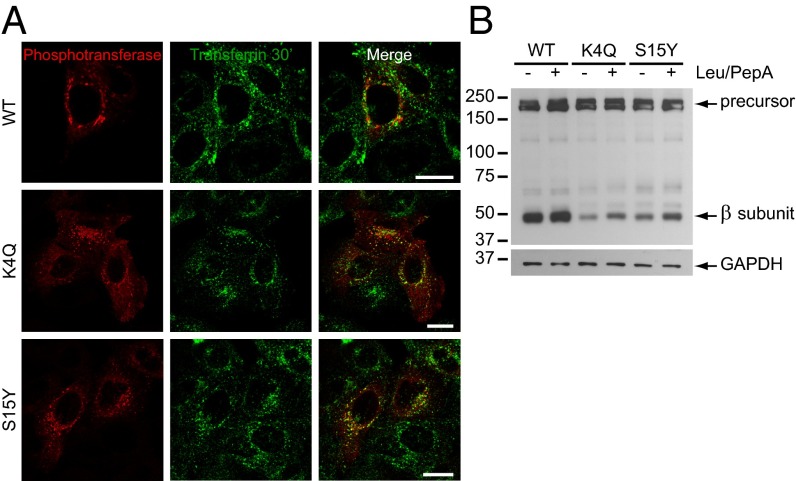
The K4Q and S15Y Mutants Undergo Lysosomal Degradation and Release into the Culture Medium.
In view of the increased turnover of the mutants, we hypothesized that the punctae represented endo-lysosomal compartments to which phosphotransferase is targeted for degradation. Consistent with this proposal, substantial colocalization was observed between the punctae and Alexa Fluor 488-conjugated transferrin after a 30-min uptake (Fig. 4A), indicating localization to early endosomes/recycling endosomes. However, the phosphotransferase-containing punctae colocalized only occasionally with the late endosomal/lysosomal marker lysosome-associated membrane protein-2 (LAMP-2) (Fig. S4). The failure to detect mutant phosphotransferase in these compartments could be a result of rapid degradation as it reaches the acidic environment of the late endosomes/lysosomes. Therefore, lysosomal degradation was blocked with leupeptin/pepstatin A for 7 h in cells overexpressing WT and mutant αβ subunits. This resulted in a ∼twofold increase in β subunit by Western blotting in the case of the mutants, but not WT (Fig. 4B). These data suggest that the mutants are targeted to the endo-lysosomal system, which results in their enhanced degradation.
Fig. 4.
K4Q and S15Y αβ phosphotransferase show increased degradation in lysosomes. (A) Confocal immunofluorescence microscopy on HeLa cells expressing WT, K4Q, or S15Y αβ phosphotransferase. The cells were incubated with transferrin-Alexa Fluor 488 for 30 min and stained with anti-α subunit. K4Q and S15Y phosphotransferase show substantial colocalization with transferrin-positive endosomes. (Scale bars, 20 μm.) (B) Western blot (anti-V5) of HEK293 cells expressing WT, K4Q, or S15Y αβ phosphotransferase treated with or without 100 μM leupeptin and 100 μM pepstatin A for 7 h (representative blot of 2 independent experiments). The levels of the mature β subunits of the mutants increase approximately twofold after inhibition of lysosomal degradation. GAPDH shows equal protein loading.
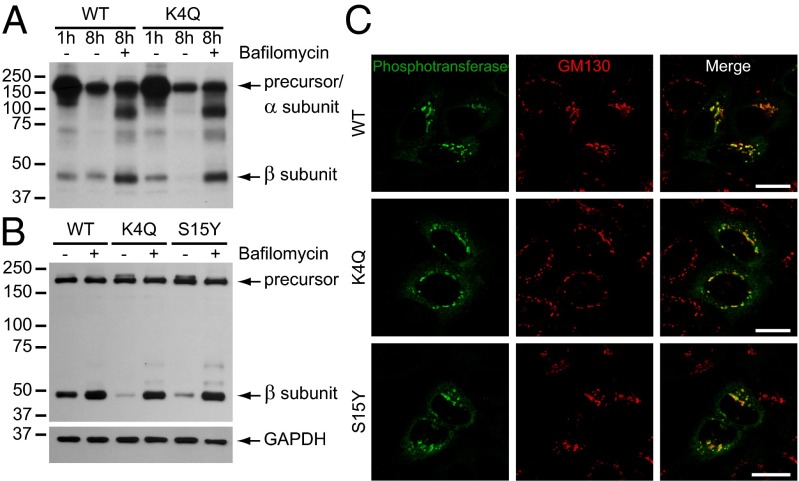
To establish a more complete block in endo-lysosomal degradation, the cells were treated with bafilomycin a1, which prevents acidification of these compartments by inhibiting the V-ATPases. Pulse-chase labeling showed a significant increase in the β subunit of WT phosphotransferase after an 8-h chase in the presence of bafilomycin (Fig. 5A), which suggests that a substantial portion of the WT enzyme is degraded in this time. Although the K4Q β subunit was hardly detectable after the 8-h chase, it was restored to a level similar to WT in the presence of bafilomycin (Fig. 5A). A 7-h treatment with bafilomycin of transiently transfected cells also showed a major increase in the β subunit levels of both mutants (Fig. 5B). These data suggest that the mutant enzyme is rapidly turned over in late endosomes and lysosomes. However, when the subcellular localization of the mutants was evaluated by immunofluorescence microscopy after a 4-h treatment with bafilomycin, they were detected in the Golgi complex with similar intensity as the WT enzyme (Fig. 5C). This finding was somewhat unexpected, although bafilomycin has been reported to affect Golgi morphology and receptor recycling between the Golgi complex and endosomes (11, 12). Therefore, our data support the conclusion that loss of the mutant phosphotransferases occurs at a site beyond the Golgi complex and, at least partially, through degradation in the endo-lysosomal system.
Fig. 5.
Bafilomycin treatment prevents the turnover of K4Q and S15Y αβ phosphotransferase (A) Transfected HEK293 cells were labeled with TRAN 35S-LABEL methionine/cysteine for 20 min, and αβ phosphotransferase was immunoprecipitated with anti-V5 after a 1- or 8-h chase with or without 0.1 μM bafilomycin a1. The samples were not treated with PNGase F. Bafilomycin treatment increases the level of the β subunits of both WT and K4Q phosphotransferase. (B) Western blot (anti-V5) of HEK293 cells expressing WT, K4Q, or S15Y αβ phosphotransferase treated with or without 0.1 μM bafilomycin a1 for 7 h (representative blot of 2 independent experiments). The mature β subunits of the mutants increase to the level of the WT β subunit after bafilomycin treatment. GAPDH shows equal protein loading. (C) Confocal immunofluorescence microscopy of HeLa cells treated for 4 h with 0.1 μM bafilomycin. The mutants (anti-α subunit) colocalize with GM130 in the cis-Golgi. (Scale bars, 20 μm.)
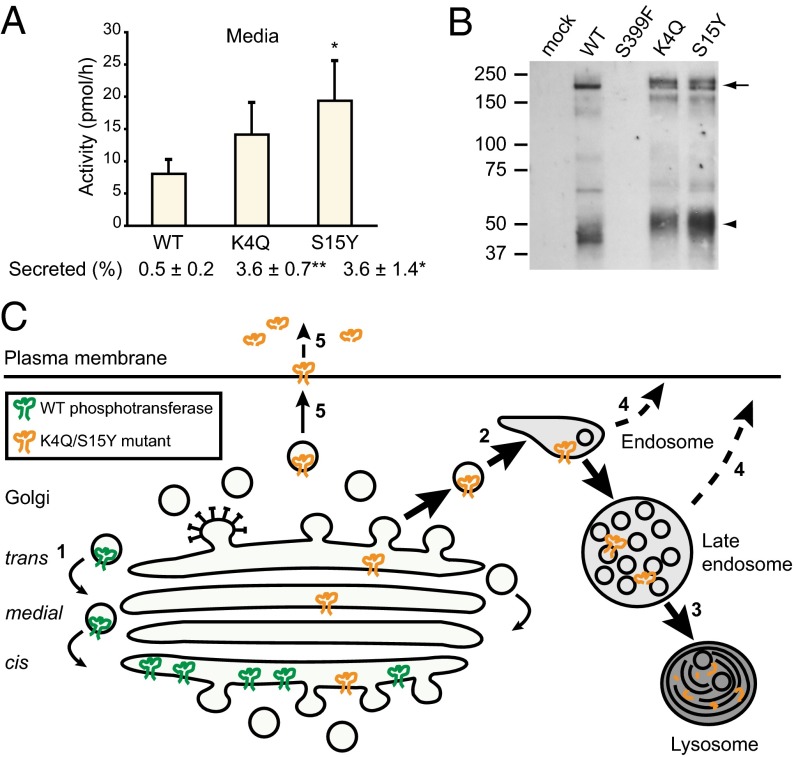
Because it has been shown that certain Golgi glycosyltransferases are secreted into the bloodstream (13), we next tested whether secretion or shedding from the plasma membrane could contribute to the rapid loss of the mutant phosphotransferases. To this end, phosphotransferase activity was measured in the culture media of transfected cells after a 15-h collection period. A low level of activity was detected in the medium of WT transfected cells (Fig. 6A). Media of cells expressing the S15Y mutant contained 2.4-fold more activity than WT media (P < 0.05), whereas the media of cells transfected with the K4Q mutant showed a 1.7-fold increase over WT, although this was not statistically significant. However, as the activity of the mutant cell lysates was much lower than WT (Fig. S5A), with a similar expression of the precursor (Fig. S5B), the percentage of total activity that was secreted was significantly increased compared with WT (i.e., 3.6 ± 0.7% for K4Q and 3.6 ± 1.4% for S15Y compared with 0.5 ± 0.2% for WT).
Fig. 6.
K4Q and S15Y αβ phosphotransferase are released into the media. (A) The activity of phosphotransferase toward α-MM was measured 48 h after transfection in HEK293 media after a 15-h incubation. The percentage secreted represents the total activity in the medium as a percentage of the total activity in cells and medium. See Fig. S5A for the activity in the cell lysates. Data are average values of 3 independent experiments, error bars represent SD. *P < 0.05; **P < 0.01 (Student t test). (B) Western blots (anti-α subunit antibody) of media from transfected HEK293 cells collected after a 6-h incubation. One-fifteenth of the total volume of medium was loaded for K4Q; all other media volumes were adjusted relative to the protein concentration of the cell lysates (see Fig. S5B for Western blot on cell lysates). The arrow indicates the αβ precursor/α subunit, and the arrowhead is a putative degradation product of the α subunit that was not observed in the cell lysates. (C) Model for the mislocalization of K4Q and S15Y αβ phosphotransferase. After synthesis and folding in the ER, the WT precursor is transported to the cis-Golgi, where it is activated by proteolytic cleavage into α and β subunits. Gradually, the enzyme progresses to the medial and trans cisternae, where it acquires complex-type N-linked glycans, but it is then recycled to the cis-Golgi (1), where the bulk of the enzyme is localized at steady state. The K4Q and S15Y mutants are transported from the ER to the Golgi complex at the same rate as the WT enzyme but fail to be retained in the Golgi. Rather, the mutants pass through the Golgi and undergo transport to endosomes (2) and lysosomes, where they are degraded (3). Some of the mutant enzyme is released into the extracellular milieu, either directly from endosomes (4) or via the plasma membrane (5).
Analysis of the media by Western blotting showed the presence of multiple bands that reacted with the anti-α subunit antibody (Fig. 6B). No reactivity was observed with an antibody to the C-terminal V5-tag, which could mean that the protein in the media represents αβ precursor/α subunit that has been shed from the plasma membrane, a process in which it loses the cytoplasmic tail that contains the V5-tag. However, at this point, we cannot exclude the possibility that phosphotransferase is released into the medium via different mechanisms; for instance, via exosome secretion. No reactivity was observed with media from mock-transfected cells or with media from cells expressing the S399F mutant (Fig. 6B). This indicates that the detected αβ subunits are not a result of cell lysis but represent enzyme that has passed through the Golgi complex.
Discussion
The data presented in this study establish that the primary abnormality resulting from the disease-causing K4Q and S15Y mutations is the failure of phosphotransferase to be retained in the Golgi. This leads to the mutant enzymes being delivered to lysosomes, where they are degraded or, to a lesser extent, released from the cell into the surrounding medium (Fig. 6C). As a consequence, the generation of Man-6-P residues on the newly synthesized acid hydrolases is insufficient for proper targeting of these degradative enzymes to lysosomes, resulting in the development of a lysosomal storage disorder.
What might be the role of the N-terminal cytoplasmic tail of the α subunit in the localization of phosphotransferase to the Golgi? There are two likely possibilities based on the current models of intra-Golgi transport (14). One model proposes that cargo moves across the Golgi complex via vesicular transport carriers in a cis to trans direction, with the Golgi-resident enzymes remaining in specific cisternae where they function. In this case, the cytoplasmic tail of phosphotransferase would engage a protein or proteins that retain the enzyme in the cis-Golgi. The alternate model proposes that the cargo remains in a cisterna that matures from cis to trans through the sequential acquisition of the appropriate Golgi enzymes. In this scenario, the Golgi-resident enzymes are transported in a retrograde manner from older to younger cisternae via vesicular carriers. Here the cytoplasmic tail of phosphotransferase would contain a motif that is recognized by an adaptor protein involved in the assembly of the retrograde vesicular carriers. In either case, the critical motifs present in the N-terminal cytoplasmic tail of phosphotransferase would include Lys4 and Ser15.
Although a number of studies using mammalian cells have documented that the cytoplasmic tails of glycosyltransferases influence the Golgi localization of these enzymes, little is known about the mechanism involved (15). In yeast, it has been shown that the cytoplasmic protein Vps74p interacts with a pentameric motif, (F/L)(L/I/V)-X-X-(R/K), present in the cytoplasmic tails of numerous Golgi-localized glycosyltransferases, as well as with components of the COPI coat (16). Deletion of VPS74 resulted in loss of Golgi localization of a subset of the glycosyltransferases (16, 17). Vps74p was therefore proposed to maintain these enzymes in the Golgi by promoting their recycling via COPI vesicles, similar to the second model discussed earlier. The mammalian ortholog of Vps74p, GOLPH3, has been shown to mediate the Golgi localization of the core 2 N-acetylglucosaminyltransferase 1 via interaction with a LLRRR motif in its cytoplasmic tail (18). In addition, GOLPH3 has been proposed to affect the subcellular distribution of exostosin glycosyltransferase 1 and 2 in certain cell types (19). It will be of interest to find out whether GOLPH3 interacts with other glycosyltransferases in the Golgi.
Recently, Franke and colleagues reported that the K4Q mutation impaired a 5LL6-dependent ER export signal in the N-terminal tail of αβ phosphotransferase (20). This was based on the finding that the level of mature β subunit was decreased relative to the αβ precursor, as detected by Western blotting and the accumulation of the mutant in the ER by immunofluorescence microscopy. The study did not include pulse-chase kinetic experiments to analyze the trafficking and turnover of the K4Q mutant. Moreover, the experiments used a 2–4-h preincubation with cycloheximide. Because our data show a significant reduction in the half-life of the K4Q mutant, this block in protein synthesis would result in a substantial loss of the mature α and β subunits and could explain why the mutant was not detected in endosomes. Although our kinetic studies indicate that the K4Q mutant exits the ER at the same rate as WT precursor, the data are not sensitive enough to exclude the possibility that the ER exit of the K4Q and S15Y mutants might be somewhat delayed.
Interestingly, a recent study reported that MLIII αβ patients with the K4Q mutation share a specific phenotype, one that is intermediate between MLII and MLIII αβ (8). Both of these lysosomal storage disorders are caused by mutations in GNPTAB, but MLII patients have a greater loss of phosphotransferase activity, which results in a more severe phenotype. Patients with the intermediate disorder show the severe bone and skeletal abnormalities that are characteristic of MLII, but their clinical course is much milder and more slowly progressive, resembling MLIII αβ. It is possible that the degree of mislocalization of K4Q phosphotransferase varies per cell type, and therefore the level of phosphotransferase activity may be more or less reduced in certain cells, which could provide an explanation for this intermediate phenotype.
Disease-causing mutations in Golgi-resident enzymes have been described in congenital disorders of glycosylation and are known to affect the expression or activity of glycosyltransferases, but none of them cause mislocalization of a catalytically active enzyme. In contrast, mutations in subunits of the conserved oligomeric Golgi complex cause mislocalization of Golgi glycosyltransferases (21–23). However, the conserved oligomeric Golgi complex is a peripheral Golgi complex that acts as a tethering factor for retrograde vesicular transport, and in this case, the mislocalization of the glycosyltransferases is a secondary effect. In contrast to these findings, this report describes a disorder caused by mutations in a Golgi enzyme that directly results in its mislocalization from that organelle.
Materials and Methods
DNA Constructs.
GNPTAB-V5/His in pcDNA6 (kind gift from W. Canfield, Genzyme, Cambridge, MA; see ref. 24) was modified by quick-change site-directed mutagenesis (Stratagene) to generate K4Q, S15Y, and S399F phosphotransferase αβ subunits. See SI Materials and Methods for the primer sequences. The full sequences were confirmed by DNA sequencing.
Phosphotransferase Activity Assays.
HEK293 cells were cultured in DMEM (Cellgro) containing sodium pyruvate and 4.5 g/L glucose, supplemented with 10% (vol/vol) FBS ( Atlanta Biologicals), 100,000 U penicillin/streptomycin (Life Technologies), and 2 mM l-glutamine (Cellgro). The cells were transfected at 50–60% confluency in six-well plates with 1–2 μg DNA and 8 μL GeneCellin transfection reagent (BioCellChallenge) according to the manufacturer's protocol. Two days after transfection, the cells were lysed in 1% Triton (T) X-100/PBS containing a protease inhibitor mixture (Complete; Roche Diagnostics GmbH). One hundred micrograms of protein were assayed in 50 mM Tris at pH 7.4, 10 mM MgCl2, 10 mM MnCl2, 2 mg/mL BSA, 2 mM ATP, 75 μM UDP-GlcNAc, and 1 μCi UDP-[3H]GlcNAc with 100 mM α-MM. The reactions were performed at 37 °C for 1 h, after which 1 mL of 2 mM Tris buffer at pH 8.0 was added. The samples were immediately applied to QAE-Sephadex columns (1 mL) that were equilibrated with the same buffer without disturbing the column. The columns were washed twice with 2 mL and once with 1 mL 2 mM Tris at pH 8.0 and eluted with 30 mM NaCl in 2 mM Tris two times with 2 mL and one time with 1 mL. The radioactivity in all fractions was measured by scintillation counting. The values obtained with mock-transfected cell lysates were subtracted from the values obtained with cell lysates transfected with WT or the mutants to correct for endogenous enzyme activity and nonspecific background counts.
To determine the activity in the culture media, the cells were washed and then incubated with 600 μL DMEM ∼30 h after transfection. After 15 h, the media were collected and centrifuged at 6,000 × g for 5 min, and subsequently at 25,000 × g for 10 min, to remove cells and debris. Eighty-one microliters medium were assayed in duplicate, incubating for 4 h at 37 °C.
To determine the relative activity of the mutants, 15 μg cell lysate in Laemmli sample buffer containing 100 mM DTT was subjected to SDS/PAGE, using a NuPAGE 4–12% Bis-Tris gel and NuPAGE Mops SDS running buffer (Life Technologies). The proteins were transferred to a 0.2-μm protran BA83 nitrocellulose membrane (Whatman GmbH) and probed with mouse anti-V5 antibody (dilution 1:1,000, Life Technologies) and sheep anti-mouse IgG Horseradish peroxidase linked whole antibody (GE Healthcare U.K. Limited) and analyzed by chemiluminescence (Immobilon Western Chemiluminescent HRP substrate, Millipore). Densities were measured with ImageJ.
Immunofluorescence Microscopy.
Confocal immunofluorescence microscopy was performed as described in ref. 24. See the SI Materials and Methods for more details. To mark early and recycling endosomes, HeLa cells were incubated with 50 μg/mL transferrin from human serum conjugated to Alexa Fluor 488 (Life Technologies) for 30 min at 37 °C. The cells were washed 4 times with PBS, fixed with 4% (wt/vol) paraformaldehyde/PBS, and stained for immunofluorescence microscopy.
When indicated, transfected HeLa cells were treated with 0.1 μM bafilomycin a1 for 4 h at 37 °C. After fixation, the cells were immediately stained as described. The cells were analyzed under a Zeiss LSM 510 Meta confocal microscope using a 63× objective with N.A. 1.4.
Analysis of Sugar Modifications.
HEK293 cell lysates (15 μg protein) expressing WT, K4Q, or S399F phosphotransferase were boiled for 10 min in glycoprotein denaturing buffer (New England Biolabs). The lysates were treated with 100 U neuraminidase in G1 buffer, 3,000 U Endo Hf in G5 buffer, or 750 U PNGase F in G7 buffer with 1% Nonidet P-40 (New England Biolabs). After incubation for 3 h at 37 °C, the samples were boiled in Laemmli sample buffer containing DTT, and the nontreated lysates were immediately boiled in G5 buffer and Laemmli sample buffer with DTT. All samples were subjected to SDS/PAGE (4–12% Bis-Tris gel) and immunoblotting, as described.
[35S]methionine Pulse-Chase.
On the day of transfection, HEK293 cells were transferred to six-well plates that had been coated with 0.5 mL 0.01% poly-l-lysine (Sigma) for 5 min, washed twice with H2O, and dried overnight. The cells were allowed to adhere for at least 3 h, after which they were transfected at 50% confluency with 1 μg DNA and Lipofectamine Plus reagent (Life Technologies), according to the manufacturer's protocol. After overnight transfection, the cells were washed with PBS, and each well was incubated with 1 mL methionine/cysteine-free DMEM (Sigma) containing 10% (vol/vol) dialyzed FBS and ∼170 μCi TRAN 35S-LABEL methionine/cysteine (MP Biomedicals). After a 20-min pulse, the media were removed and the cells were washed two times with PBS and either collected immediately or incubated for the indicated time in regular DMEM containing 10% (vol/vol) FBS (chase). When indicated, the chase was performed in the presence of 0.1 μM bafilomycin a1 (Sigma). The cells were lysed in 0.1 M Tris at pH 8.0, 0.15 M NaCl, 1% TX-100 (IP buffer) containing protease inhibitors, and phosphotransferase was immunoprecipitated by overnight incubation at 4 °C while tumbling with 2 μg mouse anti-V5 antibody (Life Technologies) coupled with 60 μL protein G agarose beads (Pierce) or with 4 μL rabbit anti-α antibody coupled to protein A agarose (RepliGen Corporation). The next day, the beads were washed once with cold IP buffer, 3 times with 0.1 M Tris at pH 8.0, 1 M NaCl, 1% TX-100, 2 mM EDTA, and one more time with IP buffer, after which the beads were boiled at 100 °C for 10 min in Laemmli sample buffer with DTT or, when treated with PNGase F, in glycoprotein denaturing buffer (New England Biolabs), and subsequently incubated with 750 U PNGase F for 3 h at 37 °C. The samples were resolved by SDS/PAGE on a 10% Bis-Tris gel, which was fixed with 10% (vol/vol) acetic acid and 30% (vol/vol) methanol for 15 min, incubated with an amplification solution (Amplify, GE Healthcare) for 10 min, and subsequently dried. The proteins were detected by autoradiography.
Inhibition of Lysosomal Degradation.
Transfected HEK293 cells were trypsinized the day after transfection and divided equally over two 6-well plates. For each construct, one well was treated either with 100 μM leupeptin (Sigma) and 100 μM pepstatin A (Sigma) or with 0.1 μM bafilomycin a1 (Sigma), and to the untreated well, DMSO was added. The cells were lysed after 7 h incubation at 37 °C, and 20–25 μg protein was subjected to SDS/PAGE and immunoblotting. To confirm equal protein loading, the blots were probed with mouse anti-GAPDH at dilution 1:10,000 (Sigma).
Phosphotransferase in Media.
Transfected HEK293 cells in poly-l-lysine-coated six-well plates were washed with PBS and incubated with 600 μL serum-free DMEM ∼44 h after transfection. After 6 h, the media were collected and centrifuged at 6,000 × g for 5 min, and subsequently at 25,000 × g for 10 min. The cells were washed with PBS and lysed in 1% TX-100/PBS containing protease inhibitors. The samples were subjected to SDS/PAGE on a 4–12% Bis-Tris gel and probed with anti-α subunit antibody (dilution 1:4,000) and donkey anti-rabbit IgG Horseradish peroxidase linked whole antibody (GE Healthcare U.K. Limited) for immunoblotting.
Supplementary Material
Acknowledgments
We thank our colleagues for helpful discussions and Dr. Balraj Doray for the affinity purification of the anti-α subunit antibody and providing the ΔCT αβ construct. This work was supported by National Institutes of Health Grant CA-008759-44.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1401417111/-/DCSupplemental.
References
- 1. Braulke T, Raas-Rothschild A, Kornfeld S (2013) The Online Metabolic & Molecular Bases of Inherited Disease, Chapter 138, eds Valle D, et al. (McGraw-Hill, New York)
- 2.Bao M, Booth JL, Elmendorf BJ, Canfield WM. Bovine UDP-N-acetylglucosamine:lysosomal-enzyme N-acetylglucosamine-1-phosphotransferase. I. Purification and subunit structure. J Biol Chem. 1996;271(49):31437–31445. doi: 10.1074/jbc.271.49.31437. [DOI] [PubMed] [Google Scholar]
- 3.Kudo M, et al. The alpha- and beta-subunits of the human UDP-N-acetylglucosamine:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase [corrected] are encoded by a single cDNA. J Biol Chem. 2005;280(43):36141–36149. doi: 10.1074/jbc.M509008200. [DOI] [PubMed] [Google Scholar]
- 4.Marschner K, Kollmann K, Schweizer M, Braulke T, Pohl S. A key enzyme in the biogenesis of lysosomes is a protease that regulates cholesterol metabolism. Science. 2011;333(6038):87–90. doi: 10.1126/science.1205677. [DOI] [PubMed] [Google Scholar]
- 5.Qian Y, et al. Functions of the alpha, beta, and gamma subunits of UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase. J Biol Chem. 2010;285(5):3360–3370. doi: 10.1074/jbc.M109.068650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cathey SS, et al. Phenotype and genotype in mucolipidoses II and III alpha/beta: A study of 61 probands. J Med Genet. 2010;47(1):38–48. doi: 10.1136/jmg.2009.067736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kudo M, Brem MS, Canfield WM. Mucolipidosis II (I-cell disease) and mucolipidosis IIIA (classical pseudo-hurler polydystrophy) are caused by mutations in the GlcNAc-phosphotransferase alpha / beta -subunits precursor gene. Am J Hum Genet. 2006;78(3):451–463. doi: 10.1086/500849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leroy JG, Sillence D, Wood T, et al. (2013) A novel intermediate mucolipidosis II/IIIalphabeta caused by GNPTAB mutation in the cytosolic N-terminal domain. Eur J Hum Genet, 10.1038/ejhg.2013.207. [DOI] [PMC free article] [PubMed]
- 9.Bargal R, et al. When Mucolipidosis III meets Mucolipidosis II: GNPTA gene mutations in 24 patients. Mol Genet Metab. 2006;88(4):359–363. doi: 10.1016/j.ymgme.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 10.De Pace R, et al. Mucolipidosis II-related mutations inhibit the exit from the endoplasmic reticulum and proteolytic cleavage of GlcNAc-1-phosphotransferase precursor protein (GNPTAB) Hum Mutat. 2013 doi: 10.1002/humu.22502. [DOI] [PubMed] [Google Scholar]
- 11.Robinson DG, Albrecht S, Moriysu Y. The V-ATPase inhibitors concanamycin A and bafilomycin A lead to Golgi swelling in tobacco BY-2 cells. Protoplasma. 2004;224(3-4):255–260. doi: 10.1007/s00709-004-0070-6. [DOI] [PubMed] [Google Scholar]
- 12.Tawfeek HA, Abou-Samra AB. Important role for the V-type H(+)-ATPase and the Golgi apparatus in the recycling of PTH/PTHrP receptor. Am J Physiol Endocrinol Metab. 2004;286(5):E704–E710. doi: 10.1152/ajpendo.00404.2003. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan HA, Woloski BM, Hellman M, Jamieson JC. Studies on the effect of inflammation on rat liver and serum sialyltransferase. Evidence that inflammation causes release of Gal beta 1 leads to 4GlcNAc alpha 2 leads to 6 sialyltransferase from liver. J Biol Chem. 1983;258(19):11505–11509. [PubMed] [Google Scholar]
- 14.Glick BS, Luini A. Models for Golgi traffic: A critical assessment. Cold Spring Harb Perspect Biol. 2011;3(11):a005215. doi: 10.1101/cshperspect.a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu L, Banfield DK. Localization of Golgi-resident glycosyltransferases. Cell Mol Life Sci. 2010;67(1):29–41. doi: 10.1007/s00018-009-0126-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tu L, Tai WC, Chen L, Banfield DK. Signal-mediated dynamic retention of glycosyltransferases in the Golgi. Science. 2008;321(5887):404–407. doi: 10.1126/science.1159411. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz KR, et al. Golgi localization of glycosyltransferases requires a Vps74p oligomer. Dev Cell. 2008;14(4):523–534. doi: 10.1016/j.devcel.2008.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ali MF, Chachadi VB, Petrosyan A, Cheng PW. Golgi phosphoprotein 3 determines cell binding properties under dynamic flow by controlling Golgi localization of core 2 N-acetylglucosaminyltransferase 1. J Biol Chem. 2012;287(47):39564–39577. doi: 10.1074/jbc.M112.346528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang WL, et al. The Drosophila GOLPH3 homolog regulates the biosynthesis of heparan sulfate proteoglycans by modulating the retrograde trafficking of exostosins. Development. 2013;140(13):2798–2807. doi: 10.1242/dev.087171. [DOI] [PubMed] [Google Scholar]
- 20.Franke M, Braulke T, Storch S. Transport of the GlcNAc-1-phosphotransferase α/β-subunit precursor protein to the Golgi apparatus requires a combinatorial sorting motif. J Biol Chem. 2013;288(2):1238–1249. doi: 10.1074/jbc.M112.407676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Foulquier F, et al. A new inborn error of glycosylation due to a Cog8 deficiency reveals a critical role for the Cog1-Cog8 interaction in COG complex formation. Hum Mol Genet. 2007;16(7):717–730. doi: 10.1093/hmg/ddl476. [DOI] [PubMed] [Google Scholar]
- 22.Foulquier F, et al. Conserved oligomeric Golgi complex subunit 1 deficiency reveals a previously uncharacterized congenital disorder of glycosylation type II. Proc Natl Acad Sci USA. 2006;103(10):3764–3769. doi: 10.1073/pnas.0507685103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu X, et al. Mutation of the COG complex subunit gene COG7 causes a lethal congenital disorder. Nat Med. 2004;10(5):518–523. doi: 10.1038/nm1041. [DOI] [PubMed] [Google Scholar]
- 24.Qian Y, Flanagan-Steet H, van Meel E, Steet R, Kornfeld SA. The DMAP interaction domain of UDP-GlcNAc:lysosomal enzyme N-acetylglucosamine-1-phosphotransferase is a substrate recognition module. Proc Natl Acad Sci USA. 2013;110(25):10246–10251. doi: 10.1073/pnas.1308453110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.